Abstract
Objective
To determine whether a simple quality improvement initiative consisting of a technical update and regular audit and feedback sessions will result in increased use of antenatal corticosteroids among pregnant women at risk of imminent preterm birth delivering at health facilities in the Philippines and Cambodia.
Design
Non-randomized, observational study using a pre-/post-intervention design conducted between October 2013 and June 2014.
Setting
A total of 12 high volume facilities providing Emergency Obstetric and Newborn Care services in Cambodia (6) and Philippines (6).
Intervention
A technical update on preterm birth and use of antenatal corticosteroids, followed by monthly audit and feedback sessions.
Main Outcome Measure
The proportion of women at risk of imminent preterm birth who received at least one dose of dexamethasone.
Results
Coverage of at least one dose of dexamethasone increased from 35% at baseline to 86% at endline in Cambodia (P < 0.0001) and from 34% at baseline to 56% at endline in the Philippines (P < 0.0001), among women who had births at 24–36 weeks. In both settings baseline coverage and magnitude of improvement varied notably by facility. Availability of dexamethasone, knowledge of use and cost were not major barriers to coverage.
Conclusions
A simple quality improvement strategy was feasible and effective in increasing use of dexamethasone in the management of preterm birth in 12 hospitals in Cambodia and Philippines.
Keywords: quality improvement, Philippines, Cambodia, premature birth, dexamethasone
Introduction
Maternal and child deaths around the world have reduced by half over the past two decades. However, reduction in neonatal deaths has been slower. Currently, 44% of under-five mortality globally is from newborn mortality [1]. Preterm birth (birth prior to 37 completed weeks gestation) complications are the leading cause of newborn mortality worldwide, and each year more than one million neonates die of preterm birth complications [1].
The administration of antenatal corticosteroids (ACS) to pregnant women at risk of imminent preterm birth is an important hospital-based intervention to reduce morbidity and mortality among babies born preterm. A Cochrane review from high- and middle-income settings concluded that ACS use before preterm birth was associated with a 34% reduction in respiratory distress syndrome, a 46% reduction in intracranial hemorrhage, a 54% reduction in necrotizing enterocolitis, and a 31% overall reduction in neonatal death [2, 3].
While the administration of ACS to women with a risk of imminent preterm birth is standard practice in high-income countries, ACS use in low- and middle-income countries is suboptimal. A recent facility-based, cross-sectional survey from 359 facilities in 29 low- and middle-income countries reported an average ACS use rate of 52% among women who gave birth between 26 and 34 weeks’ gestation in study facilities [4]. Increasing the appropriate use of ACS is an essential activity proposed under the Every Newborn Action Plan (ENAP) developed by UNICEF, the World Health Organization and more than 60 partner organizations [5]. The UN Commission on Lifesaving Commodities for Women's and Children's Health includes ACS on the list of 13 essential and overlooked commodities for women and children [6]. While a recent study raises questions about a population-based approach, WHO still recommends the use of ACS in certain circumstances [7, 8].
Cambodia and Philippines have preterm birth rates of 10.5% and 14.9%, respectively [9]. The Ministries of Health in both countries have prioritized the reduction of mortality from premature birth, including the use of ACS in both countries’ national guidelines [10]. Yet, the ACS use rate in large hospitals is 58% in Cambodia and 47% in the Philippines [4]. Reasons for those low use rates are unclear. Thus, the aim of the present study was to understand the barriers and facilitators to correct use of ACS and evaluate the effect of an intervention to increase use among women with high probability of preterm birth in 12 hospitals in Cambodia and the Philippines.
Methods
This was a non-randomized, quasi-experimental study using a pre-/post-intervention design conducted between October 2013 and June 2014. In collaboration with the Ministry of Health in Cambodia and the Philippines, 12 national and provincial public referral hospitals (six in each country) were purposefully selected based on high clinical volume, provision of emergency obstetric and newborn services and capability to manage preterm newborns. Monthly births at facilities in Cambodia ranged from 151 to 685, and from 150 to 1215 in Philippines. The study was powered to detect a minimum 20 percentage point improvement between baseline and endline monthly ACS use rates among women at risk of preterm birth who received at least one dose of dexamethasone.
