See the article by Moncayo et al pp. 621–631.
Spleen tyrosine kinase (SYK) is a cytoplasmic nonreceptor protein tyrosine kinase that plays a central role in mediating inflammatory responses by coupling immune receptors to intracellular pathways.1 SYK is mainly expressed in hematopoietic cells, including B cells, macrophages, monocytes, natural killer cells, mast cells, and neutrophils. The central paradigm of immune cell signaling involves the recruitment of SYK or ZAP70 (protein ζ–chain-associated protein kinase of 70 kDa) to active immunoreceptors, such as Fc receptors, B-cell receptors, and T-cell receptors, by binding to phosphorylated immunoreceptor tyrosine-based activation motifs.1 Therefore, SYK and ZAP70 link the activation of immunoreceptors to signaling pathways involved in survival, proliferation, phagocytosis, and differentiation. SYKs tend to function in concert with Src-family kinases which are upstream of SYK activation.2
Besides SYK’s essential role in several human diseases, including autoimmune diseases and allergies, it was also reported to be involved in the pathogenesis of hematological diseases. Specifically, SYK promoted the maintenance of B-cell malignancies by preventing apoptosis and inducing proliferation of pre-B cells, leading to their transformation. In addition, published literature suggests that overexpression of SYK might also contribute to T-cell lymphomas.1
The role of SYK in non-hematological diseases is still under investigation, and controversial results have been reported.3 SYK expression is decreased in invasive breast carcinoma tissue, and its reintroduction suppressed malignant growth and metastases.4 Although these data suggest a role for SYK as a tumor suppressor in human breast carcinomas, it was found to play an essential function in the murine mammary tumor virus-mediated transformation.5 In addition, SYK is involved in the pro-oncogenic properties of Epstein–Barr virus,6 which is strongly associated with nasopharyngeal carcinoma and in the maintenance of retinoblastoma.7
Moncayo and colleagues investigated the role of SYK and other related molecules in low- and high-grade gliomas.8 Their comprehensive study examined the relative expression of SYK, the functional characteristics of the related pathway, and the effects on the neoplastic phenotype.8 Unexpectedly, SYK is overexpressed in the non-immune cells of gliomas, specifically in the neoplastic glial cells of malignant gliomas and human pilocytic astrocytomas. Furthermore, in vitro experiments demonstrated that SYK promoted glioma cell proliferation and migration.8
As a consequence of the robust data illustrating the role of SYK in oncogenesis and hematological cancer maintenance, several SYK inhibitors have been developed and tested in preclinical and clinical settings. Fostamatinib (R788), entospletinib (GS-9973), cerdulatinib (PRT062070), and TAK-659 are in clinical trials for patients with hematological malignancies. SYK inhibition has shown promising results in patients with non-Hodgkin lymphoma and leukemias.9,10 A second generation of more selective SYK inhibitors is in development, including entospletinib (GS-9973), which has shown encouraging results in clinical trials for patients with B-cell malignancies.9,10
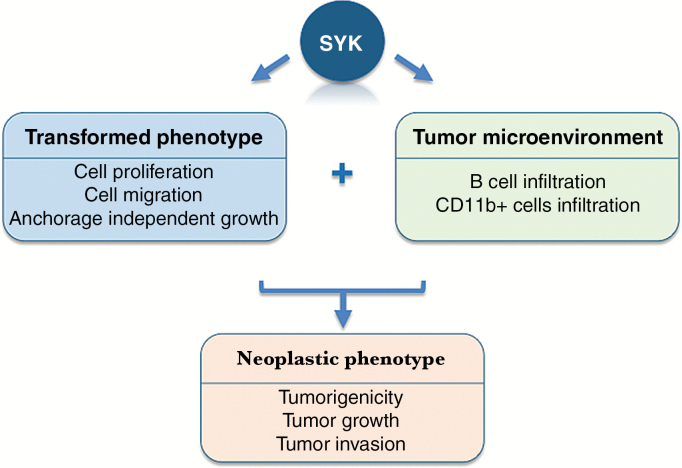
The pharmacological inhibition of SYK in preclinical glioma models decreased tumorigenicity and moderately prolonged survival.8 Moreover, SYK inhibition led to modification of the tumor microenvironment that resulted in less invasive tumors and reduced the infiltration of CD19+ and CD11b+ cells in the tumor.8 Moncayo and colleagues performed a dynamic study using multiphoton intravital microscopy to document the effect of SYK inhibition in intracranial gliomas. These studies illustrated the role of SYK in glioma invasion and in leukocyte infiltration.8 Of note, genetic manipulation of SYK that jeopardized the kinase activity of SYK (kinase domain mutations) significantly increased survival, suggesting a limited efficacy of the tested pharmacological agents and room for improvement. The effect of SYK inhibitors in diminishing the recruitment of B cells into the glioma microenvironment has important overtones for the treatment of malignant brain tumors. If these immune cells contribute necessary mechanisms for glioma growth, further studies focused on deciphering these mechanisms should be conducted (Figure 1).
