Abstract
Purpose:
The purpose was to study the retinopathy status in diabetic patients with a risk of diabetic foot (DF) syndrome visiting a tertiary care hospital in South India.
Methods:
In this cross sectional study all patients with diabetes mellitus (DM) with a risk of DF syndrome, visiting a tertiary care hospital during the study period, underwent an ophthalmological evaluation for documentation of their retinopathy status.
Results:
One hundred and eighty-two patients diagnosed to have a risk profile for DF syndrome were included in the study. Their mean age was 59.28 years and 75.27% were males. The mean duration of Type 1 and Type 2 variants of DM was 14.9 years and 10.9 years, respectively. Of the 182 patients, 67.58% had retinopathy changes. Proliferative diabetic retinopathy (DR) constituted 17.88% of the total patients with retinopathy. An increased presence of retinopathy in patients with an increased risk grade of DF was found significant by the Chi-square test (P < 0.001).
Conclusion:
Our study found an increased presence of DR in a South Indian cohort with DF syndrome. The severity of retinopathy was greater in patients with higher grades of risk for DF. The establishment of an association between DR and DF syndrome will help in developing an integrated management strategy for these two debilitating consequences of diabetes.
Keywords: Cross-sectional studies, diabetic foot, diabetic retinopathy, India, tertiary care centers
The diabetic population of the world is growing rapidly.[1] Advances in the management of this disease, by medical means as well as by lifestyle modification, have resulted in a minimal reduction, or a slight improvement of the life expectancy of diabetic patients.[2,3] However, the disability-free life expectancy has decreased considerably.[3] The increase in the duration of disease is associated with conditions resulting from changes in the microvasculature of the body including nephropathy, neuropathy, and retinopathy.[4] Diabetic foot (DF) syndrome is one of the important consequences of long-term uncontrolled diabetes, which occurs due to a combination of peripheral neuropathy and micro vasculopathy in the lower-limb extremities. It may vary from a minor ulceration to necrosis of tissues, sometimes warranting amputation.[4] Diabetic retinopathy (DR) is another consequence of diabetic microangiopathy, which may cause visual deterioration due to macular edema in any stage and vitreous hemorrhage or tractional retinal detachment in the advanced proliferative retinopathy stages.[5] The number of diabetic patients in India is estimated to increase to around 80 million in India, by the year 2030.[1] The occurrence of DF is affected by the occupation and cultural factors of the region.[6] Considering the largely agriculture-oriented, injury-prone occupations, as well as cultural factors encouraging bare foot walking, an increase in the incidence of DF is expected. The ethnicity and rising standards of living in this region also predispose to DR.[7] A combination of DF and retinopathy would affect the population in working age group the most. Early detection of both DF and DR would limit physical and visual disability in this population.
The current literature available has documented the coexistence of DF and DR as well as the association of various grades of DF ulcer and retinopathy. Our study attempts to document DR in patients from South India with a risk for DF, with or without ulceration.
Methods
This was a cross-sectional observational study conducted at a tertiary care hospital in South India, from October 2014 to March 2016. It was conducted as per the guidelines of the Declaration of Helsinki, and the Institutional ethical committee clearance was obtained prior to initiation. All patients diagnosed with diabetes mellitus (DM) who were at risk for DF syndrome were consecutively enrolled. The study was explained and written informed consent of the patients was obtained before their enrollment. Those who refused to consent, patients with gestational diabetes, and patients with type 1 diabetes for duration of fewer than 5 years were excluded. A detailed history of diabetes including duration and other demographic details were collected from the patients. The reports of blood investigations performed at the time of the study were collected from their medical records.
The grading of DF was done using the Michigan Neuropathy Scoring Instrument and methodology based on the risk classification system of the International Working Group on the Diabetic Foot (IWGDF).[8] This involved the use of a 128 Hz tuning fork to detect neuropathy, and protective sensations were assessed by 10-g monofilament. The grading was as follows:
Grade 0: Sensations intact; Grade 1: Diminution of sensation or loss of protective sensation without deformities and intact blood supply; Grade 2: Diminished sensation, foot deformities such as hammer toes, claw toes, and/or peripheral arterial disease; and Grade 3: Previous/present ulcer or amputation.
