Abstract
Background:
Stress is an invisible factor affecting modern day living and is strongly associated with many disease pathogenesis including polycystic ovarian syndrome (PCOS) in women. PCOS is the most frequent endocrinological disorder that affects women of reproductive age, leading to metabolic dysfunction and body composition alterations. Salivary amylase and cortisol are major stress mediators that have been implicated in PCOS. However, their role in altering body composition in PCOS is yet to be deciphered.
Aim:
The present study aimed at understanding the relation between stress-associated factors and alterations in body composition among PCOS patients.
Design:
This study enrolled a total of 100 patients (PCOS) and 60 age-matched controls. The female patients were of ages between 13 and 30 years.
Materials and Methods:
Standard assay kits were used to evaluate the α-amylase activity and cortisol level in saliva. The participants were chosen on the basis of the Rotterdam American Society for Reproductive Medicine/European Society of Human Reproduction criteria. Saliva was collected from each participant as per the protocol of Salimetrics, USA.
Statistical Analysis:
Statistical analysis was performed using SPSS version 20 for Windows. The quantitative variables are described as mean ± standard deviation. P < 0.05 was considered significant.
Results:
Increased salivary cortisol level and α-amylase activity were seen in the PCOS population as compared to age-matched controls suggesting patients a sustained stress scenario in their system. Moreover, overweight PCOS participants reflected higher amylase activity than the lean patients participants. Pulse rate, body mass index (BMI), visceral adiposity, and waist-hip ratio (WHR) was considerably higher in the PCOS patients participants compared to controls. A significant correlation could be drawn between the α-amylase activity and BMI or WHR, respectively, among PCOS patients. These observations indicate a strong link between the stress marker and alterations in the body composition parameters of PCOS patients participants.
Conclusion:
Higher prevalence of stress in PCOS patients participants has a critical role in their altered body composition.
KEYWORDS: Polycystic ovarian syndrome, salivary cortisol, salivary α-amylase, stress
INTRODUCTION
Polycystic ovarian syndrome (PCOS) is one of the most common endocrinopathy affecting 4%–8% of women of reproductive age.[1] Despite its heterogeneous nature, the hallmarks of the disease are hyperandrogenism and chronic anovulation.[2] According to the Rotterdam consensus, the presence of two out of the three following criteria is deemed necessary for diagnosis of PCOS: (a) oligo-ovulation or anovulation, (b) clinical and/or biochemical signs of hyperandrogenism, and (c) polycystic ovaries by ultrasound and also the exclusion of other related disorders.[3] However, in addition to its reproductive features, PCOS also has numerous metabolic consequences including increased risk of obesity,[4] insulin resistance,[5] type 2 diabetes mellitus,[6] and premature atherosclerosis.[7] Many women with PCOS meet the criteria for metabolic syndrome (MBS) as they report a higher incidence of hypertension, dyslipidemia, and visceral adiposity.[8] Recently, a transforming growth factor-β called anti-Mullerian hormone was found to be a contributor of this disease.[9] Obesity is common in PCOS, exacerbates the symptoms, and promotes negative health consequences. Obesity and fat distribution are supposed to play an important role in the etiology of PCOS.[10] About 40% of women with PCOS are obese with abdominal fat distribution.[10] These changes in body composition intensify many of the clinical manifestations associated with this disease such as hyperandrogenism and insulin resistance.
Stress plays a major role in the pathogenesis of a number of diseases.[11,12,13] It is a common and commonly underappreciated cause of reproductive dysfunction. Stress-induced anovulation leads to infertility. There are increasing reports stating the role of stress in PCOS manifestation.[14]
Salivary α-amylase (SAA) and cortisol have been implicated as sensitive biomarkers for stress-related changes in the body, with essential roles in metabolic homeostasis. These are reflective of the activation of the sympathetic-adrenal medullary (SAM) and hypothalamic–pituitary–adrenal (HPA) axis respectively.[15,16,17] Although the role of SAA and cortisol in stress has been understood, very little is known about the association of these stress mediators with altered body composition which is a major manifestation among women suffering from PCOS. In this study, we therefore tried to evaluate the role of SAA and cortisol in modulating the general body composition among PCOS patients.