Fig. 1.
Schematic illustration of the role of SYK in gliomas. The central model of immune cell signaling involves the recruitment of SYK-family kinases to the active immunoreceptors in hematological cells. Moncayo and colleagues8 reported that SYK is also expressed in glioma cells and regulates several characteristics of the neoplastic phenotype of these tumors. In addition, in vivo experiments illustrated the involvement of SYK in modulating the tumor microenvironment, with special emphasis on the recruitment of B cells.
A common drawback when utilizing pharmacological small-molecule inhibitors is that the inhibitors may not only target the molecule of interest, but have off-target effects leading to unexplainable results. Thus, data from clinical trials suggest that the first-generation SYK inhibitor R788 targets not only SYK and several SYK-dependent pathways, but also SYK-independent immune signaling networks.1 While off-target effects may be beneficial for some types of heterogeneous cancers harboring several oncogenic drivers, they might also be the cause of treatment-related adverse events.1 Current efforts are focused on the development of more specific SYK inhibitors or on inhibitors that target 2 kinases, such as cerdulatinib, which targets SYK and Janus kinase, or on the combination of SYK inhibitors with other therapeutic agents.9
In breast carcinoma, an alternative splicing of SYK jeopardizes the nuclear localization of SYK, which is required for its tumor suppressor function.11 Of note, Moncayo and collaborators described the nuclear localization of this kinase in some of the specimens and cell lines examined.8 While several tyrosine kinases are reported to be directly involved in epigenetic modifications when they traffic into the nucleus, such as tunica interna endothelial cell kinase 2 and epidermal growth factor receptor,12,13 the role of nuclear SYK in gliomas remains to be elucidated.
Another aspect to consider when implementing SYK inhibitors into clinical trials for patients with malignant gliomas relates to its tissue-specific action, whether it functions as a tumor suppressor or an oncogene. In this regard, the current body of literature suggests that in patients suffering from glioblastoma and with a family history of breast cancer or melanoma, the use of SYK inhibitors may not be the optimal treatment. If administered, they should be managed with a high degree of vigilance.
Funding
This work was supported by the National Institutes of Health/National Institute of Cancer (P50 CA127001) and the US Department of Defense (CA160525). The funding bodies were not involved in the study design, the data collection and analysis, the decision to publish, or the preparation of the manuscript.
References
- 1. Mócsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10(6):387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lowell CA. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: signaling cross talk. Cold Spring Harb Perspect Biol. 2011;3(3):doi: 10.1101/cshperspect.a002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krisenko MO, Geahlen RL. Calling in SYK: SYK’s dual role as a tumor promoter and tumor suppressor in cancer. Biochim Biophys Acta. 2015;1853(1):254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coopman PJ, Do MT, Barth M, et al. . The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature. 2000;406(6797):742–747. [DOI] [PubMed] [Google Scholar]
- 5. Lu J, Lin WH, Chen SY, et al. . Syk tyrosine kinase mediates Epstein-Barr virus latent membrane protein 2A-induced cell migration in epithelial cells. J Biol Chem. 2006;281(13):8806–8814. [DOI] [PubMed] [Google Scholar]
- 6. Katz E, Lareef MH, Rassa JC, et al. . MMTV Env encodes an ITAM responsible for transformation of mammary epithelial cells in three-dimensional culture. J Exp Med. 2005;201(3):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang J, Benavente CA, McEvoy J, et al. . A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature. 2012;481(7381):329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moncayo G, Grzmil M, Smirnova T, et al. . SYK inhibition blocks proliferation and migration of glioma cells, and modifies the tumor microenvironment. Neuro Oncol. 2018;doi: 10.1093/neuonc/noy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geahlen RL. Getting Syk: spleen tyrosine kinase as a therapeutic target. Trends Pharmacol Sci. 2014;35(8):414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu D, Mamorska-Dyga A. Syk inhibitors in clinical development for hematological malignancies. J Hematol Oncol. 2017;10(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang L, Duke L, Zhang PS, et al. . Alternative splicing disrupts a nuclear localization signal in spleen tyrosine kinase that is required for invasion suppression in breast cancer. Cancer Res. 2003;63(15):4724–4730. [PubMed] [Google Scholar]
- 12. Chou RH, Wang YN, Hsieh YH, et al. . EGFR modulates DNA synthesis and repair through Tyr phosphorylation of histone H4. Dev Cell. 2014;30(2):224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hossain MB, Shifat R, Johnson DG, et al. . TIE2-mediated tyrosine phosphorylation of H4 regulates DNA damage response by recruiting ABL1. Sci Adv. 2016;2(4):e1501290. [DOI] [PMC free article] [PubMed] [Google Scholar]