Abstract
Background
Attention and working memory symptoms are among the most common late effects in survivors of pediatric brain tumors, and are often associated with academic and psychosocial difficulties. Diagnostic and treatment approaches derived from the literature on attention-deficit hyperactivity disorder (ADHD) have frequently been applied to survivors, yet the extent of overlap in cognitive profiles between these groups is unclear. The objective of the present study is to compare neurocognition in survivors of brain tumors and children with neurodevelopmental ADHD.
Methods
Neuropsychological data were abstracted from clinically referred brain tumor survivors (n = 105, Mage = 12.0 y, 52.4% male) and children with ADHD (n = 178, Mage = 11.1 y, 64.0% male). Data consist of a battery of parent-report questionnaires and performance-based neuropsychological measures.
Results
Twenty-five survivors (23.8%) of pediatric brain tumors met symptom criteria for ADHD. Participants with neurodevelopmental ADHD and survivors who met ADHD criteria had significantly greater parent- (P < 0.001) and teacher-reported (P < 0.001) working memory and behavior regulation difficulties than survivors of tumor who did not meet criteria. Children with ADHD symptoms also performed worse on measures of sustained attention than survivors without ADHD symptoms (P < 0.001). Additionally, survivors with ADHD symptoms had greater performance-based working memory difficulties than either survivors without attention problems or children with neurodevelopmental ADHD (P = 0.002).
Conclusions
Nearly a quarter of survivors with attention symptoms have functional profiles that are similar to children with neurodevelopmental ADHD. They also experience more neurocognitive impairments than survivors without attentional difficulties, particularly in working memory. Screening for ADHD symptoms may help providers triage a subset of individuals in need of earlier or additional neuropsychological assessment.
Keywords: ADHD, attention problems, late effects, screening, survivors of pediatric brain tumors
Importance of the study
This is the first study to compare neuropsychological functioning between survivors of pediatric brain tumors with and without significant attentional concerns and children with neurodevelopmental ADHD. Results indicate that survivors with significant, parent-reported symptoms of inattention share similar psychosocial and neurocognitive difficulties with children with ADHD compared with survivors without significant attentional concerns. Findings imply that rapid screening for ADHD symptoms among survivors may be helpful in identifying those in need of additional neuropsychological evaluation.
Attention problems are among the most common neurocognitive late effects observed in survivors of pediatric brain tumors. Deficits in attention have been linked to declines in intelligence,1 academic achievement,2 social wellness,3,4 and adaptive functioning.5 Along with attention, deficits in working memory,6 processing speed,7 executive functioning,8 and visual-spatial integration9 are also prevalent. The etiology of neurocognitive deficits in survivors of pediatric brain tumors is multifactorial, arising from a combination of factors related to the tumor itself, as well as the treatments used (eg, resection, cranial radiation, chemotherapy), with imaging studies demonstrating a relationship between attention problems and white matter damage.10–12
Attention-deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by impairments in attention, organization, and impulse control. Three subtypes are differentiated by the predominant symptoms: inattentive presentation (ADHD-IN), hyperactive/impulsive presentation (ADHD-HI), and combined presentation (ADHD-C).13 Children with ADHD typically demonstrate difficulties in a number of domains, including executive functioning, academic achievement, and social functioning.14 ADHD is most effectively and commonly treated with behavioral interventions and stimulant medication,14 with recent forays into the use of computerized cognitive training programs.15,16
Given similarities in the functional impairments of survivors of pediatric brain tumors and children with ADHD, researchers have frequently consulted the ADHD literature for possible intervention options. Specifically, studies have been completed that have trialed the use of stimulant medications17,18 and computerized cognitive training programs.19–23 However, despite the prevalence of attention problems in survivors—and the efficacy of ADHD-related interventions—researchers have cautioned against the diagnosis of ADHD (acquired or secondary) in survivors.24–26 More specifically, they maintain that the diagnostic criteria do not capture the full spectrum of neuropsychological late effects observed in these patients. While there is support for this position in the literature,24 several studies have also reported that the number of survivors exhibiting symptoms consistent with the disorder is higher than would be expected in the general population.25,27 Such findings lend support to the idea that ADHD criteria may be a helpful heuristic for at least a subset of survivors. However, to date, no study has directly compared survivors of pediatric brain tumors and children with ADHD on key domains of neuropsychological functioning. This research is necessary in order to determine areas of convergence and divergence across these 2 conditions. Indeed, the argument regarding whether ADHD is a useful diagnostic framework for survivors would be strengthened by the comparison of attention-mediated neuropsychological profiles between survivors of brain tumors and children with ADHD.
A recent study suggested that symptoms of ADHD, assessed via a simple parent rating scale, may be able to differentiate survivors of pediatric cancer with and without other neurocognitive concerns, and thus may serve as an effective screening tool.27 Results indicated that 27% of clinically referred survivors of brain tumors and leukemia met criteria for ADHD-IN and that these patients evidenced greater deficits in intelligence quotient (IQ) and working memory, as well as more externalizing and social problems, than survivors who did not meet criteria.27 The authors suggested that while survivors may not fit the typical profile of children with ADHD, the presence or absence of significant inattentive symptoms provides a means for identifying those patients who are at high risk for additional neurocognitive problems. As such, this approach could be used to quickly identify those survivors who are in need of a more comprehensive evaluation and/or targeted interventions. However, this study examined only a limited range of neurocognitive skills, indicating that additional work is needed to determine whether the presence of significant ADHD symptomatology is also related to deficits in other areas, including executive functioning and memory.
