Abstract
Radiotherapy is ubiquitous in the treatment of patients with both primary brain tumors as well as disease which is metastatic to the brain. This therapy is not without cost, however, as cognitive decline is frequently associated with cranial radiation, particularly with whole brain radiotherapy (WBRT). The precise mechanisms responsible for radiation-induced morbidity remain incompletely understood and continue to be an active area of ongoing research. In this article, we review the hypothetical means by which cranial radiation induces cognitive decline as well as potential therapeutic approaches to prevent, minimize, or reverse treatment-induced cognitive deterioration. We additionally review advances in imaging modalities that can potentially be used to identify site-specific radiation-induced anatomic or functional changes in the brain and their correlation with clinical outcomes.
Keywords: cognitive deterioration, hippocampal neurogenesis, neurotoxicity, radiation-induced brain injury, whole brain radiotherapy
Up to 30% of cancer patients develop brain metastases at some point during their disease process and at least half of these receive whole brain radiotherapy (WBRT). Within the United States alone, approximately 200000 patients a year are treated with brain radiation for primary or metastatic tumors.
Cranial radiation, especially WBRT, has been shown to be associated with cognitive decline. In adult patients, DeAngelis initially reported an 11% rate of severe dementia after WBRT for patients with brain metastases, recognizing that the daily doses of radiation utilized on this retrospective study were larger than typically used clinically.1 Although historically considered a late side effect, with availability of detailed neurocognitive testing, cognitive deterioration after WBRT has been shown to appear as early as 3–4 months,2–4 affecting approximately 90% of patients, with memory and executive function preferentially affected.5,6
Recent advances in multimodality therapy have led to improved survival rates for many cancer patients. With longer survivorship, more attention has been directed toward long-term treatment-related morbidity. The effect of radiotherapy on the long-term cognitive performance of these patients is a major concern, as the morbidity can be devastating, with a significant impact on both patient and caregiver quality of life (QoL). In this article, we review the known or proposed mechanisms by which cranial radiation induces cognitive decline in patients with metastatic and primary intracranial malignancies, and the preclinical and clinical evidence to support such hypotheses. We discuss therapeutic approaches based on the underlying mechanisms that can potentially prevent, minimize, or reverse the cognitive deterioration. We will also review available imaging modalities that can potentially be used to establish correlation between imaging and clinical deterioration.
Pathophysiology of Radiation-Induced Brain Injury
Radiation Reduces Proliferation of Neural Precursors in the Hippocampus
One prevailing hypothesis for a mechanism responsible for cognitive deterioration, especially memory impairments, following cranial radiotherapy is through reduced neurogenesis after exposure of neural precursors to ionizing radiation.7 Neurogenesis is thought to occur predominantly in 2 critical regions of the developed brain: the subgranular zone (SGZ) of the hippocampus and the subventricular zone (SVZ) of the lateral ventricles.8 From here, multipotent neural stem cells give rise to neural progenitors, which are then capable of differentiating into neurons and glia.
Exposure to radiation has been shown to significantly reduce neurogenesis in the hippocampus. Work by Monje et al evaluating the effects of radiation on neural proliferation in the rat hippocampus demonstrated that, while new neuron production demonstrated a >95% reduction following a single 10-Gy dose of WBRT, the number of viable precursors one month after radiation was similar to that of non-irradiated controls.9 This suggests that early ablation of the entire pool of neural progenitors cannot solely explain the observed marked reductions in neurogenesis.7,9 Certainly, over time, there is depletion of neural progenitor cells reflective of radiation-induced mitotic catastrophes occurring over the course of multiple cell divisions. This was demonstrated in their in vitro studies confirming reduced growth of neural progenitor cells after irradiation.
Interestingly, radiotherapy has been shown to have a heterogeneous effect on neuron formation throughout the brain volume. Although neurogenesis is initially diminished in both the SGZ and SVZ equally, the SVZ has been shown to have a delayed recovery, while hippocampal neurogenesis remains stalled.10–12 It is hypothesized that lineage-specific differences in radiation sensitivity among the neural precursors are responsible for these observations.
Radiation Alters Differentiation of Neural Precursors Through Changes in the Microenvironment
Perhaps the most striking observation from Monje et al was the shunting of viable precursor cells away from a neuronal to a glial fate due to a change in the microenvironment.9 In their subsequent studies, as discussed later, Monje and colleagues suggested that radiation-induced neuro-inflammation is at least partially responsible for the altered fate of precursor cells. Radiation is known to induce significant increases in activated microglia, the resident immune cells in the brain, which are rarely observed in the un-irradiated setting.7,9 The precise mechanism for radiotherapy-induced altered neural precursor differentiation remains under active investigation, with several reported alterations in cell-cycle signaling and epigenetic modifications.13–16
Radiation Alters the Cellular Microenvironment
Radiation-induced changes in the cellular microenvironment play a major role in the inhibition of neurogenesis and are equal to, if not greater than, the direct effects of ionizing radiation on the neural precursors.17 Irradiation of neural precursors promotes differentiation in in vitro studies, leading to an increase in differentiated cells in a dose-dependent manner.9 This is believed to occur through cell-cycle arrest, which in turn stimulates differentiation of neural precursors. However, the effects of radiation on neural precursor differentiation are highly dependent upon the surrounding cellular microenvironment. For example, irradiation of neural precursors in vitro promotes differentiation into both neurons and glia, whereas exposure to ionizing radiation in vivo results in a preferential differentiation along an astrocytic lineage.18 Furthermore, transplantation of non-irradiated neural precursors into the dentate gyrus of irradiated animals does not restore neurogenesis, with persistent reductions observed in numbers of newly formed neurons.9 Taken together, these studies support alterations in the microenvironment as a major factor in the determinant of cellular fate and reductions in neurogenesis. This indicates a possible limited therapeutic benefit for interventions limited to replacement of neural progenitors, such as stem cell transplantation.
Radiation Induces an Inflammatory Response in the Brain Through Oxidative Damage
Radiation induces oxidative stress within the brain through the generation of free radicals, which in turn activates tumor necrosis factor alpha (TNF-α) and interleukin (IL)-1β and results in downstream upregulation of pro-inflammatory pathways.19,20 Due to the high baseline metabolic burden and relative lack of endogenous anti-oxidants, the brain is particularly susceptible to such oxidative damages. The inflammatory response in the brain demonstrates significant heterogeneity with differing levels of inflammatory cytokines in site-specific substructures such as the hippocampus and cortical regions, as well as distinct recovery time, with many structures exhibiting recovery following acute radiation exposure, whereas persistent inflammatory responses are observed in the hippocampus for months beyond the initial insult.
