Abstract
Natural killer (NK) cells are important effectors of innate immunity playing a key role in the eradication and clearance of viral infections. Over the recent years, several studies have shown that HIV-1 pathologically changes NK cell homeostasis and hampers their antiviral effector functions. Moreover, high levels of chronic HIV-1 viremia markedly impair those NK cell regulatory features that normally regulate the cross-talks between innate and adaptive immune responses. These pathogenic events take place early in the infection and are associated with a pathologic redistribution of NK cell subsets that includes the expansion of anergic CD56neg NK cells with an aberrant repertoire of activating and inhibitory receptors. Nevertheless, the presence of specific haplotypes for NK cell receptors as well as the engagement of NK cell antibody dependent cell cytotocity (ADCC) have been reported to control HIV-1 infection. This dichotomy can be extremely useful to both predict the clinical outcome of the infection and to develop alternative anti-viral pharmacological approaches. Indeed, the administration of antiretroviral therapy (ART) in HIV-1 infected patients restores NK cell phenotype and functions to normal levels. Thus, ART can help to develop NK cell-directed therapeutic strategies that include the use of broadly neutralizing antibodies and toll like receptor agonists. The present review discusses how our current knowledge of NK cell pathophysiology in HIV-1 infection is being translated both in experimental and clinical trials aimed at controlling the infection and disease.
Keywords: HIV-1, NK cell, pathogenesis, anti-HIV-1 therapy
Introduction
Natural Killer (NK) cells are innate lymphoid cells that provide an extended surveillance against tumor-transformed or viral-infected cells in the absence of antigen specificity [1–4]. These cells are endowed with immune-modulatory functions that regulate and link innate and adaptive immune responses via the secretion of chemokines/cytokines and by undertaking synergic cross-talks affecting the maturation and function of antigen-presenting cells (APCs) [5–7]. Under homeostatic conditions, NK cells account for up to 5–15% of all circulating lymphocytes and are divided into two distinct populations on the basis of the surface expression of CD56 and CD16 [8]. The CD56bright/CD16neg (termed CD56bright in this review) NK cell subset represent approximately 5–10 % of the whole population and mainly exerts important regulatory functions [i.e. production of soluble mediators such as interferon (IFN)-γ, tumor necrosis factor (TNF)-α and establishment of cellular interplays]. Conversely, CD56dim/CD16neg (CD56dim) NK cells (up to 90%) are primarily cytotoxic effectors eradicating tumor-transformed and viral-infected cell targets, but can also produce IFN-γ following activation [8, 9]. Moreover, unique subsets of human NK cells have been described in peripheral tissues where inflammation occurs and where the early innate immune responses pave the way for the subsequent priming of adaptive immunity. The tissue-specific human NK cell populations often carry phenotypic hallmarks that distinguish them from their circulating counterparts. These NK cells are present under homeostatic conditions in both secondary lymphoid organs [10, 11] and non-lymphoid organs such as healthy skin, gut, liver, lungs, and uterus [12, 13].
The effector functions of NK cells are controlled by a large family of NK cell receptors (NKRs), whose engagement is finely tuned by a dynamic balance between inhibitory and activating signals [14, 15]. Indeed, autologous cells are normally spared from NK cell killing via the engagement of inhibitory NKRs (iNKRs) [i.e. inhibitory Killer Ig-like receptors (iKIRs) and the C-type lectin receptors such as NKG2A] that recognize alleles of the major histocompatibility complex class I (MHC-I) (Table 1). The lack (i.e. allogeneic conditions or tumor transformation) or down-modulation (i.e. viral infections) of MHC-I expression lead to NK cell killing of cellular targets via the engagement of another family of activating NKRs (aNKRs) [i.e. Natural Cytotoxicity Receptors (NCRs) NKp30, NKp46 and NKp44, activating KIRs (aKIRs) and C-type lectin receptors such as NKG2D and NKG2C]. The latter bind to their putative ligands on stressed, viral-infected or cancer cells [16, 17]. This phenomenon is well known as the “missing self hypothesis” and explains the ability of NK cells to perform an optimal immune surveillance that malignant or virally transformed targets while sparing healthy cells [18, 19].
