Abstract
Background
Critically ill pediatric patients frequently require hemoglobin monitoring. Accurate noninvasive Hb (SpHb) would allow practitioners to decrease anemia from repeated blood draws, traumatic blood draws, and a decreased number of laboratory Hb (LabHb) medical tests. The Food and Drug Administration has approved the Masimo Pronto SpHb and associated Rainbow probes; however, its use in the pediatric intensive care unit (PICU) is controversial. In this study, we define the degree of agreement between LabHb and SpHb using the Masimo Pronto SpHb Monitor and identify clinical and demographic conditions associated with decreased accuracy.
Materials and methods
We performed a prospective, observational study in a large PICU at an academic medical center. Fifty-three pediatric patients (30-d and 18-y-old), weighing >3 kg, admitted to the PICU from January–April 2013 were examined. SpHb levels measured at the time of LabHb blood draw were compared and analyzed.
Results
Only 83 SpHb readings were obtained in 118 attempts (70.3%) and 35 readings provided a result of “unable to obtain.” The mean LabHb and SpHb were 11.1 g/dL and 11.2 g/dL, respectively. Bland–Altman analysis showed a mean difference of 0.07 g/dL with a standard deviation of ±2.59 g/dL. Pearson correlation is 0.55, with a 95% confidence interval between 0.38 and 0.68. Logistic regression showed that extreme LabHb values, increasing skin pigmentation, and increasing body mass index were predictors of poor agreement between SpHb and LabHb (P < 0.05). Separately, increasing body mass index, hypoxia, and hypothermia were predictors for undetectable readings (P < 0.05).
Conclusions
The Masimo Pronto SpHb Monitor provides adequate agreement for the trending of hemoglobin levels in critically ill pediatric patients. However, the degree of agreement is insufficient to be used as the sole indicator for transfusion decisions and should be used in context of other clinical parameters to determine the need for LabHb in critically ill pediatric patients.
Keywords: Hemoglobin, Pediatric intensive care unit, Physiologic monitoring, Transfusion
1. Introduction
Critically ill pediatric patients frequently require hemoglobin (Hb) monitoring. When compared to adults, serial Hb monitoring presents unique challenges specific to the pediatric population, including: anemia from repeated blood draws [1], difficulties with venous access, and decreased patient cooperation with blood draws [2,3]. These challenges, and the traumatizing nature of venipuncture laboratory draws in children, are balanced with a clinician’s desire to avoid unnecessary laboratory blood draws and medical tests. In an effort to provide rapid and cost-effective Hb analysis, noninvasive hemoglobin (SpHb) devices have been developed and approved by the FDA. One of these monitors is the Masimo Pronto SpHb Monitor (Masimo Corporation, Irvine, CA).
Before and since the time of Food and Drug Administration approval, several studies have examined the use of SpHb in neonates, children, and adults. The focus of these studies has been the degree of agreement between the SpHb and laboratory Hb (LabHb). Previous studies done in pediatric patients have found “acceptable” agreement between SpHb and LabHb in the outpatient [4], preoperative [5], and operating room settings [6–8]. However, the data regarding its use and implementation in critically ill children are sparse as there is only a single study examining the use of Masimo technology in a Pediatric Intensive Care Unit (PICU) [9]. However, if the accuracy of the device could be demonstrated in critically ill children, the PICU would provide an ideal setting for the use of SpHb given the need for serial monitoring in a large number of patients to address the challenges specific to pediatric patients. The present study examines the use of the Masimo Pronto SpHb Monitor, the agreement with LabHb levels in critically ill children in a busy tertiary care setting, as a first step in ultimately determining the clinical utility of the monitor in this patient population.
2. Methods
We conducted a prospective, observational study to determine the accuracy of the Masimo Pronto SpHb Monitor and associated Rainbow probes for the detection of Hb concentration in a cohort of critically ill pediatric patients admitted to the University of North Carolina PICU between January and April 2013. The study protocol was reviewed and approved by the University of North Carolina Internal Review Board (IRB #12-2019) in compliance with the guidelines on the treatment of human subjects; this study was registered at clinicaltrials.gov (NCT01750463).
