Abstract
Background
There has been significant improvement in treatment outcomes of primary central nervous system lymphoma (PCNSL) at specialized centers over the past several decades; however, it is unclear if these changes have translated to benefits in the general population.
Methods
In this study, we utilized 2 national databases to examine survival trends over time for PCNSL: the Central Brain Tumor Registry of the United States (CBTRUS, 2000–2013) and 18 registries from the Surveillance, Epidemiology, and End Results program (SEER, 1973–2013).
Results
The annual incidence of PCNSL in 2013 was 0.4 per 100000 population (CBTRUS/SEER). Incidence increased from 0.1 per 100000 in the 1970s to 0.4 per 100000 in the 1980s, correlating with an increase in the diagnosis of patients ≥70 years (1973: 0.2 vs 2013: 2.1 [SEER]). Incidence rates differed greatly between young and elderly patients (age 20–29 y: 0.08 vs 70–79 y: 4.32 [CBTRUS]). Even though the median overall survival of all patients doubled from 12.5 months in the 1970s to 26 months in the 2010s, this survival benefit was limited to patients <70 years. Survival in the elderly population has not changed in the last 40 years (6 mo in the 1970s vs 7 mo in the 2010s, P = 0.1).
Conclusion
The poor outcome seen in the particularly vulnerable elderly patient population highlights the need for clinical trials targeting the elderly in hopes of improving treatment strategies and survival.
Keywords: CBTRUS, incidence, primary CNS lymphoma (PCNSL), SEER, survival
Importance of the study
The treatment outcomes of PCNSL have significantly improved over the past decades, and we anticipate that up to half of the newly diagnosed PCNSL patients will have long-term control. Despite this significant progress, our study highlights the significant outcome disparities based on age that exist in the treatment of PCNSL. This study uses 4 decades of data from a large US population cohort to describe the lack of improvement in clinical outcome in elderly patients. Moreover, we show that the incidence of PCNSL is highest among those elderly PCNSL patients, highlighting the need for clinical trials targeting the elderly with the hopes of improving the current dismal outcomes.
Primary central nervous system lymphoma (PCNSL) is a highly aggressive non-Hodgkin lymphoma confined to the CNS, including the brain, spine, eyes, and leptomeninges. Unlike other brain tumors, it often has a favorable response to both chemotherapy and radiation therapy, but compared with lymphomas located outside the CNS, survival is inferior.
PCNSL can develop in immunosuppressed (HIV/AIDS, organ transplant, immunosuppressive agents) or immunocompetent patients. PCNSL in immunocompetent patients is rare and represents only 4% of all intracranial neoplasms and 4%–6% of all extranodal lymphomas.1 Incidence rates for PCNSL have been increasing since the 1970s.2,3 In the 1980s and early 1990s there was a steep increase in cases in younger men, most likely representing patients affected by the HIV/AIDS epidemic. Since then, incidence has remained stable at 0.4/100000.4,5 Men are slightly more affected than women and the median age is about 60.1,3,6,7
Clinical outcomes in PCNSL have significantly improved over the past several decades based on several studies performed at specialized centers.8–10 In contrast, population-based studies have explored changes in survival over time considering the significant improvement in clinical outcomes with the introduction of methotrexate-based combination chemotherapy regimens,11,12 some noting improving survival rates4,6 and others demonstrating no change.7
The goals of this study were (i) to update the incidence rate of non-HIV/AIDS PCNSL using data from Surveillance, Epidemiology, and End Results (SEER) (18 registries; 1973–2013) and the Central Brain Tumor Registry of the United States (CBTRUS) (2000–2013) and (ii) to characterize trends in clinical outcome and overall survival over the past 40 years.
