Abstract
Purpose:
Age-related macular degeneration (AMD) is a disease of the macula that significantly affects eyesight and leads to irreversible central vision loss. Recent studies have demonstrated that angiogenesis is the most important mechanism of AMD development. It is associated with extracellular remodeling involving different proteolytic systems, among them matrix metalloproteinases (MMPs), which play an essential role in the etiopathogenesis of AMD. The main objective of the present study was to determine the relationship between exudative AMD and MMP-2 (-1306 C/T) rs243865 polymorphism.
Methods:
The study enrolled 267 patients with exudative AMD and 318 controls. DNA was extracted from peripheral venous blood leukocytes by commercial kits. Genotyping of MMP-2 (-1306 C/T) rs243865 was carried out using real-time polymerase chain reaction method.
Results:
The analysis of MMP-2 (-1306 C/T) polymorphism did not reveal any differences in the distribution of CC, CT, and TT genotypes between the exudative AMD and control groups: 58.8%, 31.5% and 9.7% vs. 59.75%, 33.96% and 6.29%, respectively, P = 0.287). When the study population was subdivided into age groups, MMP-2 (-1306 C/T) rs243865 CT genotype showed 5.7-fold increased the risk of exudative AMD development compared to CC and TT genotypes together in younger (<65 years) males group (P = 0.05).
Conclusion:
MMP-2 (-1306 C/T) polymorphism is associated with exudative AMD development in younger males.
Keywords: Exudative age-related macular degeneration, gene polymorphism, matrix metalloproteinases
Age-related macular degeneration (AMD) causes significant and irreversible loss of central vision. In developed countries, AMD is the most common cause of visual loss in persons aged 60 years and older.[1]
Macular degenerative lesions in AMD include drusen formation, changes in the retinal pigment epithelium, atrophy of the retinal pigment epithelium and choroidal choriocapillary layer, lesions of Bruch's membrane, geographic atrophy of the central fovea, exudative AMD with choroidal neovascularization, detachment of the retinal pigment epithelium, or submacular disciform scarring. In normal aging, the increased thickness of Bruch's membrane,[2,3] the deposition of normal and abnormal extracellular matrix (ECM) material,[4] increased cross-link formation (oxidative and nonenzymatic glycosylation leading to advanced glycation and lipoxidation end products),[5] and the accumulation of lipid-rich debris[6,7] are the changes that imply disturbances in ECM turnover of the membrane.
Recent studies have demonstrated that angiogenesis is the most important mechanism of AMD development and is associated with extracellular remodeling involving different proteolytic systems, among which matrix metalloproteinases (MMPs) play an essential role.[8] It has been suggested that the decrease in MMP-2 activity correlates with drusen formation.[9] Irrespective of an age-related increase in levels of pro-MMP2 and pro-MMP-9 in Bruch's membrane, the amount of oxidized, denatured, and damaged collagenous substrate was observed to increase, accounting for nearly 50% of total collagen in elderly membranes.[4,10] In advanced aging associated with AMD, despite a 2-fold increase in the total level of pro-MMP-9, levels of active-MMP-2 and active-MMP-9 were reduced by 50% compared to age-matched controls.[11]
In addition, C to T allelic variation located in MMP-2 at nucleotide-1306 (National Center for Biotechnology Information single-nucleotide polymorphism [SNP] identification no. rs243865) disrupts the Sp1-binding site of a transcription factor in the promoter region, where promoter loses 50% activity.[12]
To the best of our knowledge, no studies have investigated the association between the MMP-2 (-1306 C/T) polymorphism and exudative AMD. Therefore, the aim of this study was to determine associations between the MMP-2 (-1306 C/T) gene polymorphism and the development of exudative AMD.