2.1. Eligibility criteria and recruitment
Pediatric patients (aged 30 d–18 y), who required hospitalization in the PICU with Hb monitoring during the 3 month enrollment period, were eligible for the study. Patients were excluded if they did not have exposed fingers or toes because of congenital anomalies, wound dressings, or another injury. Also patients weighing less than 3 kg were excluded.
At the time of admission to the PICU, attending physicians, nurses, or advanced care providers determined whether patients met inclusion criteria. Parents or legal guardians of eligible patients were provided with an information sheet regarding the study. Consent forms and Health Insurance Portability and Accountability Act research authorization forms were obtained by a member of the research team. Demographic data were extracted from patient charts after discharge, including date of admission, date of discharge, body mass index (BMI), admission diagnosis, race, ethnicity, date and time of LabHb and SpHb, device number, perfusion index (PI—which is the “ratio of pulsatile blood flow to nonpulsatile or static blood flow” [10]) measured by the device, oxygen saturation (SpO2), mode of ventilation or oxygen support, body temperature, heart rate, mean arterial pressure, Richmond Agitation and Sedation Score, the use of vasopressors, and the LabHb result.
The extent of each subject’s participation was dependent on the frequency of Hb monitoring required for medical management, which was determined by the pediatric intensivist on-call. Patients requiring a Hb assessment on admission were enrolled in the study and continued through four blood draws or discharge from unit.
2.2. Protocol
Nurses or phlebotomy personnel drew specimens for laboratory processing as per provider orders and standard hospital protocol. Pediatric Respiratory Therapists (RTs), received training before initiation of the study, on the use and application of the Masimo probes. Each RTdemonstrated competence in the use of the device, and a designated RT manager was on-call to answer questions regarding the function and application of the device. The RT in the PICU was called to the bedside before the blood draw (venous or arterial samples were accepted). The RT obtained noninvasive measurement of Hb using the Masimo Pronto device to correlate SpHb values with LabHb results at the time of venipuncture. The specimens were sent to the hospital’s central laboratory for processing (Adviat 120/2120 hematology system CBC/Diff)per standard operating procedures. Hb, oxygen saturation, pulse rate, and PI values measured with the Masimo Pronto device were recorded at each time point for each subject. RTs conducted measurements per manufacturer protocol and received training and practice before initiation of the study.
2.3. Sensor site
For consistency, toes or fingers were used as sensor sites, usually the nondominant ring or index finger; avoiding limbs with recent injury, surgery, constrictive dressings, or any object that may impede normal blood flow. The selected site was cleaned of debris and dried. If present, nail polish was removed before testing. The study protocol included three attempts on one extremity; and if no reading was obtained on that extremity, an additional three attempts on another extremity was performed before reporting an undetectable reading.
2.4. Sensor selection
The RainbowR1 20L sensor was used for patients weighing between 3 kg and 10 kg. The RainbowDCIP SC-200 sensor was used in patients weighing between 10 kg and 50 kg, and the RainbowDCI SC-200 was used in patients weighing >50 kg.
2.5. Study analysis
Distributions of the key variables were evaluated using standard statistics (proportion, mean, median, range, and standard deviation). Reliability of the SpHb measurements in comparison with LabHb values were evaluated using modification of the Bland–Altman plot with multiple measurements per subject [11], as implemented in MedCalc version 14.8.1 (MedCalc software, Ostend, Belgium) and Pearson correlations. Predictors of the reliability (computed as the difference between LabHb and SpHb) and predictors of being unable to detect the SpHb reading were evaluated using general linear models. Associations between categorical variables were evaluated using chi-square or Fisher exact test. Analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC). P values <0.05 were considered statistically significant.
3. Results
3.1. Degree of agreement
Fifty patients, that met inclusion criteria, were admitted to the PICU during the 3-mo study period (Table 1). The mean age of the 50 included patients was 7.6-y-old, and SpHb was detected in 83 attempts (70.3%). Examination of paired readings showed a mean LabHb of 11.1 g/dL and a mean of 11.2 g/dL for SpHb. Bland–Altman analysis showed a bias of 0.07 g/dL and precision of ±2.59 g/dL with a range from −12.0 g/dL to 3.9 g/dL. The Pearson correlation estimate is 0.55, with a 95% confidence interval between 0.38 and 0.68 (Table 1, Figure). Logistic regression analysis identified demographic and clinical factors affecting agreement between the SpHb and LabHb values, including the extremes of the Hb spectrum (<8 g/dL or >15 g/dL), P < 0.001. Increased Massey skin color scale and increased BMI also significantly affected the agreement between the SpHb and LabHb, P < 0.05 (Table 2).