Methods
Data Source
Data to determine incidence rates and median overall survival were obtained from CBTRUS (2000–2013)13 and 18 SEER registries (1973–2013). Data analysis of both SEER and CBTRUS was necessary to achieve our goals and collectively verify and strengthen the results of this study. CBTRUS includes patient data from all 50 states and Washington DC, allowing for the most accurate determination of incidence trends since 2000 but is limited, as patient survival data are not collected. On the other hand, SEER contains incidence and survival data over 40 years but is limited, as data are obtained from only a limited number of registries within the United States, currently 18. The incorporation of SEER data into our study provided the expansion of incidence trends found in CBTRUS, along with the assessment of overall survival in this patient population. PCNSL patients were identified in CBTRUS and SEER by filtering the databases based on histology codes (9590–9599; 9670–9699; 9700–9719; 9720–9729), malignant behavior, and anatomic location (primary sites: brain, spinal cord, and leptomeninges [pia/arachnoid]: C70.0, C70.1, C70.9, C70.9–C72.5, C72.8, and C72.9). Eye or ocular lymphomas were not included. To focus on non-HIV associated PCNSL within the SEER databases, patients with “other infectious and parasitic diseases including HIV” as cause of death and follow-up were excluded. The application of this code in SEER registries to define a non-HIV PCNSL patient population has been used in prior studies.6,7 The CBTRUS database does not include information about cause of death, and as a result, these analyses included HIV-associated PCNSL.
Statistical Methods
Incidence and survival analyses were performed using SEER*Stat v8.3.2 and R 3.3.2.14 Incidences were reported as per 100000 population after being age-adjusted to the 2000 US standard population. Overall incidence rates over time were calculated based on age, race, and sex. Within CBTRUS and SEER, age was defined by grouping patients by 10-year age groups. Race was defined as white, black, American Indian/Alaska Native (AIAN), and Asian/Pacific Islander (API) in CBTRUS and white, black, and other (including AIAN and API) in SEER. The CBTRUS database was used to calculate the frequency of PCNSL by site in the CNS. Kaplan–Meier and Cox proportional hazards models were used to assess median survival based on age, sex, and year of diagnosis. Corresponding P-values of 0.05 or less were considered statistically significant.
Results
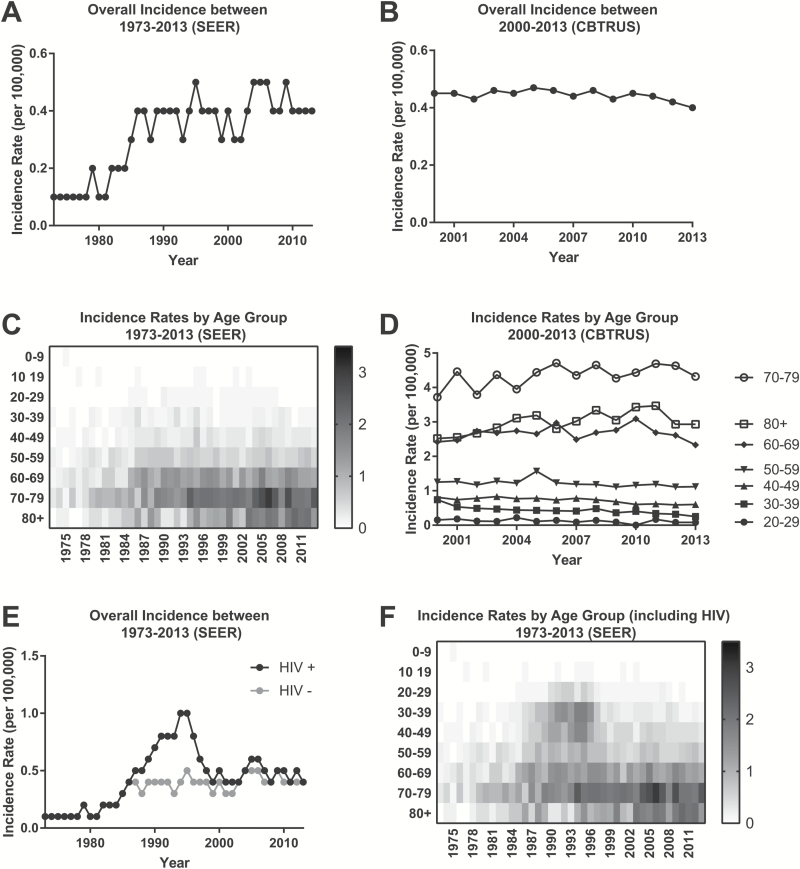
We identified 19027 patients with PCNSL in the CBTRUS and 6765 patients in the SEER databases (Table 1). PCNSL was found to be slightly more common in men than women, with a ratio of 1.14. White patients and patients older than 50 years were mostly affected (Table 1). The incidence of PCNSL was 0.4 per 100000 (SEER and CBTRUS) in 2013. Over the last decade the incidence rate of PCNSL has remained stable, ranging from 0.4 to 0.5 per 100000 in the SEER database and from 0.4 to 0.47 in CBTRUS. Incidence increased from 0.1 in the 1970s to 0.4 in the mid-1980s (SEER) and remained stable in the non-HIV population (SEER and CBTRUS) (Fig. 1A, B). The incidence of PCNSL differed greatly between age groups, ranging from 0.08 in patients 20–29 years to 4.32 in patients 70–79 years (SEER and CBTRUS) (Fig. 1C, D). The incidence in patients 70 years or older increased in the mid-1980s (SEER) (Fig. 1C). However, the incidence rate based on age did not significantly change from 2000 to 2013 (SEER and CBTRUS) (Fig. 1D). When including patients with HIV, a peak in incidence rates was observed (Fig. 1E), which has been described by others.1,5 This peak was caused by an increase in patients age 20–50 years affected by PCNSL in the late 1980s to mid-1990s (SEER) (Fig. 1F).
