Abstract
Purpose:
To compare the diagnostic ability of the ganglion cell analysis (GCA) and retinal nerve fiber layer (RNFL) protocol on optical coherence tomography (OCT), to diagnose preperimetric glaucoma.
Methods:
A prospective, cross-sectional study of 275 adult patients including 47 early glaucoma (mean deviation better than -6.0 D), 150 glaucoma suspects (106 with suspicious discs and 44 ocular hypertensive (OHT), and 78 normal controls was done. Eligible participants were scanned with the spectral domain Cirrus™ OCT (Carl Zeiss Meditec, Dublin, CA). Average peripapillary RNFL thickness and GCA measurements were obtained. Area under receiver operating characteristic (AROC) curves were used to evaluate discriminant value of both protocols to diagnose likely preperimetric glaucoma among glaucoma suspects.
Results:
Average RNFL and GCA were significantly thinner in glaucoma patients compared to glaucoma suspects and normal controls (P < 0.001). The RNFL was 92.26 ± 8.8 μ in normal controls, 87.9 ± 12.12 μ in glaucoma suspects and significantly thinner in POAG (70.29 ± 10.18 μ; P < 0.001). The GCA was 81.94 ± 6.17 μ in normal controls, 77.69 ± 9.03 μ in glaucoma suspects, and significantly thinner in POAG (69.36 ± 11.06 μ; P < 0.001). AROCs for discriminating glaucoma suspects from normal were modest, with no difference in AROC of average RNFL or GCA measurements (DeLong; P = 0.93). Average RNFL thickness had significantly greater AROC values than average GCA for discriminating glaucoma suspects (both suspicious discs and OHT) from glaucoma (P = 0.03 and 0.05, respectively. AROC for diagnosing glaucoma was significantly better (P = 0.02) for RNFL (0.88 ± 0.03) than GCA (0.77 ± 0.04).
Conclusion:
In the present time, GCA measurements, as provided by the SD-OCT, do not appear to outperform RNFL measurements in the diagnosis of preperimetric glaucoma.
Keywords: Ganglion cell analysis, preperimetric glaucoma, retinal nerve fiber layer thickness
Glaucoma is a progressive optic neuropathy which can result in irreversible blindness. A major challenge facing treating physicians is the early diagnosis of the disease and prevention of its progression. The main pathologic change in glaucoma is retinal ganglion cell (RGC) loss. This results in atrophy of all related inner retinal layers,[1,2] namely, retinal nerve fiber layer (RNFL) which are axons of ganglion cells; ganglion cell layer (GCL) which has the body of ganglion cells; and inner plexiform layer (IPL) having the dendrites of ganglion cells. The macula has the greatest density of RGCs (approx 50%).[1,2] Since the introduction of optical coherence tomography (OCT), objective quantitative analysis of RNFL thickness has become a standard tool for the detection of early glaucoma.[3,4,5,6] Good correlation has been reported between RNFL thickness attenuation and visual field loss.[7,8,9,10] Macular thickness measurements with the Stratus OCT did not were not found to be superior to RNFL measurements.[11,12,13]
The advent of spectral domain OCT (SD-OCT) technology has allowed advanced macular imaging protocols to play an important role in the diagnosis and monitoring of glaucoma.[14,15,16,17] The ganglion cell analysis (GCA) obtained by the Cirrus HD-OCT® system (Zeiss) segments and measures the thickness of the ganglion cell-IPL (GC-IPL), thereby potentially increasing its diagnostic accuracy compared to conventional peripapillary RNFL thickness measurement.
Published studies have reported comparable values for Area under Receiver Operating Characteristic Curves (AROCs) of GCA and RNFL thickness for discriminating early glaucoma from normal.[14,15,16,17] Once there is a visual field defect, such a differentiation is not difficult. The challenge comes in differentiating a glaucoma suspect from normal, in the presence of normal visual fields.
If we can demonstrate significantly lesser number of RGCs in this group of patients, it may result in earlier intervention in preperimetric disease before functional loss sets in. Although RNFL thickness measurement has proven to be useful in this regard, since RNFL loss is preceded by RGC death, measurement of this layer seems logical to detect very early disease. The macula contains 50% of RGCs in the retina and therefore measuring macular GCL appears to be a good approach to assess ganglion cell death.