In each country, a local research organization consisting of clinicians and data collectors with experience in conducting studies on maternal and newborn health in the health sector was hired to implement the study. U.S.-based study co-investigators traveled to Cambodia and the Philippines to lead a 3-day training on the study rationale, design, research protocols and tools (including informed consent) and provided a technical update on preterm birth. The local research teams were native to the country where the research was conducted but were not affiliated with the participating hospitals. Local research team members were fluent in English and the local language for each country. The intervention was implemented in two steps.
The first step was the presentation of a standardized one-day technical update for clinical managers and birth attendants, who subsequently repeated this update for all birth attendants at the hospital. Based on WHO guidelines at the time the study was implemented, the technical update recommended administration of ACS for the following conditions: (a) preterm labor, (b) preterm prelabor rupture of membranes, (c) antepartum hemorrhage or (d) severe pre-eclampsia or eclampsia among women between 24 and 36 completed weeks gestation. Providers were instructed to give 6 mg dexamethasone every 12 h for 4 doses.
The second step of the intervention consisted of a monthly audit and feedback process. Each month, members of the local research team abstracted data from birth registers and medical records, and calculated dexamethasone coverage. Monthly performance data was shared with the maternity unit director/facility champion for the intervention, strategies to improve coverage were discussed, and directors and champions were encouraged to share these data with staff.
At baseline and endline, team members conducted a structured facility assessment to document staffing levels and authority, availability of equipment and medication, and other contextual factors. Baseline performance data were abstracted from birth registers and medical records. At baseline and endline, birth attendants at each hospital completed a multiple-choice assessment of knowledge of ACS use and a confidence assessment regarding giving ACS using a 5-point Likert scale. In each country, national advisory panels were engaged to guide the research and disseminate findings.
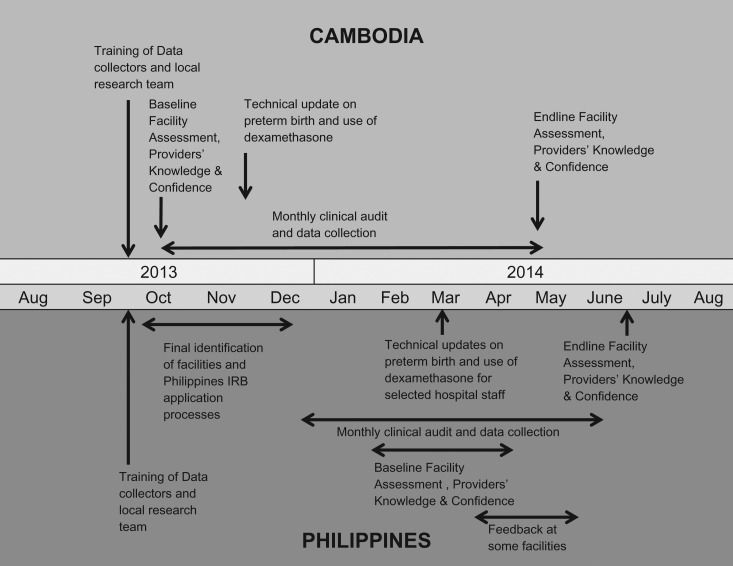
Timeline of the intervention and data collection in Cambodia
Two of the study facilities were in Phnom Penh (National Maternity Child Health Center Hospital (NMCHC) and Municipal Referral Hospital), and one facility each in Battambang, Siem Reap, Takeo and Kampong Chan. Baseline data were collected for October 2013 followed by a technical update in November 2013. Monthly data audit and feedback occurred from November 2013 to April 2014, with endline in May 2014 (Fig. 1). In response to needs identified in early feedback sessions, a member of the core study team visited five of six sites in February to provide additional technical support and refresher training. Knowledge and confidence assessments were completed by 55 providers at baseline and 64 at endline.
Figure 1.
Timeline of intervention and data collection activities by country.