Table 1.
| Receptor | Function | Impact of HIV-Viremia | Impact of Antiretroviral Therapy | References |
|---|---|---|---|---|
| CD16 (FcγRIII) | ADCC | No change | No Change | 8, 21, 22, 23, 24, 25, 26, 27, 28 |
| CD56 | Adhesion Molecules | Decrease | Restoration to normal levels | 8, 21, 22, 23, 24, 25, 26, 27, 28 |
| KIR2DL1 (CD158a) | Inhibitory | No change/Increase | Restoration to normal levels | 6, 14, 15,18, 22, 25, 31, 32, 33, 35, 107 |
| KIR2DL2 (CD158b) | Inhibitory | No change/Increase | Restoration to normal levels | 6, 14, 15,18, 22, 25, 31, 32, 33, 36, 107 |
| KIR3DL1 (CD158e1) | Inhibitory | No change/Increase | Restoration to normal levels | 6, 14, 15,18, 22, 25, 33, 82, 107 |
| KIR3DL2 (CD158k) | Inhibitory | No change | No change | 6, 14,15,18, 22, 25 |
| KIR2DL4 (CD158d) | Inhibitory | Unkown | Unkown | 6, 14,15,18, 37 |
| LIR1/ILT2 (CD85j) | Inhibitory | Increase | Restoration to normal levels | 6, 14, 15, 18, 22, 25 |
| Siglec-7 | Inhibitory | Decrease | Restoration to normal levels | 40, 41 |
| KIR2DS1 | Activating | Unkown | Unkown | 6, 14,15,18, 76, 80 |
| KIR2DS2 | Activating | Unkown | Unkown | 6, 14,15,18. |
| KIR3DS1 | Activating | Increase | Unkown | 6, 14,15,18, 33, 50, 68,71. |
| CD94 (KLRD1) | heterodimer coupled with NKG2 molecules | No change | No change | 22, 25, 93. |
| NKG2A (CD159a) | Inhibitory | Decrease | Restoration to normal levels | 6, 14, 15, 16, 18, 22, 25. |
| NKG2C (CD159c) | Activating | Increase (co-infection with HCMV) | No change | 6, 14, 15, 16, 18, 89 |
| NKG2D | Activating | No change | No change | 6, 14, 15, 16, 17, 18, 22, 25, 54, 146 |
| NKp46 (NCR1) | Activating (NCR) | Decrease | Restoration to normal levels | 6, 14, 15, 16, 17, 18, 22, 25, 34, 54, 74 |
| NKp30 (NCR2) | Activating (NCR) | Decrease | Restoration to normal levels | 6, 14, 15, 16, 17, 18, 22, 25, 34 |
| NKp44 (NCR3) | Activating (NCR) | Decrease | Restoration to normal levels | 6, 14, 15, 16, 17, 18, 22, 25, 34 |
| NKp80 | Activating (co-receptor) | No change | No change | 6, 14, 15, 16, 17, 18, 22, 25 |
| NTB-A | Activating (co-receptor) | No change | No change | 6, 14, 15, 16, 17, 18, 22, 25, 60, 61 |
| 2B4 (CD244) | Activating (co-receptor) | No change | No change | 6, 14, 15, 16, 17, 18, 22, 25, 31 |
| NKR-P1A (CD161) | Not classified | No change/Decrease | No change/Unkown | 14, 29, 62, 107 |
The dynamic balance between iNKRs and aNKRs regulate both NK cell effector functions that can employ different mechanisms for clearance of cellular targets: (i) exocytosis of cytotoxic granules containing perforin and granzymes that leads to cell lysis; (ii) signaling through TNF family death receptors such as FasL or TRAIL; (iii) production and secretion of pro-inflammatory cytokines with strong anti-viral and anti-tumor activities; and (iv) antibody-dependent cellular cytotoxicity (ADCC) occurring when the Fc portion of antibodies opsonizing target cells binds to the Fcγ receptor III on NK cells (FcγRIII or CD16). This binding then activates a downstream signal pathways ending with cytokine release, degranulation and cytotoxicity.
This review discusses the impact of HIV-1 replication on NK cell homeostasis and function as well as the clinical and therapeutic insights that these innate effector cells can exert on the natural history of HIV-associated disease.
NK cells and HIV-1 pathogenesis
Effects of HIV-1 viremia on NK cell homeostasis and phenotype
Frequencies, phenotypes and functions of NK cells are highly affected by HIV-1 viremia and undergo pathologic changes as disease progresses toward its chronic phases (Figure 1). Indeed, the absolute number of circulating NK cells first increases in the acute phase of HIV-1 infection due to the expansion of the CD56dim NK subset and the decreased frequency of the CD56bright NK cell population [20]. While the overall frequency of circulating NK cells appears to be restored as soon as HIV-1 infection enters in its chronic stage, the distribution of their different subsets undergo a pathological redistribution that deeply affects the overall NK cell anti-viral activity [21]. In particular, ongoing viral replication induces the expansion of a dysfunctional CD56neg/CD16pos (CD56neg) NK cell subset that counteracts the decrease of the cytotoxic CD56dim cells. Under homeostatic condition and in the absence of other viral infections, this population of CD56neg NK cells is either not detectable or present at very low frequency [21–29]. The distribution and activation of NK cell subsets is relevant also in the context of mother-to-child HIV-1 transmission as they influence the susceptibility to perinatal infection [30].