Table 1.
Summary characteristics of the study participants.
| Parameter | Value |
|---|---|
| Number of participants | 50 |
| SpHb readings | |
| N | 83 |
| Mean | 11.17 |
| Standard deviation | 3.08 |
| Range | 1.0–17.8 |
| Number of attempts | 118 |
| Number of being unable to detect reading, % | 35 (30) |
| All LabHb results | |
| N | 118 |
| Mean | 10.95 |
| Standard deviation | 2.03 |
| Range | 3.8–16.4 |
| LabHb results with matched SpHb readings* | |
| N | 83 |
| Mean | 11.1 |
| Standard deviation | 2.01 |
| Range | 3.8–16.4 |
| Difference (SpHb LabHb) | |
| Mean | 0.07 |
| Standard deviation | 2.59 |
| Range | −12.0 to 3.9 |
| Pearson correlation (SpHb versus LabHb) | |
| Estimate | 0.55 |
| 95% CI | 0.38–0.68 |
CI = confidence interval.
Only LabHb measurements with corresponding SpHb data.
Figure.
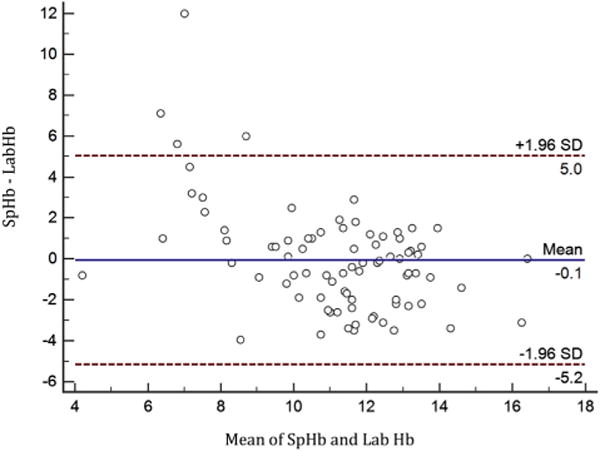
Modified Bland–Altman plot comparing (SpHb – LabHb) versus the mean SpHb and LabHb. (Color version of figure is available online.)
Table 2.
Predictors of increasing inaccuracy between Massimo Hb and LabHb value (results from a logistic regression model).
| Parameter | Regression coefficient | 95% Confidence limits | P value | |
|---|---|---|---|---|
| BMI | −0.11 | −0.29 | 0.07 | 0.226 |
| SpO2 | 0.23 | 0.00 | 0.46 | 0.053 |
| Massey skin score | −0.13 | −0.36 | 0.10 | 0.267 |
| Temperature | −1.07 | −1.90 | −0.24 | 0.012 |
The number of SpHb readings within 0–1, 1–2, and >2 g/dL of LabHb measurements (Table 3) and the difference between LabHb and SpHb at each measured LabHb value were examined (Table 4). We noted that approximately 65% of SpHb readings were within 0–2 g/dL of the measured LabHb value (Table 3). We also note the number of values obtained at each LabHb range of values (<8, 8–10, 10–12, and 12+ g/dL) and the percentage of SpHb readings within <2.0 g/dL of the LabHb value (Table 4).
Table 3.
The percentage of patients at each 1 g/dL interval difference between LabHb levels and SpHb readings.
| SpHb-LabHb (g/dL) | Number of pair readings | Percent | Cumulative frequency | Cumulative percent |
|---|---|---|---|---|
| 0–1 | 35 | 42.17 | 35 | 42.17 |
| 1–2 | 19 | 22.89 | 54 | 65.06 |
| >2 | 29 | 34.94 | 83 | 100 |
Table 4.