The objectives of the current study are twofold. First, we sought to extend previous literature by examining the relationship between significant attention problems and broader neuropsychological functioning in survivors of pediatric brain tumors. Second, we aimed to compare the neuropsychological profiles of survivors with those of children with neurodevelopmental ADHD. It was hypothesized that survivors with significant attention problems would demonstrate greater deficits in neurocognitive functioning (including working memory, processing speed, executive functioning, and memory) and psychosocial functioning (externalizing behaviors and social problems) than survivors without significant attention problems. Furthermore, it was hypothesized that survivors with significant attention problems would demonstrate a similar pattern of neurocognitive functioning as children with ADHD.
Materials and Methods
Participants and Procedures
Following approval from the institutional review board, neurocognitive and psychosocial data were abstracted from the medical records of survivors of pediatric brain tumors and children with neurodevelopmental ADHD who were assessed within the Pediatric Neuropsychology Clinic of an academic medical center in the Mid-Atlantic region between June 2013 and May 2016. All participants provided informed consent to have their records entered and stored in a database for future research purposes. Participants in the survivor group were eligible if they had had diagnoses of brain tumor, were off-treatment for at least one year, and were medically stable at the time of evaluation. The comparison sample comprised otherwise healthy children with diagnoses of neurodevelopmental ADHD (any subtype) via semi-structured interview of ADHD symptoms and questionnaire ratings completed by parents and/or teachers. Participants with neurodevelopmental ADHD were excluded if they had any history of chronic medical illness, traumatic brain injury, intellectual disability, or known genetic conditions. For both groups, participants were included if a parent or guardian had completed the ADHD Rating Scale IV (ADHD-RS-IV)28 at the time of evaluation. Survivors (n = 105; Mage = 12.0 y; SD = 3.50; range: 6–18 y; 52.4% male) were an average age of 6.05 years (SD = 3.89) at diagnosis, and were evaluated 5.64 (SD = 3.34) years later. Tumor types varied, with the most common being low-grade glioma (21.0%), medulloblastoma (19.0%), and ependymoma (17.1%). In terms of treatment, 77.1% (n = 81) underwent surgical resection, 67.6% (n = 71) were treated with chemotherapy, and 63.8% (n = 67) completed cranial radiation therapy. The comparison sample of children with neurodevelopmental ADHD (n = 178; 64.0% male) were an average of 11.1 years of age (SD = 3.33, range: 6–18 y) at evaluation. See Table 1 for all demographic and treatment information.
Table 1.
Demographic and treatment information
| Survivors without ADHD Symptoms (n = 80) | Survivors with ADHD Symptoms (n = 25) | Neurodevelopmental ADHD (n = 178) | |||||
|---|---|---|---|---|---|---|---|
| M ± SD | N (%) | M ± SD | N (%) | M ± SD | N (%) | ||
| Age, y | 12.3 ± 3.56 | 11.1 ± 3.23 | 11.1 ± 3.33 | ||||
| Age at diagnosis, y | 6.2 ± 3.92 | 5.7 ± 3.86 | — | ||||
| Time since diagnosis, y | 5.8 ± 3.20 | 5.1 ± 3.73 | — | ||||
| Sex | |||||||
| Male | 42 (52.5) | 13 (52.0) | 114 (64.0) | ||||
| Female | 38 (47.5) | 12 (48.0) | 64 (36.0) | ||||
| Race | |||||||
| Caucasian | 52 (65.0) | 19 (76.0) | 98 (55.1) | ||||
| African American | 14 (17.5) | 5 (20.0) | 34 (19.1) | ||||
| Asian American | 5 (6.3) | — | 11 (6.2) | ||||
| Hispanic | 2 (2.5) | — | 5 (2.8) | ||||
| Biracial | — | — | 3 (1.7) | ||||
| Other | 6 (7.5) | — | 17 (9.6) | ||||
| Unknown | 1 (1.3) | 1 (4.0) | 5 (2.8) | ||||
| Brain Tumor Diagnoses | |||||||
| Medulloblastoma | 17 (21.3) | 3 (12.0) | — | ||||
| Ependymoma | 16 (20.0) | 2 (8.0) | — | ||||
| Low-grade glioma | 18 (22.5) | 4 (16.0) | — | ||||
| Other (eg, germinoma, craniopharyngioma) | 28 (35.0) | 16 (64.0) | — | ||||
| Treatment | |||||||
| Surgery | 64 (80.0) | 17 (68.0) | |||||
| Chemotherapy | 53 (66.3) | 18 (72.0) | |||||
| Cranial radiation therapy | 52 (65.0) | 14 (56.0) | |||||
| Craniospinal radiation | 26 (32.5) | 8 (32.0) | |||||
| Focal radiation ADHD inattentive symptoms | 26 (32.5) | 7 (28.0) | |||||
| Parent symptom count | 0.95 ± 1.31 | 6.04 ± 2.10 | 5.80 ± 2.35 | ||||
| Teacher symptom count | 0.96 ± 1.67 | 4.88 ± 3.31 | 3.80 ± 3.17 | ||||
Note: There were no significant differences in demographic or medical variables between the 2 brain tumor groups. Survivors of brain tumors without attentional symptoms were significantly older at time of evaluation than children with developmental ADHD (P = 0.025).
Measures
Parent and Teacher Ratings
ADHD-RS-IV28
Parents of all participants completed the ADHD-RS-IV, as did teachers for 70.7% (n = 200) of participants. The ADHD-RS-IV is a well-known, widely used parent- and teacher-report measure, which assesses the 18 symptoms of ADHD29 (9 symptoms of inattention [IN] and 9 symptoms of hyperactivity/impulsivity [HI]) using a Likert response scale ranging from 0 (symptom “never” occurs) to 3 (symptom “very often” occurs). In keeping with prior literature,30,31 ratings of either a 2 (“often”) or 3 (“very often”) were considered symptomatic, and ratings of either a 0 (“never”) or 1 (“occasionally”) were considered asymptomatic. Symptoms for each domain were summed so that each participant received a score ranging from 0 to 9 for both the IN and HI domains; however, because hyperactive/impulsive behaviors are not typically areas of concern for survivors, only the total number of IN symptoms was used to classify survivors as meeting ADHD-IN symptom criteria.
