Abstract
Purpose:
This study aims to compare the effect of laser photocoagulation or observation on choroidal vascularity in acute central serous chorioretinopathy (CSCR).
Methods:
A retrospective analysis of 30 patients with acute CSCR treated either with laser photocoagulation (16 eyes) or sham laser (14 eyes) was performed. Demographic details, visual acuity (VA) assessment, and other relevant clinical data were considered from baseline to the 3rd and 6th month follow-up visits. Participants with chronic CSCR and missing follow-up or inadequate data were excluded. Choroidal analysis including choroidal thickness and choroidal vascularity index (CVI) assessment was done for each visit using Spectral Domain (SD) Optical Coherence Tomography (OCT) images.
Results:
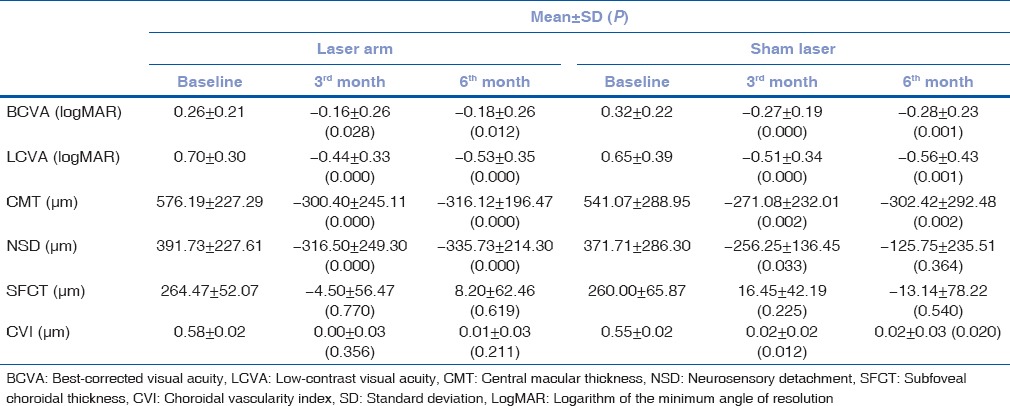
In laser arm group, there was a statistically significant change in VA, contrast sensitivity and central macular thickness (CMT) and neurosensory detachment (NSD) (P < 0.05) at the 3rd and 6th month visits. However, there was no statistically significant difference in subfoveal choroidal thickens (SFCT) and CVI (P > 0.05) at both the visits. In sham laser group, similarly, there was a significant improvement in VA, contrast sensitivity, CMT, and CVI (P < 0.05) at the 3rd and 6th month visits. There was significant reduction in NSD at the 3rd month; however, it was not statistically significant at the 6th month visit. SFCT did not change significantly at both the visits. There was no significant difference for the changes in parameters between the groups at the 6th month. Regression analysis showed no significant correlation with final VA with any of the baseline parameters.
Conclusion:
Early laser photocoagulation does lead to change in choroidal morphology, though insignificant, in comparison to observation. The present data, yet again, support no additional benefit of early laser photocoagulation in acute CSCR.
Keywords: Central serous chorioretinopathy, choroid, choroidal vascularity
Choroidal hyperpermeability is an established proposed mechanism for central serous chorioretinopathy (CSCR).[1,2] Recent studies have reported that increased choroidal thickness in eyes with acute CSCR as well as in the fellow eyes when compared with healthy eyes.[3,4,5,6,7] Recently, the concept of measuring choroidal vascular index (CVI) (ratio of luminal area to total choroidal area) using OCT images has been reported in various diseases including CSCR.[6,8,9] We reported increase in CVI in acute CSCR which was significantly more compared to chronic CSCR and age-matched healthy participants.[9,10] As conventionally reported, acute presentation of CSCR does not need any intervention. In a recent randomized trial, we reported no benefit of early laser photocoagulation, that is, within 2 months of presentation, in acute CSCR.[11] We found no significant difference between the early laser group and observation group in terms of resolution of subretinal fluid, change in retinal sensitivity, change in visual acuity (VA), and contrast VA at 6-month follow-up.
Maruko et al. reported slight decrease in choroidal thickness after laser photocoagulation along with the resolution of serous subretinal fluid, though, the change was insignificant.[12] Change in the choroidal vascularity may be a parameter to understand the response to laser photocoagulation or natural recovery in eyes with acute CSCR. In the present study, we were interested in understanding the changes in choroidal vascularity between the observation group and early laser photocoagulation group of our previous prospective study cohort.