The number of readings within each LabHb range.
| Hb range (g/dL) | N | Mean bias | Standard deviation | Difference SpHb – LabHb (g/dL)
|
||
|---|---|---|---|---|---|---|
| ≤1, % | ≤1.5, % | ≤2.0, % | ||||
| <8 | 3 | 1.23 | 2.48 | 67 | 67 | 67 |
| 8–10 | 24 | −0.06 | 2.98 | 29 | 38 | 46 |
| 10–12 | 26 | 0.7 | 2.1 | 42 | 50 | 65 |
| 12+ | 30 | −0.47 | 2.64 | 50 | 73 | 80 |
Mean results for the Modified Bland–Altman plot are then included as the mean difference between the SpHb and LabHb as the mean bias. The absolute difference between the SpHb and LabHb is also included as the percentage of readings in each Hb range within ≤1, ≤1.5, and ≤2.0 g/dL.
3.2. Ability to obtain reading
The 35 attempted readings, which did not produce a result, were designated as “unable to obtain.” Unpaired t-test and Fisher exact test showed that patients whose SpHb was not detected were significantly more likely to have increased BMI (20.3 versus 16.8 kg/m2), decreased oxygen saturation (91.3 versus 97.9%), and decreased core temperature (36.6 versus 37.0 C), P < 0.05 (Table 5). PI, mechanical ventilation, vasopressor support, and Massey skin score were also examined using Fisher exact test, but differences were not significantly associated with ability to obtain readings (Table 5). In addition to clinical parameters, the patients were grouped by admitting diagnosis, including congenital heart disease, medical, trauma, orthopedic surgery, neurosurgery, and general pediatric surgery. There were no differences between the admission diagnoses, clinical provider, device, or probe used in the detectable and undetectable groups (data not shown). Decreased oxygen saturation and decreased body temperature were confirmed to be important risk factors for an inability to obtain a reading using logistic regression analysis (Table 6).
Table 5.
The effect of examined clinical parameters on the ability to detect SpHb levels and the effect of vasopressor support and mechanical ventilation on the ability to detect SpHb levels.
| Parameter | Able to detect SpHb reading?
|
P value | |
|---|---|---|---|
| Yes
|
No
|
||
| Mean (95% CI) | Mean (95% CI) | ||
| Age, y | 6.8 (5.5–8.1) | 7.7 (5.7–9.7) | 0.4462 |
| BMI, kg/m2 | 16.8 (15.8–17.8) | 20.3 (18.7–21.9) | 0.0005 |
| Massey | 3.2 (2.6–3.7) | 3.2 (2.3–4.1) | 0.9548 |
| LabHb, g/dL | 11.1 (10.7–11.5) | 10.6 (9.9–11.3) | 0.204 |
| SpO2, % | 97.9 (96.4–99.5) | 91.3 (88.8–93.8) | <0.0001 |
| Temperature, C | 37 (36.8–37.2) | 36.6 (36.4–36.9) | 0.0277 |
| Mean BP, mm Hg | 87.7 (65.7–109.6) | 75.4 (39.9–110.8) | 0.5585 |
| PI | 3.5 (0.0–7.0) | 6.1 (4.0–8.2) | 0.21 |
| Vasopressor support, % | 21 (25.3) | 10 (28.6) | 0.8193* |
| Mechanical ventilation, % | 42 (50.6) | 20 (57.1) | 0.5504* |
| Total number of attempts | 83 | 35 | |
CI = confidence interval.
Fisher exact test.
Table 6.
Predictors of the inability to detect SpHb reading (results from a logistic regression model).
| Parameter | Odds ratio (per unit increase) | Wald 95% confidence limits | Wald chi-square P value | |
|---|---|---|---|---|
| BMI | 1.24 | 1.03 | 1.49 | 0.021 |
| SpO2 | 0.83 | 0.70 | 0.97 | 0.018 |
| Massey skin score | 0.91 | 0.69 | 1.21 | 0.511 |
| Temperature | 0.57 | 0.20 | 1.63 | 0.295 |
4. Discussion
This study demonstrates the degree of agreement between the Masimo Pronto SpHb Monitor and measured LabHb readings in the PICU of a single institution over the study period is statistically consistent with the manufacturer’s claims of accuracy [12] (Table 1, Figure). However, we noted a broader standard deviation from the difference between mean LabHb and SpHb (decreased precision) than previously presented findings [9]. The precision of the device was not affected by several examined clinical and demographic factors. However, extreme LabHb values, increased skin pigmentation, and patient BMI significantly affected agreement between the SpHb and LabHb. Our data are consistent with previous studies done in pediatric patients [4–9]. However, contrary to previous studies, broad implementation of the device in this study resulted in a significant proportion of attempted readings for which SpHb was unable to be obtained. This study identified oxygen saturation and body temperature as risk factors for an unobtainable SpHb. Although we cannot rule out technical error as a cause of unobtainable readings, this study mimics implementation of the device in a PICU setting by trained clinical providers instead of a single dedicated member of a study team. It is our opinion that this study provides a more accurate reflection of the utility of the device in a clinical setting.