The participants then underwent a detailed ophthalmological evaluation including visual acuity, anterior segment evaluation using slit-lamp biomicroscopy, intraocular pressure measurement, and fundus evaluation using a + 90 D lens and an indirect ophthalmoscope. The patients having any lesion hampering the view of the ocular fundus due to which the retinopathy could not be graded were also excluded. In patients with unequal DR, the retinopathy of greater severity was considered. The retinopathy was graded as per the International Clinical Diabetic Retinopathy and Diabetic Macular Edema Severity scale as no retinopathy changes; mild nonproliferative DR (NPDR); moderate NPDR; severe NPDR; and PDR.[9]
The statistical analysis was done using the SPSS software (Statistical Package for Social Sciences, version 15, SPSS Inc, Chicago, IL, USA). The association between DF and DR was analyzed using the Chi-square test. Kendall's tau-b test was used to assess the correlation with increasing severity of the conditions.
Results
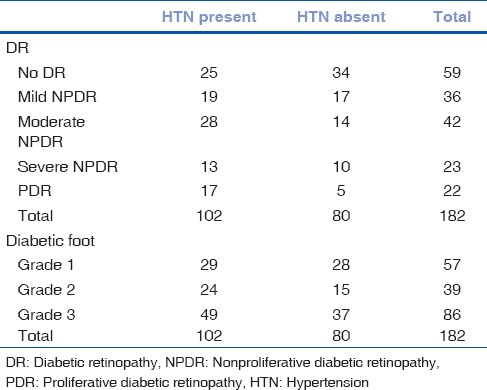
A total of 182 patients with DF were included in the study of which 75.27% were males. The mean age was 59.24 years in males and 59.42 years in females. The average duration of diabetes in the 13 patients with Type 1 disease was 14.9 years, while the rest with Type 2 DM had the disease for an average duration of 10.9 years [Table 1]. With the increase in the duration of DM, the severity of DF as well as the presence of retinopathy was observed more frequently. DR was seen in 123 patients. A significant association was found between the severity of DF grading and the presence DR, by Chi-square test (P< 0.01) [Table 2]. This implies an increased presence of retinopathy with higher grades of DF. A positive correlation was found by Kendall's tau-b test, between the increasing severity of DF and severity of the stage of retinopathy (τb = 0.262, P < 0.01). In patients with Grade 3 DF, 36.04% had severe NPDR or PDR, compared to 14.03% in Grade 1 and 15.38% in Grade 2 stages [Fig. 1]. Hypertension (HTN) was an associated condition found in 56% of the patients [Table 3]. Although there was no significant association between its presence and severity of DF, a significant association was established with increasing stage of DR (P = 0.032). Hemoglobin levels were obtained in 171 patients. The mean hemoglobin level in the cohort was 11.82 ± 2.05 g/dL. Glycosylated hemoglobin was recorded in 157 patients with a mean of 9.53 ± 2.41%. Serum urea was recorded in 174 patients with a mean of 34.09 ± 23.14 mg/dL. There was no statistically significant association found between levels of hemoglobin, glycosylated hemoglobin, or serum urea with various stages of DF or DR. The mean serum creatinine recorded in 177 patients was 1.33 ± 1.13 mg/dL. Serum creatinine had a significant association with increasing stage of DR.
Table 1.
Demographics of the study cohort (n=182)
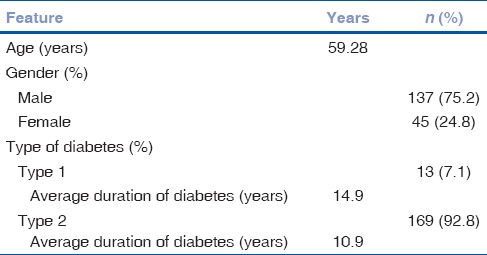
Table 2.
Association of diabetic retinopathy and diabetic foot
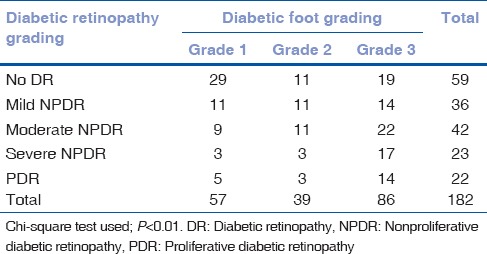
Figure 1.