One manifestation of the prolonged inflammatory response in the hippocampus is a pronounced increase in numbers of activated microglia observed following WBRT. Microglia produce TNF-α and IL-6, which contribute to ongoing inflammation and are also potent inhibitors of neurogenesis with relative sparing of gliogenesis.19 Treatment with anti-inflammatory agents following radiotherapy in animal models reduces the number of activated microglia and induces a corresponding increase in neurogenesis.21,22 Elimination of microglia by CSF receptor inhibition has been associated with avoidance of radiation-induced cognitive impairment in adult mice.23 Exploration of putative targets for intervention in relieving inflammatory-mediated damage following radiotherapy continues to be an area of active investigation.
Altered Neurovascular Relationships by Radiation
Beyond inflammation, the microvasculature plays a critical role in the maintenance of hippocampal neurogenesis. The microvasculature supports developing neural precursors and is largely responsible for neuronal recruitment and survival. Proliferative neural precursor cells in the adult hippocampus tend to be clustered around small vessels. Recruitment of the neural precursor cells is accompanied by a synchronous stimulation of angiogenesis, and this relationship is thought to be important to normal neurogenesis. However, this relationship between the microvasculature and neural precursors is disrupted in the irradiated hippocampus and is manifested morphologically by decreased size of the perivascular clusters of precursor cells and increased distance of these clusters to the nearest vessel.9 These changes persist for several months following radiation exposure and may be responsible for prolonged reductions in neurogenesis observed after cranial radiation.
Radiation-Induced Alterations in Dendritic Morphology of Mature Neurons
Although the effects of ionizing radiation have been most studied on neuronal precursor cells, cranial irradiation also leads to alterations in the function of mature neurons reflected in alterations of dendritic morphology and physiological function. Dendrites are critical for normal neuronal function and signaling, and alterations in dendritic morphology have been demonstrated in a number of neurocognitive disorders and in aging. Studies by Duman et al have demonstrated that exposure of hippocampal neurons to radiation leads to an acute proliferation of dendritic spines, followed by a progressive and persistent loss of the same.24 Dendritic spines are small projections emanating from dendrites that compromise the postsynaptic loci of most excitatory synapses in the central nervous system. Generally, increases in dendritic spines reflect increases in synapses, and spine decreases reflect synaptic decreases. Critically, both changes can lead to circuit dysfunction.
Morphological changes in the dendrites after radiation exposure are further confirmed by Limoli et al.25 They observed significant and persistent reductions in dendritic complexity, including dendritic branching, length, and area, in a dose-dependent manner, after radiation. For example, there were significant reductions in the number (20%–35%) and density (40%–70%) of dendritic spines on the hippocampal neurons of the dentate gyrus. Immature filopodia, which are small transient protrusions located along the length of the dendrites, demonstrate the most pronounced sensitivity to ionizing radiation compared with more mature spine morphologies with greatly reduced numbers following acute radiation exposure.25 The precise mechanism of dendritic injury following radiotherapy remains unknown. Alterations in the redox environment may also factor into dendritic dysfunction and impaired neuronal signaling.26
Impaired Physiological Function of Mature Hippocampal Neurons After Radiation
As noted previously, alterations in dendritic spines reflect synaptic function. Directly interrogating the effects of radiation on neuronal connectivity, Wu et al have demonstrated early ablation of long-term potentiation in the rat hippocampus after radiation.27 Long-term potentiation describes the strengthening of synaptic connections following high frequency stimulation and is a potential cellular substrate of learning and memory. Long-term depression, on the other hand, results in weakening of the synaptic strength in response to repetitive low frequency stimulation. Together, the 2 processes help to improve the signal-to-noise ratio within the system and improve the efficiency of the synaptic networks.28 Prior studies examining hippocampal slices from animal models following exposure to WBRT revealed a significant reduction in both acute and long-term synaptic efficiency in a dose-dependent manner.29,30
Vascular Hypothesis: Radiation Disrupts Microvasculature Leading to Ischemia and Toxic Neuroexcitation
Radiation-induced vascular changes are similar to small vessel disease seen with vascular dementia. Radiation causes the death of endothelial cells and platelet adherence to the exposed matrix, leading to thrombus formation and occlusion of small vessels over a period of months. Additionally radiation is associated with thickening of basement membranes and replacement of the lumen by collagen, thereby leading to vascular damage. The accelerated atherosclerosis and mineralizing microangiopathy in small vessels following radiation can lead to vascular insufficiency and infarction.31,32
Ischemia, whether caused by vascular insufficiency or radiation, results in a significant rise in the levels of extracellular glutamate. Glutamate is the principal excitatory neurotransmitter in cortical and hippocampal neurons, and a potent activator of the postsynaptic N-methyl-d-aspartate (NMDA) receptors. Under physiologic conditions, it is tightly regulated and distributed in the synaptic cleft. By binding to and activating postsynaptic NMDA receptors, glutamate promotes normal learning and memory. However, under ischemic or other pathologic states, an excessive rise in extracellular glutamate triggers neuronal excitotoxicity through persistent activation of the NMDA receptors, causing a large and prolonged influx of calcium and subsequent activation of downstream signaling pathways.33 These pathways, mediated by p38, c-Jun N-terminal kinase, and sterol regulatory element-binding protein 1, form potential targets for therapeutic intervention in the prevention of radiation-induced cerebral injury.33
Strategies to Minimize Radiation-Induced Neurotoxicity
Memantine
Memantine is a low-affinity voltage-dependent noncompetitive antagonist of NMDA receptors that preferentially acts in the excitotoxic state and relatively spares the function of glutamate signaling under normal physiologic condition. Mechanistically, memantine binds to NMDA receptors, thereby preventing the binding of glutamate when released at high levels in the ischemic or other pathologic states. This inhibits the prolonged influx of Ca2+ ions triggered by glutamate binding from extrasynaptic NMDA receptors that forms a basis of neuronal excitotoxicity.34 Due to the low affinity, noncompetitive nature, and rapid off-rate kinetics of memantine, the physiological function of NMDA receptors localized at synapses is relatively preserved, as these receptors can still be activated by high levels of glutamate released by the depolarization-induced release of glutamate from the presynapse.35 Memantine gained initial FDA approval in the treatment of Alzheimer’s and vascular dementia after 2 phase III randomized, placebo-controlled trials showed improved cognitive metrics with minimal side effects.36–38
Due to the overlapping mechanisms responsible for neurotoxicity in both vascular dementia and radiation-induced vasculopathy, there has been significant interest in the use of memantine to minimize cognitive deterioration following WBRT. The Radiation Therapy Oncology Group (RTOG) 0614 trial examined the role of memantine in the preservation of cognitive function in patients receiving WBRT. The trial randomized 554 patients to receive either WBRT (37.5 Gy in 15 fractions) plus memantine or placebo.39 Memantine was administered concurrently with radiotherapy at a dose of 5 mg daily and escalated to a final dose of 10 mg delivered twice daily and continued for a total of 24 weeks. Cognitive testing at 24 weeks post-radiotherapy demonstrated a trend toward improved memory function in the memantine arm as measured by the Hopkins Verbal Learning Test–Revised Delayed Recall (HVLT-R DR) (median decline 0 vs −0.895, P = 0.0587) with significantly less decline in the memantine arm on several secondary endpoints evaluating memory, executive function, and processing speed. Another secondary endpoint was time to cognitive decline, defined as first cognitive failure on any cognitive test, and memantine was associated with reduced cognitive function failure at 24 weeks compared with placebo (53.8% vs 64.9%, P = 0.01). Memantine was well tolerated with no significant differences in reported adverse events compared with placebo.39 Since the publication of this trial, memantine has been used in our practice as the standard of care to reduce the cognitive side effects of WBRT.