The Masimo Pronto user’s manual describes hemoglobinopathy and thalassemia as potential causes of inaccurate readings [12]. Although anemia and polycythemia are not specifically mentioned, the impact of extreme Hb values on sensor accuracy in our study was statistically significant. Our findings may be because of the small number of patients with extremely low Hb values within the study cohort, and may have improved with a larger sample size of anemic patients (Table 4). It should also be noted that of the three patients in our cohort with Hb levels <8 g/dL, 67% of the SpHb readings were within 1 g/dL of the LabHb (Table 4).
This study also identified increased skin pigmentation as a risk factor for inaccurate readings (Table 2). Another study examined the use SpHb in pediatric patients with sickle cell disease requiring outpatient Hb monitoring [4]. That study did not define the degree of skin pigmentation within their cohort. The authors noted increased bias and decreased precision when compared with previous studies because of data pairs with poor signal quality [4]. Although the cause of the poor signal quality was not identified, our results suggest that skin pigmentation of the patients in the Szmuk study may have impacted the accuracy of the readings. A Masimo Corporation funded study examined the effect of skin pigmentation using the Massey skin color scale and found that it did not affect the accuracy of the device. That study included 20 patients (12.8%) classified as having medium skin pigmentation (Massey score 4–7) and 0 patients with dark pigmentation (Massey score 8–10) [13]. A study done in rural India showed adequate agreement between SpHb and LabHb in 40 adult patients, but did not examine the effect of increasing pigmentation on study results and included no critically ill or pediatric patients [14]. Our study is unique in standardization and examination of skin pigmentation in critically ill pediatric patients and shows the effect of increased skin pigmentation on the interpretation of SpHb readings. Finally, the Masimo Pronto user’s manual states that patient exposure to dye within the serum or colored alterations on the examined digit (i.e., nail polish) may lead to inaccurate readings [12]. It appears reasonable that readings could also be affected by intrinsic differences in patient skin pigmentation.
Increasing BMI affected the degree of agreement between LabHb and SpHb (Table 2). Dewhirst et al. [5] performed a subset analysis of SpHb readings, which varied from the LabHb by <1 g/dL or >2 g/dL, and noted that the mean weight for patients in each group was similar (38.9 kg versus 36.6 kg). However, no adjustment was made for patient height [5]. Another study examining the use of the device in a PICU setting did not examine the effect of BMI on the accuracy of their results [9]. We believe that BMI is a more accurate reflection of the influence of the habitus of the examined digit because it is not influenced by patient age and height. Although BMI did not reach statistical significance by logistic or linear regression analysis (Tables 2 and 6), this may be secondary to the number of patients in our study with increased BMI, and further studies focusing on children with increased BMI are needed to clarify this point. BMI analysis is unique to our study and should inform clinical interpretation of the accuracy of SpHb.
A comparative study, performed in the PICU of another institution, showed increased bias (0.07 g/dL versus 0.66 g/dL) but more narrow precision (2.59 g/dL versus 1.4 g/dL) than the present study [9]. These differences may be related to our smaller sample size, as their study included 284 samples drawn from 80 sedated patients, whereas the present study included 84 paired samples from 50 patients. The results of the study, performed in another PICU setting, are currently only available in an abstract form and no subset analysis is described. The effect of patient sedation used in their study is also unknown, as sedation can alter patient movement, hemodynamic function, or peripheral perfusion. The use of sedatives is also likely associated with increasing severity of illness in their study cohort, all of which may have affected their study precision. We did not find that patient agitation scores affected accuracy or the ability to obtain SpHb reading. However, the Masimo Pronto owner’s manual reports that patient movement, excessive ambient light, and electrical interference from nearby equipment can potentially affect the device’s accuracy [12]. These conditions are standardized, where possible, throughout the PICU at our institution, and none were associated with decreased accuracy of, or ability to obtain, SpHb readings. Our PICU is similar to other PICUs within the United States in the ambient conditions where the SpHb device would be used. Additionally, unique to this study, we were able to analyze demographic and clinical factors affecting the accuracy of the device.