Timeline of the intervention and data collection in the Philippines
The study facilities were Region I Medical Center (R1MC) in Dagupan, Vicente Sotto Memorial Medical Center (VSMMC) in Cebu, Southern Philippines Medical Center (SPMC) in Davao, Paulino J. Garcia Memorial Research & Medical Center (PJGMRMC) in Cabanatuan, Northern Samar Provincial Hospital (NSPH) in Catarman, and Mayor Hilarion A. Ramiro Sr. Regional Training and Teaching Hospital (MHARS) in Ozamiz. Baseline data were collected in January and February 2014, the technical update was conducted in March, and 3 months of audit and feedback were completed before the endline data collection in June 2014 (Fig. 1). Knowledge and confidence assessments were completed by 64 providers at baseline and 72 at endline.
Ethical review
This study was approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board in the United States and by the National Ethics Committee for Health Research in Cambodia (October 2013). In the Philippines, the study received ethical review and approval by the University of the Philippines Manila Research Ethics Board, as well as the institutional review boards of four participating health facilities. The remaining two facilities accepted the University of the Philippines approval as adequate. Informed consent was obtained from maternity care providers at the participating facilities and key informants.
Analysis methods
Medical record review
Total number of births and preterm births in each facility were calculated from hospital registers. Information on ACS administration was available in medical records, not births registers. Births for which medical records could not be located were excluded from the analysis. From medical records data, we calculated the proportion of babies whose mothers received at least one dose of ACS by month among all preterm births, among gestational age 24–34, and among 35–36 weeks. We calculated the monthly coverage of dexamethasone for each facility and the total for all facilities combined. Since there was no specific location to indicate ACS use in either the register or the medical records, absence of information was assumed to mean that ACS was not given.
In Cambodia, 45 birth records were found to have missing gestational age. Overall, 62% of these omissions occurred in one hospital, 89% of these omissions occurred during the first 2 months of data collection, and no missing gestational ages were recorded during the last 2 months of the intervention.
Facility assessment, provider knowledge and confidence
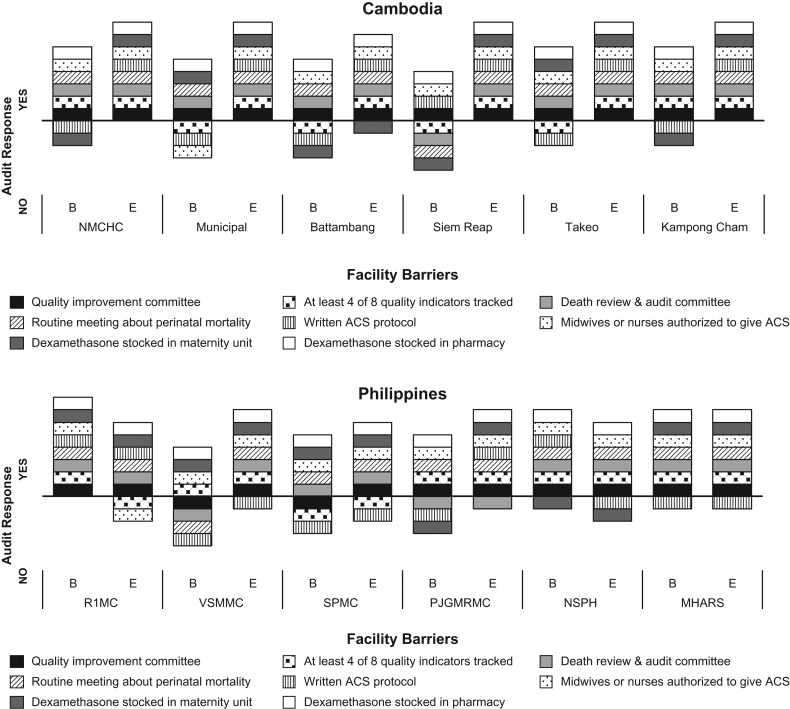
Eight binary implementation variables were assessed in each hospital and compared at baseline and endline (Fig. 2). These variables were considered to be facilitators or barriers to achievement of high coverage levels. For the knowledge assessment, the mean number of correct responses was calculated by country at baseline and endline. Similarly, average confidence score was calculated by country at baseline and endline.
