Abstract
Background
Glioblastoma (GBM) is one of the most aggressive human brain tumors, with a median survival of 15–18 months. There is a desperate need to find novel therapeutic targets. Various receptor protein kinases have been identified as potential targets; however, response rates in clinical studies have been somewhat disappointing. Targeting the spleen tyrosine kinase (SYK), which acts downstream of a range of oncogenic receptors, may therefore show more promising results.
Methods
Kinase expression of brain tumor samples including GBM and low-grade tumors were compared with normal brain and normal human astrocytes by microarray analysis. Furthermore, SYK, LYN, SLP76, and PLCG2 protein expressions were analyzed by immunohistochemistry, western blot, and immunofluorescence of additional GBM patient samples, murine glioma samples, and cell lines. SYK was then blocked chemically and genetically in vitro and in vivo in 2 different mouse models. Multiphoton intravital imaging and multicolor flow cytometry were performed in a syngeneic immunocompetent C57BL/6J mouse GL261 glioma model to study the effect of these inhibitors on the tumor microenvironment.
Results
SYK, LYN, SLP76, and PLCG2 were found expressed in human and murine glioma samples and cell lines. SYK inhibition blocked proliferation, migration, and colony formation. Flow cytometric and multiphoton imaging imply that targeting SYK in vivo attenuated GBM tumor growth and invasiveness and reduced B and CD11b+ cell mobility and infiltration.
Conclusions
Our data suggest that gliomas express a SYK signaling network important in glioma progression, inhibition of which results in reduced invasion with slower tumor progression.
Keywords: glioma, inhibitors, invasion, microenvironment, SYK
Importance of the study
GBM is one of the most aggressive human cancers and is characterized by diffuse infiltration of surrounding brain tissue with a median survival of 15–18 months. There is a desperate need to identify the molecular mechanisms which might be the Achilles heel of GBM. SYK kinase plays a key oncogenic and tumor promoter role in various cancers. While a previous study has shown that SYK is expressed at the transcript level in GBM, here we show that SYK, LYN, PLCG2, and SLP76 are expressed in glioma patient samples and patient-derived cell lines. Furthermore, in vivo multiphoton imaging and multicolor fluorescence activated cell sorting analysis suggest that targeting SYK inhibits proliferation and migration of tumor cells and affects the tumor microenvironment by partially blocking monocyte and B-cell infiltration. Therefore, SYK inhibition may represent an attractive immune modulatory therapeutic strategy.
Glioblastoma (GBM) is one of the most aggressive human cancers, characterized by diffuse infiltration of surrounding brain tissue. Current GBM therapy consists of surgical resection followed by radiation and temozolomide treatment.1 Despite intensive research, the median survival of patients with GBM remains at less than 2 years.2 Therefore, there is a desperate need to identify the molecular mechanisms and driving kinases which might be the Achilles heel of GBM. Previous studies have identified various protein kinases as targets; however, the response rates of inhibitors of platelet derived growth factor (PDGF), vascular endothelial growth factor, and epidermal growth factor (EGF) receptors (overexpressed in GBM) have been disappointing.3 Targeting specific downstream effectors common to multiple oncogenic receptors may consequently show more promising results and be applicable to other cancers.
SYK is a nonreceptor protein tyrosine kinase activated through receptors containing immunoreceptor tyrosine-based activation motifs (ITAMs), Toll like receptors, integrins, and SRC family members4–6 or through proteolytic cleavage.7 SYK activation triggers a range of signal transduction pathways modulating proliferation, differentiation, and cell survival.8 SYK was traditionally thought to be expressed exclusively in hematopoietic cells, where it acts as an essential signaling molecule.4–6,8 In myeloid cells SYK is involved in Fc gamma receptor, C-type lectin, and Toll like receptor‒mediated signaling leading to expression of inflammatory mediators and phagocytosis.9 In B cells, SYK is essential, as deficiency disrupts signaling from the pre‒B-cell receptor complex leading to complete depletion of mature B cells.10,11 Additionally, SYK is fundamental in megakaryocytic/platelet lineage development and platelet aggregation.12 However, SYK is also expressed in non-hematopoietic cells, including epithelial, breast, neuronal, and vascular endothelial cells, fibroblasts, and hepatocytes,13,14 and regulates cell adhesion and vascular development.15–17
Some studies have shown a cancer-modulating role for SYK. In leukemia, SYK is a well-known oncogene and tumor promoter.18 In breast cancer, SYK has been reported to act as a tumor suppressor,15 although SYK expression is essential for mouse mammary tumor virus and Epstein–Barr virus mediated transformation.16,19 While SYK family members have only been described in GBM leukocyte infiltrates20 (recently shown at mRNA level21), most members of the Rous sarcoma virus proto-oncogene (SRC) family (LYN, FYN, YES, and Src) have been shown to be overexpressed in GBM and contribute to GBM growth and migration.22–24 We hypothesized that SYK, which is downstream of SRC kinases, may consequently mediate the proliferative and migratory effects shown by SRC family members. SYK signaling most likely exerts cell-type and context-specific functions and therefore could play a pivotal role in gliomagenesis and progression.
Methods
Patients
Tissue samples were from neurosurgical tumor resections and processed as described previously25 in accordance with the Ethical Committee of the University Hospital of Basel with informed consent obtained for all patients. Thirty GBM samples were used for the microarray analysis, 10 freshly frozen samples were used for western blotting, and 42 paraffin samples were used for immunohistochemistry (IHC). An additional 16 paraffin-embedded pilocytic astrocytoma samples were used for IHC.
Construction of Plasmids
SYK and LK (a constitutively active truncated form of SYK with lacks the regulatory SH2 domains) SYK were gifts from M. Reth, Department of Molecular Immunology, Max Planck Institute of Immunobiology and Epigenetics. Tyrosine mutants mITAM (SYK mutated at both Src homology 2 [SH2] domains), Y131F, Y296F, Y348/352F, ALL IB (Y296/323/348/352F), K402A KD, Y525/526F, and 3F (Y629/630/631F) were generated by site-directed mutagenesis according to the manufacturer’s instructions (Clontech). NOIB (SH2 domains without interdomain), SH+IB (SH2 domains with interdomain), and nSH2D (lacks N terminal SH2 domain) were generated through site-specific primers. Small interfering (si)RNA was from Sigma Aldrich, human TRIPZ SYK from GE Healthcare, and Luc2AGFP vector was a gift from S. Gambhir.
