Abstract
Background
This phase II study was designed to determine the efficacy of the mammalian target of rapamycin (mTOR) inhibitor everolimus administered daily with conventional radiation therapy and chemotherapy in patients with newly diagnosed glioblastoma.
Methods
Patients were randomized to radiation therapy with concurrent and adjuvant temozolomide with or without daily everolimus (10 mg). The primary endpoint was progression-free survival (PFS) and the secondary endpoints were overall survival (OS) and treatment-related toxicities.
Results
A total of 171 patients were randomized and deemed eligible for this study. Patients randomized to receive everolimus experienced a significant increase in both grade 4 toxicities, including lymphopenia and thrombocytopenia, and treatment-related deaths. There was no significant difference in PFS between patients randomized to everolimus compared with control (median PFS time: 8.2 vs 10.2 mo, respectively; P = 0.79). OS for patients randomized to receive everolimus was inferior to that for control patients (median survival time: 16.5 vs 21.2 mo, respectively; P = 0.008). A similar trend was observed in both O6-methylguanine-DNA-methyltransferase promoter hypermethylated and unmethylated tumors.
Conclusion
Combining everolimus with conventional chemoradiation leads to increased treatment-related toxicities and does not improve PFS in patients with newly diagnosed glioblastoma. Although the median survival time in patients receiving everolimus was comparable to contemporary studies, it was inferior to the control in this randomized study.
Keywords: everolimus, glioblastoma, mTOR inhibition, phase II trial, radiation sensitizer
Importance of the study
Glioblastoma remains an incurable disease with an urgent need for better treatment options. Standard therapy consists of surgical resection followed by radiation and concomitant and maintenance temozolomide. Incorporating molecular targeted agents into this regimen designed to modulate tumor-specific signaling pathways offers promise in furthering clinical response. Here, we present findings from the largest study evaluating the potential for mTOR inhibition to enhance therapeutic response in patients with newly diagnosed glioblastoma. As we were not able to demonstrate a clinical benefit with this regimen, this study definitively establishes the lack of efficacy of mTOR complex 1 inhibition in glioblastoma using rapalogs, serving as a framework to develop novel strategies for targeting this oncogenic pathway. Additionally, it emphasizes the continued need for randomized studies in the phase II setting, as the clinical outcome of the control group was superior to historical controls, suggesting that survival of glioblastoma patients may be continuing to evolve.
Glioblastoma (GBM) is the most common and aggressive adult primary brain tumor.1 Despite recent advances in surgery and combined chemoradiotherapy, clinical outcomes remain poor.2 Standard treatment consists of radiation therapy with concomitant and adjuvant temozolomide (TMZ) chemotherapy, resulting in a median progression-free survival (PFS) time of 6.7 months and median survival time (MST) of 16.6 months.3 Considerable effort has been expended in better understanding the underlying biology of GBM to identify unique signaling pathways that may contribute to the aggressive phenotype of this malignancy, thereby potentially identifying novel therapeutic targets. Next-generation sequencing through The Cancer Genome Atlas has identified receptor tyrosine kinase signaling through epidermal growth factor receptor and phosphatidylinositol-3 kinase as a common, genetically altered node in GBM.4 Mammalian target of rapamycin (mTOR) serves as a downstream, regulatory hub for many of these signaling pathways, and a considerable amount of preclinical data support the potential for this axis to serve as a therapeutic target,5–8 since it may contribute to radiation resistance9–11 and angiogenesis,12 pathological hallmarks of this tumor. Further, recent findings have identified certain mutations activating the Akt/mTOR pathway to be closely associated with TMZ resistance.13 Collectively, by influencing tumor growth, therapeutic resistance, and the tumor microenvironment, the mTOR signaling axis represents an attractive therapeutic target in GBM.
Everolimus (RAD001), a derivative of rapamycin, is an oral inhibitor of mTOR that has demonstrated promising antitumor activity and has recently been approved by the FDA for several tumor types, including renal cell14 and breast cancer,15 pancreatic neuroendocrine tumors,16 and subependymal giant-cell astrocytomas associated with tuberous sclerosis.17 Our group has previously presented results of everolimus 10 mg/day with an acceptable toxicity profile from a phase I study combining everolimus with standard radiation therapy and TMZ in newly diagnosed GBM.18 The current study reports results from the subsequent randomized phase II study evaluating the efficacy of this therapeutic strategy and further defines the toxicity profile of this regimen (NCT01062399).