Behavior Rating Inventory of Executive Function (BRIEF)32
Parents (n = 277; 97.9%) and teachers (n = 231; 81.6%) completed ratings assessing participants’ everyday executive function. The BRIEF includes 86 forced-choice items rated “never,” “sometimes,” or “often,” which load onto 2 broad domains and multiple subdomains. Age- and gender-based T-scores are calculated, with higher scores reflecting greater impairment. The 2 broad domains—behavior regulation and metacognition—and one subdomain—working memory—were used for analyses.
Child Behavior Checklist (CBCL)33,34and Teacher Report Form (TRF)33The CBCL and TRF are widely used observer-report measures of a child’s emotional, behavioral, and psychosocial functioning. Parents (n = 265; 93.6%) and teachers (n = 221; 78.1%) responded to a number of open-ended and forced-choice questions. T-scores are computed, with higher scores reflecting greater impairment. The subscales for internalizing problems, externalizing problems, and social problems were used for analyses.
Performance-Based Measures
Beery Developmental Test of Visual Motor Integration (VMI)35
The VMI is an age-normed paper-and-pencil task that assesses a child’s ability to integrate visual and motor information through the copying of progressively more complex shapes. The majority of survivors (n = 86; 81.9%) and children with ADHD (n = 157; 88.2%) completed this measure.
California Verbal Learning Test–Children’s Version (CVLT-C)36
The CVLT-C is a list-learning verbal memory task that involves serial repetition and recall of a long list of everyday items. A variety of scores are calculated, including initial recall, learning slope, total recall, and short- and long-term delay scores. Total memory (T-score) and long-delay recognition memory (z-score) were used in the current analyses, with the CVLT-C completed by 95 (90.5%) survivors of brain tumors and 168 (94.4%) children with ADHD.
Test of Everyday Attention for Children (TEA-Ch)37,38
The TEA-Ch is an objective assessment of various aspects of attention—sustained, divided, and selective attention, and inhibition—for children aged 7 and older. Measures of selective or focused visual attention (Sky Search) and sustained auditory attention (Score!) were completed by 72 (68.6%) survivors and 132 (74.2%) children with ADHD.
Tower of London-DX–Drexel Version (TOL)39
The TOL is a test of visual planning and problem-solving skills administered to individuals aged 7 and older as an evaluation of executive functioning. Standard scores reflecting total correct, total moves, and total problem-solving time were used in the analyses (brain tumor n = 77, 73.3%; ADHD n = 154, 86.5%).
Wechsler Scales of Intelligence
As this study included a wide age range of participants, intellectual functioning was assessed using the age-appropriate version of the Wechsler Scales, the most widely used measure of intelligence for children and adults. Specifically, we used the Wechsler Intelligence Scale for Children, fourth edition (WISC-IV, ages 6–16)40 or fifth edition (WISC-V),41 the Wechsler Adult Intelligence Scale, fourth edition (WAIS-IV, ages 16+),42 and the Wechsler Abbreviated Scale of Intelligence, second edition (WASI-2, ages 6+).43 The majority of patients (59.0%, n = 167) completed the WISC-IV or WISC-V. From each version, the full-scale IQ score was used (brain tumor n = 99, 94.3%; ADHD n = 177, 96.2%), as well as a test of immediate/working memory (Digit Span subtest: brain tumor n = 85, 81.0%; ADHD n = 153, 83.2%) and Processing Speed Index scores when available (brain tumor n = 85, 81.0%; ADHD n = 117, 65.7%). Of note, the WASI-2 does not include indices of processing speed or working memory.
Wide-Range Assessment of Memory and Learning, 2nd edition (WRAML2)44
The WRAML2 assesses various aspects of memory, including visual and verbal memory, working memory, and attention/concentration. The Story Memory subtest was extracted (brain tumor n = 87, 82.9%; ADHD n = 128, 71.9%) as an indicator of context-based verbal memory.
Statistical Analyses
To evaluate the primary aims, univariate or multivariate analyses of variance (ANOVAs or MANOVAs, respectively) were used to examine differences between the ADHD and brain tumor samples. MANOVA procedures were utilized when analyzing subscales from the same measure to account for shared variance and to minimize the probability of type I error, while ANOVA procedures were used for scales with only one outcome. Post-hoc comparisons between children with neurodevelopmental ADHD, children with brain tumors meeting ADHD symptom criteria, and survivors of pediatric brain tumors not meeting symptom criteria were performed using Tukey–Kramer P-values. Logistical regression models were conducted to evaluate medical and demographic variables as predictors of whether or not survivors met ADHD symptom criteria. Finally, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of using ADHD symptom criteria as an indicator of the likelihood of impairment on neuropsychological outcomes among survivors were computed.
Results
Survivors Meeting ADHD Symptom Criteria
Nearly a quarter of the sample of survivors (n = 25; 23.8%) met symptom criteria of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, for ADHD-IN (defined as the presence of at least 6 of 9 symptoms as rated by parents and/or teachers). The number of parent-endorsed symptoms for all 3 groups (survivors with <6 ADHD-IN symptoms, survivors with ≥6 ADHD-IN symptoms, and children with neurodevelopmental ADHD) is provided in Table 1.