Cell Culture
GBM cell lines were from A.M. and cultured in Dulbecco’s modified Eagle’s medium with 10% fetal calf serum. GBM-derived BS287 spheres were maintained in Neurobasal media with B2, N2, and Glutamax (Invitrogen) plus 20 ng/mL EGF and fibroblast growth factor (PeproTech) as described previously.25 BAY 61-3606 was from MedChemexpress. NVP-QAB205 was a gift from Novartis. Protein phosphatase 1 and piceatannol were from Enzo Life Sciences. Normal human astrocytes were from Cambrex and cultured according to the manufacturer’s recommendations.
Immunofluorescence and Immunohistochemistry
Cells were processed for immunofluorescence (IF) as described previously.25 For IHC, paraffin sections were prepared with an automated instrument-reagent system (Discovery XT, Ventana Medical Systems). Images were captured with an LSM-700 confocal microscope and ZEN Black 2010 software (Zeiss). Hematoxylin-counterstained sections were photographed (Nikon YTHM). Glioma spontaneous mouse model slides were a gift from R. Benezra/E. C. Holland, K. Yun, and C. Krona /D. W. Cleveland. In brief, the glial fibrillary acidic protein (GFAP) tvaPDGF ARF−/− was created through gene transfer of PDGF using the replication competent avian leukosis virus splice acceptor (RCAS)/tv-a system in an ARF −/− mouse line (Gtv-a) that expresses the TVA receptor from the GFAP promoter.26 The GFAP Cre+ NF1−/+ p53−/f PTEN +/f was generated by crossing Mut3 mice (GFAP-cre;cisNf1f/+;p53−/+) with wild-type, loxP-Pten, p53f, or p53f;Ptenf females.27 The S100b-v-ErbB; p53−/− transgenic mouse model uses the S100ß-promoter to drive the expression of the vErbB gene on a Trp53−/− (p53−/−) mutant background.28 Immunohistochemistry staining was quantified as following: Neg: negative; 1–3: staining in 15%–30% of tumor; 4: strong staining in 50% of tumor (see Supplementary Fig. S1E).
Immunoblotting and Fluorescence Activated Cell Sorting
Cells were homogenized in lysis buffer (50 mM Tris-HCl pH 7.5, 120 mM NaCl, 1% NP-40, 40 mM β-glycerophosphate, 1 mM benzamidine, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 25 mM NaF, and 2 µM microcystin-LR) and subjected to western blotting as described previously.25 Fluorescence activated cell sorting (FACS) was performed using a BD FACSLSRII, FACSCalibur, and FACSDiva software (BD Biosciences) and was analyzed using FlowJo (Treestar).
Migration Assay
Wound assays were performed according to a protocol reported by Rai and Shivaji,29 taking 20 hours as the final time point.
In Vivo Experiments
L2G- or luciferase-expressing GBM cells were implanted orthotopically or in the flanks of a minimum of 5 high sucrose diet (HSD) nude mice per treatment, and tumor formation was followed by bioluminescence (IVIS Lumina XR, PerkinElmer). For intravital imaging by multiphoton microscopy (IVI-MP), GL261-L2G cells (5 × 105) were injected orthotopically 2 mm lateral, 2 mm posterior to the bregma, and 1 mm deep. A 3-mm-diameter chronic cranial window was implanted for imaging.30,31 Animals were imaged and analyzed (Fiji software) as described previously.30 Briefly, tumors expressing green fluorescent protein (GFP) were visualized by IVI-MP from the surface layer of tumor cells (0 µm) until 100 µm depth in the tumor. Each imaging field/Z-stack (minimum 45 min long) shown in examples and used for quantification consists of 21 slices per field, 5 µm apart; scale is 1 µm/pixel. In vivo experiments were performed in accordance with the Swiss Federal Animal Welfare Law.
Antibodies
Anti–α-tubulin antibody hybridoma supernatant was generated in-house (YL1/2). The following monoclonal antibodies were used: Ki67 (Thermo Scientific); PLCG2, GFAP (Sigma Aldrich); cyclin D1 (C-20), LYN (H-6), SYK (2D10 and C-20), Zap70 (M-20), SLP76 (F-7), ACTIN (I-19), GFAP (C-19 for FACS) (SCBT); goat anti-rabbit-Alexa488, goat anti-mouse-Alexa568, goat anti-rabbit-Alexa647 (Invitrogen); GFP-Alexa488, CD45APC-CY7, CD44-PE, CD4-BV785, CD8-BV711, CD19-v570, CD19-APC, CD11b-BV510, Dextran Texas Red, CD206-APC, CD25-PerCPCy5.5, CD11c-PECy5.5, CD3-Alexa700, GR-1-APC, F4/80-BV421, and MHCII IA/E-APCCy7 (Biolegend and E-Bioscience).
Statistical Analysis
As shown on each figure, n indicates the number of experiments using cells from independent experiments. Where 3 or more experiments were conducted, a 2-tailed Student’s t-test with 2 samples of unequal variance type was conducted. Means were considered significantly different when P < 0.05*, P < 0.01**. Error bars represent the SD of the mean.