Patients and Methods
Study Patients
Eligibility criteria were as follows: 18 years of age or older; Karnofsky performance status ≥70; centrally reviewed newly diagnosed, unifocal, supratentorial GBM; no prior chemotherapy, treatment with an mTOR inhibitor, or radiation to the head or neck area (except T1 glottic tumors); standard hematologic and metabolic panel within normal limits (absolute neutrophil count ≥1800 cells/mm3, platelets ≥100000 cells/m3, hemoglobin ≥10 g/dL, prothrombin time/international normalized ratio ≤1.5, blood urea nitrogen ≤30 mg/dL, creatinine ≤1.5 × normal range, bilirubin ≤1.5 × normal range, and alanine aminotransferase/aspartate aminotransferase ≤2.5 normal range); fasting cholesterol ≤300 mg/dL or ≤7.75 mmol/L; fasting triglycerides ≤2.5 × the upper limit of normal; no concurrent use of enzyme-inducing anti-epileptic drugs; no severe active comorbidity; no history of deep vein thrombosis or pulmonary embolism; no malignancy (within 3 y) except nonmelanomatous skin cancer or carcinoma in situ of the cervix or bladder; no pregnancy or lactation. Radiation therapy must have been initiated within 5 weeks after surgery. Also required was the submission of a paraffin-embedded tumor-tissue block with a minimum of 1 cm2 of tumor surface area to allow for evaluation of O6-methylguanine-DNA methyltransferase (MGMT) methylation status, which was performed centrally.
All patients provided written informed consent. This study was approved by the institutional review board or the equivalent panel at each study center before patient enrollment.
Study Treatment
Radiation therapy was delivered using intensity modulation or 3D-conformal radiation therapy (60 Gy in 30 fractions of 2 Gy each). An initial target representing the T2/axial fluid attenuated inversion recovery volume plus a tailored 2-cm margin was treated to 46 Gy in 23 fractions of 2 Gy each, followed by a 14-Gy boost (in 7 fractions of 2 Gy each) to the contrast enhancing tumor plus a 2-cm margin. During radiation therapy, daily TMZ was delivered at 75 mg/m2. Adjuvant TMZ was delivered at 150–200 mg/m2 on days 1 to 5 every 28 days for up to 12 cycles beginning 4 weeks after the completion of radiation therapy.
Patients were randomly assigned to receive either standard therapy or standard therapy combined with daily everolimus (10 mg) during concurrent chemoradiation and adjuvant TMZ. Prophylaxis against pneumocystis jiroveci pneumonitis/pneumocystis carinii pneumonitis (PJP/PCP) was strongly encouraged.
Patient Evaluation and Follow-up
At baseline, all the patients underwent a physical examination that included a neurologic assessment, complete blood counts, blood chemical analyses (including tests of renal and hepatic function and a lipid panel), and tumor imaging with either MRI (preferred) or CT, as well as a serum pregnancy test in women of child-bearing age. During radiotherapy, patients were assessed for adverse events weekly and underwent weekly complete blood counts and blood chemical analysis. During the maintenance phase of treatment, patients underwent blood counts and blood chemical analyses between days 21 and 28 of each cycle. A repeat tumor-imaging study and monitoring of adverse events were performed approximately 4 weeks after completion of radiotherapy and then before the initiation of cycle 3 of maintenance treatment (as well as before the initiation of cycles 5, 7, 9, and 11, if administered). Patients who completed adjuvant treatment underwent tumor imaging every 3 months for one year, then every 4 months for another year, then every 6 months until tumor progression. Response was assessed with the use of serial measures of the product of the 2 largest cross-sectional diameters, and progression was defined using the Response Assessment in Neuro-Oncology criteria.19 Since early reactions to radiotherapy may emulate tumor progression, investigators were encouraged not to declare tumor progression within the first 12 weeks after completion of radiotherapy unless there was a new lesion or neurologic worsening.19 Toxic effects were recorded and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Statistical Methodology
The primary endpoint of this phase II study was PFS,20 defined as the time interval from randomization to disease progression or death due to any cause, whichever occurred first. Patients who were still alive without experiencing disease progression at the time of the analysis were treated as censored observations for PFS at the date of last follow-up. Patients were stratified according to recursive partitioning analysis (RPA) class (III vs IV vs V)21 and then randomized using a permuted block design22 at the time of registration to the control or experimental arm, with a 1:1 allocation ratio.