Table 1.
Basic patient characteristics
| SEER 1973–2013 | CBTRUS 2000–2013 | |
|---|---|---|
| Patients | Patients | |
| N (%) | N (%) | |
| All Patients | 6765 (100) | 19027 (100) |
| Age at Diagnosis, y | ||
| 0–9 | 27 (0.4) | 52 (0.3) |
| 10–19 | 76 (1.1) | 137 (0.7) |
| 20–29 | 172 (2.5) | 484 (2.5) |
| 30–39 | 401 (5.9) | 1233 (6.5) |
| 40–49 | 695 (10.3) | 2181 (11.5) |
| 50–59 | 1136 (16.8) | 3190 (16.8) |
| 60–69 | 1745 (25.8) | 4617 (24.3) |
| 70–79 | 1761 (26) | 4903 (25.8) |
| 80+ | 752 (11.1) | 2230 (11.7) |
| Sex | ||
| Male | 3583 (53) | 10112 (53.1) |
| Female | 3182 (47) | 8915 (46.9) |
| Race | ||
| White | 5709 (84.4) | 16127 (84.8) |
| Black | 388 (5.7) | 1874 (9.8) |
| Other | 646 (9.5) | 906 (4.8) |
| Unknown | 22 (0.3) | 120 (0.6) |
| Year of Diagnosis | ||
| 1973–1979 | 123 (1.8) | 0 (0) |
| 1980–1989 | 534 (7.9) | 0 (0) |
| 1990–1999 | 1279 (18.9) | 0 (0) |
| 2000–2009 | 3268 (48.3) | 13264 (69.7) |
| 2010–2013 | 1561 (23.1) | 5763 (30.3) |
Fig. 1.
Incidence rates of PCNSL. (A) Incidence rates derived from the SEER registries between 1973 and 2013 and (B) CBTRUS database 2000–2013. (C) Heatmap plot of the incidence rates of PCNSL (SEER) based on patient age and year of diagnosis. (D) Change in incidence rates of PCNSL based on age groups between 2000 and 2013 (CBTRUS). (E) Incidence rates of PCNSL in non-HIV patients (gray, HIV−) and all PCNSL including HIV patients (black, HIV+) (SEER). (F) Heatmap plot of the incidence rates of PCNSL including HIV patients (SEER) based on patient age and year of diagnosis.
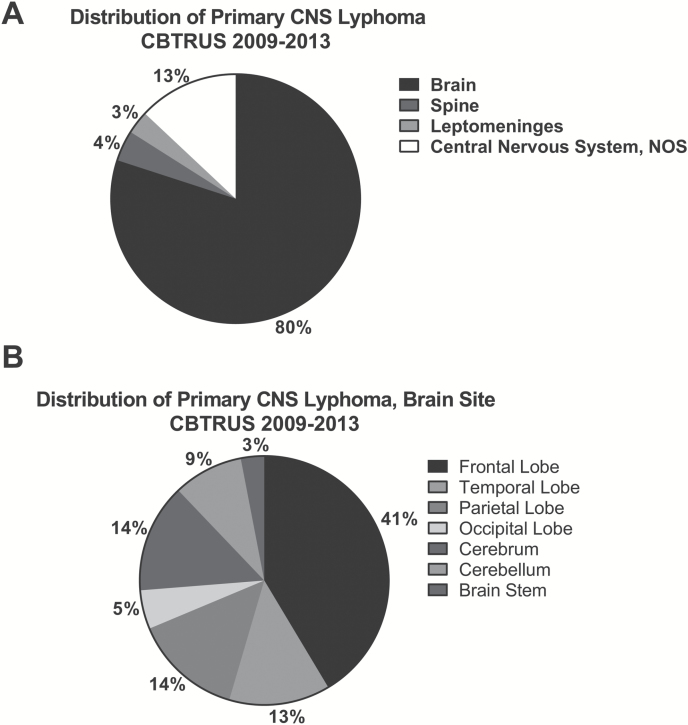
PCNSL most commonly occurred in the brain parenchyma (80%), followed by CNS not otherwise specified (13%), spine (4%), and the leptomeninges (3%) (Fig. 2A). Within the brain parenchyma, the frontal lobes (19%) were most commonly affected (Fig. 2B).