MATERIALS AND METHODS
Survey schedule
One hundred women in the reproductive age group (13–30 years) of city Kolkata and surroundings, West Bengal, India, with the primary complaints of menstrual irregularities with or without infertility were evaluated in detail for PCOS. Sixty age-matched controls were taken to compare the parameters under study. This study was approved by the Institutional Ethical Committee of the University of Calcutta, and all participants gave bilingual written informed consent. Survey was conducted on patients visiting the OPD(out patient department), Gynecology and obstetrics of IPGMER, SSKM Hospital. Necessary permission from the appropriate authority was formally taken as per rule to conduct the survey and collect the samples. Survey was done from November, 2014 to July, 2016. All those with a final diagnosis of PCOS were included in the study. PCOS was diagnosed according to the 2003 Rotterdam criteria (European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine PCOS consensus workshop group) with at least two of the following three features: (i) oligo-ovulation or anovulation, (ii) clinical and/or biochemical hyperandrogenism, and (iii) ultrasound appearance of polycystic ovaries.[3] The Rotterdam classification was used to define PCOS in the event of[1] menstrual anomalies such as (1) oligo-ovulation and/or anovulation were characterized by oligomenorrhea (intermenstrual intervals of ≥35 days) and amenorrhea (intervals >3 months), (2) clinical and/or biochemical hyperandrogenism, and (3) ultrasound sonography appearance of polycystic ovaries (multiple cysts >12 in number of 2–9 mm size). The presence of two of these three criteria was required to diagnose PCOS once all other diagnosis, such as congenital adrenal hyperplasia, virilizing tumor, Cushing syndrome, and prolactinoma were ruled out.[3,18]
Collection of anthropometric data
Weight and height were measured using digital weighing scale and portable stature meter in kg and cm, respectively, in basal condition with minimum clothing and accessories. Waist circumference was measured using a flexible fiberglass tape at the approximate midpoint between the lower margin of the last palpable rib and the top of the iliac crest without clothing and in standing position.[19] Hip circumference was measured at the around the widest portion of the buttocks.[19] A body composition analyzer (Model: OMRON-HBF-375) based on the principle of bioelectrical impedance was used for estimating body mass index (BMI) and percent body fat.[20,21]
Measurement of cardiovascular parameters
Systolic and diastolic blood pressure, O2 saturation percentage, and pulse rate were measured by sphygmomanometer and pulse oximeter, respectively.[22,23]
Survey of menstrual history
Participants were asked about the menstrual history in terms of menarche, dysmenorrhea, irregular menstruation, and other problems if any by presented questionnaires on a predesigned and pretested pro forma.
Measurement of hemoglobin
Hemoglobin (Hb) of controls and PCOS patients was measured by cyanmethemoglobin method using ready to use cyanmethaemoglobin reagent (Drabkin's solution) and standard (BeneSphera, Avantor Performance Materials India Limited, Uttarakhand, India). Whole blood was collected by red blood cell pipette and 20 μl of it was mixed with 5 ml of cyanmeth reagent in a test tube marked as test, mixed thoroughly, and incubated for 3 min at room temperature (25°C). Five milliliter of cyanmeth reagent served as blank. Absorbance of test (AT) was measured against the blank (AB) at 540 nm. Hb concentration was calculated as, Hb concentration (gm/dl) = AT/AB × Factor (Factor = 15 gm/dl, as Hb standard of 60 mg/dl was used which is equivalent to Hb concentration of 15 gm/dl).[24]
Survey of stress biomarkers
Collection of saliva
Saliva samples were collected with study participants sitting upright in a comfortable position and tilting the head forward, allowing the saliva to pool on the floor of the mouth, and then passing the saliva through the saliva collection aid (Salimetrics, Item No. 5016.02) in a polypropylene vial.[23] Samples were collected in an ice-chilled graduated saliva collector by the passive drool through a short straw into a 1.8-ml cryovial and immediately frozen.[25,26,27]
Assay of salivary α-amylase
Saliva samples were centrifuged at 3000 rpm for 15 min, and clear supernatant was processed immediately for estimation of A-A. The assay employs a chromogenic substrate, 2-chloro-4-nitrophenol linked to maltotriose (CNP-Gal-G2). A-A catalyses the hydrolysis of (CNP-Gal-G2) to CNP, and the rate of hydrolysis is measured as an increase in absorbance due to the formation of CNP which is proportional to the α-amylase activity in the sample (Direct Substrate Method, Kinetic Enzymatic, Crest Biosystem, Goa, India).