In this study, we evaluated the diagnostic accuracy of the GCA analysis by Cirrus OCT, compared to RNFL thickness measurements, for differentiating glaucoma suspects from normal controls, and those with early glaucoma.
Methods
This prospective, observational, cross-sectional study, included adult patients (>18 years) who were glaucoma suspects (including those with suspicious optic discs and ocular hypertensives [OHT]), early primary open-angle glaucoma (POAG), and normal controls. One eye of each individual was prospectively enrolled. Informed consent was taken from all participants, and the study complied by the principles of the Declaration of Helsinki. Ethical clearance was accorded by the Institute Ethics Committee of the Postgraduate Institute of Medical Education and Research (vide notification No. 71/6-Edu/14/1526).
Each enrolled participant underwent a comprehensive ophthalmic examination including best-corrected visual acuity (BCVA), intraocular pressure (IOP) measured by Goldmann applanation tonometry, slit-lamp biomicroscopy, gonioscopy, and stereoscopic fundus evaluation on the slit lamp using a 90.0-D lens. Color stereoscopic optic disc photographs and red-free nerve fiber layer photographs were taken on the Zeiss Fundus camera FF 450 with Visupac System 451 (Carl Zeiss Ophthalmic Systems, Jena, GmBH, Germany). Optic discs were assessed by 2 graders independently, who were masked to the patients' identity and other examination results. The graders were ophthalmologists, any 2 of the 3 consultants (SK, SR, and SSP). Both had to classify the disc as suspicious to be included in the study. The patients were initially examined clinically, and then, the optic disc findings were confirmed on disc photography.
All individuals underwent standard achromatic perimetry on the Humphrey's Field Analyzer 750 II (Carl Zeiss-Humphrey Systems, Dublin, CA), using the 24-2 testing protocol by SITA-Standard strategy.
Inclusion criteria
This study included glaucoma suspects, OHTs, early glaucoma patients having media clarity better than or equal to 6/12 view of the fundus, and normal controls. The criteria for diagnoses in each group were as follows:
Glaucoma suspects with optic disc suspicious for glaucoma were defined as those having all of the following features:
BCVA 20/40 or more (refractive error ± 5.0D spherical; ± 3.0D cylinder)
IOP <22 mmHg on at least 2 successive measurements spaced 2 weeks apart at approximately the same time of day
Open angles on gonioscopy
Optic disc suspicious for glaucoma defined as having features suggestive of glaucomatous optic neuropathy such as cup-disc ratio >0.6, any diffuse or focal neuroretinal rim thinning, any disc hemorrhage, and/or any RNFL defects on the red-free photograph
Normal visual fields defined as that with a mean deviation (MD) and pattern standard deviation (PSD) values within 95% normal confidence limits and a glaucoma hemifield test (GHT) classified as “within normal limits.”
OHT had to fulfill all of the following criteria in both eyes:
BCVA 20/40 or more (refractive error ± 5.0D spherical and ± 3.0D cylinder)
IOP >22 mmHg and <32 mmHg on at least 2 successive measurements spaced 2 weeks apart at approximately the same time of day
Open angles on gonioscopy and normal appearing optic disc
Normal visual fields as defined above
A normal appearing optic disc was defined as one with no features suggestive of glaucomatous optic neuropathy as defined above. Patients were included only when both observers classify the disc as “normal.”
POAG patients had to fulfill the same visual acuity and gonioscopy features as OHT in addition to
Glaucomatous optic neuropathy
Repeatable (2 consecutive) abnormal visual field tests defined as PSD outside 95% normal confidence limits and a GHT classified as ''outside normal limits.''
Only patients with early glaucoma were included, which was defined as those with MD better than − 6.00 dB; <25% of the points in total deviation plot had P = 5% and <10 points had a P = 1%; no point in the central 5° had a sensitivity <15 dB.
Normal participants were defined as those with no history of ocular or neurologic or systemic disease that might interfere with test results (e.g., diabetic retinopathy, uveitis, significant cataract, etc.), IOP ≤21 mmHg, BCVA of 20/40 or more, open angles on gonioscopy, normal optic discs, and normal visual fields.