ADHD Symptoms and Psychosocial Functioning
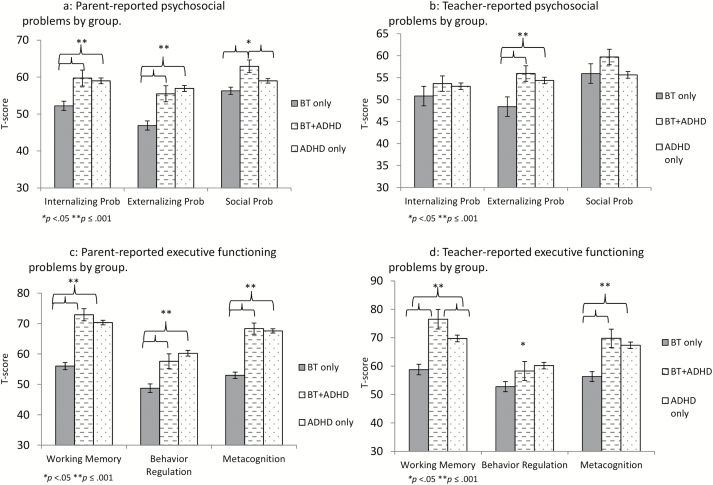
Both the subset of survivors meeting ADHD criteria and the neurodevelopmental ADHD group exhibited significantly greater parent-reported internalizing symptoms (F = 10.69, P < 0.001), externalizing symptoms (F = 22.26, P < 0.001), and social problems (F = 5.85, P = 0.003; Fig. 1A). In contrast, teacher ratings suggested no significant differences among the groups with regard to internalizing symptoms (F = 0.82, P = 0.44) or social problems (F = 2.51, P = 0.08), but survivors meeting ADHD symptom criteria and children with neurodevelopmental ADHD had significantly greater externalizing difficulties than survivors who did not meet ADHD criteria by teacher report (F = 8.32, P < 0.001; Fig. 1B).
Fig. 1.
Comparison of survivors meeting vs not meeting ADHD symptom criteria with children with developmental ADHD on observer-reported executive and psychosocial functioning.
ADHD Symptoms and Neurocognitive Functioning
Analyses were completed to examine group differences on parent- and teacher-reported executive functioning (ie, subscales from the BRIEF) and measures of neurocognitive ability (eg, IQ, working memory, processing speed). On the BRIEF, both parent (Wilks’s lambda = 0.64, P < 0.001) and teacher (Wilks’s lambda = 0.85, P < 0.001) ratings reflected significant group differences. Parents and teachers alike viewed children with significant ADHD symptoms—regardless of brain tumor diagnosis—as having significantly greater difficulties with behavior regulation (parent rating F = 22.26, P < 0.001; teacher rating F = 5.69, P = 0.004), working memory (parent rating F = 59.68, P < 0.001; teacher rating F = 16.27, P < 0.001), and metacognition (parent rating F = 69.31, P < 0.001; teacher rating F = 15.37, P < 0.001) than survivors without ADHD symptoms (Fig. 1C, D for parent and teacher ratings, respectively).
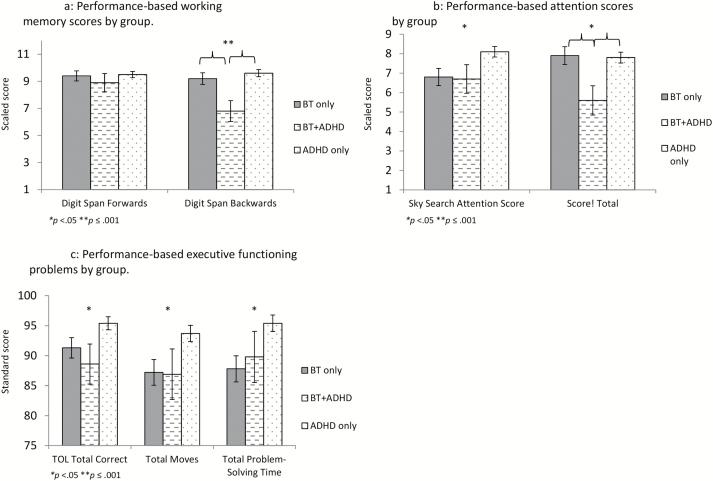
For performance-based tasks measuring skills often impacted by ADHD inattentive symptoms, MANOVAs indicated a significant difference between groups on working memory (Wilks’s lambda = 0.95, P = 0.024), attention (Wilks’s lambda = 0.93, P = 0.007), and executive functioning (Wilks’s lambda = 0.93, P = 0.013). Specifically, survivors who met ADHD-IN symptom criteria had lower scores on Digit Span backward (F = 5.77, P = 0.004) than either survivors without ADHD symptoms or children with neurodevelopmental ADHD (Fig. 2A). Of interest, both survivor groups scored significantly lower on measures of visual attention (F = 3.99, P = 0.02) and on a task measuring accurate and efficient problem-solving (TOL total correct F = 4.57, P = 0.011; TOL total problem-solving time F = 4.88, P = 0.008) than did children with neurodevelopmental ADHD (Fig. 2B, C). In contrast, survivors with ADHD-IN symptoms scored more poorly on a measure of auditory attention than both other groups (F = 3.45, P = 0.034; Fig. 2B).
Fig. 2.
Comparison of survivors meeting vs not meeting ADHD symptom criteria with children with developmental ADHD on performance-based working memory, attention, and executive functioning tasks.