Assay of salivary cortisol
Saliva cortisol was designed to be assayed by salivary cortisol ELISA assay kit from Diametra (using ELISA reader-Bio-Rad-680). During the course of analysis,first, the wells of the ELISA plates were marked as calibrators, samples, and blank. Then, 25 μL of each of the calibrators and samples was taken in the respective wells and 200 μL of diluted conjugate was added to each of the wells leaving the blank empty. This was then incubated at 37°C for 1 h. Following this, the contents of the wells were removed and washed with 300 μl of diluted wash solution for 3 times. A volume of 100 μl of tetramethylbenzidine substrate was added to all the wells including the blank and incubated at room temperature for 15 min in the dark with gentle shaking. At the end of incubation, 100 μl of stop solution was added and the absorbance was read at 450 nm against blank within 5 min. The mean value of absorbance of the calibrators was plotted against concentration and a best fit curve was obtained. The absorbance values of the samples were interpolated on the calibration curve to obtain the corresponding values of the concentrations expressed in ng/ml.
Statistical analysis
The collected data were analyzed using the Statistical Package for the Social Sciences, version 20 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) for all variables. Results were expressed as mean ± standard deviation (SD). The group means were compared by one-way ANOVA followed by Turkey's post hoc test, and Pearson correlation was performed to evaluate the R (Pearson correlation coefficient) between respective parameters, with 95% confidence intervals, and P < 0.05 is deemed statistically significant. To maintain data quality, all anthropometric measurements were taken in duplicate and had a high coefficient of reliability (R = 0.99). The technical error of measurement for them was well within the limits.[28]
RESULTS
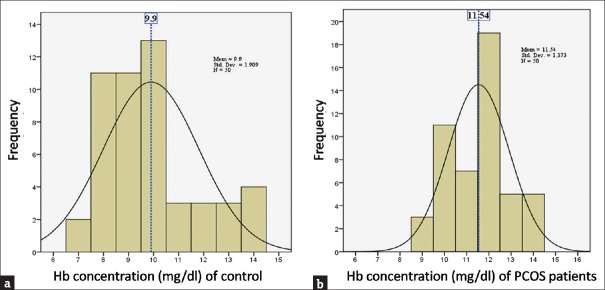
Anthropometric, biochemical, and clinical parameters were screened in patients with PCOS and in healthy controls as shown in Table 1. We studied 100 patients with PCOS (mean age: 21.87 ± 4.67 years, range: 13–30 years) and 60 age-matched healthy controls (mean age: 20.69 ± 1.38 years, range: 18–24 years). However, during the course of the survey, several participants dropped out. Table 2 reflects the menstrual history of the PCOS patients (N = 98). The Gaussian distribution of Hb concentration [Figure 1] shows that the mean Hb concentrations were 11.54 g/dl (SD ± 1.373) and 9.9 g/dl (SD ± 1.909) in PCOS patients and controls, respectively.
Table 1.
Summary of anthropometric, clinical, and biochemical parameters of polycystic ovarian syndrome women and controls
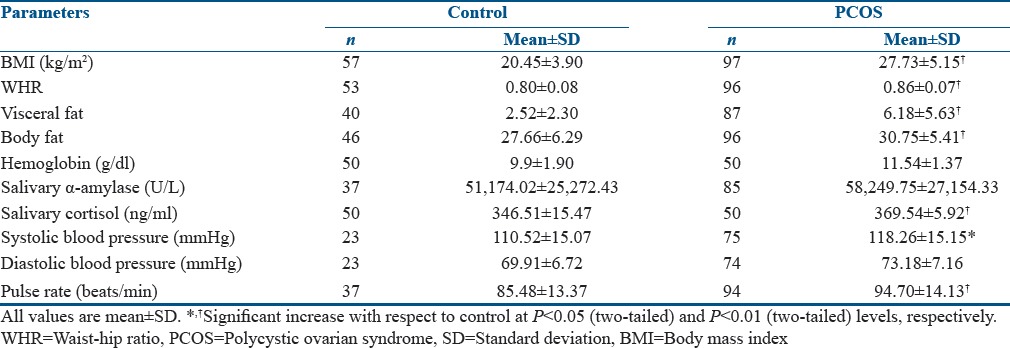
Table 2.
Menstrual history of polycystic ovarian syndrome patients (n=98)

Figure 1.
Hemoglobin concentration of (a) controls and (b) polycystic ovarian syndrome patients
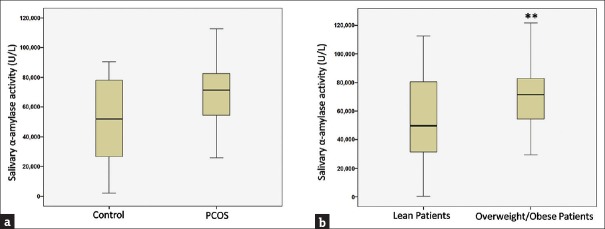
There is alteration in SAA activity in PCOS patients. It is evident from our study that stress is critically associated with PCOS. An increase of 13.82% in SAA activity was noted among PCOS women with respect to age-matched controls [Figure 2a]. Furthermore, a subgroup analysis revealed heightened salivary amylase activity (39.92%; P < 0.01) among overweight/obese patients (BMI >25 kg/m2) in comparison to lean patients (BMI <25 kg/m2) [Figure 2b].