Exclusion criteria
Patients with media opacity precluding good-quality OCT scans such as corneal opacity, cataracts, etc., history of intraocular diseases, complicated intraocular surgery, nonglaucomatous secondary causes of elevated IOP, (for example, iridocyclitis and trauma), coexisting retinal disease (for example, diabetic retinopathy), other diseases affecting visual field (for example, pituitary lesions, demyelinating diseases, HIV positive or AIDS, or diabetic retinopathy), with medications known to affect visual field sensitivity or with problems other than glaucoma affecting color vision, were excluded from this study.
Patients with moderate or advanced glaucoma (MD worse than -6 dB) were excluded from the study.
Instrumentation
All included individuals were scanned with the SD Cirrus™ OCT (Carl Zeiss Meditec, Dublin, CA; software version-3.0.0.64). All OCT scans were acquired by an experienced OCT operator. The investigators measuring the OCT parameters were masked to the patients' diagnosis.
Optical coherence tomography scanning
After dilating the pupils, the individual was seated with his/her chin in a chin rest and the machine properly aligned. The individual was instructed to fixate with the eye being measured on the internal fixation target to bring the optic nerve head within view of the examiner real-time. The Z-offset was adjusted to bring the OCT image into view.
Scanning Protocols
For RNFL thickness measurements: The optic disc 200 × 200 scan was used to acquire a cube of side 6 mm, while the patient was fixated so that the optic disc was near the center of the scan. Each optic disc scan captures a 6 mm × 6 mm × 2-mm “cube” of data consisting of 200 A-scans from 200 linear B-scans (40,000 points) in ~1.5 s (27,000 A-scans/sec). Cirrus OCT® extracts the RNFL thickness values in a circle centered on the optic disc. The machine does not depend on the operator correctly placing the scan reproducibly because it includes an automated graph-based algorithm (AutoCenter™) that identifies the center and border of the optic disc in peripapillary images. This allows the RNFL thickness to be measured at the same location each time
For macular GCA: The macular cube 200 × 200 protocol was used for GCA. The GCL is thickest in the perimacular region and decreased total macular thickness has been observed in glaucomatous eyes likely due to thinning of the GCL in this region. However, since segmenting the GCL alone is very difficult based on reflectivity alone. In the Cirrus OCT, GCA consists of the combined GCL and IPL. GCC images are obtained as an elliptical annulus (area 14.13 mm2) centered over the macula.
Criteria for acceptable scans
To be acceptable for inclusion, the OCT scans had to fulfill the following criteria: The fundus image must have been clear enough to see the optic disc and scan circle or spokes, the scan must have been properly centered on the optic disc or macula, respectively, the signal strength had to have been >6, color saturation must have been even and dense across the entire scan. Care was taken to ensure no missing areas in the scan due to blinks or eye motion.
Statistical methods
The results were analyzed using the SPSS for Windows software, Version 10.0, ©SPSS Inc., Chicago, US. Descriptive statistics were computed for all the variables measured. Patient characteristics were compared among all 3 groups using ANOVA with post hoc Bonferroni correction. The best modality for discriminating glaucoma suspects from POAG and normal controls was evaluated by calculating areas under receiver operating characteristic curves (AROCs). AROCs of GCA and RNFL parameters in each group were compared to each other by the method of DeLong et al. Results were considered statistically significant at P < 0.05.
Results
Forty-seven patients with early glaucoma, 150 glaucoma suspects (106 with suspicious discs and 44 OHT), and 78 normal controls were included. Descriptive characteristics are shown in Table 1. Patients with glaucoma were significantly older than normal controls and those with suspicious discs (P< 0.001) but were similar in age to OHT (P = 0.21). The IOP in OHT was significantly greater than all other groups (P< 0.001). The central cornea was significantly thicker in OHT compared to glaucoma (P = 0.024) and suspicious discs (0.036).
Table 1.