A substantial portion (30%) of patient readings were reported as “unable to detect” (Table 1), despite multiple attempts on multiple digits. Analysis in the “unable to detect” group further identified increased BMI, hypoxia, and hypothermia as potential risk factors for undetectable Hb levels (Table 5). The undetectable readings were also not associated with a specific Masimo device, probe, clinical provider/user, or known improper use of the device. There were 18 patients in whom SpHb was “unable to detect” during at least one attempted reading. In nine of those patients, SpHb readings were obtained at one or more other time points during their admission, raising the previously stated concerns of interference from ambient conditions or technical error. Others have reported the inability to detect readings in 2.6%–12.3% of patients [13,15]. These studies used similar spot check techniques with either the Masimo Pronto or the Masimo Pronto-7 device [13,15]. Although there is no obvious explanation for our high rate of undetectable readings, the rate at which Hb could not be detected should be considered during widespread implementation of the device.
The confirmation of agreement in the PICU setting demonstrates clinical value of the device; however, its exact role remains unclear. A recent review of the accumulated data from previous Masimo Pronto studies, performed almost exclusively in adults, reports that the device does not provide sufficient precision to be used as the sole clinical indicator for transfusion [16]. This is consistent with the manufacturer’s recommendation [12]. In studies examining the use of the device in surgical patients, the device may be used to monitor trends in Hb levels and guide clinical decision-making regarding timing of LabHb analysis [17]. The use of the device may decrease unnecessary medical testing and may spare pediatric patients the trauma of venipuncture laboratory draws. However, the current cost for laboratory supplies and overhead for a complete blood count (CBC) at our institution is $5.86, meaning that the device would need to eliminate several LabHb tests to be cost-effective. Previous studies demonstrate that the cost of intraoperative transfusion is between $522 and $1183 per unit transfused, and implementation of the device significantly decreased the rate of blood transfusion during orthopedic surgical procedures in adults [18], offsetting the cost of the device. A similar prospective study should be performed to demonstrate the cost-effectiveness of the device in a PICU setting.
5. Conclusions
In conclusion, the Masimo device provides adequate agreement between SpHb and LabHb for the trending of Hb levels in the PICU setting, with 95% of patient SpHb readings within 2.59 g/dL of the corresponding LabHb. However, the device does not provide sufficient accuracy to be used as the sole basis for transfusion requirements. The strength of our study is that it was performed in a normal clinical setting, by on duty providers and examined several clinical factors that influence the degree of agreement between SpHb and LabHb. Identification of the clinical scenarios in which the monitor can be effectively used in the critically ill pediatric patients remains unclear. Future studies are needed to identify the number of LabHb levels that can be replaced by this technology, as well as a cost-benefit analysis to see what impact the device would have on transfusion requirements in the PICU setting. We highlight the importance of using the information supplied by the device in context with other laboratory and clinical factors when evaluating patients with altered Hb levels as instructed by the manufacturer [12].
Acknowledgments
The University of North Carolina School of Medicine provided all funding for this research.
Footnotes
Authors’ contributions: M.R.P., S.E.M., B.L.J., and A.G.C. contributed to all aspects of the project, including conception and data collection. M.R.P., S.E.M., B.L.J., A.G.C., and A.V.B. did the article drafting and revisions. A.L.K., A.M., K.A.S., and B.A.C. contributed to the data gathering. M.R.P., S.E.M., B.L.J., A.G.C., A.L.K., A.M., K.A.S., A.V.B., and B.A.C. contributed to the statistical analysis and the finalization of the article.