Figure 2.
Salivary α-amylase activity (a) control and polycystic ovarian syndrome women (b) lean and obese polycystic ovarian syndrome patients. The box plot represents 25%, 50%(median), and 75% quartile values, and the error bar represents the standard deviation. **Significant change with respect to control at P < 0.01 level
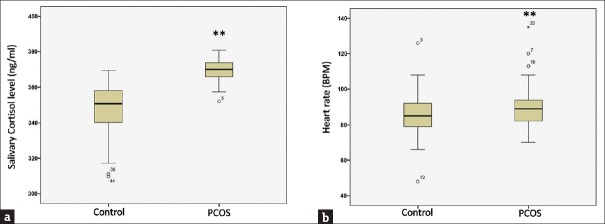
Deregulation of the HPA axis is associated with PCOS. Cortisol, which is the major effector hormone of the HPA axis and a strong regulator of systemic stress pathway,[29] showed 6.64% (P < 0.01) increase in PCOS patients as compared to age-matched controls [Figure 3a]. It has been previously reported that heart rate is strongly associated with HPA axis activation and body composition alterations;[30] a heart rate rise of 9.37% (P < 0.01) was observed among the patients as compared to age-matched controls [Figure 3b].
Figure 3.
Activation of hypothalamic–pituitary–adrenal axis along with heart rate in polycystic ovarian syndrome patients in comparison to age-matched control (a) salivary cortisol level (b) heart rate. All values in the respective box plots show 25%, 50% (median), and 75% quartile values, and the error bar indicates the standard deviation. **The significant change with respect to control at P < 0.01 level
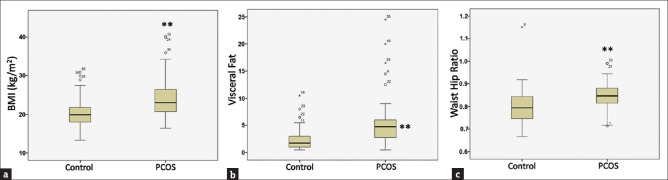
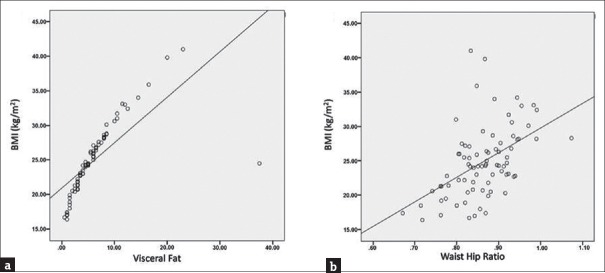
Relevant studies have shown that body composition alterations are preponderant among PCOS cases. To further ascertain this, BMI, visceral fat, and waist-hip ratio (WHR) were compared between PCOS patients and controls. It was observed that women with PCOS had significantly higher BMI (20.9%; P < 0.01) and visceral fat (144.9%; P < 0.01), respectively [Figure 4a and b]. Furthermore, there was 7.27% (P < 0.01) increase in WHR in PCOS patients as compared to control women [Figure 4c]. A comparison was also studied between WHR of lean controls and lean PCOS patients, and the results reflected a significant difference between the two groups (P = 0.002; P < 0.01) [Table 3a]. A strong positive association between BMI and visceral fat composition (Pearson correlation coefficient, R = 0.726; P < 0.01) and WHR (R = 0.488; P < 0.01), respectively, was evident in PCOS patients [Figure 5a, b and Table 4], suggesting increased alterations in body composition in these women.
Figure 4.
Altered body composition in polycystic ovarian syndrome patients (a) body mass index (b) visceral fat (c) waist-hip ratio. The respective box plots are representing the 25%, 50% (median), and 75% quartiles, whereas the error bars indicate the standard deviation. **Significant change with respect to control at P < 0.01 level
Table 3a.
T-test between waist-hip ratio of lean control and lean polycystic ovarian syndrome patients
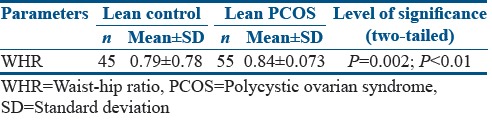
Figure 5.