Characteristics of all study participants
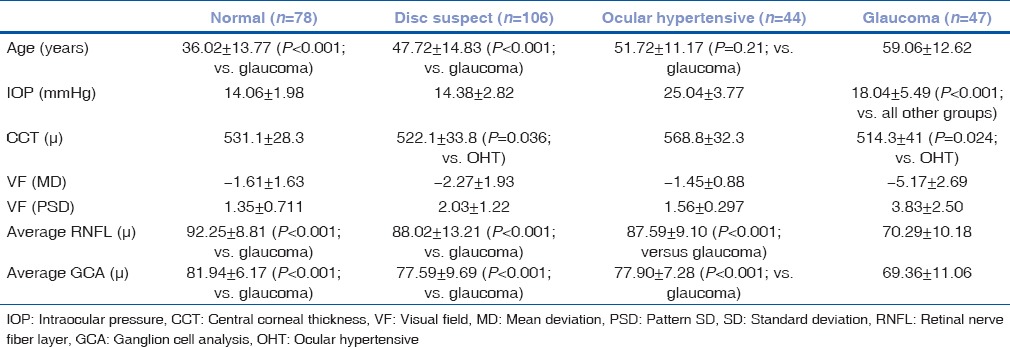
The average RNFL was significantly thinner in glaucoma patients compared to OHTs (P< 0.001), those with suspicious discs (P< 0.001), and normal controls (P< 0.001). There was no difference in RNFL thickness between normal controls and glaucoma suspects. The average GCA was significantly thinner in early glaucoma patients compared with both OHT and suspicious discs (P< 001). There was no significant difference of GCA between OHT and suspicious discs (P = 1.00), suspicious discs and normal (P = 0.76), and OHT versus normal (P = 0.56).
Area under receiver operating curves for average RNFL and Average GCA measurements were calculated to determine the best discriminator between glaucoma suspects and normal controls and glaucoma suspects and glaucoma [Table 2]. AROCs for discriminating glaucoma suspects from normal were modest, with no difference in AROC of average RNFL or GCA measurements [Fig. 1]. Average RNFL thickness had significantly greater AROC values than average GCA for discriminating glaucoma suspects (both suspicious discs and OHT) from glaucoma [P = 0.03 and 0.05, respectively, [Fig. 2].
Table 2.
Area under receiver operating characteristic values of ganglion cell analysis and retinal nerve fiber layer thickness between different groups
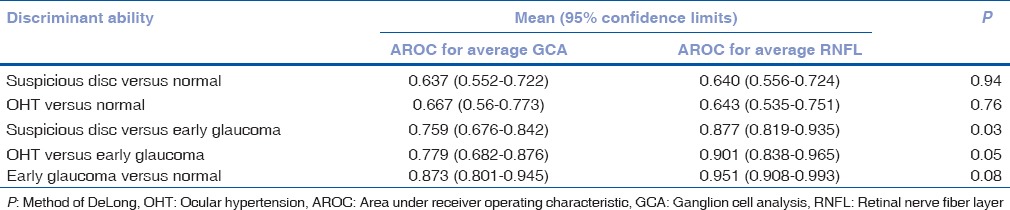
Figure 1.
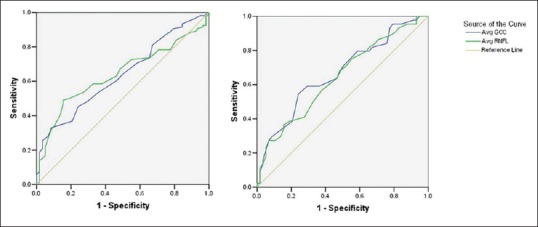
Area under receiver operating characteristic curves (AROC) for discriminating between glaucoma suspects and normal controls. (Left: AROC for suspicious discs; Right: AROC for ocular hypertensive)
Figure 2.
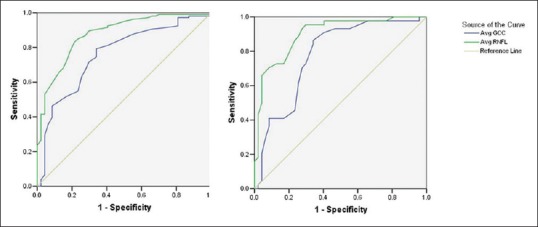
Area under receiver operating characteristic curves (AROC) for discriminating between glaucoma suspects and glaucoma patients. (Left: AROC for suspicious discs; Right: AROC for ocular hypertensive)
Average RNFL thickness measurements also outperformed GCA in discriminating early glaucoma from normal controls [P = 0.08; Fig. 3].
Figure 3.