Results
SYK, LYN, PLCG2, and SLP76 Are Overexpressed in GBM and Gliomas
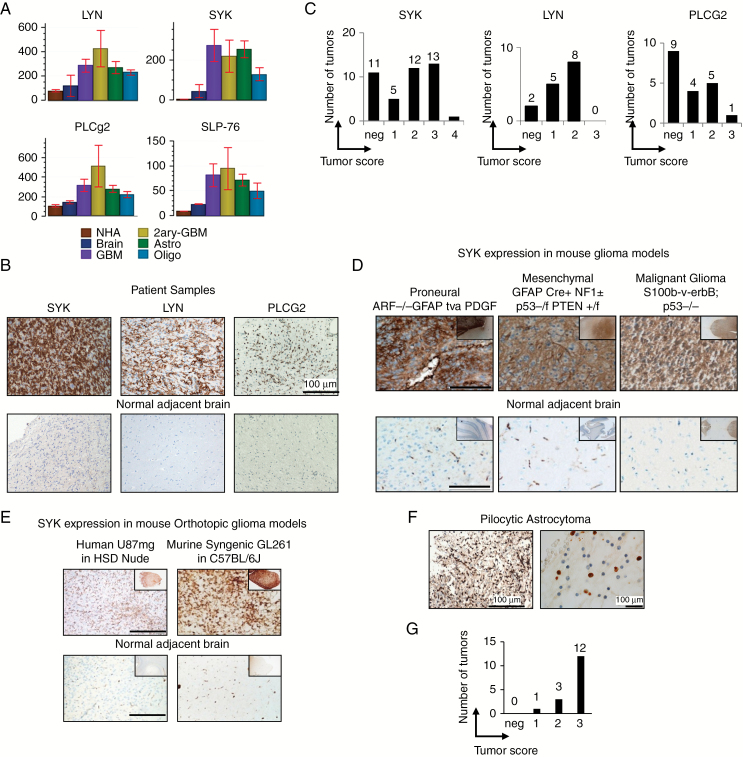
To identify molecules which may be important in GBM progression, we analyzed gene expression of 30 brain tumor samples, including 15 high-grade gliomas (12 primary and 3 secondary GBM) and 15 low-grade tumors (8 astrocytomas and 7 oligodendrogliomas) and compared the expression values to 3 normal brain and 3 NHAs (GSE15824).25 A range of molecules involved in B-cell and macrophage signaling pathways were found upregulated in gliomas, mainly in the SYK signaling pathway including the Src family members LYN, SYK, and downstream signaling factors such as PLCG2 and SLP76 (Fig. 1A and Supplementary Table S1).
Fig. 1.
Immune signaling molecules are expressed by glioma cells in vivo. (A) Microarray analysis of SYK, LYN, PLCG2, and SLP76 in NHAs, normal brain (Brain), glioblastoma (GBM), secondary glioblastoma (2ary-GBM), lower-grade astrocytoma (Astro), and oligodendrogliomas (Oligo) using Genedata Expressionist analyst software. (B) Representative IHC staining of GBM samples for SYK, LYN, and PLCG2; n > 15. (C) Tumor score of GBM samples stained for SYK, LYN, and PLCG2. (D) SYK expression in glioma mouse models (GFAP tvaPDGF ARF−/−; GFAP Cre+ NF1−/+ p53−/f PTEN +/f; S100b-v-ErbB; p53−/−). (E) SYK expression in 2 orthotopic glioma mouse models (U87MG-luc in HSD nude; GL261-L2G in C57BL/6J). (F) SYK expression of pilocytic astrocytoma n = 16 and (G) tumor score.
A second set of patient samples with GBM were analyzed by IHC to confirm if the genes identified by the microarray screen were expressed (Fig. 1B). The kinases SYK and LYN and the adaptor protein PLCG2 were found expressed on 74% (31/42), 86% (13/15), and 52% (10/19) of tumors, respectively (Fig. 1C). SYK expression was further confirmed in 3 spontaneous (Fig. 1D) and 2 orthotopic mouse glioma models (Fig. 1E) and was absent in normal human and murine brain (normal adjacent brain on the same slide). Additionally, we analyzed SYK expression on lower-grade pilocytic astrocytomas, which are known to have a better prognosis.32 Unexpectedly, it was found expressed in all (16/16) human pilocytic astrocytomas tested (Fig. 1F and 1G).
Visual analysis of SYK staining showed that SYK is found on the tumor mass, surrounding tumor cells, pseudopalisading cells, and the infiltrating front (Supplementary Fig. S1A). Intracellularly it was found in the nucleus and cytoplasm (Supplementary Fig. S1B). In pilocytic astrocytoma SYK was localized mainly in the nucleus, in 12 of 16 cases (Fig. 1G). Specificity of the SYK IHC staining was confirmed through siRNA (Supplementary Fig. S1C), while ZAP70 expression was negative (Supplementary Fig. S1D). Tumor score was analyzed as shown in Fig. S1E.
SYK Is Expressed by Glioma Tumor Cells
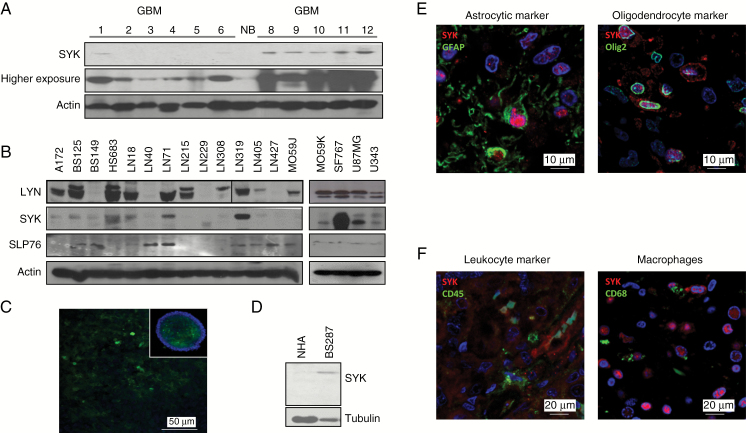
To confirm that SYK was expressed by GBM cells themselves, we analyzed protein expression in freshly frozen samples of patients with GBM (Fig. 2A) and 18 established in vitro glioma cell lines devoid of any leukocytes (Fig. 2B). We observed that most cell lines expressed SYK, LYN, and SLP76. We also confirmed SYK staining of glioma cancer spheres by IF (Fig. 2C) and western blot (Fig. 2D), while it was absent in NHAs. In addition, we performed co-IF with leukocyte, astrocyte, and oligodendrocyte markers in GBM patient samples (Fig. 2E) and found that SYK was expressed by CD45 and CD68 negative glioma tumor cells (Fig. 2F). In summary, in glioma, SYK signaling components are expressed not only by leukocytes, but also by glioma tumor cells themselves. These signaling molecules, therefore, may serve as biomarkers for both GBM and lower-grade glioma.
Fig. 2.