Correlation plots of association between (a) body mass index and visceral fat (b) body mass index and waist-hip ratio
Table 4.
Correlation between BMI and body composition parameters (Visceral Fat and Waist Hip Ratio respectively) among PCOS patients

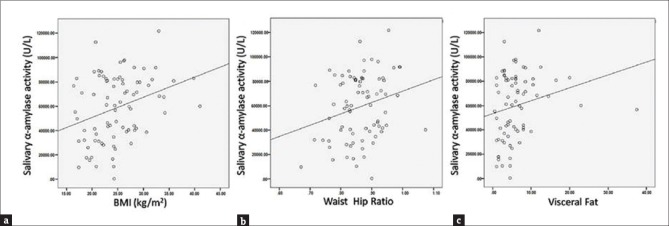
In this present study, the prevalence of increased salivary amylase activity being higher in overweight and obese PCOS women [Figure 2b] suggested an association between stress mediators such as amylase and altered body composition in these women. Further elaboration on this finding led us to the observation that there is a significant association between the BMI of these women and their salivary amylase activity (R = 0.314; P < 0.01) [Figure 6a and Table 5]. A t-test was performed to evaluate if BMI is the dictating factor for the heightened amylase activity in the PCOS patients. Interestingly, results showed no significant change in the amylase activity between the lean and overweight/obese patients (P = 0.154) [Table 3b], indicating that BMI is not the only contributing factor to increased amylase activity in the PCOS women. Furthermore, a strong positive correlation could be drawn between their WHR and amylase activity (R = 0.244; P < 0.05) indicating the impact of stress in altering body fat distribution [Figure 6b and Table 5]. A positive association was also observed between their visceral fat index and the amylase activity (R = 0.216), but it failed to achieve statistical significance [Figure 6c and Table 4], possibly because of the inability of this present study to accommodate a larger number of samples. Hence, salivary cortisol levels and α-amylase activity may be linked to alterations in body composition among PCOS patients.
Figure 6.
Correlation plots of association between (a) salivary α-amylase activity and body mass index (b) salivary α-amylase activity and waist.hip ratio (c) salivary α-amylase activity and visceral fat
Table 5.
Correlation of salivary alpha-amylase activity with BMI, WHR and VF among PCOS patients
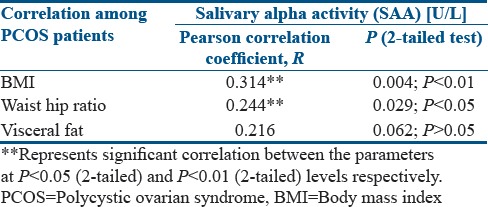
Table 3b.
T-test of amylase activity between polycystic ovarian syndrome patients with body mass index <25 kg/m2 and above 25kg/m2

DISCUSSION
PCOS is the most frequent endocrine abnormality in women of reproductive age.[1] Menstrual irregularity is a cardinal feature of PCOS that may manifest as anovulation with erratic bleedings. Ovarian dysfunction is usually associated with oligomenorrhea/amenorrhea resulting from chronic oligo-ovulation/anovulation.[31,32] In our study, 42.4% of the patients reported of oligomenorrhea (menstrual cycles lasting >35 days with 4–9 cycles/year) and 13.6% of amenorrhea [Table 2]. Irregular menses can be painful because the infrequent occurrence often leads to increased cramping with the heavier flow.[33] Nearly 57.14% and 71.4% of the patients [Table 2] in our study complained of dysmenorrhea and passage of clots, respectively. Besides, the mean Hb value (g/dl) was also elevated in women suffering from PCOS as compared to the age-matched controls [Figure 1 and Table 1], an observation that might be attributed to the fewer number of menstrual cycles in the afflicted women.[34]
There are multiple factors which contribute to the pathogenesis of the disease, stress being one of them. It has been hypothesized that stress plays an important role in modulating the different clinical symptoms in PCOS patients.[14] In our study, we tried to evaluate the association of stress-related factors such as SAA and cortisol with alterations in body fat composition among PCOS patients. Stress responsiveness is primarily regulated by two neuroendocrine axes: HPA and SAM systems.[35,36] Salivary cortisol, a noninvasive index of free circulating levels of cortisol, is used as a measure of the HPA axis activation.[37] SAA, an enzyme that hydrolyzes starch in the oral cavity, is secreted by the parotid gland in response to adrenergic activity.[35] Studies have provided evidence that stimulation of the SAM system could significantly increase SAA levels rendering it a sensitive indicator of sympathetic activity.