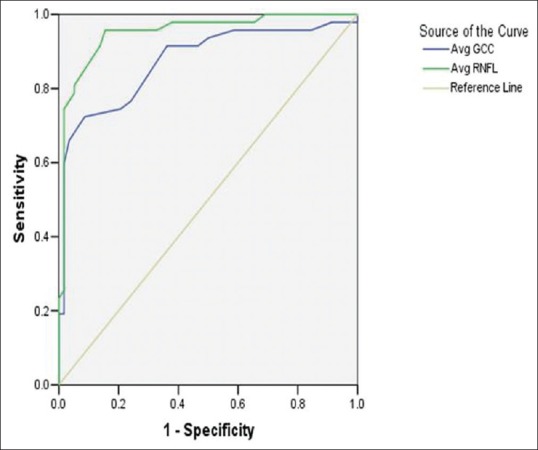
Area under receiver operating characteristic curves (AROC) for discriminating between glaucoma patients and normal controls
Discussion
Glaucoma typically goes through several stages, from clinically nonapparent disease to irreversible blindness.[18,19] The diagnosis of glaucoma can often be difficult, especially in the very early stages when structural damage and functional changes are not obvious. Often the diagnosis in early stages involves a constellation of signs and risk factors. This has the potential both for missed diagnosis leading to failure to treat glaucoma or a waste of expensive and potentially harmful treatment on individuals who do not have glaucoma. This has led to the development of newer diagnostic modalities to reliably diagnose the disease as early as possible.
Glaucoma being caused due to death of RGCs, and RGCs being concentrated at the macula have led investigators to explore the possibility of using macular thickness as a useful discriminator for glaucoma.[20] Although the GCA and RNFL are measured at different locations of the retina, since the macula contains 50% of RGCs in the retina, measuring macular GCL appears to be a good approach to assess ganglion cell death. The RNFL measured around the disc are axons of ganglion cells which converge on the optic disc before leaving the eye. Measuring RGC elsewhere in the retina might be fallacious as the GCL elsewhere is thin and spread out. It is unlikely, that with the present instrumentation, any meaningful measurements could have been obtained.
The Cirrus OCT GCA protocol automatically segments the ganglion cell–IPL (GC-IPL) from the remaining retinal layers and measures it within an elliptical annulus 14.13 mm,[2] centered on the fovea, which corresponds to the area of thickest RGC density in nonglaucomatous eyes. Good reproducibility of macular ganglion cell measurements determined by OCT has been reported,[21,22,23] and has been suggested as a potential alternative to RNFL thickness assessment to explore structural changes in patients with glaucoma. However, earlier reports found that macular thickness evaluation did not outperform RNFL thickness assessment in terms of glaucoma detection.[24,25,26]
The few studies that have evaluated GCA for preperimetric glaucoma diagnosis have reported comparable diagnostic capability to that of RNFL thickness measurements.[27,28] Kim et al.[26] reported that the inner directional angle of RNFL defects affected the diagnostic sensitivity of macular ganglion cell-inner plexiform layer parameters in their study. Karti et al.[28] reported significantly lower RNFL and ganglion cell complex measurements in normal looking discs of individuals who had a history of POAG in their first-degree relatives, compared to individuals without a family history. Jung et al.,[29] in a structure-function relationship study, reported that preperimetric glaucoma patients with structural loss in the macula also had functional loss revealed by 10-2 short-wavelength automated perimetry, even though 24-2 visual fields were normal. However, they did not measure RNFL thickness in their study, which makes it unclear whether the macular GCA analysis was better than conventional structural testing.
We looked at the utility of using GCA for early diagnosis of glaucoma suspects compared to the discriminating ability of RNFL thickness. Our results indicate moderate AROC for discriminating between glaucoma suspects and normals and high AROC for discriminating between glaucoma suspects and early glaucoma. However, average RNFL thickness measurements outperformed average GCA measurements in our study.