Donepezil
Several neurocognitive disorders are characterized by reductions in the activity of choline acetyltransferase, which leads to decreased levels of acetylcholine and impaired neuronal signaling.40 Donepezil is a reversible noncompetitive inhibitor of acetylcholinesterase, which enhances cholinergic-dependent neural communication. It is typically well tolerated and has shown efficacy in improving cognitive function in patients with Alzheimer’s disease, vascular dementia, multiple sclerosis, and Parkinson’s disease, among others.41
Donepezil has been explored in a double-blinded, placebo-controlled trial in patients with a history (≥6 mo prior) of either whole brain or partial brain radiotherapy of at least 30 Gy for treatment of either primary or metastatic brain tumors.42 A total of 198 patients were randomized to receive either donepezil (5 mg daily for 6 wk, then 10 mg daily for 18 wk) or placebo with the primary outcome evaluating cognitive function at 24 weeks. Both interim and final evaluation failed to demonstrate a significant difference in overall cognitive function with the addition of donepezil, although there were modest improvements in several cognitive functions, especially in those patients with greater pretreatment impairments. Overall, donepezil was well tolerated, with only the presence of diarrhea (25% vs 9%, P = 0.005) significantly different from the placebo arm.42
Hippocampal Avoidance WBRT
Despite the success with memantine in preserving cognitive function in patients receiving WBRT, over 50% of patients still experienced cognitive decline, arguing for the need for additional measures to further reduce cognitive toxicity from radiotherapy. The hippocampus is critical in memory formation and learning, and is extraordinarily sensitive to cranial radiotherapy, with even low doses of ionizing radiation demonstrating a significant reduction in neurogenesis and deficits in memory.43 As the hippocampus is an infrequent site for metastatic brain involvement, RTOG 0933 sought to answer the question whether avoidance of the hippocampus via highly conformal radiotherapy techniques resulted in preserved memory function compared with historical controls.44
RTOG 0933 enrolled a total of 113 patients, of whom 42 were alive and analyzable at the study endpoint of 4 months. Per protocol, 30 Gy in 10 fractions was delivered to the brain parenchyma via an intensity modulated radiation therapy technique. The dose to 100% of the hippocampus was constrained to 9 Gy with a maximal point dose of 16 Gy with central review of treatment plans prior to initiation of therapy. At 4 months, patients on trial experienced significantly less mean decline on the HVLT-R DR compared with historical controls (7% vs 30%, P < 0.001). Hippocampal avoidance furthermore did not appear to convey a heightened risk of intracranial progression, with only 4.5% of patients who developed intracranial progression experiencing progression within the hippocampal avoidance region.44 Despite the promising cognitive outcomes of this trial, it is a nonrandomized phase II study and the cognitive benefits of hippocampal avoidance need to be proven in a phase III trial. Currently 2 phase III randomized studies are being conducted investigating the role of hippocampal sparing in the setting of WBRT (NRG CC001) and prophylactic cranial irradiation for small cell lung cancer patients (NRG CC003).
Anti-Inflammatories
As discussed earlier, radiation causes chronic inflammation in the hippocampus, contributing to reduced neurogenesis. In animal models, treatment of inflammation with nonsteroidal anti-inflammatory drugs (NSAIDs) or elimination of inflammation by other means has shown preservation of memory and learning capability.21 This seems to be a mechanistically sound and potentially viable clinical approach to reduce radiation-induced cognitive decline. However, clinical trials are needed to establish the role of NSAIDs in reducing radiation-induced cognitive deterioration.
Other Strategies to Avoid or Delay Whole Brain Radiotherapy
Stereotactic radiosurgery
Another increasingly popular approach by which to spare radiation dose to the hippocampus as well as other areas of uninvolved brain parenchyma is by deferring WBRT in favor of stereotactic radiosurgery (SRS) to the sites of known disease or surgical cavity. WBRT has previously been shown to reduce the risk of local and distant brain failure following either surgical resection45 or SRS,46,47 although it has not been shown to convey a significant survival benefit and additionally has been associated with a significant longitudinal decline in cognitive function following treatment.3 Given this knowledge, several randomized trials have been conducted to address the question of whether the potential cognitive deficits due to an increased risk of tumor recurrence in the brain with the omission of WBRT outweigh the cognitive dysfunction caused by treatment of the entire brain parenchyma. At least 3 studies have shown a cognitive benefit by using SRS to treat patients with limited brain metastases without compromising survival outcome, establishing SRS as the standard of care in this patient population.2,3,48
The N0574 trial, the largest of these trials, enrolled a total of 213 patients with 1–3 brain metastases who were randomized to SRS alone versus the addition of WBRT. The primary endpoint was cognitive deterioration as defined by a decline of >1 standard deviation on at least 1 of 7 administered cognitive tests. As expected, there was a significant reduction in the incidence in the time to intracranial failure with the addition of WBRT (hazard ratio [HR] 3.6, P < 0.001), although there was no difference in overall survival between the study arms. The patients receiving SRS alone demonstrated significantly less cognitive deterioration at 3 months compared with the addition of WBRT (63.5% vs 91.7%, P < 0.001), which remained significant at 1 year following treatment in long-term survivors. In addition, there was better QoL at 3 months with SRS alone compared with those treated with WBRT. As the largest trial to systematically employ comprehensive cognitive testing between these 2 treatment arms, N0574 cast doubt on the overall benefit of WBRT in patients with oligometastatic disease. The relative lack of a benefit in terms of meaningful clinical endpoints other than the incidence of new metastases for WBRT in these patients is likely due in part to the availability of salvage therapies in patients who are initially treated with SRS alone. Any benefit procured by up-front WBRT for this patient population also appears to be outweighed by the potential significant cognitive toxicities imparted by treatment of the entire brain volume.