Fig. 2.
Distribution of PCNSL by site in the CNS. (A) The distribution of PCNSL within the central nervous system as well as (B) the anatomic site within the brain.
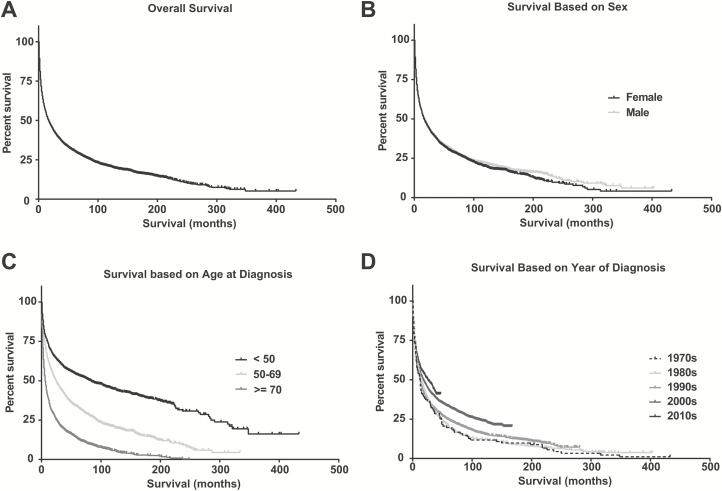
The median overall survival of all 6765 patients within SEER was 17 months (Fig. 3A). No overall sex-related survival differences were observed based on Kaplan–Meier curves (Fig. 3B). In concordance to the established prognostic scores of Memorial Sloan Kettering Cancer Center15 and the International Extranodal Lymphoma Study Group,16 both of which include age as a prognostic factor, survival differed greatly between age groups (Fig. 3C). Median overall survival was 83 months in younger patients (<50 y) in comparison to 25 months in patients age 50–69 years and 6 months in elderly patients (≥70 y). Moreover, improved overall survival was observed in patients treated in the 2010s in comparison to patients treated in the 1970s (Fig. 3D). When analyzing changes in clinical outcomes over the last 4 decades based on age, there was an increase in overall survival in younger patients (<50 y) and patients age 50–69 years, 35.5 to 134 months and 8 to 35 months, respectively. In contrast, survival of the elderly population (≥70 y) did not change (6 to 7 mo) over the past 40 years (Table 2).
Fig. 3.
Clinical outcome of PCNSL. Kaplan–Meier curves demonstrating clinical outcome in PCNSL (SEER registries 1973–2013). Overall survival of (A) all PCNSL patients, based on (B) gender, (C) age at diagnosis grouped by age <50, 50–69, and ≥70, and (D) decade of diagnosis.
Table 2.
Median overall survival
| Median Overall Survival Based on Age, mo | ||||
|---|---|---|---|---|
| Time of Diagnosis | <50 y | 50–69 y | ≥70 y | All Patients |
| 1970s | 35.5 | 8 | 6 | 12.5 |
| 1980s | 19.5 | 18 | 8.5 | 14 |
| 1990s | 23 | 18 | 6 | 13 |
| 2000s | 134 | 25 | 6 | 18 |
| 2010s | NR | 35 | 7 | 26 |
| 1973–2013 | 83 | 25 | 6 | 17 |
NR, not reached.