One reason for this could be in the inherent pathophysiology of glaucomatous RGC loss. The spatial distribution of different ganglion cells in the retina[30,31] is such that predominantly large cells which map to the magnocellular region of the lateral geniculate body (LGB) are designated “M” cells and are found mainly outside the central foveal region. A far greater number of smaller cells which map to the parvocellular region of the LGB are designated “P” cells, represent the majority of RGCs, and are distributed mainly in the parafoveal area. Earlier reports have confirmed the selective loss of large-diameter “M” RGCs in early glaucoma.[1,32] This has been supported by selective loss of predominantly larger axons in early glaucoma, and a significantly lower magnocellular cell density in the LGB, compared to parvocellular layers.[33]
Although RGCs are maximally concentrated at the macula, they are predominantly the smaller “P” cells, and if the larger “M” cells are lost in early glaucoma, it can be understood why GCA by OCT may not detect very early glaucoma. Our results suggest that RNFL thickness measurements may, in fact, be a better indicator of very early RGC loss since it takes into account average thickness of all axons converging on the optic nerve and is more likely to include axons subserving the M cells also.
Indeed, recent reports of the utility of macular GCC analysis for advanced glaucoma[34] support the postulation that this modality may not be a good tool for preperimetric glaucoma assessment. The papillomacular bundle is known to be resistant to glaucomatous structural damage and usually remains intact until the final stages of the disease are attained. In patients with moderate-to-advanced glaucoma, detection of changes in the macular ganglion cell area may be more useful for evaluation of patients with advanced glaucoma.
As for all technology, the GCA analysis also needs to be interpreted with caution, keeping the clinical setting in mind. In a cohort of healthy eyes, Kim et al.[35] reported abnormal diagnostic classifications in 40.4% and 30.8% on GCA and RNFL maps, respectively, especially in eyes with long axial lengths, large fovea-disc angles, and small optic discs. It is also important to keep in mind that conditions such as diabetic macular edema and age-related macular degeneration, which are common comorbidities in the age group of glaucoma patients, may affect the macular RGC thickness. Even after excluding such patients from our study, we found no significant advantage of macular GCA over RNFL thickness. In the clinical situation, one must likely be even more cautious before relying on macular GCA for preperimetric glaucoma diagnosis.
Conclusion
In the present time, the GCA measurements do not appear to outperform average RNFL measurements to discriminate between glaucoma suspects and established early glaucoma or normal controls. It appears to have more of a role in monitoring progression of established glaucoma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107:453–64. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 2.Quigley HA, Miller NR, George T. Clinical evaluation of nerve fiber layer atrophy as an indicator of glaucomatous optic nerve damage. Arch Ophthalmol. 1980;98:1564–71. doi: 10.1001/archopht.1980.01020040416003. [DOI] [PubMed] [Google Scholar]
- 3.Kanamori A, Nakamura M, Escano MF, Seya R, Maeda H, Negi A, et al. Evaluation of the glaucomatous damage on retinal nerve fiber layer thickness measured by optical coherence tomography. Am J Ophthalmol. 2003;135:513–20. doi: 10.1016/s0002-9394(02)02003-2. [DOI] [PubMed] [Google Scholar]
- 4.Sihota R, Sony P, Gupta V, Dada T, Singh R. Diagnostic capability of optical coherence tomography in evaluating the degree of glaucomatous retinal nerve fiber damage. J Glaucoma. 2007;16:1–8. doi: 10.1167/iovs.05-1102. [DOI] [PubMed] [Google Scholar]