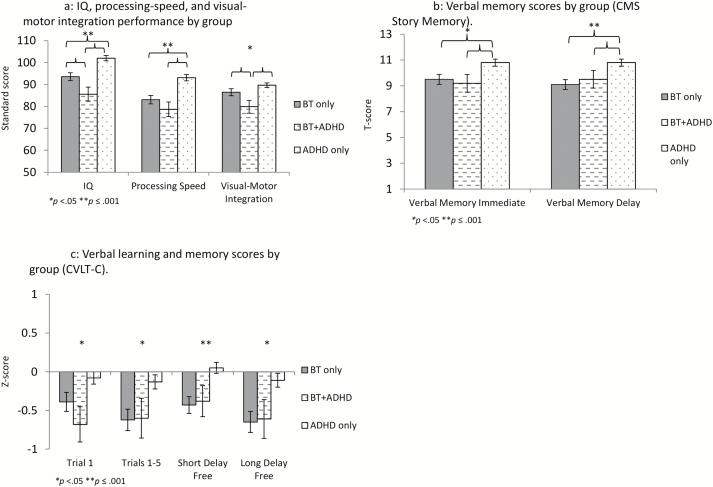
Group differences were also observed for other tasks measuring neurocognitive domains typically impacted in children treated for brain tumors. Full-scale IQ and processing speed scores were lowest for survivors with ADHD symptoms (F = 16.56, P < 0.001 and F = 13.64, P < 0.001, respectively), as were visual-motor integration scores (F = 5.75, P = 0.004; Fig. 3A). For measures assessing memory, however, both survivor groups were impaired relative to children with neurodevelopmental ADHD; this was the case for immediate memory (Story Memory Immediate F = 4.92, P = 0.008; CVLT-C Trial 1 F = 3.65, P = 0.028), verbal learning (CVLT-C Trials 1–5 F = 5.41, P = 0.005), and delayed memory (Story Memory Delay F = 6.93, P = 0.001; CVLT-C Long Delay Free F = 4.29, P = 0.015) (Fig. 3B, C).
Fig. 3.
Comparison of survivors meeting vs not meeting ADHD symptom criteria with children with developmental ADHD on other cognitive tasks.
ADHD Symptom Status and Medical/Demographic Predictors
Binary logistic regression analyses were performed to determine the potential association between medical variables and ADHD symptoms in the sample of survivors. There was no significant effect for any medical or demographic variables, including time since diagnosis (P = 0.58), age at diagnosis (P = 0.61), field of radiation therapy (P = 0.90), or gender (P = 0.77). Because these findings were somewhat unexpected, we examined whether or not there was a difference in the mean number of ADHD symptoms as a function of radiation field (ie, craniospinal radiation, focal radiation, or no radiation treatment), categorical age at diagnosis (≤3 y at diagnosis vs >3 y), and their interaction. However, children who were 3 years or younger at diagnosis did not differ in their reported number of ADHD symptoms compared with children who were older at diagnosis (F = 0.28, P = 0.60); similarly, children who received craniospinal radiation did not significantly differ from those who received focal or no radiation therapy (F = 0.01, P = 0.99). Moreover, the interaction between categorical age at diagnosis and radiation field was also nonsignificant (F = 0.10, P = 0.91).
Predictive Utility of ADHD Symptoms Among Survivors
Finally, predictive utility of using parent ratings of ADHD inattentive symptoms to predict impairment on the various neuropsychological outcomes examined in the study was evaluated, with the understanding that attention symptoms would not be expected to perfectly predict performance in all other domains of functioning. Specifically, survivors’ performance on each variable was categorized as either impaired (ie, ≤1 SD below the mean for performance measures or ≥1 SD above the mean for parent and teacher ratings) or not impaired. Specificity, sensitivity, PPV, and NPV of parent ratings of inattentive symptoms for each outcome were then calculated. As seen in Table 2, the sensitivity of ADHD ratings to predict impairment on neuropsychological variables was quite variable (range = 15.0%–79.0%) and best for performance-based processing speed and auditory attention tasks. In contrast, specificity values tended to be higher, particularly with regard to psychosocial outcomes (range = 46.8%–92.6%). Whereas PPVs were low for most outcomes (range = 13.6%–50.0%), NPVs were adequate for all but memory tasks (range = 73.4%–85.7%).
Table 2.
Sensitivity, specificity, PPV, and NPV associated with using attention-deficit hyperactivity disorder symptoms to predict rates of neuropsychological dysfunction
| Sensitivity | Specificity | PPV (95% CI) | NPV (95% CI) | |
|---|---|---|---|---|
| Neurocognitive Outcome | ||||
| IQ | 50.0% | 67.6% | 33.3% | 80.7% |
| Processing Speed | 75.0% | 46.8% | 31.3% | 85.3% |
| Visual-Motor Integration | 60.0% | 56.1% | 29.3% | 82.2% |
| Digit Span Backward | 58.8% | 65.5% | 34.5% | 83.7% |
| Digit Span Forward | 41.2% | 72.7% | 31.8% | 80.0% |
| CVLT Total Trials | 21.1% | 63.6% | 14.3% | 73.7% |
| CVLT Trial 1 | 40.9% | 60.3% | 23.7% | 77.2% |
| CVLT Short Delay Free | 30.0% | 69.1% | 22.2% | 77.1% |
| CVLT Long Delay Free | 40.9% | 54.8% | 21.4% | 75.5% |
| Stories Immediate | 15.0% | 71.2% | 13.6% | 73.4% |
| Stories Delay | 25.0% | 68.8% | 20.0% | 74.6% |
| TOL Total Correct | 43.8% | 70.5% | 28.0% | 82.7% |
| TOL Total Moves | 46.7% | 55.0% | 20.6% | 80.5% |
| TOL Total Problem-Solving Time | 43.8% | 53.5% | 20.6% | 77.5% |
| TEA-Ch Sky Search Total | 50.0% | 56.9% | 31.3% | 74.4% |
| TEA-Ch Score! Total | 79.0% | 48.0% | 36.6% | 85.7% |
| Psychosocial Outcome | ||||
| Parent-reported Internalizing Problems | 52.2% | 78.3% | 44.4% | 83.1% |
| Parent-reported Externalizing Problems | 34.8% | 87.0% | 47.1% | 80.0% |
| Parent-reported Social Problems | 56.5% | 68.6% | 37.1% | 82.8% |
| Teacher-reported Internalizing Problems | 17.7% | 81.5% | 23.1% | 75.9% |
| Teacher-reported Externalizing Problems | 23.5% | 92.6% | 50.0% | 79.4% |
| Teacher-reported Social Problems | 47.1% | 72.7% | 34.8% | 81.6% |
Discussion
The current paper is the first to directly compare neuropsychological profiles between samples of clinically referred survivors of pediatric brain tumors and those of clinically referred physically healthy children diagnosed with neurodevelopmental ADHD. Consistent with prior work,27 results indicated that almost a quarter of the brain tumor sample (23.8%) met symptom criteria for clinically significant attention problems. Moreover, in accordance with previous literature, this subgroup of survivors exhibited a pattern of neurocognitive impairments—including deficits in IQ, processing speed, executive functioning, and psychosocial concerns—that are more severe than survivors whose parents did not endorse significant attention concerns. When this subgroup of survivors was compared with children with neurodevelopmental ADHD, results indicated similar behavioral profiles, including difficulties with externalizing and social problems. However, survivors who met ADHD criteria demonstrated even more significant performance impairments across domains of functioning than children with neurodevelopmental ADHD or survivors without ADHD. This was particularly apparent on measures of working memory and auditory attention.