On multivariable analysis of SEER patients, age <50 years and diagnosis after the 1970s were associated with improved survival (Table 3). Although no sex-related survival difference was observed on the Kaplan–Meier curve, multivariable analysis found that women <50 years had better outcomes, but patients <50 years constituted only 20% of the total population. Patients ≥70 years had a 214% (odds ratio [OR] 3.1) increase in probability of death compared with patients <50 years (P < 0.0001). Men had a 10% (OR 1.1) increase in probability of death (P = 0.0007). Race was not a significant predictor of death. The probability of death decreased over the last 40 years: A patient with a diagnosis of PCNSL in the 2010s had a 47% (OR 0.5) decrease in the probability of death compared with a patient with that diagnosis in the 1970s (P < 0.0001). There was no significant change in the probability of death in the past 40 years in patients ≥70 years of age, whereas patients age <50 years and 50–69 years experienced a reduction in probability of death by 68% (OR 0.3) and 53% (OR 0.5), respectively.
Table 3.
Multivariate analysis
| Odds Ratio | 95% CI | P-value | |
|---|---|---|---|
| Age (50–69 y vs <50 y) | 1.7 | (1.5, 1.8) | <0.0001 |
| Age (≥70 y vs <50 y) | 3.1 | (2.9, 3.4) | <0.0001 |
| Sex (male vs female) | 1.1 | (1.0, 1.2) | 0.0007 |
| Race (black vs white) | 0.9 | (0.8, 1.1) | 0.3813 |
| Year of diagnosis (80s vs 70s) | 0.8 | (0.7, 1.0) | 0.0378 |
| Year of diagnosis (90s vs 70s) | 0.8 | (0.7, 1.0) | 0.0152 |
| Year of diagnosis (00s vs 70s) | 0.6 | (0.5, 0.7) | <0.0001 |
| Year of diagnosis (10s vs 70s) | 0.5 | (0.4, 0.6) | <0.0001 |
| Stratified by Age | |||
| Age <50 y | |||
| Sex (male vs female) | 1.3 | (1.1, 1.5) | 0.001 |
| Race (black vs white) | 1 | (0.8, 1.3) | 0.8817 |
| Year of diagnosis (80s vs 70s) | 1 | (0.7, 1.5) | 0.9988 |
| Year of diagnosis (90s vs 70s) | 1 | (0.7, 1.4) | 0.8757 |
| Year of diagnosis (00s vs 70s) | 0.5 | (0.3, 0.8) | 0.0007 |
| Year of diagnosis (10s vs 70s) | 0.3 | (0.2, 0.5) | <0.0001 |
| Age 50–69 y | |||
| Sex (male vs female) | 1 | (1.0, 1.1) | 0.3598 |
| Race (black vs white) | 0.9 | (0.7, 1.1) | 0.246 |
| Year of diagnosis (80s vs 70s) | 0.7 | (0.5, 1.0) | 0.0293 |
| Year of diagnosis (90s vs 70s) | 0.6 | (0.5, 0.8) | 0.0011 |
| Year of diagnosis (00s vs 70s) | 0.5 | (0.4, 0.7) | <0.0001 |
| Year of diagnosis (10s vs 70s) | 0.5 | (0.4, 0.6) | <0.0001 |
| Age ≥70 y | |||
| Sex (male vs female) | 1.1 | (1.0, 1.2) | 0.0499 |
| Race (black vs white) | 1 | (0.8, 1.3) | 0.9112 |
| Year of diagnosis (80s vs 70s) | 0.9 | (0.6, 1.3) | 0.5067 |
| Year of diagnosis (90s vs 70s) | 0.9 | (0.7, 1.3) | 0.7226 |
| Year of diagnosis (00s vs 70s) | 0.8 | (0.5, 1.1) | 0.1737 |
| Year of diagnosis (10s vs 70s) | 0.7 | (0.5, 1.0) | 0.0785 |
Discussion
Our study shows that the overall incidence rate of PCNSL has remained stable since the mid-1980s at approximately 0.4/100000. Other than the sharp spike in PCNSL incidence in the early 1990s due to the HIV epidemic, the overall increase in PCNSL seen in the 1980s is a reflection of a growing elderly population with a longer life expectancy, as there was an increase in the incidence in patients 70 years or older in the mid-1980s. Improved imaging techniques with more frequent use of MRI and more aggressive diagnostic workups of neurologic complaints in older patients could also be contributing factors.