- 5.Anton A, Moreno-Montanes J, Blaquez F, Alvarez A, Martin B, Molina B, et al. Usefulness of optical coherence tomography parameters of the optic disc and the retinal nerve fiber layer to differentiate glaucomatous, ocular hypertensive, and normal eyes. Invest Ophthalmol Vis Sci. 2006;47:2006–10. doi: 10.1097/01.ijg.0000212215.12180.19. [DOI] [PubMed] [Google Scholar]
- 6.Badalà F, Nouri-Mahdavi K, Raoof DA, Leeprechanon N, Law SK, Caprioli J, et al. Optic disk and nerve fiber layer imaging to detect glaucoma. Am J Ophthalmol. 2007;144:724–32. doi: 10.1016/j.ajo.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ajtony C, Balla Z, Somoskeoy S, Kovacs B. Relationship between visual field sensitivity and retinal nerve fiber layer thickness as measured by optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48:258–63. doi: 10.1167/iovs.06-0410. [DOI] [PubMed] [Google Scholar]
- 8.Cvenkel B, Kontestabile AS. Correlation between nerve fibre layer thickness measured with spectral domain OCT and visual field in patients with different stages of glaucoma. Graefes Arch Clin Exp Ophthalmol. 2011;249:575–84. doi: 10.1007/s00417-010-1538-z. [DOI] [PubMed] [Google Scholar]
- 9.Schlottmann PG, De Cilla S, Greenfield DS, Caprioli J, Garway-Heath DF. Relationship between visual field sensitivity and retinal nerve fiber layer thickness as measured by scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2004;45:1823–9. doi: 10.1167/iovs.03-0692. [DOI] [PubMed] [Google Scholar]
- 10.Leung CK, Chan WM, Chong KK, Yung WH, Tang KT, Woo J, et al. Comparative study of retinal nerve fiber layer measurement by StratusOCT and GDx VCC, I: Correlation analysis in glaucoma. Invest Ophthalmol Vis Sci. 2005;46:3214–20. doi: 10.1167/iovs.05-0294. [DOI] [PubMed] [Google Scholar]
- 11.Wollstein G, Schuman JS, Price LL, Aydin A, Beaton SA, Stark PC, et al. Optical coherence tomography (OCT) macular and peripapillary retinal nerve fiber layer measurements and automated visual fields. Am J Ophthalmol. 2004;138:218–25. doi: 10.1016/j.ajo.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Leung CK, Chan WM, Yung WH, Ng AC, Woo J, Tsang MK, et al. Comparison of macular and peripapillary measurements for the detection of glaucoma: An optical coherence tomography study. Ophthalmology. 2005;112:391–400. doi: 10.1016/j.ophtha.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Tan O, Chopra V, Lu AT, Schuman JS, Ishikawa H, Wollstein G, et al. Detection of macular ganglion cell loss in glaucoma by Fourier-Domain Optical Coherence Tomography. Ophthalmology. 2009;116:2305–140. doi: 10.1016/j.ophtha.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotowski J, Folio LS, Wollstein G, Ishikawa H, Ling Y, Bilonick RA, et al. Glaucoma discrimination of segmented cirrus spectral domain optical coherence tomography (SD-OCT) macular scans. Br J Ophthalmol. 2012;96:1420–5. doi: 10.1136/bjophthalmol-2011-301021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan O, Li G, Lu AT, Varma R, Huang D, et al. Advanced Imaging for Glaucoma Study Group. Mapping of macular substructures with optical coherence tomography for glaucoma diagnosis. Ophthalmology. 2008;115:949–56. doi: 10.1016/j.ophtha.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganekal S. Ganglion cell complex scan in the early prediction of glaucoma. Nepal J Ophthalmol. 2012;4:236–41. doi: 10.3126/nepjoph.v4i2.6538. [DOI] [PubMed] [Google Scholar]
- 17.Morooka S, Hangai M, Nukada M, Nakano N, Takayama K, Kimura Y, et al. Wide 3-dimensional macular ganglion cell complex imaging with spectral-domain optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci. 2012;53:4805–12. doi: 10.1167/iovs.12-9870. [DOI] [PubMed] [Google Scholar]
- 18.Weinreb RN, Friedman DS, Fechtner RD, Cioffi GA, Coleman AL, Girkin CA, et al. Risk assessment in the management of patients with ocular hypertension. Am J Ophthalmol. 2004;138:458–67. doi: 10.1016/j.ajo.2004.04.054. [DOI] [PubMed] [Google Scholar]