SYK is expressed by glioma cells. (A) SYK and beta actin western blot of GBM and normal brain samples. (B) Western blot of SYK, LYN, SLP76, and beta actin expression in 18 GBM cell lines. (C) IF of SYK (green) and nuclei (blue) in BS287. (D) Western blot of SYK and tubulin on BS287 cancer spheres and NHA. (E) IF of GBM sections co-stained for SYK (red) and glioma markers GFAP and oligodendrocyte transcription factor (green). (F) IF of GBM sections co-stained for SYK (red), leukocyte marker CD45 (green), and monocyte/macrophage/microglia marker CD68 (green).
Role of SYK in Proliferation and Migration
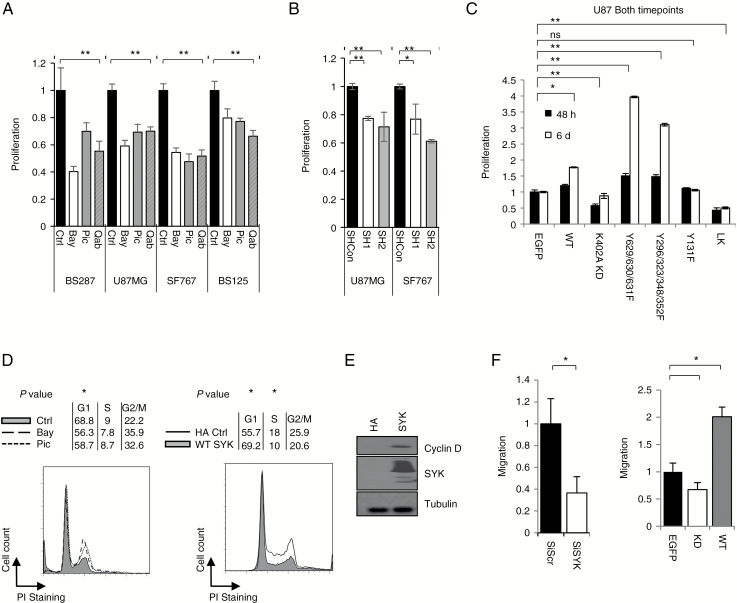
The role of SYK in proliferation in a range of GBM cell lines was analyzed next. The primary cancer sphere line BS287, which closely recapitulates gliomas; U87MG and SF767 cell lines, which express high levels of SYK; and the cell line BS125, which expresses lower levels of SYK were treated with SYK inhibitors (BAY 61-3606 [BAY], piceatannol [Pic], and NVP-QAB205 [QAB]), and proliferation was measured by an MTT assay. Fig. 3A shows that inhibiting SYK with a range of inhibitors blocks proliferation. Cell lines expressing high levels of SYK (U87MG and SF676) were treated with SYK tetracycline inducible short hairpin (sh)RNA to knock down SYK, which also blocked proliferation (Fig. 3B). Transfection of activatory or inhibitory SYK constructs were also used to specifically modulate SYK kinase signaling in U87MG cells (Fig. 3C). Transfection with the active wild type, the C-terminal 3F overactive SYK mutant, and the Y296/323/348/352F interdomain B autoinhibitory site mutant showed increased proliferation. In contrast, transfection with kinase dead (KD) SYK resulted in reduced proliferation compared with the control. Cell cycle analysis showed that SYK inhibition stalled cells at the G2/M phase, whereas overexpression increased cells in G1 (Fig. 3D) and induced the expression of cyclin D (Fig. 3E). Furthermore, SYK inhibition by Pic (Fig. 3F), KD overexpression (Fig. 3G), or siRNA also blocked GBM migration (Fig. 3H). Thus, we conclude that SYK kinase activity plays a significant role in GBM proliferation and migration.
Fig. 3.
SYK inhibition blocks proliferation and migration. MTT proliferation assays of GBM cell lines (BS287, U87MG, SF767, and BS125), after treatment with (A) SYK inhibitors BAY 61-3606 (BAY) (1 µM), piceatannol (Pic) (10 µM), or NVP-QAB205 (QAB) (1 µM). (B) Tetracycline inducible shRNA (U87MG and SF767 with higher SYK expression) or (C) SYK constructs (U87MG). (D) FACS and cell cycle quantification analysis (figures represent % of total population) of cells treated with SYK inhibitors Pic or BAY (SF767, high SYK expression) or overexpressing wild-type empty vector containing the human influenza hemagglutinin (HA) tag SYK or HA control (LN229, SYK negative). (E) Western blot of SYK and cyclin D after SYK overexpression (LN229, SYK negative) or in HA control. (F) Scratch assay analysis of SF767 cells transfected with siScramble (siScr) or SYK siRNA. (G) Scratch assay analysis of U87MG cells transfected with either wild-type or KD SYK overexpressing constructs or eGFP control. Error bars show SD. *P < 0.05, **P < 0.01.
Role of SYK Inhibition in Tumor Formation
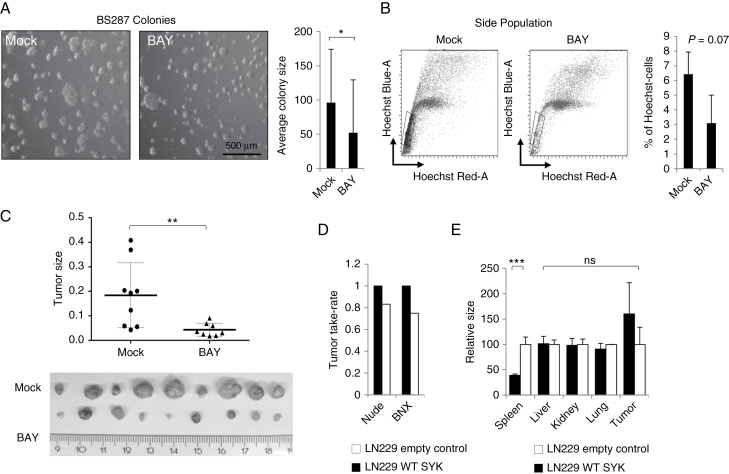
We further investigated how SYK inhibition affects tumor formation. BS287 cancer sphere cells were treated for 5 days with the SYK inhibitor BAY. Analysis of colony size showed that the cancer spheres were significantly smaller following BAY treatment (Fig. 4A). These cells were also stained and analyzed for side population cells which represent tumor-initiating cells (Fig. 4B). There was a smaller side population after SYK inhibitory treatment, although the difference was not statistically significant. While side population staining is used to identify “stem cells” based on the dye efflux properties of ATP-binding cassette transporters, we wanted to confirm that SYK inhibition also reduced tumor initiation in vivo. Indeed, BS287 cells pretreated for 5 days with BAY and then injected into nude mice were slower to form tumors, and these were significantly smaller than controls (Fig. 4C).