Incidence rates varied greatly by age group. The highest rates were observed in patients 60 years and older, with the incidence in those 70–79 years being 10 times higher than the general population. However, the median age in most recent first-line prospective trials8,9,17–20 in PCNSL ranged from 56 to 61 years, with only a few trials targeting the elderly population.21,22
This study shows that the median overall survival of the general population has increased from 12.5 months in the 1970s to 26 months in the last decade, potentially representing advances in the treatment of PCNSL. However, patients 70 years and older are the exception. Not only does this group have an inferior median survival but there has been no improvement in survival over the last 40 years. This could be explained by a less aggressive treatment approach, limited functional status, and greater comorbidities in this patient population. Our data are supportive of a recent smaller population-based Dutch study showing no improvement in survival in elderly PCNSL patients.23 Another factor may be the lack of prospectively collected data to guide treatment decisions in this patient population. Our data underscore the need for prospective clinical trials in elderly patients with PCNSL. Many older PCNSL patients tolerate aggressive multi-agent chemotherapy regimens24 and should be considered for enrollment in clinical trials whenever possible.
There are several limitations to our study that must be taken into consideration when assessing our findings. Due to the constraints of the SEER database, we were unable to identify and, therefore, exclude every HIV-associated PCNSL. On the other hand, the rise in incidence from the mid-1980s with a peak in the mid-1990s disappears with the application of our HIV exclusion parameter and, therefore, suggests that the majority of HIV-associated cases were excluded. We were also not able to filter out PCNSL due to other immunocompromised status (eg, posttransplant). The observation of reduced median overall survival of patients age <50 years in the 1980s and 1990s might be a reflection of not being able to exclude all HIV- and immunocompromised PCNSL cases. Other inherent limitations within SEER registries were the inability to assess known prognostic factors (Karnofsky/Eastern Cooperative Oncology Group performance statuses, serum lactate dehydrogenase, CSF protein, and lesions within deep structures)15,16 and treatment histories of patients. Lastly, we speculate that outcomes may be dependent upon the treating institution, considering that clinical trials showing improved survival were conducted primarily at specialized care centers. However, we were unable to provide evidence supporting our hypothesis, as data regarding treatment location are limited within SEER.
The strength of this study is that analysis of 2 population-based registries, CBTRUS and SEER, demonstrates concordant PCNSL incidence rates over the last decade. PCNSL incidence has largely remained unchanged since the mid-1980s. Furthermore, they also corroborate that patients 70 years or older have the highest incidence of PCNSL but have not benefited from the changes in treatment regimens over the past 4 decades. Identification of this vulnerable patient population underscores the need for clinical trials targeting elderly patients, in hopes of improving treatment strategies and outcomes.
Funding
This research was supported by an NIH/NCI Cancer Center Support Grant (P30-CA008748) and by grants from the Memorial Sloan Kettering Brain Tumor Center (C.G.), the Society of Memorial Sloan Kettering Cancer Center (C.G.), the American Brain Tumor Association Basic Research Fellowship Award (C.G.), the Lymphoma Research Foundation Career Development Award (C.G.), Susan and Peter Solomon Divisional Fund (C.G.), and Cycle for Survival Equinox Innovation Award (C.G.). Funding in support of CBTRUS was provided by the Centers for Disease Control and Prevention (CDC) under contract no. 200-2016-M-90304, the Sontag Foundation, Genentech, Novocure, AbbVie, along with the Musella Foundation, the Children’s Brain Tumor Foundation, National Brain Tumor Society, Pediatric Brain Tumor Foundation, and the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in-kind donations. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC.
Acknowledgments
The authors would like to thank Judith Lampron for her editorial assistance. Previous presentation of this work was made in part at the 2017 American Society of Clinical Oncology Meeting in Chicago, Illinois.
Conflict of interest statement. None declared.