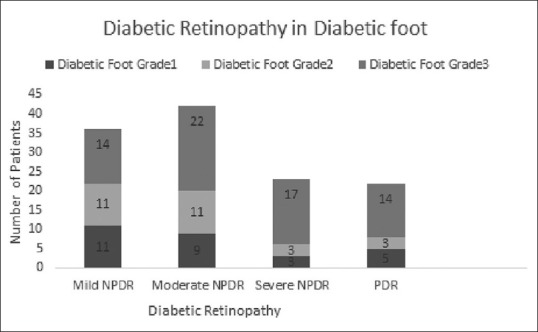
Graph depicting the proportionately higher number of Grade 3 diabetic foot cases with severe retinopathy. NPDR: Nonproliferative diabetic retinopathy; PDR: Proliferative diabetic retinopathy
Table 3.
Association of hypertension
Discussion
The prevalence of DM is increasing rapidly.[1] It is but a matter of time till the complications of this disease also grow to epidemic proportions. This can be avoided by improving methods for early detection and treatment of these complications. DF and DR are two such complications which can be controlled well if detected early. DF is a major cause of loss of working hours and in some cases disability due to diabetes.[6] DR is one of the leading causes of blindness in the world.[10] An association between them, if found, could help initiate better screening protocols, enabling early detection of both diseases.
We studied the retinopathy status of 182 patients presenting with DF at a tertiary care center. The mean age of the patients was 59.28 years and 75.27% were males. An increased occurrence of diabetic neuropathy in males was observed by Olsen et al. in a Danish population,[11] as well as by Viswanathan et al. in a South Indian population.[12]
The prolonged duration of DM has been associated with increased frequency of these complications.[13] More than half of our patients with DF (60.43%) and DR (62.6%) had been suffering from diabetes for over 10 years.
We chose to adopt the grading of the risk for DF based on the IWGDF guidelines, to include the study of preulcerative stages of DF. In our study, DR was seen in 67.58% of patients, with 17.88% of retinopathy being proliferative. This contrasts with Hwang et al. who found most of their DF patients to have retinopathy (90%), nearly one-half of which was proliferative (55%).[14] This difference may be on account of a difference in classification of DF between our studies. Their patients were similar to the patients classified as having Grade 3 DF in our cohort. In the 86 patients with Grade 3 DF in our study, retinopathy was present in 77.9% of which 36.04% had sight-threatening stages of severe and proliferative retinopathy. A difference in the ethnicity of the study population may be another factor for the disparity of results.
Retinopathy is considered to be a risk factor for worsening of DF.[15] Conversely, the presence of DF is a predictor for progress to the proliferative stages of DR.[16]
A statistically significant association between the presence of retinopathy in advanced grades of DF was noted in the present study. The severity of the retinopathy was also found to be greater in patients with higher grades of DF. Hwang et al. too noted a similar association of proliferative retinopathy and DF. They speculate it to be a result of increased oxidative stress and endothelial damage occurring in vascular disease in the later stages of diabetes.[14]
Venkatesh et al. studied the comorbidities associated with DR in an Indian cohort and reported an increased presence of nephropathy and HTN among other factors.[17] This is comparable with our study in terms of a significant association of serum creatinine and HTN. In our study too, we found a significant association with serum creatinine levels and HTN with DR. However, a similar association was not established with DF.
This is a cross-sectional study which found an association between DF and DR. However, a prospective cohort study with longitudinal follow-up would have been ideal to establish a stronger pathogenic correlation between the two conditions. A study of diabetic patients with retinopathy prior to clinically evident DF (Grade 0) would also aid in studying the correlation between the two diabetic complications. The absence of objective modalities for establishing the presence of certain variables such as the use of nerve conduction studies to determine neuropathy[17] is also a limitation of our study.