The past 5–10 years have seen a general shift toward using SRS for patients with >3 brain metastases with the intent of eliminating or avoiding WBRT in as many patients for as long as possible. Can we extrapolate the results from trials in patients with 1–3 brain metastases and expand the use of SRS alone to patients with 4 or more brain metastases? The answer may be yes, based on several retrospective studies and one prospective observational study (JLGK0901) that compared outcomes for patients with 2–4 versus 5–10 brain metastases.49 In this study, SRS for patients with 5–10 brain metastases was not inferior to SRS for patients with 2–4 brain metastases in terms of overall survival (10.8 vs 10.8 mo), local recurrence (6.5% vs 7.0%, P = 0.70), appearance of new brain lesions (63.8% vs 54.5%, P = 0.067), or risk of neurologic death (4.3% vs 1.7%, P = 0.25) at 1 year. This is the best evidence available to support the use of SRS in patients with 5–10 brain metastases. One ongoing phase III trial for patients with 4–15 brain metastases, NCT01592968, in which patients are randomized to receive WBRT versus SRS, with neurocognitive function and intracranial control as primary endpoints, will hopefully provide high-level evidence to guide the therapy for patients with multiple brain metastases.
Systemic therapy
In the era of targeted therapy (eg, inhibitors of epidermal growth factor receptor [EGFR], Braf, anaplastic lymphoma kinase [ALK]) and immunotherapy, the notion of using WBRT to control microscopic disease is further questioned. One emerging concept is to use SRS to treat gross disease and targeted agents and/or immunotherapy to control microscopic disease, thereby avoiding or delaying the need for WBRT for as long as possible. In that regard, multiple studies have demonstrated the intracranial activity of inhibitors for EGFR, ALK, and Braf. As for immunotherapy, retrospective evidence from MD Anderson in which melanoma patients with 4 or more brain metastases given ipilimumab and SRS had better intracranial control than a propensity-matched cohort who received WBRT suggests that anti–cytotoxic T lymphocyte antigen 4 has similar or better ability to control microscopic disease in the brain.50 It should be noted that delaying radiation due to CNS activity of systemic agents should be handled with caution. Two recent retrospective studies showed that in patients with EGFR-mutant non–small cell lung cancer, delaying brain radiation, either SRS or WBRT, until intracranial disease progression was associated with increased neurologic death and worse survival.51,52 The authors of these studies noted that SRS followed by tyrosine kinase inhibitors resulted in the longest overall survival and allowed patients to avoid the potential cognitive sequelae of WBRT.
Tumor-treating fields
Tumor-treating fields (TTFields) are an antimitotic treatment that selectively disrupts the division of cells by delivering low-intensity, intermediate-frequency alternating electric fields via transducer arrays applied to the shaved scalp. TTFields have been shown in a phase III trial to improve survival in patients with newly diagnosed glioblastoma53 without compromising cognitive outcomes. In patients with recurrent glioblastoma, TTFields have also been shown to lead to similar survival rates as systemic therapy—however, with better patient reported outcomes.54 The device is currently being evaluated in a randomized trial for patients with lung cancer who develop brain metastases (NCT02831959). In this phase III trial, patients are randomized to TTFields or observation after SRS for 1–10 brain metastases. The primary outcome is time to first cerebral progression to see if TTFields could be a less toxic alternative to WBRT to improve intracranial control.
Imaging of Radiation-Induced Brain Injury
Conventional Diagnostic Imaging
Conventional neuroimaging modalities have demonstrated anatomic changes in a variety of neurologic diseases, some of which are pathognomonic for specific neurodegenerative disorders. Likewise, changes in cerebral anatomy can also be observed following radiation exposure, although these tend to be more subtle and not necessarily specific to radiotherapy. Reductions in hippocampal volume following high-dose partial brain radiotherapy have been demonstrated in patients with primary brain tumors, with these changes remaining significant on multivariate analysis even after accounting for a variety of patient factors, disease laterality, and systemic therapy.55
The hippocampus, though, is not the only cerebral structure to demonstrate changes in anatomic appearance following radiotherapy, with changes in cerebral cortex anatomy also observed following cranial irradiation.56 Karunamuni et al have shown a dose-dependent reduction in cortical thickness 1 year after partial brain irradiation in the treatment of patients with high-grade gliomas. The degree of cortical thickness loss was most pronounced in the temporal and limbic lobes and parallel patterns are observed in neurodegeneration caused by Alzheimer’s disease.56 Additional studies in patients undergoing high-dose partial brain radiotherapy have shown changes in the underlying white matter that are significantly correlated with radiation dose.57 Clinically, these changes have been shown to be associated with the development of cognitive disabilities.58
Diffusion Tensor Imaging
Diffusion tensor imaging (DTI) is a form of magnetic resonance imaging that measures the anisotropy of structures in relation to the diffusion of water. Water molecules readily diffuse along the length of the neuron but are much more limited in their ability to move perpendicularly to the neuronal axis across the myelin sheath. In the brain, this differential diffusion capability can be exploited to visualize white matter tracts. Many different pathologies can cause changes in white matter DTI-identified fiber tracts, with several studies in particular exploring DTI-identified changes following cranial radiotherapy.