Fig. 4.
SYK regulates tumor formation. (A) Representative image and colony size quantification of BS287 cells either mock-treated or treated with BAY for 5 days. (B) Flow cytometric analysis of side population and quantification of BS287 cells either mock-treated or treated with BAY for 5 days. (C) Images and sizes of tumors of mock- or BAY-treated BS287 cells injected into nude mice at a threshold size of 1 cm3. (D) Tumor take rates of LN229 empty vector control containing the human influenza hemagglutinin (HA) tag or wild-type SYK tumors in nude and BNX mice. (E) Organ sizes of tumor-positive mice implanted with LN229 empty vector control or wild-type SYK tumors.
To further investigate the effect of SYK expression on GBM in vivo, stably wild-type SYK transfected LN229 (low basal SYK expression) cells were injected into the flanks of beige/nude/x-linked immunodeficient (BNX) or nude mice (Fig. 4D). There was no difference in tumor size between the mock transfected and SYK overexpressing cells, although take rate was higher for SYK overexpressing tumors. Surprisingly, spleens of BNX mice bearing SYK-overexpressing tumors were significantly smaller than the normally expected increased size (splenomegaly) observed in empty vector controls. This absence of splenomegaly suggests that tumors overexpressing SYK may have a paracrine effect on extratumoral leukocytes. Therefore, SYK expression may increase the tumorigenic potential of cells and have a systemic effect through a reduction in immunogenicity.
SYK Inhibition Increases Survival
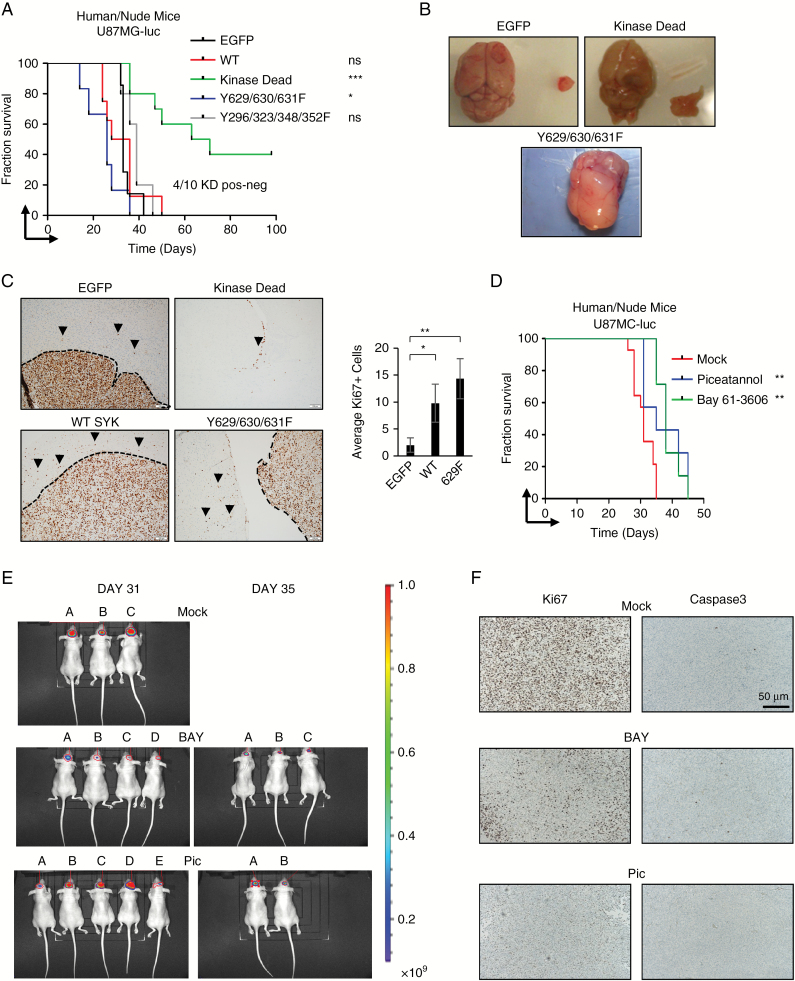
To genetically study the role of SYK kinase tumor activity in vivo, we injected nude mice with SYK mutants expressed in human U87MG (Fig. 5A). This allowed us to study the effects of inhibiting SYK directly on tumor cells and not immune infiltrates. The prognosis of mice injected with tumors expressing wild-type SYK was not significantly worse compared with enhanced (e)GFP–overexpressing cells, although tumors were vascularized and visually more invasive (Fig. 5B). More striking was the poorer prognosis of tumors bearing the 3F SYK overactive mutant. These tumors were not encapsulated and completely invaded the normal brain (Fig. 5B). In contrast, 4 out of 10 SYK KD tumors regressed by day 100. Importantly, these tumors were luciferase positive for at least 2 weeks, confirming implantation. The KD tumors that did progress were completely non-invasive and grew ectopically (Fig. 5B). These tumors were also analyzed for Ki67 expression. To evaluate the invasiveness, the amount of Ki67-positive cells outside the tumor mass was quantified (Fig. 5C).
Fig. 5.
SYK regulates tumor proliferation and progression in vivo. (A) Kaplan–Meier survival analysis of immune-compromised mice orthotopically injected with U87MG cells overexpressing eGFP or SYK mutants; n > 5. (B) Representative images of brains and tumors from orthotopically injected U87MG cells overexpressing eGFP, wild type, KD, Y629/630/631F, or Y296/323/348/352F. (C) Ki67 expression of eGFP, wild type, KD, and Y629/630/631F tumor. Bar chart depicts amount of Ki67-positive cells outside the tumor mass in relation to every 10 cells at the tumor front. (D) Kaplan–Meier survival analysis of mice orthotopically injected with U87MG cells either mock-treated (red) or treated with Pic 20 mg/kg 3 times per week (blue) or BAY 5 mg/kg/daily (green). (E) Bioluminescence of mice orthotopically injected with U87MG cells either mock-treated or treated with Pic 20 mg/kg 3 times per week or BAY 5 mg/kg/daily on day 31 (mock: 1.480e+10, 4.911e+09, 1.215e+10, and 1.857e+08; BAY: 1.730e+09, 4.156e+08, 2.493e+08, and 1.857e+08; piceatannol: 6.576e+08, 8.871e+09, 1.1138e+10, 2.262e+10, and 4.933e+08) and day 35 (BAY: 3.729e+08, 1.352e+09, and 4.860e+08; piceatannol: 9.792e+08 and 5.803e+08); n > 8. (F) Caspase-3 and Ki67 IHC of mice orthotopically injected with U87MG cells either mock-treated or treated with Pic 20 mg/kg 3 times per week or BAY 5 mg/kg/daily.