[37] The findings of our present study showed that both SAA activity [Figure 2a] and salivary cortisol level [Figure 3a] were higher in PCOS patients as compared to age-matched controls implicating an exaggerated response of the central stress stations among the affected women. Pulse rate associated with HPA activation was also elevated among these women [Figure 3b]. WHR of lean PCOS patients and controls [Table 3a] reflected the prevalence of androgenic influence on body fat distribution among the PCOS women compared to their age-matched controls.[10] On considering their BMI, the SAA activity was significantly higher among the overweight/obese PCOS patients than the lean patients [Figure 2b]; also, the overall BMI was found to be significantly higher [Figure 4a] in the PCOS-affected women compared to their age-matched controls. These observations suggest that a strong association exists between stress or stress-related factors and altered body composition.[8] Obesity is a common finding in women with PCOS, and between 40% and 80% of women with this condition are overweight or obese.[10] Fat distribution is not a homogeneous phenotype, and the android pattern of body fat with disproportionate adipose tissue distribution in the abdominal visceral depots is a key feature prevalent in 50%–60% of women with PCOS.[10] Such upper body fat distribution in the affected women per se contributes to hyperandrogenism and consequent hyperinsulinemia.[38] Hyperinsulinemia itself contributes to obesity by the anabolic effect on fat metabolism through adipogenesis.[39] Therefore, a vicious cycle operates in which android fat produces more android fat and exacerbates the predisposition toward weight gain.[39] The WHR is an index for assessing the android and gynoid obesity (android >0.8; gynoid <0.8).[40] Our study showed that both visceral fat and WHR were significantly altered [Figure 4b and c] in the patients compared to the control group which is in concordance to previous reports.[41,42,43] A significant correlation could be established between SAA activity and BMI and WHR, respectively. Table 3b indicates that in spite of differences in BMI, there is not any significant change in the amylase activity of the patients. It can thus be inferred that obesity and a higher BMI is not the only factor that dictates heightened amylase activity in the PCOS patients. Visceral fat also showed positive correlation with SAA activity although a statistical significance was not achieved [Figure 6 and Table 3]. These findings indicate that stress might play a pivotal role in modifying body fat distribution in PCOS patients which, in turn, predisposes them to MBS and cardiovascular diseases.
CONCLUSION
It can be concluded from the results that stress or more precisely stress-associated factors are positively associated with body composition alterations in PCOS individuals, as compared to age-matched controls, which needs to be further validated in future studies with a mechanistic and molecular viewpoint. In our future endeavors, we aim to develop animal models to understand the contribution of exposure to stress on the development of this syndrome and how they compare with the phenotype seen in women with PCOS.
Financial support and sponsorship
This study was financially supported by the University Grants Commission, Government of India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors take full responsibility for the content of this article and wish to thank the Department of Obstetrics and Gynecology, IPGME and R (SSKM Hospital), Kolkata, India, for giving permission to collect the sample and data from the outdoor patients. Authors are thankful to Dr. Sreya Chattopadhyay, Department of Physiology, University of Calcutta, and Dr. Nilansu Das, Department of Molecular Biology, Surendranath College, for giving technical support and also grateful to the Principal, Surendranath College, for providing infrastructural facility to carry out the research work. The authors would like to thank all the women who participated in this study.