DTI studies following radiotherapy have demonstrated the capability to detect radiation-induced white matter changes that are often not readily apparent on conventional diagnostic imaging. One study evaluating a group of patients receiving WBRT ± chemotherapy revealed significant differences in the spatiotemporal disturbances in diffusion indices potentially indicating differing pathologic mechanisms of injury and sensitivity to ionizing radiotherapy dependent upon anatomic location.59 Specifically, there was a significant decrease in fractional anisotropy, which is a marker of white matter density and integrity, most prominently observed in the fornix, cingula, and corpus callosum. It was hypothesized that these regions are particularly susceptible to damage from ionizing radiation and give rise to the functional impairments observed following cranial radiotherapy.60
Many DTI studies exploring white matter injury following cranial radiotherapy are confounded by the fact that most patients are also treated with systemic therapy, with some receiving numerous lines of several cytotoxic agents. Numerous studies have demonstrated that patients receiving systemic therapy alone61,62 or surgery without additional adjuvant therapy63 also have DTI-identified changes associated with white matter injury and neurotoxicity. As DTI visualizes white matter tracts predominantly on the macrocellular level, it is difficult to discern from imaging findings alone as to the mechanisms and microenvironmental alterations responsible for the visualized white matter damage. Further work is needed to elucidate the role of DTI changes following chemotherapy or radiation and their ability to predict later cognitive injury.
Functional Imaging of Radiation-Induced Toxicity
While conventional imaging and DTI measure anatomic changes within the brain, functional imaging techniques have also been used to assess radiation-induced neurotoxicity. Functional MRI, which indirectly measures neural activity through the measurement of changes in blood flow, has been widely utilized in the clinical setting during surgical treatment planning in order to spare vital eloquent cortical regions.64,65 After high doses of radiotherapy, decreased neural activations during motor and sensory tasks have been observed with suppressed output observed several months following treatment.66 Functional imaging studies have also been utilized clinically to differentiate radiation-induced damage such as radiation necrosis from recurrent intracranial tumors.67–70
Spectroscopy is a powerful functional imaging tool to detect and characterize alterations in metabolism in vivo in the absence of any overt anatomic pathology. Following high-dose partial brain radiation therapy, spectroscopy tools have shown intracranial molecular derangements even in unexposed brain regions.71,72 This is hypothesized to arise from the release of cytokine cascades following radiotherapy exposure, which may also account for functional neural changes observed outside of the focal radiotherapy field.73 These metabolic changes have been observed for months following radiation exposure even in the absence of significant overt anatomic changes.74,75 Clinically, alterations detected by functional imaging have also been correlated with long-term cognitive dysfunction in pediatric brain tumor survivors.76
Conclusions
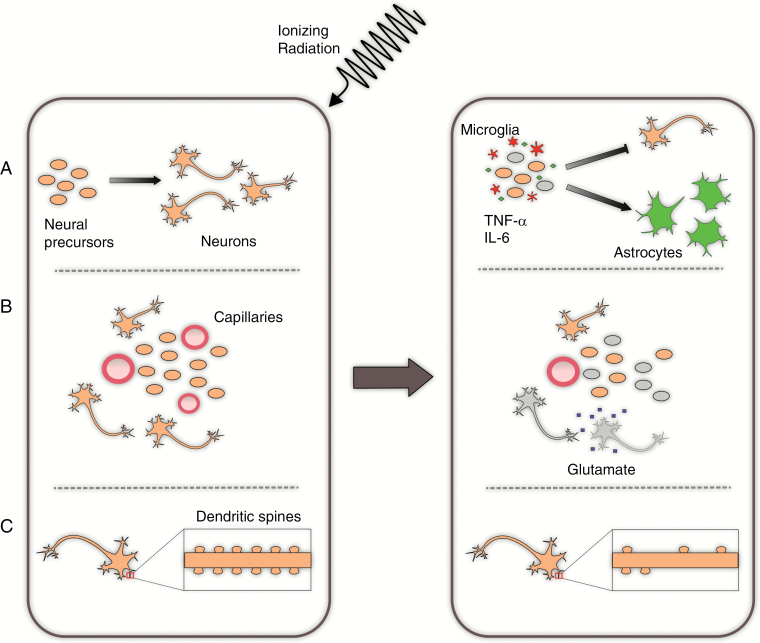
Cranial radiation is associated with significant cognitive deterioration which can have a devastating impact on patients’ QoL. Possible mechanisms of radiation-induced cognitive decline are multifactorial, including (i) reduced neurogenesis in the hippocampus and altered neural stem cell differentiation, chronic inflammation/abnormal relationship between neural stem cells and microvasculature; (ii) altered neuron morphology, with reduced dendritic spines and filopodia reflective of impaired synaptic function; and (iii) vascular insufficiency with ischemia-induced excitotoxicity as seen in vascular dementia (Fig. 1).
Fig. 1.
Selected proposed mechanisms of radiation-induced neurocognitive dysfunction. (A) Pro-inflammatory changes following radiotherapy result in an increase in the numbers of microglia which produce TNF-α and IL-6. This contributes to an ongoing inflammatory state and alteration in the microenvironment, which preferentially drive differentiation of neural precursors to an astrocytic lineage. (B) Radiation disrupts the vascular niche of the neural precursors and additionally leads to ischemia and toxic neuroexcitation among mature neurons. (C) Radiation exposure reduces the number of dendritic spines on mature neurons, which in turn disrupts synaptic efficiency.
Based on these mechanisms supported by preclinical and clinical evidence, there are multiple targets for therapeutic intervention with the goal to prevent, minimize, or reverse radiation-induced cognitive decline, some of which have been proven to be successful (Table 1). These include: (i) memantine to prophylactically reduce radiation-induced cognitive decline, supported by level 1 evidence; (ii) hippocampal avoidance WBRT, supported by a promising phase II trial with 2 phase III trials ongoing; (iii) use of SRS to delay or avoid WBRT, supported by at least 3 phase III trials in patients with limited brain metastases, with phase III trials in patients with 5+ brain metastases ongoing; (iv) an ongoing phase III trial in lung cancer patients with 1–10 brain metastases assessing the efficacy of TTFields to reduce intracranial recurrence without the side effects of WBRT; and (v) use of systemic therapies that especially target agents against EGFR, ALK, and Braf and immunotherapy that has demonstrated intracranial activities to delay cranial radiation. Retrospective studies have cast doubt on whether patients’ survival is compromised by delaying cranial radiation, and this will need to be studied in a prospective setting. Other potential preventive or therapeutic interventions include intrahippocampal transplantation of neural stem cells or microvesicles and the use of NSAIDs or other agents to reduce chronic inflammation. These are currently limited to the preclinical setting, although they remain areas of active investigation. Finally, advances in imaging allowing for more accurate assessment of radiation-induced brain injury may help to further refine our understanding of critical brain structures which are especially sensitive to ionizing radiotherapy. Thanks to improvements in multimodality therapies and a corresponding increase in long-term cancer survivorship, mitigation of treatment-related toxicities affecting QoL will continue to become an ever-increasingly important consideration in the care of this patient population.