The primary objective was to determine whether the addition of everolimus would improve PFS in newly diagnosed GBM patients. Assuming exponential distributions for the PFS times with a median PFS time of 6.7 months for the control arm, it was hypothesized that there would be a 43% improvement in the median PFS time for the experimental arm, corresponding to a median PFS time of 9.6 months. This is equivalent to a hazard ratio (HR) of 0.7 for PFS for the experimental arm with respect to the control arm. With a one-sided significance level of 0.15, a total of 134 PFS events out of 180 eligible patients were required to detect the projected effect size with an 85% statistical power. Guarding against up to a 20% rate for ineligibility due to insufficient tissue, progression, death prior to randomization, or other reasons, the projected accrual for the phase II part was 225 patients.
Secondary endpoints included overall survival (OS), treatment-related toxicity, and correlation between MGMT methylation status and survival outcomes.
OS was defined as the time interval from randomization to death due to any cause. Patients who were still alive at the time of the analysis were treated as censored observations for OS at the date of last follow-up. PFS and OS rates by treatment arm were estimated using the Kaplan–Meier method.23 The HRs for the treatment effect on PFS and OS, respectively, were calculated using the Cox proportional hazards model24 and tested using the log-rank test.25 Multivariate analyses were conducted using the Cox proportional hazards model to assess the adjusted treatment effects with the stratification factor and other patient pretreatment characteristics included as covariates. Differences in the severities of the reported treatment-related toxicities (grades <3 vs ≥3) between the treatment arms were tested using the chi-square test. The PFS and OS rates by MGMT methylation status (methylated vs unmethylated) were estimated using the Kaplan–Meier method, for the experimental arm, the control arm, and the study as a whole; the HRs on the effect of MGMT methylation status were computed using the Cox proportional hazards model and were tested using the log-rank test. Multivariate analyses were also conducted.
For all secondary endpoints, 2-sided tests with a significance level of 0.05 were used in the analyses.
Results
Patient Characteristics
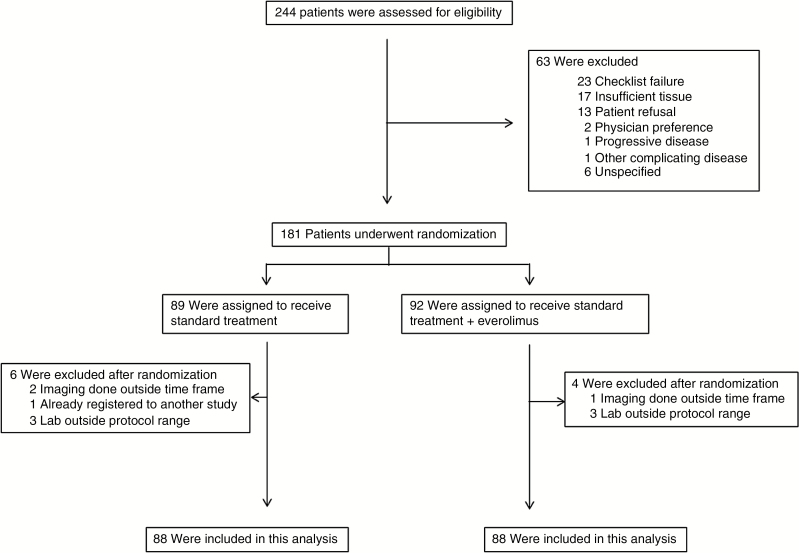
Between December 19, 2012 and September 12, 2013, a total of 244 patients were initially registered to this study. One hundred and eighty-one (74.2%) patients were randomized, and 10 of these were subsequently found ineligible. Reasons for not being randomized and ineligibility after randomization by treatment arm are listed in Fig. 1. Therefore, for the subsequent statistical analyses, there were 83 and 88 randomized and eligible patients for the control and experimental arms, respectively. Table 1 shows the distribution of patient pretreatment characteristics by treatment arm for all the randomized and eligible patients. The stratification factor, RPA class, was balanced between the treatment arms. Sixty-four (77.1%) of the patients in the control arm went on to receive adjuvant TMZ, while only 53 (60.2%) in the experimental arm went on to receive adjuvant chemotherapy. The median number of adjuvant cycles was 4 for patients randomized to receive everolimus, compared with 6 cycles in the control arm. MGMT promoter methylation status was available on 78.3% and 71.6% of the patients in the control and experimental arms, respectively (Supplementary Table S1).