Methods
The present retrospective study included 30 eyes of 30 patients underwent either early focal (16 eyes) or sham laser (14 eyes) photocoagulation in eyes with acute CSCR. This study was conducted at a tertiary eye care center in India to compare the effect of early focal laser photocoagulation with that of sham laser photocoagulation for the treatment of acute CSCR with focal leak. The informed consent was taken from all the patients and ethical approval was obtained from Institutional review board.
Inclusion criteria
Subjects diagnosed with acute CSCR and with complete visits during the acute phase along with natural history (baseline to the 6th month visit) and eyes with visible choroidal outer boundaries on spectral domain OCT (SD-OCT) in both groups from previous data.
Exclusion criteria
(i) History of prior treatment for CSCR; (ii) absence of any leak on fundus fluorescein angiography (FFA); (iii) subfoveal leak on FFA; (iv) diffuse leak on FFA, retinal pigment epithelium (RPE) atrophy, or any other signs of chronic CSC; (v) vitreoretinal/macular disorders other than CSC currently or in the past; (vi) any intraocular procedure in the last 6 months; (vii) currently on steroid therapy; (viii) any media opacity likely to cause attenuation of signal strength in OCT; (ix) history of malignant hypertension; (x) pregnancy; (xi) currently, on any systemic medication such as pioglitazones which can cause macular edema; (xii) evidence of glaucoma; and (xiii) spherical equivalent ≥ ±6 D. Participants with poor visibility of choroidal boundaries were excluded from the study.
All the participants underwent a comprehensive ophthalmic examination including best-corrected VA (BCVA) tested using Snellen charts, slit-lamp biomicroscopy, intraocular pressure measurement using Goldmann applanation tonometer, and dilated fundoscopic examination. BCVA was assessed using early treatment diabetic retinopathy study (ETDRS) letter count and low-contrast VA (LCVA) was assessed using COMPlog.
Fundus fluorescein angiography
FFA was performed using fluorescein sodium 20% on Navilas® system (OD-OS GmbH, Teltow, Germany) to determine the site of leakage at baseline, and at 3 and 6 months from baseline.
Laser photocoagulation
Participants in laser group were treated with navigated laser photocoagulation as per the protocol reported before. In sham laser group, only the laser beam was turned on, but not the source and fundus photographs were obtained.
Spectral domain optical coherence tomography
The SD-OCT scans were obtained using Cirrus high-definition (HD)-OCT (Carl Zeiss Meditec, Inc., Dublin, CA, USA) after dilatation of pupil with 0.8% tropicamide and 5% phenylephrine eye drops at both the visits. The scanning protocol included HD 5-line raster, HD single line raster, enhanced depth imaging, and macular cube. Central macular thickness (CMT) was determined automatically and analyzed by OCT software, by generating images using the Macular Cube 512 × 128 scan over 6 mm × 6 mm area, the cube being composed of 128 horizontal examination lines of 512 A-scans each. The CMT was obtained from the 1-mm central retinal thickness area as described in the ETDRS fields corresponding to the CMT. The subfoveal choroidal thickness (SFCT) was obtained using the EDI-OCT technique previously described by Spaide et al. The vertical distance between the hyperreflective line of Bruch's membrane and the innermost hyperreflective line of the chorioscleral interface was taken, and the average of the two scans (vertical and horizontal) was considered as the SFCT. The height of neurosensory detachment (NSD) was measured by the vertical distance between the outermost hyperreflective margin of the detached neurosensory retina at the fovea and the hyperreflective line of the RPE.
Choroidal vascularity index calculation
The CVI calculation of the single HD scan, passing through fovea, was done using previously reported algorithm.[13] Briefly, choroidal stroma and vessel area analysis involved (i) automated binarization of a HD horizontal 6-mm OCT B-scan and (ii) automated segmentation of the binarized choroid layer as reported previously. The task of automated binarization in turn involved (a) preprocessing, (b) exponential and nonlinear enhancement, and (c) thresholding.
Statistical analysis
Statistics for Windows, Version 24 (IBM Corp. Released 2016, IBM SPSS) was used to analyze the results. The BCVA and LCVA were converted to logarithm of the minimum angle of resolution equivalent for statistical analysis. The change in BCVA, LCVA, CMT, NSD, SFCT, and CVI from baseline, 3rd and 6th month follow-up visits, was analyzed using paired samples t-test to compare the means (within the group and between the group). P <0.05 was considered as statistically significant. Correlation between the variables was estimated using Pearson's correlation.