Methods
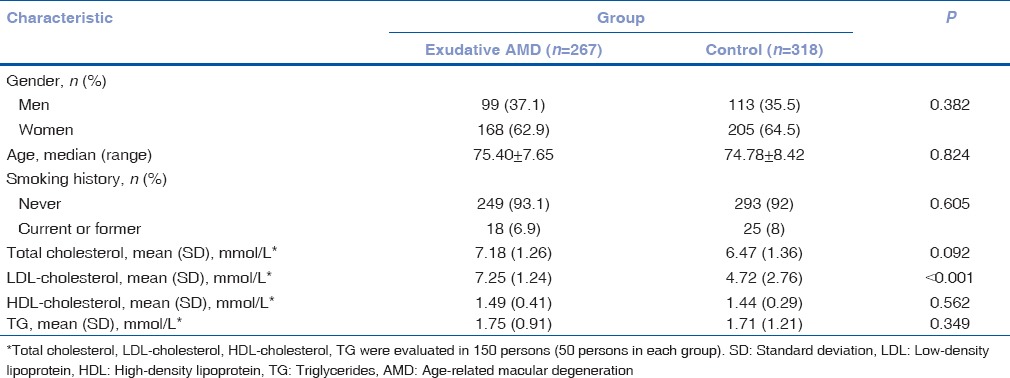
Permission (Number BE-2-/13) to undertake the study was obtained from Kaunas Regional Biomedical Research Ethics Committee. The study was conducted in the Department of Ophthalmology, Hospital of Lithuanian University of Health Sciences and Laboratory of Ophthalmology, Neuroscience Institute, Lithuanian University of Health Sciences. The study enrolled 267 patients with exudative AMD: 99 males (37.1%) and 168 females (62.9%). The control group consisted of 113 males (35.5%) and 205 (64.5%) females and the proportion of males and females between the study groups did not differ (P = 0.382). Individuals in the exudative AMD group were not significantly older than control group individuals, 75.4 and 74.78 years, respectively (P = 0.824). During the study, the study population was subdivided into two groups by age: persons younger than 65 years and aged 65 years and older. Risk factors as smoking and lipid profile were included in our manuscript. There was no significant difference in gender and age between the two groups (P > 0.05). Smoking history was not a risk factor for AMD in this study population either (P = 0.605). However, we found that low-density lipoprotein-cholesterol was significantly higher in exudative AMD patients than in controls (7.25% vs. 4.72%, P < 0.001).
Demographic and clinical characteristics of the study population are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the study groups
Control group formation
The age- and gender-matched control group comprised 318 selected participants who had no ophthalmologic pathology on examination and matched inclusion criteria.
The random sample population was selected from the following projects:
The International Health, Alcohol and Psychosocial Factors in Eastern Europe project[13] involving Kaunas population aged 45–74 years run by the Laboratory of Population Research, Institute of Cardiology, LUHS
The International Countrywide Integrated Noncommunicable Disease Intervention project[14] involving Lithuanian population aged 25–65 years run by the Laboratory of Preventive Medicine, Institute for Biomedical Research, LUHS
The Kaunas Healthy Ageing Study[15] involving Kaunas population older than 65 years run by the Clinic of Geriatrics and the Laboratory of Molecular Cardiology, Institute of Cardiology, LUHS.
Gene polymorphisms did not differ between healthy controls and a random sample of the population. Hence, only healthy controls were included in the further research.
All patients were consulted by a general practitioner and a neurologist. Patients meeting the inclusion criteria were further examined at the Department of Cardiology, LUHS, to exclude ischemic heart disease (IHD). All patients completed a questionnaire about the risk factors of IHD and clinical symptoms. Patients with no symptoms of typical chest angina and with no typical ischemic changes on the ECG were included into the final study group.
Ophthalmologic examination
All study patients were evaluated by slit-lamp biomicroscopy to assess corneal and lenticular transparency. At each examination, the intraocular pressure was measured. Pupils were dilated with 1% tropicamide, and then the fundoscopy using a direct monocular ophthalmoscope and slit-lamp biomicroscopy using a double aspheric lens of +78 diopters were performed. The results of ophthalmologic examinations were recorded on specially standardized forms. For the detailed evaluation of the macula, stereoscopic color fundus photographs of the macula centered at 45° and 30° to the fovea were obtained.
The classification system of AMD proposed by the Age-Related Eye Disease Study[16] was used. The diagnosis of exudative AMD was based on optical coherence tomography examination results. Fluorescein angiograms were performed if necessary.
The following exclusion criteria were used: (i) unrelated eye disorders, for example, high refractive error, cloudy cornea, lens opacity (nuclear, cortical, or posterior subcapsular cataract), keratitis, acute or chronic uveitis, glaucoma, or diseases of the optic nerve; (ii) systemic illnesses, for example, diabetes mellitus, malignant tumors, systemic connective tissue disorders, chronic infectious diseases, or conditions after organ or tissue transplantation; and (iii) ungraded color fundus photographs resulting from obscuration of the ocular optic system or because of fundus photograph quality.
DNA extraction and genotyping
The DNA extraction and analysis of the gene polymorphism of MMP-2 were carried out in the Laboratory of Molecular Cardiology at the Institute of Cardiology of the LUHS for the control group, and in the Laboratory of Ophthalmology at the Institute of Neuroscience of the LUHS for the AMD patient group. DNA was extracted from the venous blood of patients using the genomic DNA purification kit (GeneJET Genomic DNA Purification Kit, Thermo Fisher Scientific Inc., Waltham, United States), according to the recommendations of the manufacturer.