REFERENCES
- 1.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–3. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bremer AA. Polycystic ovary syndrome in the pediatric population. Metab Syndr Relat Disord. 2010;8:375–94. doi: 10.1089/met.2010.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 4.Anderson AD, Solorzano CM, McCartney CR. Childhood obesity and its impact on the development of adolescent PCOS. Semin Reprod Med. 2014;32:202–13. doi: 10.1055/s-0034-1371092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakkakula BV, Thangavelu M, Godla UR. Genetic variants associated with insulin signaling and glucose homeostasis in the pathogenesis of insulin resistance in polycystic ovary syndrome: A systematic review. J Assist Reprod Genet. 2013;30:883–95. doi: 10.1007/s10815-013-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Leo V, Musacchio MC, Cappelli V, Massaro MG, Morgante G, Petraglia F, et al. Genetic, hormonal and metabolic aspects of PCOS: An update. Reprod Biol Endocrinol. 2016;14:38. doi: 10.1186/s12958-016-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughan KS, Tfayli H, Warren-Ulanch JG, Barinas-Mitchell E, Arslanian SA. Early biomarkers of subclinical atherosclerosis in obese adolescent girls with polycystic ovary syndrome. J Pediatr. 2016;168:104–10. doi: 10.1016/j.jpeds.2015.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glueck CJ, Papanna R, Wang P, Goldenberg N, Sieve-Smith L. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism. 2003;52:908–15. doi: 10.1016/s0026-0495(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 9.Wiweko B, Susanto CA. The effect of metformin and cinnamon on serum anti-mullerian hormone in women having PCOS: A Double-blind, randomized, controlled trial. J Hum Reprod Sci. 2017;10:31–6. doi: 10.4103/jhrs.JHRS_90_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Legro RS. Obesity and PCOS: Implications for diagnosis and treatment. Semin Reprod Med. 2012;30:496–506. doi: 10.1055/s-0032-1328878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conrad CD, Mauldin-Jourdain ML, Hobbs RJ. Metyrapone reveals that previous chronic stress differentially impairs hippocampal-dependent memory. Stress. 2001;4:305–18. doi: 10.3109/10253890109014754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deak T, Quinn M, Cidlowski JA, Victoria NC, Murphy AZ, Sheridan JF, et al. Neuroimmune mechanisms of stress: Sex differences, developmental plasticity, and implications for pharmacotherapy of stress-related disease. Stress. 2015;18:367–80. doi: 10.3109/10253890.2015.1053451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanghez V, Cubuk C, Sebastián-Leon P, Carobbio S, Dopazo J, Vidal-Puig A, et al. Chronic subordination stress selectively downregulates the insulin signaling pathway in liver and skeletal muscle but not in adipose tissue of male mice. Stress. 2016;19:214–24. doi: 10.3109/10253890.2016.1151491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel A, Sharma PS, Narayan P, Binu VS, Dinesh N, Pai PJ, et al. Prevalence and predictors of infertility-specific stress in women diagnosed with primary infertility: A clinic-based study. J Hum Reprod Sci. 2016;9:28–34. doi: 10.4103/0974-1208.178630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Booij SH, Bos EH, Bouwmans ME, van Faassen M, Kema IP, Oldehinkel AJ, et al. Cortisol and α-amylase secretion patterns between and within depressed and non-depressed individuals. PLoS One. 2015;10:e0131002. doi: 10.1371/journal.pone.0131002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Vente W, van Amsterdam JG, Olff M, Kamphuis JH, Emmelkamp PM. Burnout is associated with reduced parasympathetic activity and reduced HPA axis responsiveness, predominantly in males. Biomed Res Int 2015. 2015:431725. doi: 10.1155/2015/431725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnar J, Dunlop W, editors. Recent Advances in Obstetrics and Gynecology. Vol. 23. London: Royal Society of Medicine Press Limited; 2005. [Google Scholar]
- 18.Kala K, Datti SN, Sujatha C, Dayanand R, Kumar Guruprasad GA. A study of clinical manifestations of PCOS among obese and non- obese rural women. Indian J Basic Appl Med Res. 2013;8:946–51. [Google Scholar]
- 19.World Health Organization. STEPwise Approach to Surveillance (STEPS) Geneva: World Health Organization; 2008b. [Google Scholar]
- 20.Martinsen OG, Grimnes S. Bioimpedance and Bioelectricity Basics. United States: Academic Press; 2011. [Google Scholar]
- 21.Paksaichol A, Chaturapanich G, Pholpramool C. Validation of bioelectric impedance analysis for the assessment of body composition in young adult Thai women. J Physiol Biomed Sci. 2013;26:69–75. [Google Scholar]