Disclosure
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Sztefko K, Beba J, Mamica K, Tomasik P. Blood loss from laboratory diagnostic tests in children. Clin Chem Lab Med. 2013;51:1623. doi: 10.1515/cclm-2012-0672. [DOI] [PubMed] [Google Scholar]
- 2.Slifer KJ, Babbitt RL, Cataldo MD. Simulation and counterconditioning as adjuncts to pharmacotherapy for invasive pediatric procedures. J Dev Behav Pediatr. 1995;16:133. [PubMed] [Google Scholar]
- 3.Slifer KJ, Hankinson JC, Zettler MA, et al. Distraction, exposure therapy, counterconditioning, and topical anesthetic for acute pain management during needle sticks in children with intellectual and developmental disabilities. Clin Pediatr (Phila) 2011;50:688. doi: 10.1177/0009922811398959. [DOI] [PubMed] [Google Scholar]
- 4.Szmuk P, Pickering RB, Farris L, Rogers ZR, Buchanan GR. Evaluation of noninvasive and continuous hemoglobin monitoring in children with sickle cell disease. The Proceedings of the Annual Meeting of the American Society of Anesthesiologists. 2011 [Google Scholar]
- 5.Dewhirst E, Naguib A, Winch P, et al. Accuracy of noninvasive and continuous hemoglobin measurement by pulse co-oximetry during preoperative phlebotomy. J Intensive Care Med. 2013;29:238. doi: 10.1177/0885066613485355. [DOI] [PubMed] [Google Scholar]
- 6.Gill H, Navaratnarajah J, Naik M, Tallach R, Fernandez E, Sury M. Assessment of a noninvasive hemoglobin monitor (Masimo SpHb) in infants and small children undergoing craniofacial surgery. Paediatr Anaesth. 2012;22:916. [Google Scholar]
- 7.Agrawal A, Sullivan JN, Zink MA, Jones BM. Evaluation of the Masimo((R)) Rainbow SET Radical-7 in a 6-month-old pediatric multivisceral organ transplant. J Anesth. 2012;26:629. doi: 10.1007/s00540-012-1383-9. [DOI] [PubMed] [Google Scholar]
- 8.Jou F, Kurth C, Beckman E, Istaphanous GK. Anesth Analg. Honolulu, HI: 2010. Absolute and trend accuracy of continuous and noninvasive hemoglobin in pediatric surgery patients. [Google Scholar]
- 9.Garcia-Soler P, Camacho-Alonso JM, Milano-Manso G. Noninvasive monitoring of hemoglobin concentration in pediatric critical patients; 24th Annual Meeting of the European Society of Paediatric and Neonatal Intensive Care; Rotterdam, Netherlands. 2013. [Google Scholar]
- 10.Clinical applications of perfusion index. Irvine, CA: Masimo Corporation; 2007. p. 1. [Google Scholar]
- 11.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 12.Pronto Operator’s manual. USA: Masimo Corporation; 2011. p. 52. [Google Scholar]
- 13.Raikhel M. Accuracy of noninvasive and invasive point-of-care total blood hemoglobin measurement in an outpatient setting. Postgrad Med. 2012;124:250. doi: 10.3810/pgm.2012.07.2584. [DOI] [PubMed] [Google Scholar]
- 14.Shah N, Shah K. Evaluation of a pulse CO-Oximeter for noninvasive hemoglobin measurement in adult population in rural India; Annual Meeting of the American Society of Anesthesiologists; Chicago, IL. 2011. [Google Scholar]
- 15.Joseph B, Aziz H, Pandit V, et al. Evaluation of the noninvasive spot check for hemoglobin in the trauma intensive care unit. Crit Care Med. 2012;40:231. [Google Scholar]
- 16.Rice MJ, Gravenstein N, Morey TE. Noninvasive hemoglobin monitoring: how accurate is enough? Anesth Analg. 2013;117:902. doi: 10.1213/ANE.0b013e31829483fb. [DOI] [PubMed] [Google Scholar]
- 17.Park YH, Lee JH, Song HG, et al. The accuracy of noninvasive hemoglobin monitoring using the radical-7 pulse CO-Oximeter in children undergoing neurosurgery. Anesth Analg. 2012;115:1302. doi: 10.1213/ANE.0b013e31826b7e38. [DOI] [PubMed] [Google Scholar]
- 18.Ehrenfeld JM, Henneman JP, Sandberg WS. Impact of continuous and noninvasive hemoglobin monitoring on intraoperative blood transfusions. Am Soc Anesthesiologists. 2010;2010:LB05. [Google Scholar]