The genotyping of MMP-2 (-1306 C/T, rs243865) was carried out using the real-time polymerase chain reaction (RT-PCR) method by the HT 7900 RT-PCR quantification system (Applied Biosystems, USA). An appropriate RT-PCR mixture of MMP-2 (-1306 C/T, rs243865) was prepared using SNP genotyping assay (rs243865: C_3225943-10, Applied Biosystems, Foster City, CA, USA), 2X TaqMan® Universal Master Mix (Applied Biosystems, Foster City, CA, USA), and nuclease-free water (Thermo Fisher Scientific Inc., Waltham, United States), according to manufacturer's recommendations, under the following PCR conditions: 10 min at 95°C; 40 cycles (15 s at 95°C, and 1 min at 60°C).
Genotyping quality control
Five percentage of randomly chosen samples for each of the two SNPs were selected for repetitive analysis. Replication experiments revealed a 100% concordance rate of genotypes and alleles with the initial genotyping results.
Statistical analysis
Statistical analysis was performed using the SPSS/W 20.0 software (Statistical Package for the Social Sciences for Windows, Inc., Chicago, Illinois, USA). Data are expressed as absolute numbers with percentages, mean value, and standard deviation as well. Frequencies of genotypes are expressed in percentages.
Hardy–Weinberg analysis was performed to compare the observed and expected frequencies of MMP-2 (-1306 C/T) rs243865 genotypes and alleles using the Chi-square test in the exudative AMD and control groups. The distribution of MMP-2 (-1306 C/T) rs243865 genotypes in the exudative AMD and control groups was compared using the Chi-square test or the Fisher exact test. Binomial logistic regression analysis was performed to estimate the impact of genotypes on the exudative AMD development. Odds ratios (OR) and 95% confidence intervals (CIs) are presented. The selection of the best genetic model was based on the Akaike information criterion (AIC); therefore, the best genetic models were those with the lowest AIC values.
Differences were considered statistically significant when P < 0.05.
Results
Hardy–Weinberg equilibrium
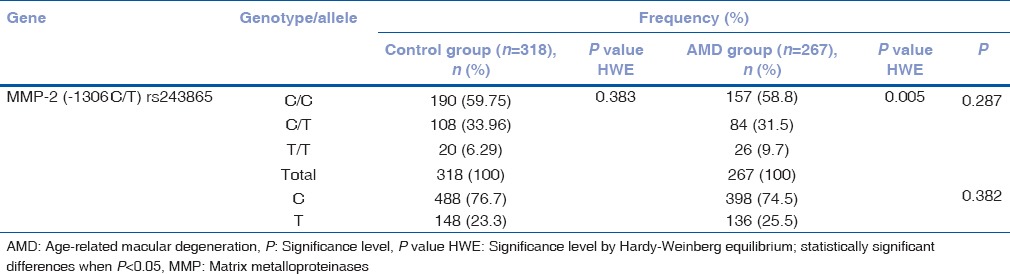
The observed frequencies of MMP-2 (-1306 C/T) rs243865 genotypes were in Hardy–Weinberg equilibrium in the control group (P = 0.383) but not in the exudative AMD group (P = 0.005).
Matrix metalloproteinases-2 (-1306 C/T) rs243865 genotype distribution in exudative age-related macular degeneration and control groups
Frequencies of MMP-2 (-1306 C/T) rs243865 genotype and allele distribution are presented in Table 2. Statistical analysis did not show significant differences comparing genotype (C/C, C/T, and T/T) and allele (C and T) distribution between the study groups (P = 0.287 for genotypes; P = 0.382 for alleles) [Table 2].
Table 2.
Frequency of matrix metalloproteinases-2 (-1306 C/T) rs243865 genotypes in exudative age-related macular degeneration and control groups
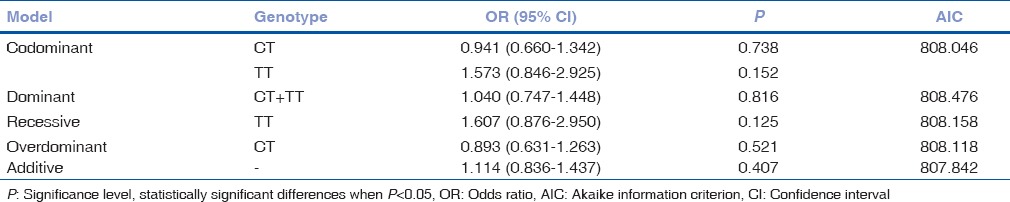
We only observed a tendency of TT genotype to be linked with exudative AMD development when compared to CC genotype, but binomial logistic regression analysis did not reveal statistically significant results [Table 3].