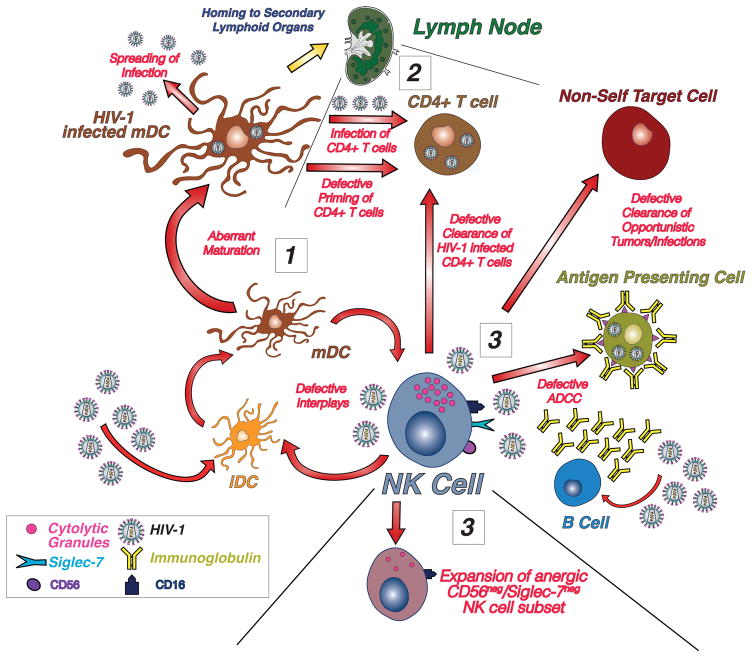
Figure 1. Impact of HIV-1 on NK cell homeostasis and functions.
Since from the early stages of HIV-1 infection when tissue-resident antigen presenting cells (APCs) such as immature Dendritic Cells (iDCs) uptake the virus, the defective crosstalk between NK cells and DCs lead to an aberrant maturation and to the infection of these APCs (1). Once migrated to secondary lymphoid organs, these aberrant mature DCs (mDCs) contribute to infect autologous CD4+ T cells instead of ensuring an optimal activation and priming of adaptive immune responses (2). High levels of HIV-1 replication also markedly affects NK cell phenotype and function by inducing the expansion of an anergic subset that lack bot CD56 and Siglec-7 and that is highly impaired in killing autologous and viral infected CD4+ T cells, in the clearance of opportunistic infections and tumors and in performing antibody dependent cell cytotoxicity (ADCC) (3). This aberrant and highly pathogenic vicious loop is completely reversible following the administration of antiretroviral therapy that can restore NK cell homeostasis and anti-viral functions, thus making them an ideal cellular tool to follow-up disease progression and treatment efficacy as well as to develop alternative curative strategies to clear the infection.
The down-modulation of CD56 is not the only NK surface marker altered by HIV-1 viremia. The expressions as well as the functional relevance of several aNKRs and iNKRs are pathologically modified by high levels of viral replication [22, 25, 31–38]. CD56neg NK cells expanded in HIV-1 infected patients express high levels of iNKRs and low levels of aNKRs, a condition that leads to a defective control of viral replication and to disease progression (Table 1) [4, 39]. The HIV-1-induced changes in the NK cell expression of CD56 and NKRs require a chronic exposure to the virus [25]. The only exception to this rule is represented by Siglec-7, a iNKRs constitutively expressed on the majority of NK cells. It is highly sensitive to HIV-1 viremia in the earliest phases of the infection and is down-modulated on the surface of NK cells in HIV-1 infected patients before CD56 [40–42]. This phenomenon makes it possible to identify two distinct subsets characterizing different stages of the disease: the Siglec-7neg/CD56dim NK cells only present in the early stages of HIV-1 infection and the Siglec-7neg/CD56neg NK cells that become detectable several months later in the chronic state of the disease.
All these HIV-1-induced phenotypic changes are reversible following the administration of a successful antiretroviral therapy (ART) under which the restoration of Siglec-7 expression on NK cells is much faster compared to that of CD56 and the NKRs. The impact of viral replication in inducing these phenotypic abnormalities has also been confirmed by experimental evidence. NK cells from those HIV-1 infected patients with a low/undetectable levels of viral replication that do not progress to AIDS (i.e. long-term non progressors or LTNP) are similar and undistinguishable from the ones from uninfected healthy individuals [40]. These NK cell features in response to viral exposure make it possible to easily monitor the clinical outcome and progression of the infection. Furthermore, they can also be used to assess the adherence and the efficacy of ART (Table 1).