Overall, the results of the current study identified areas of convergence and divergence between the typical profile of survivors of pediatric brain tumors with significant attention problems and the typical profile of children with neurodevelopmental ADHD. Specifically, children with neurodevelopmental ADHD were found to exhibit a more developmentally appropriate neurocognitive profile than survivors of pediatric brain tumors (eg, average intelligence, verbal memory). However, both survivors and non–survivors with attention problems demonstrate similar impairments in observer-reported working memory, attention, and executive functioning. The existing literature suggests that problems with core attention and working memory skills contribute to declines in IQ over time in survivors of pediatric brain tumor,10 which may explain the lower intellectual functioning and increased experience of inattention in daily life observed in our sample. It is important to note that attention is not a unitary construct, and may be influenced by processing speed weaknesses and impaired visual or auditory processing. Collectively, difficulties in these areas may go on to impact working memory functions, as well as long-term storage and retrieval. Questionnaire ratings of inattention are unlikely to fully characterize or differentiate possible impairments in this cluster of symptoms, yet our results suggest that attention symptoms may be a salient indicator of a cluster of late effects that will need to be further evaluated by comprehensive neuropsychological testing.
Notably, none of the medical or demographic factors that often are associated with increased risk for neuropsychological late effects were predictive of ADHD symptoms in our sample. Use of radiation therapy, particularly craniospinal treatment, and younger age at diagnosis are well-documented risk factors for neurocognitive problems in survivors,45–47 but participants with significant ADHD symptoms in our sample were no more likely to have received radiation treatment or to be treated at a younger age than survivors with fewer attention problems. It is possible that survivors without these risk factors are underrepresented in our sample, as they may be less likely to be referred for testing unless they present with specific neurocognitive concerns. In this case, a population-based study would be more likely to identify medical predictors of attention concerns. We also had limited power to evaluate additional factors—and their interactions—that may contribute to the development of late effects. Future studies with larger (or more homogeneous) samples may elucidate contributing factors such as radiation dose and field, use of proton therapy, tumor location, and age at radiation. However, use of this method of screening may also be helpful in identifying children with fewer risk factors who may be less likely to have participated in neuropsychological surveillance but would likely benefit from this service.
Most importantly, the present study provides additional evidence for the clinical utility of an ADHD symptom rating scale in identifying some of the most at-risk survivors with regard to neurocognitive deficits. Results showed that levels of impairment across several neurocognitive and psychosocial domains in survivors of pediatric brain tumors were differentiated by the presence or absence of at least 6 symptoms of ADHD. Indeed, survivors with attention problems demonstrated significantly worse performance than survivors without these concerns across almost all domains assessed. Moreover, specificity and negative predictive value analyses indicated that survivors with fewer than 6 inattentive symptoms are less likely to demonstrate impairments in other neurocognitive domains. Ultimately, this suggests that the administration of a simple 5-minute questionnaire to parents during routine medical visits may help to triage survivors who are in need of additional neuropsychological assessment and/or targeted intervention from survivors who may defer evaluation to a later timepoint.48–50 This type of clinic-based, rapid assessment approach has been recently recommended by several authors who argue that survivors of pediatric brain tumors require regular neurocognitive surveillance,49 but that such monitoring need not always be in the form of comprehensive neuropsychological evaluations.48,50 Rather, there is growing consensus that neuro-oncologists may be in the best position to conduct clinical surveillance of neuropsychological functioning as patients are seen in clinic for routine care, and then consulting with neuropsychologists or referring for further evaluation when there is evidence of potential difficulty. Since providers are often required to justify referrals for neuropsychological evaluations for the purpose of insurance coverage, use of a brief and inexpensive questionnaire may facilitate critical referrals without burdening providers or families.
In addition, identification of a subgroup of children with significant ADHD symptoms may help direct providers to effective interventions. Specifically, the use of stimulant medication has been associated with improvements in attention and academic and social functioning in prior trials with survivors,17,18 and it may be that individuals with a clear profile of inattention will benefit most from this approach. Alternatively, researchers have also focused on the use of cognitive training programs that target specific neurocognitive impairments in attention, working memory, and executive functioning through focused practice,19–23 and this subgroup may benefit from this type of intervention as well. Ultimately, further research will be needed to determine whether survivors with more significant concerns may benefit from these more targeted approaches to intervention.
These important findings should be considered in light of a few limitations. The current study used data derived from clinically referred samples; both the survivors and children with neurodevelopmental ADHD were referred for neuropsychological testing. It is possible that a population-based sample may have reflected a lower percentage of attentional difficulties among the survivor group. In addition, some of the survivors and children with neurodevelopmental ADHD were being treated with stimulant medication at the time of testing, which may also have altered their symptom presentation as well as their neuropsychological assessment results. Although low numbers of treated children precluded analysis of differences in neuropsychological profiles associated with stimulant medication in the 2 groups, it is important to note that none of the survivors in the group of children reporting few attentional symptoms were treated with stimulants. Thus, whereas the magnitude of differences in the profiles of survivors and children with neurodevelopmental ADHD may have been reduced in our sample, the overall pattern of strengths and weaknesses across groups is likely to be replicated, even in samples without stimulant use.