Table 1.
Selected studies exploring techniques to prevent or minimize radiation-induced cognitive dysfunction
| Intervention | Authors | # Patients | Treatment Arms | Radiotherapy | Overall Survival | Progression-Free Survival | Outcomes | Toxicity |
|---|---|---|---|---|---|---|---|---|
| Memantine | Brown et al (RTOG 0614) | 554 | Memantine x24 weeks | WBRT 37.5 Gy / 15 fractions (fx) |
4.7 mo | 6.7 mo | Time to cognitive failure at 24 weeks | 14% grade 3–4 toxicity attributed to treatment in each arm (NS) |
| Placebo | 5.5 mo (not significant [NS]) | 7.8 mo (NS) | HR 0.78 (CI 0.62–0.99, P = 0.01) | |||||
| Donepezil | Rapp et al (Wake Forest) | 198 | Donepezil x24 weeks | At least 30 Gy | Not reached | Not reached | No significant difference among cognitive composite scores (P = 0.48) | Increased diarrhea in donepezil group (25% vs 9%, P = 0.005) |
| Whole brain (40%) | ||||||||
| Placebo | Partial brain (59%) | Improvement on memory (recognition, P = 0.027; discrimination, P = 0.007) and motor speed and function (P = 0.016) with donepezil | ||||||
| Hippocampal avoidance (HA) | Gondi et al (RTOG 0933) | 113 | Hippocampal avoidance WBRT | HA-WBRT 30 Gy / 10 fx |
6.8 mo | 5.9 mo | 7% mean decline in HVLT-R DR from baseline to 4 mo | Two grade 3 toxicities (fatigue, headache) |
| 42 analyzed | Historically 30% on PCI-P-120–9801 (P < 0.01) | No grade 4–5 toxicities | ||||||
| SRS | Brown et al (NCCTG N107C) | 194 | WBRT | WBRT: 30 Gy / 10 fx or 37.5 Gy / 15 fx |
11.6 mo | 27.5 mo | Cognitive deterioration-free survival | Grade 3+ toxicity SRS (12%) vs WBRT (18%) |
| SRS | SRS: 12–20 Gy |
12.2 mo (NS) | 6.4 mo | SRS 3.7 mo WBRT 3.0 mo |
4% grade 2+ CNS necrosis in SRS arm | |||
| HR 2.45 (1.62–3.72, P < 0.0001) | HR 0.47 (0.35–0.63, P < 0.0001) | |||||||
| Brown et al (NCCTG N0574) | 213 | SRS alone | WBRT: 30 Gy / 12 fx |
10.4 mo | 3 mo CNS control SRS 75.3% |
3 mo cognitive deterioration | CNS necrosis (2.9% SRS + WBRT; 4.5% SRS alone) | |
| SRS + WBRT | SRS: 18–22 Gy (prior WBRT) 20–24 Gy (SRS alone) |
7.4 mo (NS) | SRS + WBRT 93.7% (P < 0.001) |
SRS 63.5% SRS + WBRT 91.7% (P < 0.001) |
||||
| Chang et al (MD Anderson) | 58 | SRS alone | WBRT: 30 Gy / 12 fx |
15.2 mo | 1 year freedom from CNS relapse | Decline in 4 mo recall | SRS alone: 3 grade 3+ toxicities | |
| SRS + WBRT | SRS: 15–20 Gy |
5.7 mo (P = 0.003) | SRS 27% SRS + WBRT 73% P = 0.0003 |
SRS 24% SRS + WBRT 52% |
SRS + WBRT: 1 grade 3+ toxicity |
Funding
None.
Conflict of interest statement. Jing Li received research funding from Bristol-Myers Squibb to support an ongoing clinical trial. The rest of the authors declare no conflict of interest.
References
- 1. DeAngelis LM, Mandell LR, Thaler HT et al. The role of postoperative radiotherapy after resection of single brain metastases. Neurosurgery. 1989;24(6):798–805. [DOI] [PubMed] [Google Scholar]
- 2. Brown PD, Jaeckle K, Ballman KV et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang EL, Wefel JS, Hess KR et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 4. Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25(10):1260–1266. [DOI] [PubMed] [Google Scholar]
- 5. Meyers CA, Smith JA, Bezjak A et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22(1):157–165. [DOI] [PubMed] [Google Scholar]
- 6. Laack NN, Brown PD. Cognitive sequelae of brain radiation in adults. Semin Oncol. 2004;31(5):702–713. [DOI] [PubMed] [Google Scholar]
- 7. Monje M. Cranial radiation therapy and damage to hippocampal neurogenesis. Dev Disabil Res Rev. 2008;14(3):238–242. [DOI] [PubMed] [Google Scholar]
- 8. Gonzalez-Perez O. Neural stem cells in the adult human brain. Biol Biomed Rep. 2012;2(1):59–69. [PMC free article] [PubMed] [Google Scholar]
- 9. Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. [DOI] [PubMed] [Google Scholar]
- 10. Hellström NA, Björk-Eriksson T, Blomgren K, Kuhn HG. Differential recovery of neural stem cells in the subventricular zone and dentate gyrus after ionizing radiation. Stem Cells. 2009;27(3):634–641. [DOI] [PubMed] [Google Scholar]
- 11. Hellström NA, Lindberg OR, Ståhlberg A et al. Unique gene expression patterns indicate microglial contribution to neural stem cell recovery following irradiation. Mol Cell Neurosci. 2011;46(4):710–719. [DOI] [PubMed] [Google Scholar]
- 12. Gage FH. Mammalian neural stem cells. Science. 2000;287(5457): 1433–1438. [DOI] [PubMed] [Google Scholar]
- 13. Chen H, Goodus MT, de Toledo SM, Azzam EI, Levison SW, Souayah N. Ionizing radiation perturbs cell cycle progression of neural precursors in the subventricular zone without affecting their long-term self-renewal. ASN Neuro. 2015;7(3):175909141557802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bajinskis A, Lindegren H, Johansson L, Harms-Ringdahl M, Forsby A. Low-dose/dose-rate γ radiation depresses neural differentiation and alters protein expression profiles in neuroblastoma SH-SY5Y cells and C17.2 neural stem cells. Radiat Res. 2011;175(2):185–192. [DOI] [PubMed] [Google Scholar]
- 15. Eom HS, Park HR, Jo SK et al. Ionizing radiation induces altered neuronal differentiation by mGluR1 through PI3K-STAT3 signaling in C17.2 mouse neural stem-like cells. PLoS One. 2016;11(2):e0147538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li YQ, Cheng ZC, Liu SW, Aubert I, Wong CS. P53 regulates disruption of neuronal development in the adult hippocampus after irradiation. Cell Death Discov. 2016;2:16072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fike JR, Rosi S, Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Semin Radiat Oncol. 2009;19(2):122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: a review. Front Oncol. 2012;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hong JH, Chiang CS, Campbell IL, Sun JR, Withers HR, McBride WH. Induction of acute phase gene expression by brain irradiation. Int J Radiat Oncol Biol Phys. 1995;33(3):619–626. [DOI] [PubMed] [Google Scholar]