Table 3.
Binomial logistic regression analysis in exudative age-related macular degeneration and control groups
Matrix metalloproteinases-2 (-1306 C/T) rs243865 genotype distribution in exudative age-related macular degeneration and control groups by gender and age
Further MMP-2 (-1306 C/T) rs243865 analysis was made in exudative AMD and the control groups by gender [Table 4] and age [Table 5]. The same tendency for TT genotype to be linked with exudative AMD development was observed in males and females and in the groups of persons younger than 65 years and 65 years and older. Unfortunately, the results did not reach statistical significance.
Table 4.
Frequency of matrix metalloproteinases-2 (-1306 C/T) rs243865 genotypes in exudative age-related macular degeneration and control groups by gender
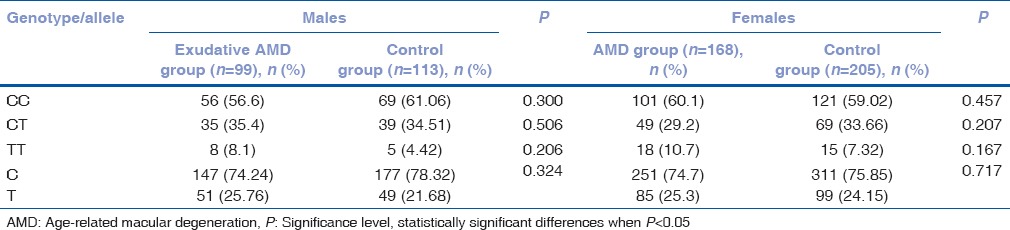
Table 5.
Frequency of matrix metalloproteinases-2 (-1306 C/T) rs243865 genotypes in exudative age-related macular degeneration and control groups by age
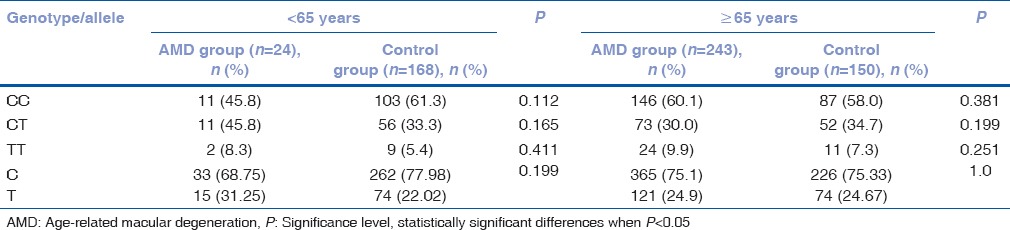
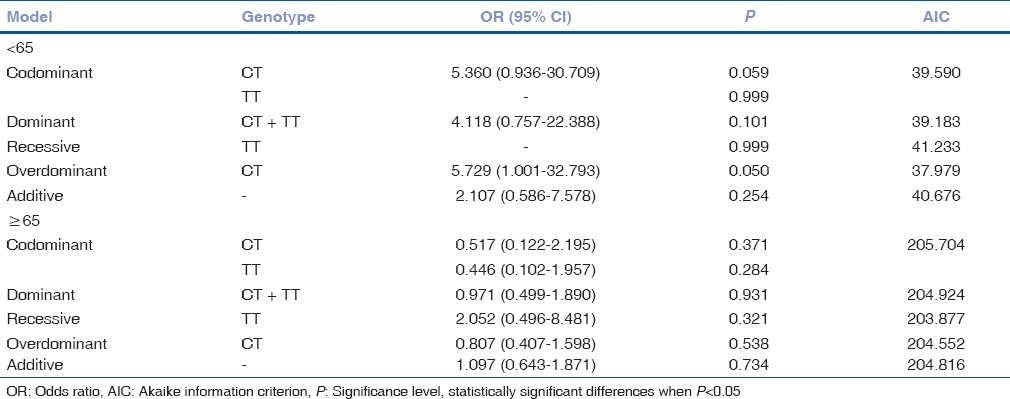
MMP-2 (-1306 C/T) rs243865 genotype distribution analysis in male and female groups by age was performed [Tables 6 and 7]. Analysis of rs243865 genotype distribution in younger and older females did not reveal any statistically significant results. On the other hand, we found CT genotype association with 5.7-fold increased risk of exudative AMD development in younger males (OR = 5.729; 95% CI: 1.001; 32.793; P = 0.05) [Table 8].