Fig. 1.
CONSORT diagram.
Table 1.
Patient and tumor characteristics for all eligible patients
|
Phase II:
RT + TMZ |
Phase II:
RT + Everolimus + TMZ |
Total | ||||
|---|---|---|---|---|---|---|
| Patient or Tumor Characteristic | n | % | n | % | n | % |
| Age, y | ||||||
| ≤49 | 23 | 27.7 | 19 | 21.6 | 42 | 24.6 |
| 50–59 | 27 | 32.5 | 19 | 21.6 | 46 | 26.9 |
| 60–69 | 20 | 24.1 | 35 | 39.8 | 55 | 32.2 |
| ≥70 | 13 | 15.7 | 15 | 17.0 | 28 | 16.4 |
| Sex | ||||||
| Male | 46 | 55.4 | 56 | 63.6 | 102 | 59.6 |
| Female | 37 | 44.6 | 32 | 36.4 | 69 | 40.4 |
| Race | ||||||
| Black or African American | 4 | 4.8 | 0 | 0.0 | 4 | 2.3 |
| Native Hawaiian or Other Pacific Islander | 0 | 0.0 | 1 | 1.1 | 1 | 0.6 |
| White | 76 | 91.6 | 86 | 97.7 | 162 | 94.7 |
| More than one race | 2 | 2.4 | 0 | 0.0 | 2 | 1.2 |
| Unknown or not reported | 1 | 1.2 | 1 | 1.1 | 2 | 1.2 |
| Ethnicity | ||||||
| Hispanic or Latino | 2 | 2.4 | 5 | 5.7 | 7 | 4.1 |
| Not Hispanic or Latino | 77 | 92.8 | 78 | 88.6 | 155 | 90.6 |
| Unknown (individuals not reporting ethnicity) | 4 | 4.8 | 5 | 5.7 | 9 | 5.3 |
| KPS | ||||||
| 70–80 | 25 | 30.1 | 32 | 36.4 | 57 | 33.3 |
| 90–100 | 58 | 69.9 | 56 | 63.6 | 114 | 66.7 |
| Surgery | ||||||
| Subtotal | 34 | 41.0 | 42 | 47.7 | 76 | 44.4 |
| Total (gross) | 48 | 57.8 | 45 | 51.1 | 93 | 54.4 |
| Other | 1 | 1.2 | 1 | 1.1 | 2 | 1.2 |
| Neurologic Function | ||||||
| No symptoms | 26 | 31.3 | 32 | 36.4 | 58 | 33.9 |
| Minor symptoms | 45 | 54.2 | 40 | 45.5 | 85 | 49.7 |
| Moderate symptoms | 12 | 14.5 | 16 | 18.2 | 28 | 16.4 |
| RPA class* | ||||||
| III | 16 | 19.3 | 15 | 17.0 | 31 | 18.1 |
| IV | 57 | 68.7 | 61 | 69.3 | 118 | 69.0 |
| V | 10 | 12.0 | 12 | 13.6 | 22 | 12.9 |
RT = radiotherapy. *Stratification factor.
Toxicities
There was a statistically significant increase in treatment-related grade 3–5 adverse events in patients randomized to receive everolimus (n = 68, 80.0%) compared with the control arm (n = 33, 42.3%; P < 0.0001). For adverse events regardless of attribution to protocol treatment, there were 14 (17.9%) patients with reported grade 4 events and 1 (1.3%) with a reported grade 5 event in the control arm, compared with 26 (30.6%) and 10 (11.8%), respectively, in the experimental arm. The majority of the grade 4 events reported in the experimental arm involved bone marrow suppression, including lymphopenia (11.8% vs 3.8% in control) and thrombocytopenia (16.5% vs 5.1% in control). Of the 10 grade 5 events observed in patients randomized to receive everolimus, 4 events (respiratory failure, lung infection, scrotal infection, and meningitis) were deemed to be definitely, probably, or possibly related to protocol treatment, compared with 1 grade 5 event (sepsis) in the control arm. An expected increase in grade 1–3 hypertriglyceridemia was observed in the experimental arm (n = 53, 62.4%), compared with 16 (20.5%) in the control arm.