To test if we could effectively recapitulate the genetic inhibition pharmaceutically, orthotopically injected U87MG cells were treated in vivo with 2 different SYK inhibitors (Pic and BAY). These SYK inhibitor treatments prolonged the life of treated mice (Fig. 5D) and reduced GBM cell proliferation (analyzed for Ki67 expression in Fig. 5E).
SYK Inhibition Blocks Tumor and Leukocyte Migration and Infiltration
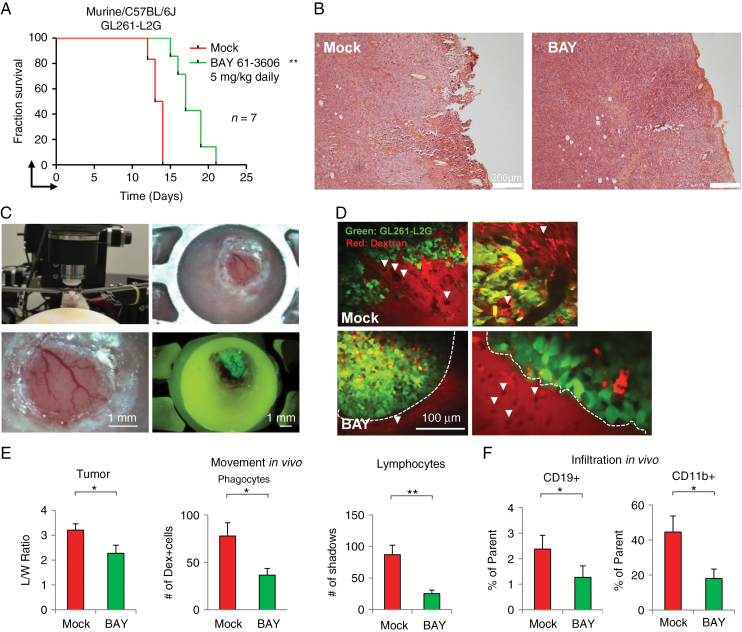
We have shown that blocking SYK activity directly on tumor cells genetically or with inhibitors prolonged the life of treated mice. However, SYK can also be found expressed on infiltrated leukocytes. Therefore, to further understand any systemic effect of SYK inhibition in an immune-competent tumor microenvironment, we treated established GL261-L2G murine tumors orthotopically injected into C57BL/6J mice with the SYK inhibitor BAY. Survival was also increased by inhibitor treatment (Fig. 6A).
Fig. 6.
SYK modulates the tumor microenvironment in immune-competent GL261-L2G/C57BL/6J mice model. (A) Kaplan–Meier survival analysis of mock- (red) or BAY-treated (green) C57BL/6J mice orthotopically injected with the GL261-L2G murine glioma cell line; n = 7. (B) Hematoxylin and eosin staining of mock- and BAY-treated tumors. (C) Multiphoton setup, window, and epifluorescence of injected GL261-L2G cells after 7 days of implantation. (D) Multiphoton imaging of mock- or BAY-treated GL261-L2G tumors from depths 20–45 µm below the tumor surface. Lymphocyte “shadows” are marked with a white arrowhead. Bar represents 100 µm. (E) Multiphoton analysis of mock- (red) or BAY-treated (green) GL261-L2G tumors showing morphology (or invasiveness) of tumor cells (length/width ratio), number of dextran-labeled phagocytic cells, and unlabeled “shadows” (lymphocytes); n > 3 mice, n = 6–10 fields, 21 Z-slices per field (0–100 µm depth), 5 µm apart. (F) FACS analysis of infiltrated CD19+ and CD11b+ cells showing percentage of parent cells; n = 4. Error bars show SD. *P < 0.05, **P < 0.01.
To investigate the effects of SYK inhibition on tumor cells and host cell motility in vivo, we analyzed treated and mock-treated tumors by hematoxylin and eosin (Fig. 6B) and by IVI-MP (Fig. 6C). Intravital imaging supported our earlier observations by showing that mock-treated tumors appear invasive (Supplementary Videos 1–4), while BAY-treated tumors appeared encapsulated (Fig. 6D and Supplementary Videos 5–7). In addition, motion analysis at tumor depths ranging from 0 to 100 µm suggests that single tumor cells, unstained cells representing lymphocytes, and red phagocytic cells moved less in SYK inhibitor–treated mice (Fig. 6E). We then analyzed immune infiltration using a 9-color panel of antibodies to identify lymphocytes and a 7-color panel of antibodies to identify monocytic cells. We could discriminate that the proportions of CD19+ (B cells) and total CD11b+ infiltrative leukocytes were reduced in BAY-treated animals (Fig. 6F and Supplementary Figure S2). Therefore, SYK inhibition affected tumor proliferation and invasiveness in vivo, as well as leukocyte infiltration.
SYK inhibition has a range of effects on tumor cells (proliferation and migration) and infiltrating leukocytes (motion of leukocytes, and infiltration of B and CD11b cells), which may contribute to the encapsulated phenotype observed in treated tumors.
Discussion
The immune system is intricately linked to cancer development and control, playing a key role in cancer prevention and eradication.33 However, inflammation has been implicated in promoting tumorigenesis and progression. In addition, cancer cells and leukocytes have the highest proliferative capacity of all cells. Although the immune system and cancer are highly interrelated,34 the expression and function of hematopoietic molecules in solid cancers have widely been neglected.