Table 6.
Frequency of matrix metalloproteinases-2 (-1306 C/T) rs243865 genotypes in females by age
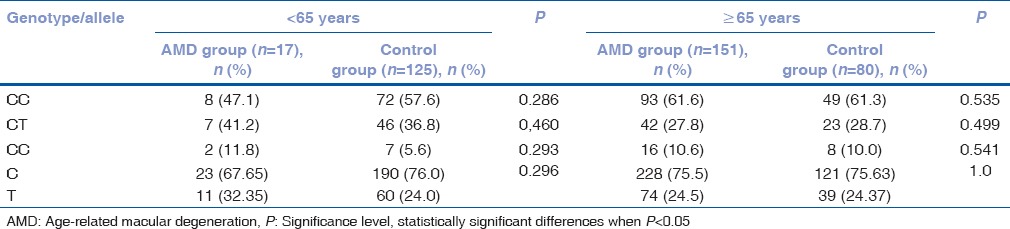
Table 7.
Frequency of matrix metalloproteinases-2 (-1306 C/T) rs243865 genotypes in males by age
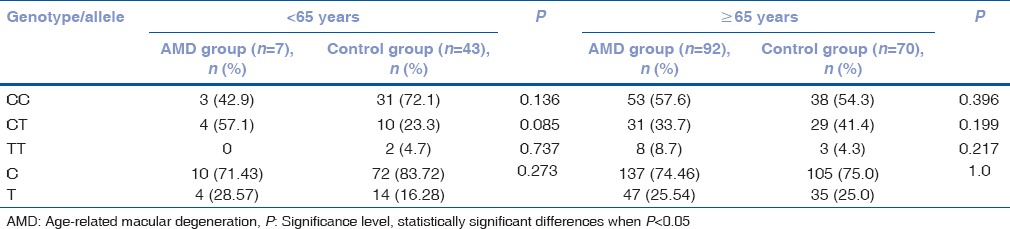
Table 8.
Binomial logistic regression analysis in males of exudative age-related macular degeneration and control groups by age
Discussion
Our results revealed that MMP-2 (-1306 C/T) rs243865 CT genotype showed 5.7-fold increased risk of exudative AMD development compared to CC and TT genotypes together in younger (<65 years) males group (P = 0.05), so we concluded that MMP-2 (-1306 C/T) rs243865 polymorphism may be associated with exudative AMD development in younger males. To the best of our knowledge, currently, there are only three studies analyzing MMP-2 (-1306) C/T gene polymorphism influence on AMD development.[17,18,19] There is only one study[17] analyzing MMP-2 (-1306 C/T) rs243865 gene polymorphism association and only with the early AMD development. Our study analyzed 387 patients with early AMD and a random sample of 682 healthy persons (control group). This study showed a significantly greater prevalence of the C/C and C/T genotypes in the patients with AMD younger than 65 years and those aged ≥65 years, respectively. Moreover, the AMD women aged <65 years were the carriers of the C/C genotype significantly more frequently than their control counterparts.[17] This study draws attention to the limitation of this study as the replication of MMP-2 gene polymorphism in patients with exudative AMD is needed. The study by Seitzman et al. analyzed MMP-2 (-1306) C/T gene polymorphism in females with AMD[19] where association between MMP-2 and early or late AMD in older women was not found.[19] The following study done by Ortak et al. also analyzed genotype distributions and allelic frequencies of MMP2 (-1306C >T). This case-controlled prospective study included 144 AMD patients and 172 control subjects.[18] No significant differences in either genotype distribution or allelic frequencies of MMP2 (-1306C >T) were found among the patients with dry AMD, wet AMD, and control group.[18]
Age and gender interactions with mutations were reported for several genes, such as ApoE, apoA1, lipoprotein lipase, and apoC3, associated with cardiovascular diseases.[20,21,22,23] Russo et al. reported the relationship of the genotype with male gender and younger age.[24] Edelman et al. also revealed several differences associated with the genotype and gender.[25] However, the authors agree that additional studies are required to further explore the relationships between genotypes and gender.[25]
Another study[26] analyzing MMP-2 (-735) C/T gene polymorphism association with the early AMD did not find any statistically significant differences between the patients with early AMD and the control group. A study done by Seitzman et al. proved that MMP-2 rs2287074 SNP (G-->A) was associated with age-related maculopathy (ARM). The A allele was present in 47%, 43%, and 30% of participants with no, early, and late ARM, respectively (P = 0.01) and was associated with lower odds of any ARM.