Finally, it is important to recognize that survivors with significant attentional difficulties represent only one of perhaps several subgroups of children with different patterns of cognitive late effects. Only about a quarter of survivors in our sample met symptom criteria, whereas the majority of survivors did not exhibit this pattern of significant attention symptoms and would not be differentiated by the ADHD rating scale. There may be other subgroups—perhaps those identified by slowed processing or those with predominantly psychosocial concerns, who show few neurocognitive deficits and thus were not detected in our sample—who may benefit from alternative approaches to intervention than those whose predominant symptoms include attentional concerns.
As the survivor population grows, it will be important to have multiple targeted intervention approaches that can be offered to survivors based on their individual neurocognitive profiles. In other words, while a quarter of patients identified by significant attention symptoms may benefit from stimulant medication, patients with predominant memory or processing speed concerns could benefit from alternative approaches. Additional research is needed to identify subgroups of patients based on unique neurocognitive and psychosocial profiles and to link these subgroups to appropriate intervention. Many of the evidence-based interventions for survivors have been derived from the ADHD literature,17,19,22 but it is not known if these approaches work best for subsets of survivors whose pattern of late effects is more consistent with neurodevelopmental ADHD. A better understanding of the efficacy of ADHD-based interventions for those survivors identified as meeting criteria for ADHD-IN may provide further support for the clinical utility of a rapid assessment of ADHD symptoms as part of routine neurocognitive surveillance.
Funding
None.
Acknowledgments
Portions of this manuscript were presented at the 21st Annual Meeting of the Society for Neuro-Oncology in Scottsdale, Arizona (November 2016) and the 44th Annual Meeting of the International Neuropsychological Society in Boston, Massachusetts (February 2016).
Conflict of interest statement. None.
References
- 1. Schatz J, Kramer JH, Ablin A, Matthay KK. Processing speed, working memory, and IQ: a developmental model of cognitive deficits following cranial radiation therapy. Neuropsychology. 2000;14(2):189–200. [DOI] [PubMed] [Google Scholar]
- 2. Mabbott DJ, Spiegler BJ, Greenberg ML, Rutka JT, Hyder DJ, Bouffet E. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23(10):2256–2263. [DOI] [PubMed] [Google Scholar]
- 3. Moyer KH, Willard VW, Gross AM et al. . The impact of attention on social functioning in survivors of pediatric acute lymphoblastic leukemia and brain tumors. Pediatr Blood Cancer. 2012;59(7):1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel SK, Lai-Yates JJ, Anderson JW, Katz ER. Attention dysfunction and parent reporting in children with brain tumors. Pediatr Blood Cancer. 2007;49(7):970–974. [DOI] [PubMed] [Google Scholar]
- 5. Papazoglou A, King TZ, Morris RD, Krawiecki N. Parent report of attention problems predicts later adaptive functioning in children with brain tumors. Child Neuropsychol. 2009;15(1):40–52. [DOI] [PubMed] [Google Scholar]
- 6. Conklin HM, Ashford JM, Howarth RA et al. . Working memory performance among childhood brain tumor survivors. J Int Neuropsychol Soc. 2012;18(6):996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kahalley LS, Conklin HM, Tyc VL et al. . Slower processing speed after treatment for pediatric brain tumor and acute lymphoblastic leukemia. Psychooncology. 2013;22(9):1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Winter AL, Conklin HM, Tyc VL et al. . Executive function late effects in survivors of pediatric brain tumors and acute lymphoblastic leukemia. J Clin Exp Neuropsychol. 2014;36(8):818–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22(4):706–713. [DOI] [PubMed] [Google Scholar]
- 10. Reddick WE, White HA, Glass JO et al. . Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer. 2003;97(10):2512–2519. [DOI] [PubMed] [Google Scholar]
- 11. Reddick WE, Shan ZY, Glass JO et al. . Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer. 2006;106(4):941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reddick WE, Taghipour DJ, Glass JO et al. . Prognostic factors that increase the risk for reduced white matter volumes and deficits in attention and learning for survivors of childhood cancers. Pediatr Blood Cancer. 2014;61(6):1074–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. American Psychiatric Association. Diagnositc and Statistical Manual of Mental Disorders (DSM-5). 5th ed Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 14. Barkley RA. Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. 2nd ed New York, NY: Guilford; 1998. [Google Scholar]
- 15. Klingberg T, Fernell E, Olesen PJ et al. . Computerized training of working memory in children with ADHD—a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44(2):177–186. [DOI] [PubMed] [Google Scholar]
- 16. Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. J Clin Exp Neuropsychol. 2002;24(6):781–791. [DOI] [PubMed] [Google Scholar]
- 17. Mulhern RK, Khan RB, Kaplan S et al. . Short-term efficacy of methylphenidate: a randomized, double-blind, placebo-controlled trial among survivors of childhood cancer. J Clin Oncol. 2004;22(23):4795–4803. [DOI] [PubMed] [Google Scholar]
- 18. Conklin HM, Reddick WE, Ashford J et al. . Long-term efficacy of methylphenidate in enhancing attention regulation, social skills, and academic abilities of childhood cancer survivors. J Clin Oncol. 2010;28(29):4465–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hardy KK, Willard VW, Allen TM, Bonner MJ. Working memory training in survivors of pediatric cancer: a randomized pilot study. Psychooncology. 2013;22(8):1856–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hardy KK, Willard VW, Bonner MJ. Computerized cognitive training in survivors of childhood cancer: a pilot study. J Pediatr Oncol Nurs. 2011;28(1):27–33. [DOI] [PubMed] [Google Scholar]