Results
In the present study, laser group had 16 eyes of 16 participants (53.3%), of which 13 were male and 3 were female. Sham laser group had 14 eyes of 14 paricipants (46.6%), of which 13 were male and only 1 female. Baseline characteristics of both the groups are shown in Table 1.
Table 1.
Baseline, 3rd, and 6th month visit characteristics of laser arm and sham laser groups
Laser group
There was a statistically significant change in BCVA, LCVA, CMT, and NSD (P< 0.05) at the 3rd and 6th month visits. However, there was no statistically significant difference in SFCT and CVI (P > 0.05) at both the visits [Table 1 and Fig. 1].
Figure 1.
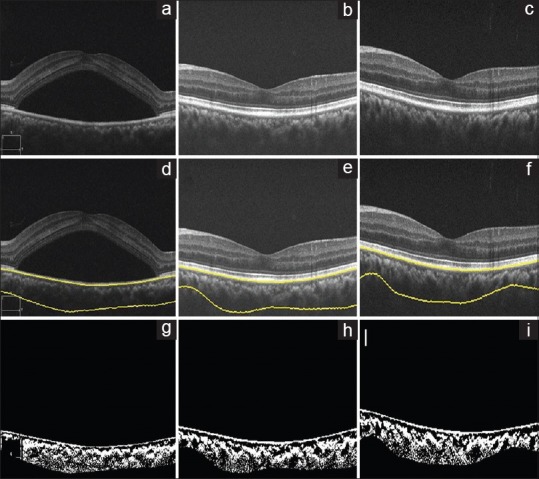
Laser arm, optical coherence tomography of the left eye of a 32-year-old female using spectral domain optical coherence tomography at baseline, 3 months, and 6 months, (a-c), respectively. (d-f) Images show choroid segmentation using an automated algorithm. (g-i) are the binarized images, which is used to calculate the choroidal vascularity index (0.57, 0.57, and 0.57, respectively)
Sham laser group
Similarly, there was a statistically significant improvement in BCVA, LCVA, CMT, and CVI (P< 0.05) at the 3rd and 6th month visits. Whereas, there was significant reduction in NSD at the 3rd month; however, it was not statistically significant at the 6th month visit [Fig. 2]. SFCT did not change significantly at both the visits [Table 1].
Figure 2.
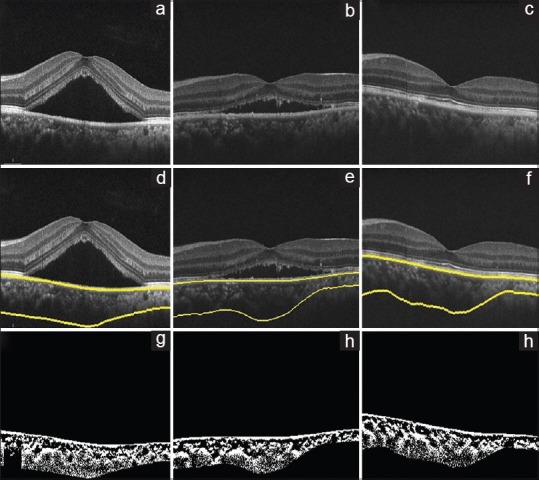
Sham laser, optical coherence tomography of the right eye of a 40-year-old male using spectral domain optical coherence tomography at baseline, 3 months, and 6 months, (a-c), respectively. (d-f) Images show choroid segmentation using an automated algorithm. (g-i) are the binarized images, which is used to calculate the choroidal vascularity index (0.56, 0.58, and 0.59, respectively)
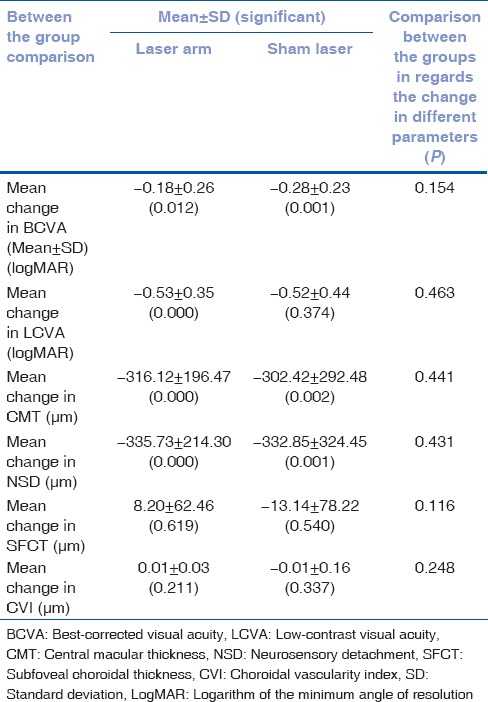
Comparison between the groups for change in parameters at 6 months showed no statistical significance with any of the parameters [Table 2].