[19] It is known that the MMP2 promoter consists of several cis-acting regulatory elements, which can regulate MMP2 expression through transcription factors, such as p53 and Sp1[27,28] and one of the SNPs in the MMP2 promoter site (C/T transition at nucleotide − 1306) disrupts the Sp1-binding position at the T allele (CCACC BOX) and has significantly lower transcription activity when compared to the C allele.[12] In a study done by Guo et al., some levels of pro-MMP-2 were detected in healthy human Bruch's membrane and choroid, which increased with age.[10] Increased levels of inactive forms of MMP and scarcity of active forms of MMP-2 suggested possible involvement of impaired extracellular degradation in both aging and macular degeneration. The study authors stated that reduction in the levels of activated MMP-2 and MMP-9 may be responsible for the impaired matrix degradation of Bruch's membrane, leading to the pathology associated with AMD.[10] Furthermore, in another study, the activity of gelatinase A (MMP-2), the most abundant MMP in IPM (interphotoreceptor matrix) and vitreous, was measured with respect to age in normal human donor eyes and compared to donors with AMD. Comparing normal and AMD donors, there was no significant difference in the gelatinase A levels in vitreous or in retina-associated IPM. However, the level of gelatinase A was nearly doubled in retinal pigment epithelium-associated IPM, compared to the eyes with AMD. It was concluded by a research team, that gelatinase A may be associated with neovascularization.[29] Chau et al. analyzed circulating MMP-2 levels in AMD patients and healthy individuals and did not reveal any differences.[30] However, Steen et al. detected active tissue MMP-2 in the areas of new vessels formation and in the enveloping of the Bruch-like membrane, suggesting that MMP-2 and MMP-9 may be cooperatively involved in the progressive growth of choroidal neovascular membranes in AMD.[31] In contrast to the positive association observed between MMP-2 expression and choroidal neovascularization in mice study showed that estrogen depletion in ovariectomized mice resulted in a loss of MMP-2 expression and subsequent changes associated with dry ARM, such as sub-retinal pigmented epithelial (sub-RPE) deposit formation and Bruch's membrane thickening.[32] Both male and female 16-month-old mice showed qualitatively similar basal laminar deposit morphology, but the severity of thickness, continuity, and content were significantly greater in female mice. Aged female mice also demonstrated a trend toward more severe endothelial changes and increased Bruch's membrane thickening compared with age-matched male mice. It was concluded that estrogen deficiency may increase susceptibility to formation of sub-RPE deposits by dysregulating turnover of Bruch's membrane contributing to collagenous thickening and endothelial changes. Estrogen supplementation at the dosages used in this study did not appear to protect against formation of sub-RPE deposits.[32] Hussain et al.[11] determined that the free and total level of pro-MMP-2 was reduced in AMD (P< 0.05). Also, there was reduction in bound and total levels of active MMP-2 (P < 0.005 and P < 0.05, respectively) in AMD samples. Total levels (bound plus free) of active MMP-2 were significantly reduced in AMD donors (age range, 71–95 years) (P< 0.05).[11]
Conclusion
Our results showed that MMP-2 (-1306 C/T) rs243865 CT genotype showed 5.7-fold increased risk of exudative AMD development compared to CC and TT genotypes together in younger (<65 years) males group.