Microarray analysis of low-grade glioma and GBM showed that a range of immune signaling molecules were found overexpressed. Although Gene Ontology terms of the mesenchymal subgroup include “immune system process,” “immune response,” and “inflammatory response,” this was assumed to reflect infiltrative cells,35,36 while earlier work on immune markers37 has been neglected. Therefore, it was of great interest to confirm whether this immune signaling molecule expression was derived from glioma cells themselves. Western blot and IHC analyses clearly showed that SYK was expressed by glioma cells. We also found LYN, PLCG2, and SLP76 protein expression in GBM cells, showing that the whole immunogenic signaling repertoire is present.
SYK has a dual reputation—in some cells SYK acts as a tumor promoter, while in others as a tumor suppressor.18 In leukemia, SYK is a well-known oncogene and target.38 Conversely, it is considered to be a tumor suppressor in some solid tumors where loss of expression correlates with worse prognosis.18 SYK can be found both in the cytoplasm and in the nucleus due to an unconventional shuttling sequence,39 while during receptor activation it can localize to the membrane.6 Localization is relevant, as nuclear expression correlates with better prognosis.40–42 Possible explanations for the divergent roles in cancer may be due to phosphorylation and receptor or SYK interaction partner availability in those tissues; however, this needs further investigation. Therefore, it is important to understand if SYK could be a target in glioblastoma.
We first demonstrated that SYK inhibition blocked glioma cell proliferation and migration in vitro. We then showed that this could be mirrored in vivo on U87MG human glioma cells through intrinsic genetic inhibition, where 4 out of 10 SYK KD tumors regressed. This was also the case when using 2 different chemical SYK inhibitors in 2 different mouse models. Strikingly, overexpression of the 3F SYK overactive mutant could recapitulate many features, such as high invasiveness and vascularization observed in human GBM. These are important characteristics which are sometimes lacking in the U87MG orthotopic mouse model. Since SYK is also expressed by infiltrating leukocytes, we were interested to understand how SYK inhibition would affect the tumor microenvironment. Multiphoton imaging suggested that SYK inhibition had a negative effect on tumor cell motility, as well as a significant negative effect on dextran-negative leukocyte motility and dextran-positive phagocytic leukocytes. One technical caveat for the IVI experiments is that highly aggressive tumors, while implanted as described (minimum 1 mm below the surface of the brain), could only be imaged to a maximum of about 100 µm depth due to limitations of the imaging system. Hence, the observations do not perfectly reflect a true brain microenvironment but may represent only the surface compartment. Nevertheless, this approach still provides a unique opportunity to observe in vivo cell behavior compared with in vitro invasion or motility assays.43–45 Multicolor flow cytometry could confirm that SYK inhibition modified the tumor microenvironment, reducing CD11b+ monocytic cells and CD19+ B cells. This is important, as SYK may be a promising therapeutic target, which also affects leukocyte infiltration and mobility.
We also demonstrate that SYK inhibition affects tumor establishment and reduces the number of tumor-initiating cells. Overexpression of SYK in LN229 cells in BNX mice did not elicit the typical splenomegaly seen after xenograft implantation. This suggests that SYK plays a role in extratumoral leukocytes or in the immunogenic properties of the tumor. While this needs further investigation, the expression of immunological molecules by cancer cells may be a form of immune evasion, acting to camouflage cancer cells from the immune system. The question then arises whether this is a glioma-specific characteristic or might also apply to other cancer entities in which SYK activity is important, such as head and neck cancer, retinoblastoma, or mammary cancer.15,17,46
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This research was funded by Oncosuisse Collaborative Cancer Research Projects (CCRP) grants from The Swiss Cancer League KFP OCS-01613-12-2004, KLS 02787-02-2011, KFS 02528-02-2010, and KFS 3000-08-2012. The Friedrich Miescher Institute is affiliated with the Novartis Institutes for Biomedical Research.
Acknowledgments
We thank S. Bichet and M. Espino for IHC; J. Gill and L. Kennins for antibodies; and H-G. Zerwes for discussions.
Conflict of interest statement. The present invention relates to a method for treating cancer, for instance brain tumors, in a subject by inhibiting spleen tyrosine kinase (Syk) by administering to said subject a therapeutically effective amount of a modulator of Syk, for example a specific antibody or a siRNA.
References
- 1. Ostermann S, Csajka C, Buclin T, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10(11):3728–3736. [DOI] [PubMed] [Google Scholar]
- 2. Perry JR, Laperriere N, O’Callaghan CJ, et al. ; Trial Investigators Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 3. Lo HW. EGFR-targeted therapy in malignant glioma: novel aspects and mechanisms of drug resistance. Curr Mol Pharmacol. 2010;3(1):37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lowell CA. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: Signaling cross talk. Cold Spring Harb Perspect Biol. 2010;3. doi: 10.1101/cshperspect.a002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jabara HH, McDonald DR, Janssen E, et al. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat Immunol. 2012;13(6):612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flaswinkel H, Barner M, Reth M. The tyrosine activation motif as a target of protein tyrosine kinases and SH2 domains. Semin Immunol. 1995;7(1):21–27. [DOI] [PubMed] [Google Scholar]
- 7. Taniguchi T, Kobayashi T, Kondo J, et al. Molecular cloning of a porcine gene syk that encodes a 72-kDa protein-tyrosine kinase showing high susceptibility to proteolysis. J Biol Chem. 1991;266(24):15790–15796. [PubMed] [Google Scholar]
- 8. Mócsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10(6):387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yi YS, Son YJ, Ryou C, Sung GH, Kim JH, Cho JY. Functional roles of Syk in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014;2014:270302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Turner M, Mee PJ, Costello PS, et al. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378(6554):298–302. [DOI] [PubMed] [Google Scholar]