Conclusion
The data on DF, DR, and an association between the two from an Indian population are limited. With India headed to be the diabetes capital of the world in the near future, an increase in these two complications would affect the quality of life of the working population to a large extent.[18,19] A determined and well-planned strategy incorporating the existing health-care structure toward the provision of an integrated approach to diabetics in the early stages to prevent complications is of utmost importance. The results of our study support the need for a system of ophthalmological referral in case of detection of DF, as well as a prompt referral of DR patients to a DF specialist.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ. Improvements in the life expectancy of type 1 diabetes: The Pittsburgh epidemiology of diabetes complications study cohort. Diabetes. 2012;61:2987–92. doi: 10.2337/db11-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huo L, Shaw JE, Wong E, Harding JL, Peeters A, Magliano DJ, et al. Burden of diabetes in Australia: Life expectancy and disability-free life expectancy in adults with diabetes. Diabetologia. 2016;59:1437–45. doi: 10.1007/s00125-016-3948-x. [DOI] [PubMed] [Google Scholar]
- 4.Alavi A, Sibbald RG, Mayer D, Goodman L, Botros M, Armstrong DG, et al. Diabetic foot ulcers: Part I. Pathophysiology and prevention. J Am Acad Dermatol. 2014;70:1.e1–8. doi: 10.1016/j.jaad.2013.06.055. [DOI] [PubMed] [Google Scholar]
- 5.Ding J, Wong TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 2012;12:346–54. doi: 10.1007/s11892-012-0283-6. [DOI] [PubMed] [Google Scholar]
- 6.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–24. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 7.Raymond NT, Varadhan L, Reynold DR, Bush K, Sankaranarayanan S, Bellary S, et al. Higher prevalence of retinopathy in diabetic patients of South Asian ethnicity compared with white Europeans in the community: A cross-sectional study. Diabetes Care. 2009;32:410–5. doi: 10.2337/dc08-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Working Group on the Diabetic Foot Guidelines. 2015. [Last accessed on 2017 Jul 10]. Available from: http://www.iwgdf.org/guidelines/definitionscriteria 2015/
- 9.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 10.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–36. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 11.Olsen BS, Sjølie A, Hougaard P, Johannesen J, Borch-Johnsen K, Marinelli K, et al. A 6-year nationwide cohort study of glycaemic control in young people with type 1 diabetes. Risk markers for the development of retinopathy, nephropathy and neuropathy. Danish Study Group of Diabetes in Childhood. J Diabetes Complications. 2000;14:295–300. doi: 10.1016/s1056-8727(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 12.Viswanathan V, Madhavan S, Rajasekar S, Chamukuttan S, Ambady R. Urban-rural differences in the prevalence of foot complications in South-Indian diabetic patients. Diabetes Care. 2006;29:701–3. doi: 10.2337/diacare.29.03.06.dc05-1777. [DOI] [PubMed] [Google Scholar]
- 13.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–88. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 14.Hwang DJ, Lee KM, Park MS, Choi SH, Park JI, Cho JH, et al. Association between diabetic foot ulcer and diabetic retinopathy. PLoS One. 2017;12:e0175270. doi: 10.1371/journal.pone.0175270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Rubeaan K, Al Derwish M, Ouizi S, Youssef AM, Subhani SN, Ibrahim HM, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10:e0124446. doi: 10.1371/journal.pone.0124446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris Nwanyanwu K, Talwar N, Gardner TW, Wrobel JS, Herman WH, Stein JD, et al. Predicting development of proliferative diabetic retinopathy. Diabetes Care. 2013;36:1562–8. doi: 10.2337/dc12-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkatesh P, Tibrewal S, Bhowmik D, Tripathi M, Ramakrishnan S, Vashist N, et al. Prevalence of systemic co-morbidities in patients with various grades of diabetic retinopathy. Indian J Med Res. 2014;140:77–83. [PMC free article] [PubMed] [Google Scholar]
- 18.Sekhar MS, Thomas RR, Unnikrishnan MK, Vijayanarayana K, Rodrigues GS. Impact of diabetic foot ulcer on health-related quality of life: A cross-sectional study. Semin Vasc Surg. 2015;28:165–71. doi: 10.1053/j.semvascsurg.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Pereira DM, Shah A, D'Souza M, Simon P, George T, D'Souza N, et al. Quality of life in people with diabetic retinopathy: Indian study. J Clin Diagn Res. 2017;11:NC01–6. doi: 10.7860/JCDR/2017/24496.9686. [DOI] [PMC free article] [PubMed] [Google Scholar]