Figure 2.
Baseline and endline scores of facility barriers and provision of care assessment by facility and country.
Qualitative data
The study team prepared notes from monthly feedback sessions. Study authors summarized key themes from feedback notes across facilities, and checked this interpretation with the field implementation team.
Quantitative data
Quantitative data were entered into MS Excel and analyzed using STATA 12. Tabulation and descriptive analysis was conducted to estimate ACS coverage, facility level factors and knowledge and confidence related ACS use. Chi square (χ2) tests adjusted for clustering at facility level were used to assess differences between baseline and endline ACS coverage at national level. Chi square test was also used to assess differences between baseline and endline coverage at each hospital. A P-value of <0.05 was considered statistically significant.
Results
Medical record review
The overall proportion of preterm births in the study facilities was 8% in Cambodia and 12% in the Philippines during the study period, with variation between facilities (Table 1). The proportion of births for which medical records could be obtained increased from 88% in Cambodia during the first month of the intervention to 99% during the last month, and in the Philippines it ranged from 89% to 97%.
Table 1.
Description of births and completeness of medical records in the study facility, by country
| Number of births | Number of preterm births | Number and percentage of births 24–34 weeks | Number and percentage of births 35–36 weeks | Number and percentage of patient charts located, among all preterm births | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cambodia | Philippines | Cambodia | Philippines | Cambodia | Philippines | Cambodia | Philippines | Cambodia | Philippines | |||||||
| No. | Rate | No. | Rate | No. | Rate | No. | Rate | No. | Rate | No. | Rate | |||||
| Month | ||||||||||||||||
| 1 | 1626 | 3671 | 172 | 410 | 117 | 7.2 | 215 | 5.9 | 55 | 3.4 | 195 | 5.3 | 152 | 88.4 | 364 | 88.8 |
| 2 | 1811 | 3190 | 145 | 347 | 86 | 4.7 | 181 | 5.7 | 59 | 3.3 | 166 | 5.2 | 123 | 84.8 | 301 | 86.7 |
| 3 | 1992 | 3655 | 131 | 455 | 91 | 4.6 | 241 | 6.6 | 40 | 2 | 214 | 5.9 | 124 | 94.7 | 439 | 96.5 |
| 4 | 1859 | 3616 | 137 | 432 | 101 | 5.4 | 236 | 6.5 | 36 | 1.9 | 196 | 5.4 | 124 | 90.5 | 418 | 96.8 |
| 5 | 1676 | 4013 | 131 | 532 | 96 | 5.7 | 287 | 7.2 | 35 | 2.1 | 245 | 6.1 | 129 | 98.5 | 485 | 91.2 |
| 6 | 1783 | 3671 | 164 | 467 | 123 | 6.9 | 236 | 6.4 | 41 | 2.3 | 231 | 6.3 | 153 | 93.3 | 426 | 91.2 |
| 7 | 1885 | 150 | 121 | 6.4 | 29 | 1.5 | 148 | 98.7 | ||||||||
| Total | 12 632 | 21 816 | 1030 | 2643 | 735 | 5.8 | 1396 | 6.4 | 295 | 2.3 | 1247 | 5.7 | 953 | 92.5 | 2433 | 92.1 |
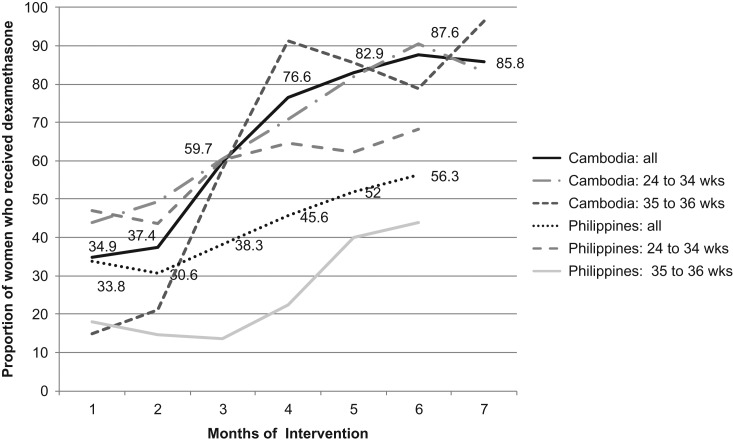
In Cambodia, the proportion of preterm births that received at least one dexamethasone dose increased from 35% at baseline to 86% at endline (P < 0.0001), and the gains in coverage were comparable for both the 24–34 week and 35–36 week gestational age groups (Fig. 3). In the Philippines, overall coverage increased from 34% at baseline to 56% at endline. Coverage of a majority of births was achieved in the 24–34-week group, which increased from 47% at baseline to 68% at endline, while the 35–36-week age group increased from 18% to 44%.