- 11. Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378(6554):303–306. [DOI] [PubMed] [Google Scholar]
- 12. Finney BA, Schweighoffer E, Navarro-Núñez L, et al. CLEC-2 and Syk in the megakaryocytic/platelet lineage are essential for development. Blood. 2012;119(7):1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yanagi S, Inatome R, Takano T, Yamamura H. Syk expression and novel function in a wide variety of tissues. Biochem Biophys Res Commun. 2001;288(3):495–498. [DOI] [PubMed] [Google Scholar]
- 14. Duta F, Ulanova M, Seidel D, et al. Differential expression of spleen tyrosine kinase Syk isoforms in tissues: Effects of the microbial flora. Histochem Cell Biol. 2006;126(4):495–505. [DOI] [PubMed] [Google Scholar]
- 15. Coopman PJ, Do MT, Barth M, et al. The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature. 2000;406(6797):742–747. [DOI] [PubMed] [Google Scholar]
- 16. Lu J, Lin WH, Chen SY, et al. Syk tyrosine kinase mediates Epstein-Barr virus latent membrane protein 2A-induced cell migration in epithelial cells. J Biol Chem. 2006;281(13):8806–8814. [DOI] [PubMed] [Google Scholar]
- 17. Luangdilok S, Box C, Patterson L, et al. Syk tyrosine kinase is linked to cell motility and progression in squamous cell carcinomas of the head and neck. Cancer Res. 2007;67(16):7907–7916. [DOI] [PubMed] [Google Scholar]
- 18. Krisenko MO, Geahlen RL. Calling in SYK: SYK’s dual role as a tumor promoter and tumor suppressor in cancer. Biochim Biophys Acta. 2015;1853(1):254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katz E, Lareef MH, Rassa JC, et al. MMTV Env encodes an ITAM responsible for transformation of mammary epithelial cells in three-dimensional culture. J Exp Med. 2005;201(3):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hussain SF, Kong LY, Jordan J, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007;67(20):9630–9636. [DOI] [PubMed] [Google Scholar]
- 21. Nijaguna MB, Patil V, Urbach S, et al. Glioblastoma-derived macrophage colony-stimulating factor (MCSF) induces microglial release of insulin-like growth factor-binding protein 1 (IGFBP1) to promote angiogenesis. J Biol Chem. 2015;290(38):23401–23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu KV, Zhu S, Cvrljevic A, et al. Fyn and SRC are effectors of oncogenic epidermal growth factor receptor signaling in glioblastoma patients. Cancer Res. 2009;69(17):6889–6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kleber S, Sancho-Martinez I, Wiestler B, et al. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell. 2008;13(3):235–248. [DOI] [PubMed] [Google Scholar]
- 24. Stettner MR, Wang W, Nabors LB, et al. Lyn kinase activity is the predominant cellular SRC kinase activity in glioblastoma tumor cells. Cancer Res. 2005;65(13):5535–5543. [DOI] [PubMed] [Google Scholar]
- 25. Grzmil M, Morin P Jr, Lino MM, et al. MAP kinase-interacting kinase 1 regulates SMAD2-dependent TGF-β signaling pathway in human glioblastoma. Cancer Res. 2011;71(6):2392–2402. [DOI] [PubMed] [Google Scholar]
- 26. Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15(15):1913–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kwon CH, Zhao D, Chen J, et al. Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res. 2008;68(9):3286–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harris MA, Yang H, Low BE, et al. Cancer stem cells are enriched in the side population cells in a mouse model of glioma. Cancer Res. 2008;68(24):10051–10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rai P, Shivaji S. The role of DJ-1 in the pathogenesis of endometriosis. PLoS One. 2011;6(3):e18074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smirnova T, Zhou ZN, Flinn RJ, et al. Phosphoinositide 3-kinase signaling is critical for ErbB3-driven breast cancer cell motility and metastasis. Oncogene. 2012;31(6):706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wyckoff JB, Wang Y, Lin EY, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67(6):2649–2656. [DOI] [PubMed] [Google Scholar]
- 32. Kayama T, Tominaga T, Yoshimoto T. Management of pilocytic astrocytoma. Neurosurg Rev. 1996;19(4):217–220. [DOI] [PubMed] [Google Scholar]
- 33. Greene W, Kuhne K, Ye F, et al. Molecular biology of KSHV in relation to AIDS-associated oncogenesis. Cancer Treat Res. 2007;133:69–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 35. Beier CP, Kumar P, Meyer K, et al. The cancer stem cell subtype determines immune infiltration of glioblastoma. Stem Cells Dev. 2012;21(15):2753–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Doucette T, Rao G, Rao A, et al. Immune heterogeneity of glioblastoma subtypes: extrapolation from the cancer genome atlas. Cancer Immunol Res. 2013;1(2):112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Tribolet N. Immunological markers of brain gliomas. Bull Schweiz Akad Med Wiss. 1981:113–121. [PubMed] [Google Scholar]
- 38. Efremov DG, Laurenti L. The Syk kinase as a therapeutic target in leukemia and lymphoma. Expert Opin Investig Drugs. 2011;20(5):623–636. [DOI] [PubMed] [Google Scholar]
- 39. Zhou F, Hu J, Ma H, Harrison ML, Geahlen RL. Nucleocytoplasmic trafficking of the Syk protein tyrosine kinase. Mol Cell Biol. 2006;26(9):3478–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hong J, Yuan Y, Wang J, et al. Expression of variant isoforms of the tyrosine kinase SYK determines the prognosis of hepatocellular carcinoma. Cancer Res. 2014;74(6):1845–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prinos P, Garneau D, Lucier JF, et al. Alternative splicing of SYK regulates mitosis and cell survival. Nat Struct Mol Biol. 2011;18(6):673–679. [DOI] [PubMed] [Google Scholar]
- 42. Wang L, Duke L, Zhang PS, et al. Alternative splicing disrupts a nuclear localization signal in spleen tyrosine kinase that is required for invasion suppression in breast cancer. Cancer Res. 2003;63(15):4724–4730. [PubMed] [Google Scholar]
- 43. Askoxylakis V, Badeaux M, Roberge S, et al. A cerebellar window for intravital imaging of normal and disease states in mice. Nat Protoc. 2017;12(11):2251–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Osswald M, Winkler F. Insights into cell-to-cell and cell-to-blood-vessel communications in the brain: in vivo multiphoton microscopy. Cell Tissue Res. 2013;352(1):149–159. [DOI] [PubMed] [Google Scholar]
- 45. Bonapace L, Wyckoff J, Oertner T, Van Rheenen J, Junt T, Bentires-Alj M. If you don’t look, you won’t see: intravital multiphoton imaging of primary and metastatic breast cancer. J Mammary Gland Biol Neoplasia. 2012;17(2):125–129. [DOI] [PubMed] [Google Scholar]
- 46. Zhang J, Benavente CA, McEvoy J, et al. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature. 2012;481(7381):329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.