Table 2.
Comparison between the groups for change in parameters at 6 months
Correlations
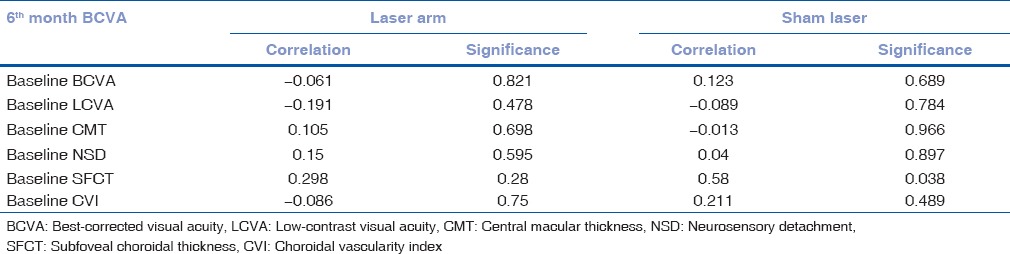
To evaluate the correlation of BCVA at final visit with baseline parameters, Pearson's correlation was performed [Table 3a] which showed no significant correlation with any of the baseline parameters. Similarly, CVI at the final visit with baseline parameters showed no significant correlation with any baseline parameters in laser arm group, but in sham laser group, only baseline SFCT was closely correlating with final CVI (<0.05) [Table 3b].
Table 3a.
Correlation of baseline parameters with final visit best-corrected visual acuity of both the groups
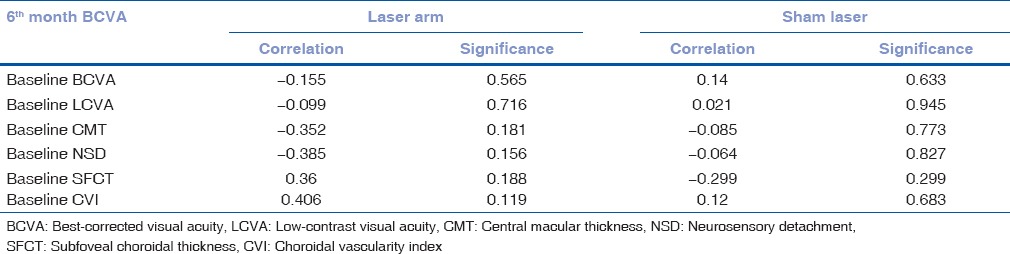
Table 3b.
Correlation of baseline parameters with final visit choroidal vascularity index of both the groups
Discussion
In our previous study, we reported increased CVI in acute CSCR compared to fellow eye and age-matched healthy participants.[9,10] CVI could be a parameter demonstrating the choroidal vessel congestion, which is the primary pathogenic mechanism for CSCR. Therefore, CVI could also be a useful to understand the effect of therapeutic intervention. The present study shows the change in CVI in acute CSCR with or without intervention.
Interestingly, there was a slight increase in CVI, though it is statistically insignificant, in sham laser group, which could be because of persistent choroidal congestion in the absence of any intervention. Such increase was not noted in laser arm; rather a slight decrease in choroidal thickness and CVI was noted. There was an increase in CVI in both the groups at 6 months, which may be suggestive of reappearance of choroidal congestion, however, which was not associated with the recurrence of subretinal fluid. This probably could be associated with recurrence in subretinal fluid in the future in sham group. However, this cannot be answered with the present study data.