- 22.Iyriboz Y, Powers S, Morrow J, Ayers D, Landry G. Accuracy of pulse oximeters in estimating heart rate at rest and during exercise. Br J Sports Med. 1991;25:162–4. doi: 10.1136/bjsm.25.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomczyk R, Ociepka A, Kiałka M, Milewicz T, Migacz K, Kowalczuk A, et al. Metformin and changes in blood pressure and heart rate in lean patients with polycystic ovary syndrome (PCOS) – Preliminary study. Przegl Lek. 2015;72:302–5. [PubMed] [Google Scholar]
- 24.Ranganathan H, Gunasekaran N. Simple method for estimation of hemoglobin in human blood using color analysis. IEEE Trans Inf Technol Biomed. 2006;10:657–62. doi: 10.1109/titb.2006.874195. [DOI] [PubMed] [Google Scholar]
- 25.Saliva Collection and Handling Advice Book. 3rd ed. Salimetrics, LLC, SalivaBio, LLC; 2015. [Google Scholar]
- 26.Out D, Granger DA, Sephton SE, Segerstrom SC. Disentangling sources of individual differences in diurnal salivary α-amylase: Reliability, stability and sensitivity to context. Psychoneuroendocrinology. 2013;38:367–75. doi: 10.1016/j.psyneuen.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Weerth C, Jansen J, Vos MH, Maitimu I, Lentjes EG. A new device for collecting saliva for cortisol determination. Psychoneuroendocrinology. 2007;32:1144–8. doi: 10.1016/j.psyneuen.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Ulijaszek SJ, Kerr DA. Anthropometric measurement error and the assessment of nutritional status. Br J Nutr. 1999;82:165–77. doi: 10.1017/s0007114599001348. [DOI] [PubMed] [Google Scholar]
- 29.Beehner JC, Bergman TJ. The next step for stress research in primates: To identify relationships between glucocorticoid secretion and fitness. Horm Behav. 2017;91:68–83. doi: 10.1016/j.yhbeh.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Filipovský J, Ducimetiére P, Eschwége E, Richard JL, Rosselin G, Claude JR, et al. The relationship of blood pressure with glucose, insulin, heart rate, free fatty acids and plasma cortisol levels according to degree of obesity in middle-aged men. J Hypertens. 1996;14:229–35. doi: 10.1097/00004872-199602000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Brassard M, AinMelk Y, Baillargeon JP. Basic infertility including polycystic ovary syndrome. Med Clin North Am. 2008;92:1163–92, xi. doi: 10.1016/j.mcna.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Coulam CB, Annegers JF, Kranz JS. Chronic anovulation syndrome and associated neoplasia. Obstet Gynecol. 1983;61:403–7. [PubMed] [Google Scholar]
- 33.Sheehan MT. Polycystic ovarian syndrome: Diagnosis and management. Clin Med Res. 2004;2:13–27. doi: 10.3121/cmr.2.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim I, Yetley EA, Calvo MS. Variations in iron-status measures during the menstrual cycle. Am J Clin Nutr. 1993;58:705–9. doi: 10.1093/ajcn/58.5.705. [DOI] [PubMed] [Google Scholar]
- 35.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–95. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCarty R, Horwatt K, Konarska M. Chronic stress and sympathetic-adrenal medullary responsiveness. Soc Sci Med. 1988;26:333–41. doi: 10.1016/0277-9536(88)90398-x. [DOI] [PubMed] [Google Scholar]
- 37.Symons FJ, Wolff JJ, Stone LS, Lim TK, Bodfish JW. Salivary biomarkers of HPA axis and autonomic activity in adults with intellectual disability with and without stereotyped and self-injurious behavior disorders. J Neurodev Disord. 2011;3:144–51. doi: 10.1007/s11689-011-9080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Figurová J, Dravecká I, Petríková J, Javorský M, Lazúrová I. The effect of alfacalcidiol and metformin on metabolic disturbances in women with polycystic ovary syndrome. Horm Mol Biol Clin Investig. 2017;29:85–91. doi: 10.1515/hmbci-2016-0039. [DOI] [PubMed] [Google Scholar]
- 39.Vilmann LS, Thisted E, Baker JL, Holm JC. Development of obesity and polycystic ovary syndrome in adolescents. Horm Res Paediatr. 2012;78:269–78. doi: 10.1159/000345310. [DOI] [PubMed] [Google Scholar]
- 40.Kang SM, Yoon JW, Ahn HY, Kim SY, Lee KH, Shin H, et al. Android fat depot is more closely associated with metabolic syndrome than abdominal visceral fat in elderly people. PLoS One. 2011;6:e27694. doi: 10.1371/journal.pone.0027694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kar S. Anthropometric, clinical, and metabolic comparisons of the four Rotterdam PCOS phenotypes: A prospective study of PCOS women. J Hum Reprod Sci. 2013;6:194–200. doi: 10.4103/0974-1208.121422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majumdar A, Singh TA. Comparison of clinical features and health manifestations in lean vs. Obese Indian women with polycystic ovarian syndrome. J Hum Reprod Sci. 2009;2:12–7. doi: 10.4103/0974-1208.51336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chitme HR, Al Azawi EA, Al Abri AM, Al Busaidi BM, Salam ZK, Al Taie MM, et al. Anthropometric and body composition analysis of infertile women with polycystic ovary syndrome. J Taibah Univ Med Sc. 2017;12:139–45. doi: 10.1016/j.jtumed.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]