- 19.Medeiros FA, Zangwill LM, Bowd C, Vessani RM, Susanna R, Jr, Weinreb RN, et al. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol. 2005;139:44–55. doi: 10.1016/j.ajo.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa H, Stein DM, Wollstein G, Beaton S, Fujimoto JG, Schuman JS, et al. Macular segmentation with optical coherence tomography. Invest Ophthalmol Vis Sci. 2005;46:2012–7. doi: 10.1167/iovs.04-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearce JG, Maddess T. Inter-visit test-retest variability of OCT in glaucoma. Optom Vis Sci. 2017;94:404–10. doi: 10.1097/OPX.0000000000001022. [DOI] [PubMed] [Google Scholar]
- 22.Kim KE, Yoo BW, Jeoung JW, Park KH. Long-term reproducibility of macular ganglion cell analysis in clinically stable glaucoma patients. Invest Ophthalmol Vis Sci. 2015;56:4857–64. doi: 10.1167/iovs.14-16350. [DOI] [PubMed] [Google Scholar]
- 23.Kim MJ, Park KH, Yoo BW, Jeoung JW, Kim HC, Kim DM, et al. Comparison of macular GCIPL and peripapillary RNFL deviation maps for detection of glaucomatous eye with localized RNFL defect. Acta Ophthalmol. 2015;93:e22–8. doi: 10.1111/aos.12485. [DOI] [PubMed] [Google Scholar]
- 24.Hwang YH, Ahn SI, Ko SJ. Diagnostic ability of macular ganglion cell asymmetry for glaucoma. Clin Exp Ophthalmol. 2015;43:720–6. doi: 10.1111/ceo.12545. [DOI] [PubMed] [Google Scholar]
- 25.Jeoung JW, Choi YJ, Park KH, Kim DM. Macular ganglion cell imaging study: Glaucoma diagnostic accuracy of spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:4422–9. doi: 10.1167/iovs.12-11273. [DOI] [PubMed] [Google Scholar]
- 26.Kim MJ, Jeoung JW, Park KH, Choi YJ, Kim DM. Topographic profiles of retinal nerve fiber layer defects affect the diagnostic performance of macular scans in preperimetric glaucoma. Invest Ophthalmol Vis Sci. 2014;55:2079–87. doi: 10.1167/iovs.13-13506. [DOI] [PubMed] [Google Scholar]
- 27.Saha M, Bandyopadhyay S, Das D, Ghosh S. Comparative analysis of macular and peripapillary retinal nerve fiber layer thickness in normal, glaucoma suspect and glaucomatous eyes by optical coherence tomography. Nepal J Ophthalmol. 2016;8:110–8. doi: 10.3126/nepjoph.v8i2.16991. [DOI] [PubMed] [Google Scholar]
- 28.Karti O, Yuksel B, Uzunel UD, Karahan E, Zengin MO, Kusbeci T, et al. The assessment of optical coherence tomographic parameters in subjects with a positive family history of glaucoma. Clin Exp Optom. 2017;100:663–7. doi: 10.1111/cxo.12523. [DOI] [PubMed] [Google Scholar]
- 29.Jung Y, Park HY, Jeong HJ, Choi SY, Park CK. The ability of 10-2 short-wavelength perimetry in detecting functional loss of the macular area in preperimetric glaucoma patients. Invest Ophthalmol Vis Sci. 2015;56:7708–14. doi: 10.1167/iovs.15-17819. [DOI] [PubMed] [Google Scholar]
- 30.Hebel R, Holländer H. Size and distribution of ganglion cells in the human retina. Anat Embryol (Berl) 1983;168:125–36. doi: 10.1007/BF00305404. [DOI] [PubMed] [Google Scholar]
- 31.Asai T, Katsumori N, Mizokami K. Retinal ganglion cell damage in human glaucoma. 2. Studies on damage pattern. Nippon Ganka Gakkai Zasshi. 1987;91:1204–13. [PubMed] [Google Scholar]
- 32.Quigley HA, Dunkelberger GR, Green WR. Chronic human glaucoma causing selectively greater loss of large optic nerve fibers. Ophthalmology. 1988;95:357–63. doi: 10.1016/s0161-6420(88)33176-3. [DOI] [PubMed] [Google Scholar]
- 33.Chaturvedi N, Hedley-Whyte ET, Dreyer EB. Lateral geniculate nucleus in glaucoma. Am J Ophthalmol. 1993;116:182–8. doi: 10.1016/s0002-9394(14)71283-8. [DOI] [PubMed] [Google Scholar]
- 34.Sung KR, Sun JH, Na JH, Lee JY, Lee Y. Progression detection capability of macular thickness in advanced glaucomatous eyes. Ophthalmology. 2012;119:308–13. doi: 10.1016/j.ophtha.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 35.Kim KE, Jeoung JW, Park KH, Kim DM, Kim SH. Diagnostic classification of macular ganglion cell and retinal nerve fiber layer analysis: Differentiation of false-positives from glaucoma. Ophthalmology. 2015;122:502–10. doi: 10.1016/j.ophtha.2014.09.031. [DOI] [PubMed] [Google Scholar]