References
- 1. Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105(9):1414–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eby NL, Grufferman S, Flannelly CM, Schold SC Jr, Vogel FS, Burger PC. Increasing incidence of primary brain lymphoma in the US. Cancer. 1988;62(11):2461–2465. [DOI] [PubMed] [Google Scholar]
- 3. Olson JE, Janney CA, Rao RD et al. . The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma: a Surveillance, Epidemiology, and End Results analysis. Cancer. 2002;95(7):1504–1510. [DOI] [PubMed] [Google Scholar]
- 4. Shiels MS, Pfeiffer RM, Besson C et al. . Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br J Haematol. 2016;174(3):417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Neill BP, Decker PA, Tieu C, Cerhan JR. The changing incidence of primary central nervous system lymphoma is driven primarily by the changing incidence in young and middle-aged men and differs from time trends in systemic diffuse large B-cell non-Hodgkin’s lymphoma. Am J Hematol. 2013;88(12):997–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Norden AD, Drappatz J, Wen PY, Claus EB. Survival among patients with primary central nervous system lymphoma, 1973–2004. J Neurooncol. 2011;101(3):487–493. [DOI] [PubMed] [Google Scholar]
- 7. Panageas KS, Elkin EB, DeAngelis LM, Ben-Porat L, Abrey LE. Trends in survival from primary central nervous system lymphoma, 1975–1999: a population-based analysis. Cancer. 2005;104(11):2466–2472. [DOI] [PubMed] [Google Scholar]
- 8. Omuro A, Correa DD, DeAngelis LM et al. . R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125(9):1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rubenstein JL, Hsi ED, Johnson JL et al. . Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol. 2013;31(25):3061–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morris PG, Correa DD, Yahalom J et al. . Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol. 2013;31(31):3971–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeAngelis LM, Yahalom J, Thaler HT, Kher U. Combined modality therapy for primary CNS lymphoma. J Clin Oncol. 1992;10(4):635–643. [DOI] [PubMed] [Google Scholar]
- 12. Glass J, Gruber ML, Cher L, Hochberg FH. Preirradiation methotrexate chemotherapy of primary central nervous system lymphoma: long-term outcome. J Neurosurg. 1994;81(2):188–195. [DOI] [PubMed] [Google Scholar]
- 13. Ostrom QT, Gittleman H, Xu J et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 2016;18(suppl 5):v1–v75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. R Core Team. R: A language and environment for statistical computing. 2016; http://www.R-project.org/. [Google Scholar]
- 15. Abrey LE, Ben-Porat L, Panageas KS et al. . Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24(36):5711–5715. [DOI] [PubMed] [Google Scholar]
- 16. Ferreri AJ, Blay JY, Reni M et al. . Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21(2):266–272. [DOI] [PubMed] [Google Scholar]
- 17. Ferreri AJ, Cwynarski K, Pulczynski E et al. ; International Extranodal Lymphoma Study Group (IELSG) Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3(5):e217–e227. [DOI] [PubMed] [Google Scholar]
- 18. Illerhaus G, Kasenda B, Ihorst G et al. . High-dose chemotherapy with autologous haemopoietic stem cell transplantation for newly diagnosed primary CNS lymphoma: a prospective, single-arm, phase 2 trial. Lancet Haematol. 2016;3(8):e388–e397. [DOI] [PubMed] [Google Scholar]
- 19. Glass J, Won M, Schultz CJ et al. . Phase I and II study of induction chemotherapy with methotrexate, rituximab, and temozolomide, followed by whole-brain radiotherapy and postirradiation temozolomide for primary CNS lymphoma: NRG Oncology RTOG 0227. J Clin Oncol. 2016;34(14):1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thiel E, Korfel A, Martus P et al. . High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11(11):1036–1047. [DOI] [PubMed] [Google Scholar]
- 21. Omuro A, Chinot O, Taillandier L et al. . Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: an intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol. 2015;2(6):e251–e259. [DOI] [PubMed] [Google Scholar]
- 22. Fritsch K, Kasenda B, Schorb E et al. . High-dose methotrexate-based immuno-chemotherapy for elderly primary CNS lymphoma patients (PRIMAIN study). Leukemia. 2017;31(4):846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van der Meulen M, Dinmohamed AG, Visser O, Doorduijn JK, Bromberg JEC. Improved survival in primary central nervous system lymphoma up to age 70 only: a population-based study on incidence, primary treatment and survival in the Netherlands, 1989–2015. Leukemia. 2017;31(8):1822–1825. [DOI] [PubMed] [Google Scholar]
- 24. Welch MR, Omuro A, Deangelis LM. Outcomes of the oldest patients with primary CNS lymphoma treated at Memorial Sloan-Kettering Cancer Center. Neuro Oncol. 2012;14(10):1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]