- 20. Lee WH, Sonntag WE, Mitschelen M, Yan H, Lee YW. Irradiation induces regionally specific alterations in pro-inflammatory environments in rat brain. Int J Radiat Biol. 2010;86(2):132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. [DOI] [PubMed] [Google Scholar]
- 22. Jenrow KA, Brown SL, Lapanowski K, Naei H, Kolozsvary A, Kim JH. Selective inhibition of microglia-mediated neuroinflammation mitigates radiation-induced cognitive impairment. Radiat Res. 2013;179(5):549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Acharya MM, Green KN, Allen BD et al. Elimination of microglia improves cognitive function following cranial irradiation. Sci Rep. 2016;6:31545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duman JG, Tzeng CP, Tu YK et al. The adhesion-GPCR BAI1 regulates synaptogenesis by controlling the recruitment of the Par3/Tiam1 polarity complex to synaptic sites. J Neurosci. 2013;33(16):6964–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parihar VK, Limoli CL. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc Natl Acad Sci U S A. 2013;110(31):12822–12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zou Y, Corniola R, Leu D et al. Extracellular superoxide dismutase is important for hippocampal neurogenesis and preservation of cognitive functions after irradiation. Proc Natl Acad Sci U S A. 2012;109(52):21522–21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu PH, Coultrap S, Pinnix C et al. Radiation induces acute alterations in neuronal function. PLoS One. 2012;7(5):e37677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Massey PV, Bashir ZI. Long-term depression: multiple forms and implications for brain function. Trends Neurosci. 2007;30(4):176–184. [DOI] [PubMed] [Google Scholar]
- 29. Pellmar TC, Schauer DA, Zeman GH. Time- and dose-dependent changes in neuronal activity produced by X radiation in brain slices. Radiat Res. 1990;122(2):209–214. [PubMed] [Google Scholar]
- 30. Pellmar TC, Lepinski DL. Gamma radiation (5-10 Gy) impairs neuronal function in the guinea pig hippocampus. Radiat Res. 1993;136(2):255–261. [PubMed] [Google Scholar]
- 31. Orrego F, Villanueva S. The chemical nature of the main central excitatory transmitter: a critical appraisal based upon release studies and synaptic vesicle localization. Neuroscience. 1993;56(3):539–555. [DOI] [PubMed] [Google Scholar]
- 32. Danysz W, Parsons CG, Karcz-Kubicha M et al. GlycineB antagonists as potential therapeutic agents. Previous hopes and present reality. Amino Acids. 1998;14(1-3):235–239. [DOI] [PubMed] [Google Scholar]
- 33. Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol. 2014;115:157–188. [DOI] [PubMed] [Google Scholar]
- 34. Tozzi A, Costa C, Di Filippo M et al. Memantine reduces neuronal dysfunctions triggered by in vitro ischemia and 3-nitropropionic acid. Exp Neurol. 2007;207(2):218–226. [DOI] [PubMed] [Google Scholar]
- 35. Barnes CA, Danysz W, Parsons CG. Effects of the uncompetitive NMDA receptor antagonist memantine on hippocampal long-term potentiation, short-term exploratory modulation and spatial memory in awake, freely moving rats. Eur J Neurosci. 1996;8(3):565–571. [DOI] [PubMed] [Google Scholar]
- 36. Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I; Memantine Study Group Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–324. [DOI] [PubMed] [Google Scholar]
- 37. Wilcock G, Möbius HJ, Stöffler A; MMM 500 group A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500). Int Clin Psychopharmacol. 2002;17(6):297–305. [DOI] [PubMed] [Google Scholar]
- 38. Orgogozo JM, Rigaud AS, Stöffler A, Möbius HJ, Forette F. Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial (MMM 300). Stroke. 2002;33(7):1834–1839. [DOI] [PubMed] [Google Scholar]
- 39. Brown PD, Pugh S, Laack NN et al. ; Radiation Therapy Oncology Group (RTOG) Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Malouf R, Birks J. Donepezil for vascular cognitive impairment. Cochrane Database Syst Rev. 2004;(1):CD004395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seltzer B. Donepezil: an update. Expert Opin Pharmacother. 2007;8(7):1011–1023. [DOI] [PubMed] [Google Scholar]
- 42. Rapp SR, Case LD, Peiffer A et al. Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. J Clin Oncol. 2015;33(15):1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gondi V, Hermann BP, Mehta MP, Tomé WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2013;85(2):348–354. [DOI] [PubMed] [Google Scholar]
- 44. Gondi V, Pugh SL, Tome WA et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Patchell RA, Tibbs PA, Regine WF et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485–1489. [DOI] [PubMed] [Google Scholar]
- 46. Aoyama H, Shirato H, Tago M et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. [DOI] [PubMed] [Google Scholar]
- 47. Kocher M, Soffietti R, Abacioglu U et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brown PD, Ballman KV, Cerhan JH et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yamamoto M, Serizawa T, Shuto T et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–395. [DOI] [PubMed] [Google Scholar]
- 50. Fang P, Jiang W, Allen P et al. Radiation necrosis with stereotactic radiosurgery combined with CTLA-4 blockade and PD-1 inhibition for treatment of intracranial disease in metastatic melanoma. J Neurooncol. 2017;133(3):595–602. [DOI] [PubMed] [Google Scholar]
- 51. Magnuson WJ, Yeung JT, Guillod PD, Gettinger SN, Yu JB, Chiang VL. Impact of deferring radiation therapy in patients with epidermal growth factor receptor-mutant non-small cell lung cancer who develop brain metastases. Int J Radiat Oncol Biol Phys. 2016;95(2):673–679. [DOI] [PubMed] [Google Scholar]