One of the limitations of our study is the small sample size for the present analysis. Due to poor visibility of choroidal boundaries, we could not include all the eyes of our previous cohort. As the change in CVI is very small, therefore, study with larger sample size in two groups would be required to evaluate the significance.” However, our study provides the change in CVI in a prospective manner, which is one of the main strengths of our study. We did not evaluate the CVI changes at 1 month; it is possible the change in CVI could have been more significant at 1 month from baseline.
Conclusion
Our study provides a change in choroidal vascularity in eyes with acute CSCR with or without laser intervention. Early laser photocoagulation does lead to change in choroidal morphology though insignificant, in comparison to observation. The present data, yet again, support no additional benefit of early laser photocoagulation in acute CSCR. However, further, prospective evaluation of larger sample of acute CSCR may expand our understanding about the changes in choroidal morphology with different treatment modalities.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Peyman GA. Choroidal hyperpermeability in central serous choroidopathy: A new concept? Arch Ophthalmol. 1995;113:701–2. doi: 10.1001/archopht.1995.01100060023012. [DOI] [PubMed] [Google Scholar]
- 2.Yanagi Y, Ting DSW, Ng WY, Lee SY, Mathur R, Chan CM, et al. Choroidal Vascular Hyperpermeability as a Predictor of Treatment Response for Polypoidal Choroidal Vasculopathy. Retina. 2017 doi: 10.1097/IAE.0000000000001758. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Chhablani J, Wong IY, Kozak I. Choroidal imaging: A review. Saudi J Ophthalmol. 2014;28:123–8. doi: 10.1016/j.sjopt.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan KA, Gupta P, Agarwal A, Chhablani J, Cheng CY, Keane PA, et al. State of science: Choroidal thickness and systemic health. Surv Ophthalmol. 2016;61:566–81. doi: 10.1016/j.survophthal.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Arora S, Pyare R, Sridharan P, Arora T, Thakar M, Ghosh B, et al. Choroidal thickness evaluation of healthy eyes, central serous chorioretinopathy, and fellow eyes using spectral domain optical coherence tomography in Indian population. Indian J Ophthalmol. 2016;64:747–51. doi: 10.4103/0301-4738.194999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agrawal R, Salman M, Tan KA, Karampelas M, Sim DA, Keane PA, et al. Choroidal vascularity index (CVI) – A novel optical coherence tomography parameter for monitoring patients with panuveitis? PLoS One. 2016;11:e0146344. doi: 10.1371/journal.pone.0146344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei X, Ting DS, Ng WY, Khandelwal N, Agrawal R, Cheung CM, et al. Choroidal Vascularity Index: A Novel optical coherence tomography based parameter in patients with exudative age-related macular degeneration. Retina. 2017;37:1120–5. doi: 10.1097/IAE.0000000000001312. [DOI] [PubMed] [Google Scholar]
- 8.Tan KA, Laude A, Yip V, Loo E, Wong EP, Agrawal R, et al. Choroidal vascularity index – A novel optical coherence tomography parameter for disease monitoring in diabetes mellitus? Acta Ophthalmol. 2016;94:e612–16. doi: 10.1111/aos.13044. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal R, Gupta P, Tan KA, Cheung CM, Wong TY, Cheng CY, et al. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci Rep. 2016;6:21090. doi: 10.1038/srep21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agrawal R, Chhablani J, Tan KA, Shah S, Sarvaiya C, Banker A, et al. Choroidal vascularity index in central serous chorioretinopathy. Retina. 2016;36:1646–51. doi: 10.1097/IAE.0000000000001040. [DOI] [PubMed] [Google Scholar]
- 11.Ambiya V, Khodani M, Goud A, Narayanan R, Tyagi M, Rani PK, et al. Early focal laser photocoagulation in acute central serous chorioretinopathy: A Prospective, randomized study. Ophthalmic Surg Lasers Imaging Retina. 2017;48:564–71. doi: 10.3928/23258160-20170630-07. [DOI] [PubMed] [Google Scholar]
- 12.Maruko I, Iida T, Sugano Y, Ojima A, Ogasawara M, Spaide RF, et al. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology. 2010;117:1792–9. doi: 10.1016/j.ophtha.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Vupparaboina KK, Nizampatnam S, Chhablani J, Richhariya A, Jana S. Automated estimation of choroidal thickness distribution and volume based on OCT images of posterior visual section. Comput Med Imaging Graph. 2015;46(Pt 3):315–27. doi: 10.1016/j.compmedimag.2015.09.008. [DOI] [PubMed] [Google Scholar]