- 21. Kesler SR, Lacayo NJ, Jo B. A pilot study of an online cognitive rehabilitation program for executive function skills in children with cancer-related brain injury. Brain Inj. 2011;25(1):101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Conklin HM, Ogg RJ, Ashford JM et al. . Computerized cognitive training for amelioration of cognitive late effects among childhood cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33(33):3894–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Conklin HM, Ashford JM, Clark KN et al. . Long-term efficacy of computerized cognitive training among survivors of childhood cancer: a single-blind randomized controlled trial. J Pediatr Psychol. 2017;42(2):220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kahalley LS, Conklin HM, Tyc VL et al. . ADHD and secondary ADHD criteria fail to identify many at-risk survivors of pediatric ALL and brain tumor. Pediatr Blood Cancer. 2011;57(1):110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krull KR, Khan RB, Ness KK et al. . Symptoms of attention-deficit/hyperactivity disorder in long-term survivors of childhood leukemia. Pediatr Blood Cancer. 2011;57(7):1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alderson RM, Mullins LL. Theoretical and clinical implications of using an ADHD framework to understand attention, concentration, and executive functioning deficits in pediatric cancer survivors. Pediatr Blood Cancer. 2011;57(1):4–5. [DOI] [PubMed] [Google Scholar]
- 27. Hardy KK, Willard VW, Wigdor AB, Allen TM, Bonner MJ. The potential utility of parent-reported attention screening in survivors of childhood cancer to identify those in need of comprehensive neuropsychological evaluation. Neurooncol Pract. 2015;2(1):32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DuPaul GJ, Power TJ, Anastopoulos AD, Reid R.. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. New York, NY: The Guilford Press; 1998. [Google Scholar]
- 29. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4th ed Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 30. Conners CK. Conners’ Rating Scales—Revised. Toronto, ON: Multi-Health Systems Inc; 1997. [Google Scholar]
- 31. Conners CK. Conners Rating Scales—3rd edition. Toronto, ON: Multi-Health Systems Inc; 2008. [Google Scholar]
- 32. Gioia GA, Isquith PK, Guy SC, Kenworthy L.. Behavior Rating Inventory of Executive Function (BRIEF). Lutz, FL: PAR, INC; 2000. [Google Scholar]
- 33. Achenbach TM. Manual for the CBCL. Burlington: University of Vermont; 1991. [Google Scholar]
- 34. Achenbach TM, Rescorla LA.. Manual for the ASEBA School-Age Forms & Profiles. Burlington: University of Vermont, Research Center for Children, Youth & Families; 2001. [Google Scholar]
- 35. Beery KE. The Beery-Buktenica Developmental Test of Visual-Motor Integration: Administration, Scoring and Teaching Manual, 4th edition, revised. Parsippany, NJ: Modern Curriculum Press; 1997. [Google Scholar]
- 36. Delis DC, Kramer KH, Kaplan E, Ober BA.. Manual for the California Verbal Learning Test—Children’s Version. San Antonio, TX: Psychological Corporation; 1994. [Google Scholar]
- 37. Manly T, Anderson V, Nimmo-Smith I, Turner A, Watson P, Robertson IH. The differential assessment of children’s attention: the test of everyday attention for children (TEA-Ch), normative sample and ADHD performance. J Child Psychol Psychiatry. 2001;42(8):1065–1081. [DOI] [PubMed] [Google Scholar]
- 38. Manly T, Robertson IH, Anderson V, Nimmo-Smith I.. Test of Everyday Attention for Children (TEA-Ch). Bury St. Edmunds, UK: Thames Valley Test Company; 1999. [Google Scholar]
- 39. Culbertson WC, Zillmer EA.. Tower of LondonDx™.2nd ed North Tonawanda, NY: MHS Assessments; 2005. [Google Scholar]
- 40. Wechsler D. Wechsler Intelligence Scale for Children—4th Edition. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 41. Wechsler D. Wechsler Intelligence Scale for Children, 5th edition. San Antonio, TX: Pearson; 2014. [Google Scholar]
- 42. Wechsler D. Wechsler Adult Intelligence Scale—4th edition. San Antonio, TX: The Psychological Corporation; 2008. [Google Scholar]
- 43. Wechsler D. Wechsler Abbreviated Scale of Intelligence, 2nd edition. San Antonio, TX: Pearson; 2011. [Google Scholar]
- 44. Sheslow D, Adams W.. Manual for the Wide Range Assessment of Memory and Learning. Wilmington, DE: Jastak; 1990. [Google Scholar]
- 45. Tonning Olsson I, Perrin S, Lundgren J, Hjorth L, Johanson A. Long-term cognitive sequelae after pediatric brain tumor related to medical risk factors, age, and sex. Pediatr Neurol. 2014;51(4):515–521. [DOI] [PubMed] [Google Scholar]
- 46. Holmquist LA, Scott J. Treatment, age, and time-related predictors of behavioral outcome in pediatric brain tumor survivors. J Clin Psychol Med Settings. 2002;9(4):315–321. [Google Scholar]
- 47. Schreiber JE, Gurney JG, Palmer SL et al. . Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro Oncol. 2014;16(8):1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hardy KK, Olson K, Cox SM, Kennedy T, Walsh KS. A prevention-based model of neuropsychological assessment for children with medical illness. J Pediatr Psychol. 2017;doi:10.1093/jpepsy/jsx060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Annett RD, Patel SK, Phipps S. Monitoring and assessment of neuropsychological outcomes as a standard of care in pediatric oncology. Pediatr Blood Cancer. 2015;62(Suppl 5):S460–S513. [DOI] [PubMed] [Google Scholar]
- 50. Baum KT, Powell SK, Jacobson LA et al. . Implementing guidelines: proposed definitions of neuropsychology services in pediatric oncology. Pediatr Blood Cancer. 2017;64(8):doi:10.1002/pbc.26446. [DOI] [PubMed] [Google Scholar]