Figure 3.
Coverage of dexamethasone (≥1 dose) in each country by month, among all women at risk of preterm birth and by gestational age categories.
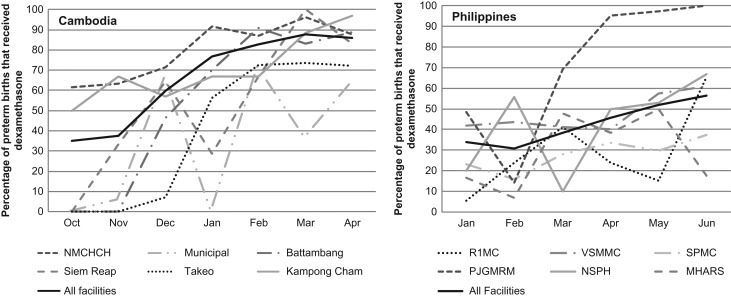
Four facilities in Cambodia increased coverage of ACS from zero at baseline to more than 60% at endline (Fig. 4).
Figure 4.
Proportion of women that received at least one dose of dexamethasone in each facility by month and country.
One facility in the Philippines (Municipal) had wide monthly fluctuations, explained in part by few (less than 10) monthly preterm births. Statistically significant increases were measured at all but one Philippine facility (Fig. 4), and coverage was above 60% at four of the six hospitals at endline.
Facility assessment, providers’ knowledge and confidence
In Cambodia, four of the hospitals did not stock dexamethasone in the maternity unit at baseline, and three of these did so at endline (Fig. 2). Four of the hospitals tracked fewer than four quality of care indicators at baseline and more than four at endline. Two hospitals showed improvement through establishment of a quality improvement or death review and audit committee, and one hospital began stocking dexamethasone in the maternity unit.
In Cambodia, mean provider knowledge scores increased from baseline (4.1) to endline (7.4), out of nine points maximum (data not shown). The mean confidence score was 3.0 at baseline and 3.7 at endline, out of five points maximum. In the Philippines, knowledge was similar at baseline (6.6) and endline (6.4) out of 10 possible, and no change in confidence seen between baseline (3.4) and endline (3.4).
Qualitative data
In Cambodia, gestational age was not calculated universally at baseline, either because women were unable to report the date of their last menstrual period (LMP), or providers did not routinely record this information. The need for accurate gestational age estimation was emphasized during the second technical visit (including provision of job aids and pregnancy wheels) and during monthly feedback sessions. Endline data showed improvement in gestational age calculation and in providers’ awareness of the need to provide timely treatment with ACS. In the Philippines, although gestational age was usually recorded, respondents were not confident in the accuracy of estimates.
In Cambodia, monthly ACS performance charts were displayed where all staff could see them; in contrast, in the Philippines, some hospitals shared results only with those staff involved in the study or on the management committee, while other facilities shared results during staff meetings with doctors, residents, and nurses. Inconsistent application of the audit and feedback methodology by facility leadership, particularly in broad sharing of performance data, may have limited increases in ACS uptake in the Philippines.
Facility-wide dexamethasone stockouts were described as rare in all facilities. In Cambodia, key informants said that cost was rarely a barrier and patients are not charged for the medication. In some facilities in both countries, dexamethasone availability was a challenge at baseline because the drug was inconsistently stocked in the maternity unit or emergency department.