Supplementary Table S2 lists the reported highest grade adverse events regardless of relationship to protocol treatment by specific adverse events term, and Supplementary Table S3 lists all reported grade 5 events with their relationship to protocol treatment.
Efficacy Outcomes
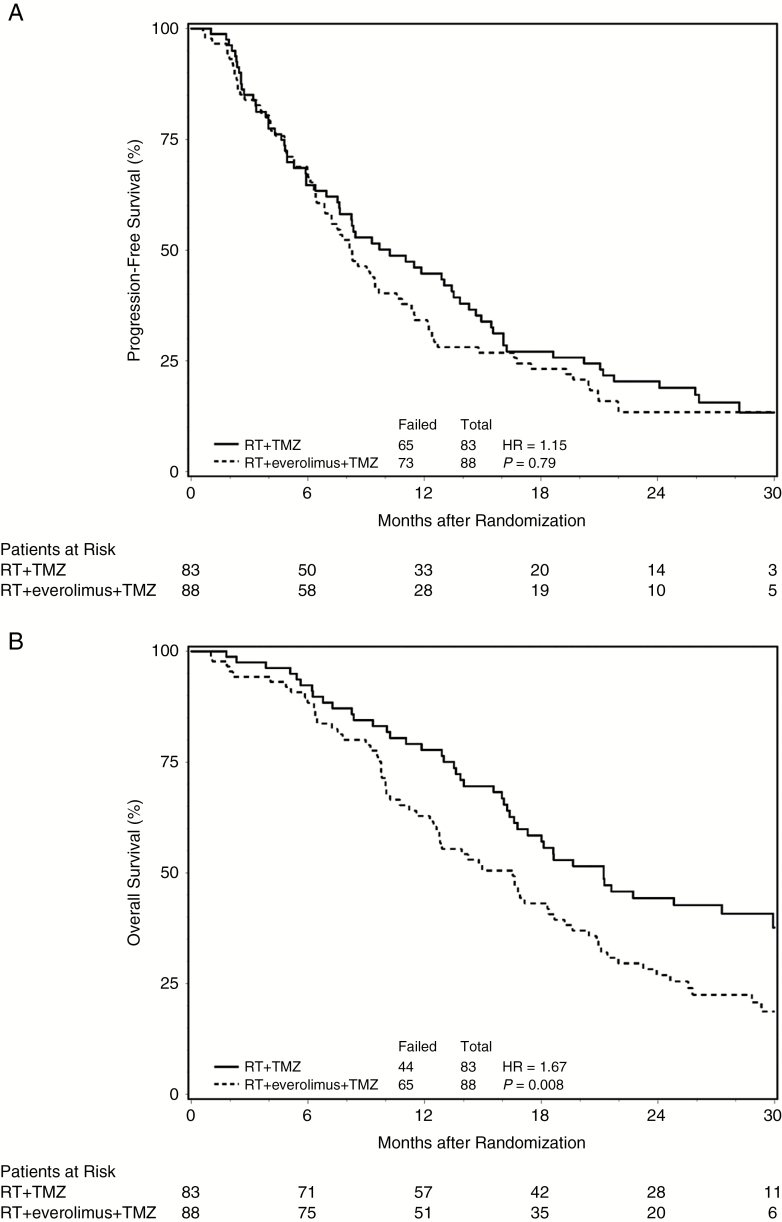
Of all the randomized and eligible patients for both arms, 36.3% were still alive at the time of this analysis, and the median follow-up time for these patients was 27.7 months, with a range of 0.1 to 36.7 months. The median PFS time for the control arm was 10.2 months with a 95% CI of 7.5 to 13.8 months, compared with a median PFS time of 8.2 months with a 95% CI of 6.5 to 10.6 months for patients randomized to receive everolimus (Fig. 2A). The HR for the treatment effect on PFS was 1.15 for the experimental arm with respect to the control arm, with a 95% CI of 0.82 to 1.60, and a one-sided P-value of 0.79. These results suggest that there is not a significant difference on PFS between the 2 treatment arms. The MST for the control arm was 21.2 months with a 95% CI of 16.6 to 29.9 months, compared with an MST of 16.5 months with a 95% CI of 12.5 to 18.7 months for patients randomized to receive everolimus (Fig. 2B). The HR for the treatment effect on OS was 1.67 in favor of the control arm, with a 95% CI of 1.14 and 2.45 and a P-value of 0.008. The MST for patients in the control arm with MGMT promoter hypermethylated tumors was not reached and 18.6 months in unmethylated tumors (HR = 2.05; P = 0.06), compared with 18.4 and 14.2 months, respectively, in patients randomized to receive everolimus (HR = 1.48; P = 0.20). Similar results were obtained from the multivariate analyses, which also suggested no significant treatment and MGMT status interaction effects on the survival outcomes.