The novelty and strengths of our study include a thorough clinical examination of patients. First, we used very strict inclusion/exclusion criteria: all patients were examined to exclude IHD and completed a questionnaire about the risk factors of IHD and clinical symptoms. Patients with no symptoms of typical chest angina and with no typical ischemic changes on the ECG were included in the final study population. All patients were consulted by a general practitioner and a neurologist. Patients with malignant tumors, rheumatoid diseases, and end-stage liver or renal diseases were excluded from the study as well. Second, only healthy controls were included into our research as control group. Third, compared to other three studies, we included more patients with AMD and more healthy controls.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–95. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 2.Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT, et al. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857–64. [PubMed] [Google Scholar]
- 3.Okubo A, Rosa RH, Jr, Bunce CV, Alexander RA, Fan JT, Bird AC, et al. The relationships of age changes in retinal pigment epithelium and Bruch's membrane. Invest Ophthalmol Vis Sci. 1999;40:443–9. [PubMed] [Google Scholar]
- 4.Karwatowski WS, Jeffries TE, Duance VC, Albon J, Bailey AJ, Easty DL, et al. Preparation of Bruch's membrane and analysis of the age-related changes in the structural collagens. Br J Ophthalmol. 1995;79:944–52. doi: 10.1136/bjo.79.10.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Handa JT, Verzijl N, Matsunaga H, Aotaki-Keen A, Lutty GA, te Koppele JM, et al. Increase in the advanced glycation end product pentosidine in Bruch's membrane with age. Invest Ophthalmol Vis Sci. 1999;40:775–9. [PubMed] [Google Scholar]
- 6.Pauleikhoff D, Zuels S, Sheraidah GS, Marshall J, Wessing A, Bird AC, et al. Correlation between biochemical composition and fluorescein binding of deposits in Bruch's membrane. Ophthalmology. 1992;99:1548–53. doi: 10.1016/s0161-6420(92)31768-3. [DOI] [PubMed] [Google Scholar]
- 7.Holz FG, Sheraidah G, Pauleikhoff D, Bird AC. Analysis of lipid deposits extracted from human macular and peripheral Bruch's membrane. Arch Ophthalmol. 1994;112:402–6. doi: 10.1001/archopht.1994.01090150132035. [DOI] [PubMed] [Google Scholar]
- 8.Tatar O, Adam A, Shinoda K, Eckert T, Scharioth GB, Klein M, et al. Matrix metalloproteinases in human choroidal neovascular membranes excised following verteporfin photodynamic therapy. Br J Ophthalmol. 2007;91:1183–9. doi: 10.1136/bjo.2007.114769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyman L, Neborsky R. Risk factors for age-related macular degeneration: An update. Curr Opin Ophthalmol. 2002;13:171–5. doi: 10.1097/00055735-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Guo L, Hussain AA, Limb GA, Marshall J. Age-dependent variation in metalloproteinase activity of isolated human Bruch's membrane and choroid. Invest Ophthalmol Vis Sci. 1999;40:2676–82. [PubMed] [Google Scholar]
- 11.Hussain AA, Lee Y, Zhang JJ, Marshall J. Disturbed matrix metalloproteinase activity of Bruch's membrane in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:4459–66. doi: 10.1167/iovs.10-6678. [DOI] [PubMed] [Google Scholar]
- 12.Price SJ, Greaves DR, Watkins H. Identification of novel, functional genetic variants in the human matrix metalloproteinase-2 gene: Role of sp1 in allele-specific transcriptional regulation. J Biol Chem. 2001;276:7549–58. doi: 10.1074/jbc.M010242200. [DOI] [PubMed] [Google Scholar]
- 13.Peasey A, Bobak M, Kubinova R, Malyutina S, Pajak A, Tamosiunas A, et al. Determinants of cardiovascular disease and other non-communicable diseases in Central and Eastern Europe: Rationale and design of the HAPIEE study. BMC Public Health. 2006;6:255. doi: 10.1186/1471-2458-6-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabauskas V, Miseviciene I, Klumbiene J, Petkeviciene J, Milasauskiene Z, Plieskiene A, et al. Prevalence of dyslipidemias among lithuanian rural population (CINDI program) Medicina (Kaunas) 2003;39:1215–22. [PubMed] [Google Scholar]
- 15.Lesauskaite V, Macijauskiene J, Rader E. Challenges and opportunities of health care for the aging community in Lithuania. Gerontology. 2006;52:40–4. doi: 10.1159/000089824. [DOI] [PubMed] [Google Scholar]