Discussion
This study demonstrates that it is feasible to rapidly increase coverage of ACS for women at risk of preterm birth using a short technical update followed by a monthly audit and feedback schedule. Moreover, routine recording of gestational age improved in Cambodia, and the completeness of documentation improved in both countries. Providers’ knowledge of appropriate ACS use may have been a limiting factor at baseline in Cambodia, but it did not seem to be a significant factor in the Philippines. In both countries, the significant change in ACS administration, in the face of a modest change in knowledge, suggests that regular audit and feedback of ACS data was a more significant influence than the provision of updated technical information.
Facility management issues appear to have played a role in low initial rates of use, including limited authority for administration by midwives and availability of dexamethasone on the maternity unit. This intervention focused indirectly on addressing those issues through the transparent use of data. No specific measures were taken to address these management issues, yet the facility teams themselves addressed them when they saw that their monthly performance data was not as desired. This appears to support the idea that regular audit and measurement of achievement of a desired outcome can be a potent driver of improved performance [12, 13].
This study was not powered to measure impact on mortality and morbidity indicators, because at the time it was thought that the mortality impact of ACS administration was well-established. While this increase in utilization rates at the facility level is encouraging, it focused on those women who presented in time to receive at least one dose. In light of the recent study by Althabe et al. [7], however, ACS administration should likely not be encouraged at lower level facilities unable to meet the recently established safe administration criteria [8]. However, it is important to highlight key differences between this study and the Althabe study. The Althabe study included ACS administration at all levels of the health system. Gestational age estimation was problematic in those settings, possibly contributing to overtreatment since 84% of mothers who received ACS did not deliver prematurely [13]. In the Althabe study, those who were born preterm often lacked access to neonatal intensive care. In contrast, this study was conducted in tertiary facilities providing neonatal intensive care.
As noted previously, Vogel et al. found that the median rate of use of ACS among women at risk of preterm birth between 26 and 34 weeks gestation in facilities in 29 countries was 54%, the average rate in the Philippines of administration was 47%, and the average rate in Cambodia was 58% [4], much higher than the rates found at baseline in this study. The Vogel study excluded births that occurred within 3 h of arrival at the facility, whereas this study included all births, which could explain some of the discrepancy observed.
The four-country SEA-ORCHID study, which included the Philippines but not Cambodia, reported an increase from 15% to 31% in women who gave birth at <34 weeks and received ACS [14]. The comparatively greater magnitude of change measured in this study may be because it focused on only one intervention, while the SEA-ORCHID trial promoted change in more than a dozen interventions.
Limitations
One limitation of the data is that completeness of course and dosing was not captured adequately. Gestational age was most commonly estimated by LMP; first trimester GA estimates either through ultrasound or LMP were lacking from patient records. Direct observation of care for data collection was not feasible; data were instead abstracted from medical records which may have been incomplete. It was not possible to capture timing of birth relative to start of ACS. Hospital key informants reported that women were often referred from lower levels of the health system and were very close to delivery when they reached the study hospital. It is unknown whether improvements demonstrated during the intervention were sustained after the audit and feedback cycles ended. Additionally, impact of administration to women whose babies delivered at term was not measured. Future studies should include interviews with patients to better understand their experiences accessing care for preterm births.
Conclusions
This study contributed to the understanding of quality of care in Southeast Asia, a subject of relatively few published studies [15]. This simple audit-feedback intervention was able to demonstrate clinical change in a relatively short time period. The approach also drove improvement on measures of facility barriers (Fig. 2), including decisions by facility teams to maintain a stock of dexamethasone directly in the maternity unit. Confidence in gestational age estimation, access to estimation tools (e.g. pregnancy wheels), and recording of GA estimates in medical records, appear to support provider confidence in administering ACS. This study provides lessons for quality improvement that may be applicable beyond ACS use. Baseline understanding of facility barriers and enablers can be useful in understanding the environment for change. But the greater driver of change was clinical teams’ awareness of their own performance and their ability to measure and monitor efforts to improve performance. Leadership and engagement of facility staff played a key role in the feedback process, enabling teams to address barriers and identify solutions.