Fig. 2.
Principal efficacy outcomes per treatment.
Discussion
A significant body of preclinical data, further strengthened by recent next-generation sequencing efforts, has provided a strong rationale for developing therapeutic strategies designed to target the mTOR pathway in GBM. Accordingly, several cooperative groups have attempted to translate clinical successes achieved in other tumor sites with rapamycin analog mTOR inhibitors to newly diagnosed GBM. The North Central Cancer Treatment Group recently presented phase II findings with weekly everolimus in nearly 100 newly diagnosed GBM patients, demonstrating no improvement in survival when compared with historical controls, with a median PFS and survival times of 6.4 and 15.8 months, respectively.26 As daily dosing has evolved as the standard schedule for everolimus,14,15 which is supported by clinical pharmacokinetic/pharmacodynamic data,27,28 and in an effort to take advantage of the radiation sensitization potential of this agent, we performed this randomized phase II study using a daily everolimus schedule with standard chemoradiation that was deemed to be safe in a recent phase I study.18 Unfortunately, this strategy did not lead to improved clinical outcomes, highlighting the clear challenge of targeting this signaling hub in GBM.
Several factors may have contributed to the observed lack of efficacy of mTOR inhibitors in GBM. Although it is possible that there may be a limited capacity of these agents to cross the blood–brain barrier, molecular29 and radiographic26,30 changes observed following administration of mTOR inhibitors do suggest intratumoral activity. Another possibility for the lack of efficacy may be attributed to compensatory Akt activation as a result of the S6 kinase/insulin receptor substrate 1 feedback loop driven by unregulated mTOR complex C2 signaling.31 Therefore, one strategy to overcome this is through dual inhibition of mTOR complex 1 and mTOR complex 2, thereby preventing feedback loop activation of Akt. Further, identifying a specific molecular subtype that accurately predicts response to mTOR inhibitors and enriching for such patients in subsequent clinical trials may be another strategy to achieve a therapeutic benefit from these agents.
As an initial application of mTOR inhibitors involved their role as an effective immunosuppressive therapy following organ transplant,32 the potential of these agents to increase the risk of infection is a clear concern when used in cancer therapy, as has been demonstrated with the mTOR inhibitor temsirolimus (CCI-779) in newly diagnosed GBM.33 Similarly, in a phase I study, significant myelosuppression was observed when combining everolimus with the maximal dose of TMZ in GBM patients.34 In our study, we observed a significant increase in treatment-related mortality in patients randomized to everolimus. Of the 10 reported cases, 4 were deemed potentially related to treatment and 6 of the events were described as either being infectious or respiratory in etiology. These findings, along with the noted increases in generalized grade 3–4 toxicities associated with everolimus in combination with TMZ and radiation tempers further study of this regimen in newly diagnosed GBM, even if a select molecular subtype sensitive to this regimen were to be identified. The European Organisation for Research and Treatment of Cancer recently presented data evaluating the combination of temsirolimus with radiation alone in newly diagnosed GBM patients without MGMT promoter hypermethylation,35 thereby obviating the need to include TMZ and the toxicities associated with this combination. This regimen was determined to be well tolerated, and although this study did not improve survival compared with patients randomized to receive standard radiotherapy and TMZ, phosphorylated mTOR was identified as a potential predictive factor of temsirolimus and worthy of further investigation.