- 16.Age-Related Eye Disease Study Research Group. The age-related eye disease study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: The Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol. 2001;132:668–81. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- 17.Liutkeviciene R, Lesauskaite V, Zaliaduonyte-Peksiene D, Sinkunaite-Marsalkiene G, Zaliuniene D, Mizariene V, et al. Role of MMP-2 (-1306 C/T) polymorphism in age-related macular degeneration. Ophthalmic Genet. 2016;37:170–6. doi: 10.3109/13816810.2015.1020556. [DOI] [PubMed] [Google Scholar]
- 18.Ortak H, Demir S, Ateş Ö, Benli İ, Söǧüt E, Sahin M, et al. The role of MMP2 (-1306C>T) and TIMP2 (-418 G<C) promoter variants in age-related macular degeneration. Ophthalmic Genet. 2013;34:217–22. doi: 10.3109/13816810.2013.781192. [DOI] [PubMed] [Google Scholar]
- 19.Seitzman RL, Mahajan VB, Mangione C, Cauley JA, Ensrud KE, Stone KL, et al. Estrogen receptor alpha and matrix metalloproteinase 2 polymorphisms and age-related maculopathy in older women. Am J Epidemiol. 2008;167:1217–25. doi: 10.1093/aje/kwn024. [DOI] [PubMed] [Google Scholar]
- 20.Schaefer EJ, Lamon-Fava S, Johnson S, Ordovas JM, Schaefer MM, Castelli WP, et al. Effects of gender and menopausal status on the association of apolipoprotein E phenotype with plasma lipoprotein levels. Results from the framingham offspring study. Arterioscler Thromb. 1994;14:1105–13. doi: 10.1161/01.atv.14.7.1105. [DOI] [PubMed] [Google Scholar]
- 21.Peacock RE, Temple A, Gudnason V, Rosseneu M, Humphries SE. Variation at the lipoprotein lipase and apolipoprotein AI-CIII gene loci are associated with fasting lipid and lipoprotein traits in a population sample from Iceland: Interaction between genotype, gender, and smoking status. Genet Epidemiol. 1997;14:265–82. doi: 10.1002/(SICI)1098-2272(1997)14:3<265::AID-GEPI5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Russo GT, Meigs JB, Cupples LA, Demissie S, Otvos JD, Wilson PW, et al. Association of the sst-I polymorphism at the APOC3 gene locus with variations in lipid levels, lipoprotein subclass profiles and coronary heart disease risk: The Framingham Offspring Study. Atherosclerosis. 2001;158:173–81. doi: 10.1016/s0021-9150(01)00409-9. [DOI] [PubMed] [Google Scholar]
- 23.Hegele RA, Evans AJ, Tu L, Ip G, Brunt JH, Connelly PW, et al. Agene-gender interaction affecting plasma lipoproteins in a genetic isolate. Arterioscler Thromb. 1994;14:671–8. doi: 10.1161/01.atv.14.5.671. [DOI] [PubMed] [Google Scholar]
- 24.Russo GT, Friso S, Jacques PF, Rogers G, Cucinotta D, Wilson PW, et al. Age and gender affect the relation between methylenetetrahydrofolate reductase C677T genotype and fasting plasma homocysteine concentrations in the Framingham Offspring Study Cohort. J Nutr. 2003;133:3416–21. doi: 10.1093/jn/133.11.3416. [DOI] [PubMed] [Google Scholar]
- 25.Edelman EA, Girirajan S, Finucane B, Patel PI, Lupski JR, Smith AC, et al. Gender, genotype, and phenotype differences in smith-magenis syndrome: A meta-analysis of 105 cases. Clin Genet. 2007;71:540–50. doi: 10.1111/j.1399-0004.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- 26.Liutkeviciene R, Lesauskaite V, Sinkunaite-Marsalkiene G, Zaliuniene D, Zaliaduonyte-Peksiene D, Mizariene V, et al. The role of matrix metalloproteinases polymorphisms in age-related macular degeneration. Ophthalmic Genet. 2015;36:149–55. doi: 10.3109/13816810.2013.838274. [DOI] [PubMed] [Google Scholar]
- 27.Bian J, Sun Y. Transcriptional activation by p53 of the human type IV collagenase (gelatinase A or matrix metalloproteinase 2) promoter. Mol Cell Biol. 1997;17:6330–8. doi: 10.1128/mcb.17.11.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin H, Sun Y, Benveniste EN. The transcription factors sp1, sp3, and AP-2 are required for constitutive matrix metalloproteinase-2 gene expression in astroglioma cells. J Biol Chem. 1999;274:29130–7. doi: 10.1074/jbc.274.41.29130. [DOI] [PubMed] [Google Scholar]
- 29.Plantner JJ, Jiang C, Smine A. Increase in interphotoreceptor matrix gelatinase A (MMP-2) associated with age-related macular degeneration. Exp Eye Res. 1998;67:637–45. doi: 10.1006/exer.1998.0552. [DOI] [PubMed] [Google Scholar]
- 30.Chau KY, Sivaprasad S, Patel N, Donaldson TA, Luthert PJ, Chong NV, et al. Plasma levels of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye (Lond) 2008;22:855–9. [PubMed] [Google Scholar]
- 31.Steen B, Sejersen S, Berglin L, Seregard S, Kvanta A. Matrix metalloproteinases and metalloproteinase inhibitors in choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1998;39:2194–200. [PubMed] [Google Scholar]
- 32.Cousins SW, Marin-Castaño ME, Espinosa-Heidmann DG, Alexandridou A, Striker L, Elliot S, et al. Female gender, estrogen loss, and sub-RPE deposit formation in aged mice. Invest Ophthalmol Vis Sci. 2003;44:1221–9. doi: 10.1167/iovs.02-0285. [DOI] [PubMed] [Google Scholar]