Acknowledgements
The authors would like to acknowledge the contributions of Mary Burner, Caitlin Warthin, Gbaike Ajayi, Human Development Research Cambodia (including Kai-Lih Liu, Pheap Sambath, and Chien Samphoas), and the Nutrition Center of the Philippines (including Emaluz Z. Parian, Eusebeia Joy B. Mendoza, Cherry C. Maramag, Helen V. Madamba, Ernesto Gregorio, Jr.).
Funding
This work was supported by the United States Agency for International Development (USAID) through the Maternal and Child Health Integrated Program (MCHIP) and the Maternal and Child Survival Program (MCSP) (grant numbers GHS-A-00-08-00002-00, AID-OAA-A-14-00028).
References
- 1. Lawn JE, Kinney MV, Belizan JM et al. Born too soon: accelerating actions for prevention and care of 15 million newborns born too soon. Reprod Health 2013;10:S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2006: CD004454. DOI: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 3. Brownfoot FC, Crowther CA, Middleton P. The Cochrane Collaboration Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth In: Brownfoot FC. Cochrane Database of Systematic Reviews [Internet]. Chichester, UK: John Wiley & Sons, Ltd., 2008. http://doi.wiley.com/10.1002/14651858.CD006764.pub2, (July 15 2013, date last accessed). [DOI] [PubMed] [Google Scholar]
- 4. Vogel JP, Souza JP, Gülmezoglu AM et al. Use of antenatal corticosteroids and tocolytic drugs in preterm births in 29 countries: an analysis of the WHO Multicountry Survey on Maternal and Newborn Health In: The Lancet [Internet] 2014. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(14)60580-8/abstract, (September 26 2014, date last accessed). [DOI] [PubMed] [Google Scholar]
- 5. Mason E, McDougall L, Lawn JE et al. From evidence to action to deliver a healthy start for the next generation. Lancet 2014;384:455–67. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization WHO Model List of Essential Medicines 18th List April 2013 [Internet]. Geneva: World Health Organization, 2013. http://www.who.int/medicines/publications/essentialmedicines/18th_EML_Final_web_8Jul13.pdf. [Google Scholar]
- 7. Althabe F, Belizán JM, McClure EM et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet 2014;385:629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization WHO Recommendations on Interventions to Improve Preterm Birth Outcomes. Geneva: World Health Organization, 2015. http://apps.who.int/iris/bitstream/10665/183037/1/9789241508988_eng.pdf?ua=1. [PubMed] [Google Scholar]
- 9. March of Dimes, PMNCH, Save the Children, World Health Organization Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization, 2012. [Google Scholar]
- 10. Philippine Department of Health (DOH), Philippine Obstetric and Gynecological Society (POGS ). Clinical Practice Guidelines on Intrapartum and Immediate Postpartum Care, 2012. [Internet]. 2012. Accessed online at http://www.wpro.who.int/philippines/publications/clinical_practical_guidelines_einc.pdf
- 11. Wagner C, Thompson C A, Arah O A et al. A checklist for patient safety rounds at the care pathway level. Int J Quality in Health Care 2014;26:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Althabe F, Buekens P, Bergel E et al. A behavioral intervention to improve obstetrical care. N Engl J Med 2008;358:1929–40. [DOI] [PubMed] [Google Scholar]
- 13. Hodgins S. Caution on corticosteroids for preterm delivery: learning from missteps. Glob Health Sci Pract 2014;2:371–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. SEA-ORCHID Study Group Lumbiganon P, McDonald SJ, Laopaiboon M et al. Impact of increasing capacity for generating and using research on maternal and perinatal health practices in South East Asia (SEA-ORCHID Project). PloS One 2011;6:e23994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harrison R, Wai A, Cohen S et al. Patient safety and quality of care in developing countries in Southeast Asia: a systematic literature review. Int J Qual Health Care 2015;27:240–54. [DOI] [PubMed] [Google Scholar]