Although the survival of patients randomized to receive everolimus was comparable to contemporary results, the control arm had an unexpectedly high PFS and OS that interestingly would have signaled a positive study if achieved in the experimental arm. These findings reiterate the lack of reliability of single-arm phase II studies in newly diagnosed GBM. As the treatment arms were well matched, including stratification by RPA class and post-hoc evaluation of MGMT hypermethylation status and therapy after progression (Supplementary Table S4), it is difficult to reconcile this difference. Factors that may have contributed to this discrepancy are actively being investigated, as identifying and considering additional prognostic factors may inform better phase II trial designs in the future. However, as this was a randomized study, the possibility of everolimus contributing to a detriment in survival needs to be considered, which could be supported by the increased toxicities, including increased lymphopenia, and decreased number of adjuvant TMZ cycles received in these patients.
Conclusion
The combination of everolimus with standard radiation therapy and TMZ led to increased toxicity and no clinical benefit in patients with newly diagnosed GBM. Based on the observed increase in toxicities when combined with TMZ, continued efforts in targeting this pathway may potentially be performed in patients with MGMT unmethylated tumors, where TMZ may be excluded or patients enriched with a specific molecular subtype predicted to respond to this combination. Further, trials testing dual mTOR complex 1/2 inhibitors36 or combining an mTOR inhibitor with an additional targeted agent designed to overcome acquired resistance may also be worthy of further investigation in this subset of GBM patients.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by the National Cancer Institute, NRG Oncology Operations (U10CA180868), NRG Oncology SDMC (U10CA180822), and Novartis.
Conflict of interest statement. Dr Ahluwalia reports grants and personal fees from Monteris Medical, Abbvie, BMS, AstraZeneca, Novocure, Incyte; personal fees from Datar Genetics, CBT Pharmaceuticals, Kadmon Pharmaceuticals, Elsevier; grants from Novartis, Pharmacyclics, Tracon Pharmaceuticals; personal fees from Prime Oncology and Caris Lifesciences, outside the submitted work. Dr Fiveash reports other from Varian, outside the submitted work. Dr Mehta reports other from Novartis, Cavion, Novocure, Varian, Agenus, Insys, Remedy, IBA, Oncoceutics, AstraZeneca, Pharmacyclics, outside the submitted work; and Monteris, Data Safely, Monitory Board. Dr Wen reports grants, personal fees, and nonfinancial support from Agios, Angiochem, AstraZeneca, Genentech/Roche, GlaxoSmithKline, Immunocellular Therapeutics, Karyopharm, Merck, Novartis, Oncoceutics, Sanofi-Aventis, Vascular Biogenics, Cavion, Insys, Monteris, Novogen, Regeneron, VBI Vaccines, Tocagen, outside the submitted work. The other authors have no disclosures.
References
- 1. Ostrom QT, Gittleman H, Fulop J et al. . CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. [DOI] [PubMed] [Google Scholar]
- 3. Gilbert MR, Wang M, Aldape KD et al. . Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cancer Genome Atlas Research Network. TCGA: comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choe G, Horvath S, Cloughesy TF et al. . Analysis of the phosphatidylinositol 3ʹ-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63(11):2742–2746. [PubMed] [Google Scholar]
- 6. Laks DR, Crisman TJ, Shih MY et al. . Large-scale assessment of the gliomasphere model system. Neuro Oncol. 2016;18(10):1367–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pachow D, Wick W, Gutmann DH, Mawrin C. The mTOR signaling pathway as a treatment target for intracranial neoplasms. Neuro Oncol. 2015;17(2):189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang MY, Lu KV, Zhu S et al. . Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN-deficient and PTEN-intact glioblastoma cells. Cancer Res. 2006;66(16):7864–7869. [DOI] [PubMed] [Google Scholar]
- 9. Albert JM, Kim KW, Cao C, Lu B. Targeting the Akt/mammalian target of rapamycin pathway for radiosensitization of breast cancer. Mol Cancer Ther. 2006;5(5):1183–1189. [DOI] [PubMed] [Google Scholar]
- 10. Cao C, Subhawong T, Albert JM et al. . Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 2006;66(20):10040–10047. [DOI] [PubMed] [Google Scholar]