- 52. Magnuson WJ, Lester-Coll NH, Wu AJ et al. Management of brain metastases in tyrosine kinase inhibitor-naïve epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35(10):1070–1077. [DOI] [PubMed] [Google Scholar]
- 53. Stupp R, Taillibert S, Kanner AA et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543. [DOI] [PubMed] [Google Scholar]
- 54. Stupp R, Wong ET, Kanner AA et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192–2202. [DOI] [PubMed] [Google Scholar]
- 55. Seibert TM, Karunamuni R, Bartsch H et al. Radiation dose-dependent hippocampal atrophy detected with longitudinal volumetric magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2017;97(2):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Karunamuni R, Bartsch H, White NS et al. Dose-dependent cortical thinning after partial brain irradiation in high-grade glioma. Int J Radiat Oncol Biol Phys. 2016;94(2):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Corn BW, Yousem DM, Scott CB et al. White matter changes are correlated significantly with radiation dose. Observations from a randomized dose-escalation trial for malignant glioma (Radiation Therapy Oncology Group 83-02). Cancer. 1994;74(10):2828–2835. [DOI] [PubMed] [Google Scholar]
- 58. Douw L, Klein M, Fagel SS et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. [DOI] [PubMed] [Google Scholar]
- 59. Chapman CH, Nazem-Zadeh M, Lee OE et al. Regional variation in brain white matter diffusion index changes following chemoradiotherapy: a prospective study using tract-based spatial statistics. PLoS One. 2013;8(3):e57768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nazem-Zadeh MR, Chapman CH, Lawrence TL, Tsien CI, Cao Y. Radiation therapy effects on white matter fiber tracts of the limbic circuit. Med Phys. 2012;39(9):5603–5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Deprez S, Amant F, Yigit R et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp. 2011;32(3):480–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Koppelmans V, de Groot M, de Ruiter MB et al. Global and focal white matter integrity in breast cancer survivors 20 years after adjuvant chemotherapy. Hum Brain Mapp. 2014;35(3):889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rueckriegel SM, Driever PH, Blankenburg F, Lüdemann L, Henze G, Bruhn H. Differences in supratentorial damage of white matter in pediatric survivors of posterior fossa tumors with and without adjuvant treatment as detected by magnetic resonance diffusion tensor imaging. Int J Radiat Oncol Biol Phys. 2010;76(3):859–866. [DOI] [PubMed] [Google Scholar]
- 64. Håberg A, Kvistad KA, Unsgård G, Haraldseth O. Preoperative blood oxygen level-dependent functional magnetic resonance imaging in patients with primary brain tumors: clinical application and outcome. Neurosurgery. 2004;54(4):902–914; discussion 914. [DOI] [PubMed] [Google Scholar]
- 65. Lehéricy S, Duffau H, Cornu P et al. Correspondence between functional magnetic resonance imaging somatotopy and individual brain anatomy of the central region: comparison with intraoperative stimulation in patients with brain tumors. J Neurosurg. 2000;92(4):589–598. [DOI] [PubMed] [Google Scholar]
- 66. Kovács Á, Emri M, Opposits G et al. Changes in functional MRI signals after 3D based radiotherapy of glioblastoma multiforme. J Neurooncol. 2015;125(1):157–166. [DOI] [PubMed] [Google Scholar]
- 67. Lupo JM, Nelson SJ. Advanced magnetic resonance imaging methods for planning and monitoring radiation therapy in patients with high-grade glioma. Semin Radiat Oncol. 2014;24(4):248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Larsen VA, Simonsen HJ, Law I, Larsson HB, Hansen AE. Evaluation of dynamic contrast-enhanced T1-weighted perfusion MRI in the differentiation of tumor recurrence from radiation necrosis. Neuroradiology. 2013;55(3):361–369. [DOI] [PubMed] [Google Scholar]
- 69. Covarrubias DJ, Rosen BR, Lev MH. Dynamic magnetic resonance perfusion imaging of brain tumors. Oncologist. 2004;9(5):528–537. [DOI] [PubMed] [Google Scholar]
- 70. Verma N, Cowperthwaite MC, Burnett MG, Markey MK. Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro Oncol. 2013;15(5):515–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jyothi Lakshmi R, Kartha VB, Murali Krishna C, R Solomon JG, Ullas G, Uma Devi P. Tissue Raman spectroscopy for the study of radiation damage: brain irradiation of mice. Radiat Res. 2002;157(2):175–182. [DOI] [PubMed] [Google Scholar]
- 72. Estève F, Rubin C, Grand S, Kolodié H, Le Bas JF. Transient metabolic changes observed with proton MR spectroscopy in normal human brain after radiation therapy. Int J Radiat Oncol Biol Phys. 1998;40(2):279–286. [DOI] [PubMed] [Google Scholar]
- 73. Feiock C, Yagi M, Maidman A, Rendahl A, Hui S, Seelig D. Central nervous system injury—a newly observed bystander effect of radiation. PLoS One. 2016;11(9):e0163233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chan KC, Khong PL, Cheung MM, Wang S, Cai KX, Wu EX. MRI of late microstructural and metabolic alterations in radiation-induced brain injuries. J Magn Reson Imaging. 2009;29(5):1013–1020. [DOI] [PubMed] [Google Scholar]
- 75. Kaminaga T, Shirai K. Radiation-induced brain metabolic changes in the acute and early delayed phase detected with quantitative proton magnetic resonance spectroscopy. J Comput Assist Tomogr. 2005;29(3):293–297. [DOI] [PubMed] [Google Scholar]
- 76. Davidson A, Tait DM, Payne GS et al. Magnetic resonance spectroscopy in the evaluation of neurotoxicity following cranial irradiation for childhood cancer. Br J Radiol. 2000;73(868):421–424. [DOI] [PubMed] [Google Scholar]