- 11. Shinohara ET, Cao C, Niermann K et al. . Enhanced radiation damage of tumor vasculature by mTOR inhibitors. Oncogene. 2005;24(35):5414–5422. [DOI] [PubMed] [Google Scholar]
- 12. Lee DF, Kuo HP, Chen CT et al. . IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130(3):440–455. [DOI] [PubMed] [Google Scholar]
- 13. Johnson BE, Mazor T, Hong C et al. . Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Motzer RJ, Escudier B, Oudard S et al. ; RECORD-1 Study Group Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. [DOI] [PubMed] [Google Scholar]
- 15. Baselga J, Campone M, Piccart M et al. . Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yao JC, Shah MH, Ito T et al. ; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krueger DA, Care MM, Holland K et al. . Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363(19):1801–1811. [DOI] [PubMed] [Google Scholar]
- 18. Chinnaiyan P, Won M, Wen PY et al. . RTOG 0913: a phase 1 study of daily everolimus (RAD001) in combination with radiation therapy and temozolomide in patients with newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys. 2013;86(5):880–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wen PY, Macdonald DR, Reardon DA et al. . Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 20. Reardon DA, Galanis E, DeGroot JF et al. . Clinical trial end points for high-grade glioma: the evolving landscape. Neuro Oncol. 2011;13(3):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J, Wang M, Won M et al. . Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81(3):623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27(7-8):365–375. [DOI] [PubMed] [Google Scholar]
- 23. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 24. Cox DR. Regression models and life-tables. J Royal Statistical Society Series B. 1972;34:187–202. [Google Scholar]
- 25. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163–170. [PubMed] [Google Scholar]
- 26. Ma DJ, Galanis E, Anderson SK et al. . A phase II trial of everolimus, temozolomide, and radiotherapy in patients with newly diagnosed glioblastoma: NCCTG N057K. Neuro Oncol. 2015;17(9):1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shiah HS, Chen CY, Dai CY et al. . Randomised clinical trial: comparison of two everolimus dosing schedules in patients with advanced hepatocellular carcinoma. Aliment Pharmacol Ther. 2013;37(1):62–73. [DOI] [PubMed] [Google Scholar]
- 28. Tanaka C, O’Reilly T, Kovarik JM et al. . Identifying optimal biologic doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data. J Clin Oncol. 2008;26(10):1596–1602. [DOI] [PubMed] [Google Scholar]
- 29. Cloughesy TF, Yoshimoto K, Nghiemphu P et al. . Antitumor activity of rapamycin in a phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5(1):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sarkaria JN, Galanis E, Wu W et al. . North Central Cancer Treatment Group phase I trial N057K of everolimus (RAD001) and temozolomide in combination with radiation therapy in patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2011;81(2):468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O’Reilly KE, Rojo F, She QB et al. . mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66(3):1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schaffer SA, Ross HJ. Everolimus: efficacy and safety in cardiac transplantation. Expert Opin Drug Saf. 2010;9(5):843–854. [DOI] [PubMed] [Google Scholar]
- 33. Sarkaria JN, Galanis E, Wu W et al. . Combination of temsirolimus (CCI-779) with chemoradiation in newly diagnosed glioblastoma multiforme (GBM) (NCCTG trial N027D) is associated with increased infectious risks. Clin Cancer Res. 2010;16(22):5573–5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mason WP, Macneil M, Kavan P et al. . A phase I study of temozolomide and everolimus (RAD001) in patients with newly diagnosed and progressive glioblastoma either receiving or not receiving enzyme-inducing anticonvulsants: an NCIC CTG study. Invest New Drugs. 2012;30(6):2344–2351. [DOI] [PubMed] [Google Scholar]
- 35. Wick W, Gorlia T, Bady P et al. . Phase II study of radiotherapy and temsirolimus versus radiochemotherapy with temozolomide in patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation (EORTC 26082). Clin Cancer Res. 2016;22(19):4797–4806. [DOI] [PubMed] [Google Scholar]
- 36. Kahn J, Hayman TJ, Jamal M et al. . The mTORC1/mTORC2 inhibitor AZD2014 enhances the radiosensitivity of glioblastoma stem-like cells. Neuro Oncol. 2014;16(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.