Human cytomegalovirus (HCMV) is a major cause of co-morbidity in patients with HIV-1 infection and is associated with the expansion of NK cell subset expressing NKG2C [4, 43]. This phenotypic feature, together with the HIV-1-mediated down-modulation of NKG2A, pathologically reverses the ratio of NKG2A/NKG2C on NK cells in HIV-1 and co-infected with HCMV-infected patients [44]. Interestingly, HCMV infection is also associated with an epigenetic diversification of NK cells, thus demonstrating that these innate lymphocytes can be reprogrammed in response to this pathogen [45, 46]. An additional NK cell subset lacking the aNKR FcRy is expanded in chronically HIV-1 infected individuals. Similar to what it has been observed with HCMV-induced NK cells, this latter population expresses low levels of NKp30 and is endowed with remarkably high ADCC activity [47]. Taken together, these data indicate that NK cells from HIV and HCMV co-infected patients have unique immunologic features that can be potentially targeted for developing anti-viral therapeutic approaches.
Effects of HIV-1 viremia on NK cell effector-functions
The long exposure to HIV-1 viremia and the prolonged cellular activation represent the major factors likely driving the expansion of then “exhausted” and “functional compromised” CD56neg NK cell subset. However, a direct correlation between NK cell activation and disease progression remains controversial and depends on the patient’s disease state [48–51]. Different activation markers have been tested to monitor NK cells in HIV-1 infected patients and one study reported no correlation between NK cell activation and disease progression [49]. Yet, other groups have shown that levels of expression of CD38 and HLA-DR on CD56dim NK cells are associated with progression to AIDS, high plasma viral load and low CD4+ T cell count [50, 52]. Moreover, CD38 and HLA-DR have been reported not to be increased in expression on NK cells from HIV-1 elite controllers (ECs), a rare group of healthy individuals who are able to naturally control viremia as well as to maintain low levels of innate immune activation similar to those of uninfected individuals [50].
Chronic HIV-1 infection also causes a defect in NKG2D-mediated NK cell killing due to the reduced expression on HIV-1 infected CD4+ T cells of the NKG2D-ligand MIC-A. Instead, it is present at high level in the plasma of HIV-1 infected individuals [25, 53]. This finding explains, at least in part, the poor NK cell-mediated clearance of HIV-1 endogenously infected CD4+ T cells [39] and transformed cell targets [21]. Such aberrancies limit NK cell immune surveillance and allow the development of opportunistic infections and cancers. Another aNKR involved in the lysis of HIV-1-infected autologous CD4+ T cells is the NCR NKp44 [54, 55]. Indeed, the surface expression of NKp44 ligand on HIV-1 infected CD4+ T cells is induced by the viral protein gp41 and could theoretically induce NKp44-mediated killing. Hence, as suggested by some studies, this mechanism could be involved in the CD4+ T cell depletion in HIV-1 infection and in the impaired immunologic recovery in ART treated patients [55–57]. However, this possibility does not represent the main pathogenic event associated with the drastic reduction of CD4+ T cell count in advanced HIV-1 infection: the expression levels of NKp44 on NK cells from viremic HIV-1 infected patients is very low, if not undetectable, even after in vitro stimulation [22, 25].
Pathologic CD56neg NK cells are also defective in the production and secretion of important immune regulatory cytokines such as IFN-γ, TNF-α and Granulocyte-macrophage colony-stimulating factor (GM-CSF) [20, 25]. These latter NK cell dysfunctions have a strong negative impact on their interplay with autologous DCs. In fact, the expansion of CD56neg NK cells in chronic HIV-1 infection is associated with: i) a reduced ability of NK cells to induce an optimal maturation of autologous DCs; ii) an impaired NK cell-mediated clearance of HIV-1 infected and immature DCs (iDCs); iii) the lack of T cell priming against HIV-1; and iv) the infection of CD4+ T cells through a mechanism associated with cellular interactions with HIV-1 infected and aberrant mature DCs (mDCs) [58, 59]. In turn, dysfunctional and HIV-1 infected mDCs fail to secrete adequate amounts of important regulatory factors such as IFN-α and interleukin (IL)-15. The lack of these important cytokines limits the priming of NK cells that then fail to kill HIV-1-infected CD4+ T cells through NKp46- and NKG2D-mediated signaling [60, 61]. However, it is not clear if these phenotypic and functional abnormalities of NK cells are due to the direct effect of HIV-1 on NK cells or are rather associated with the establishment of chronic inflammation affecting the homeostasis of the immune system. In this regard, NK cells express HIV-1 receptor and co-receptors such as CD4, CXCR4/CCR5 and Siglec-7 [42, 62–64], thus implying that a direct interaction between NK cells and HIV-1 occurs. However, controversial results were obtained regarding the susceptibility of NK cells to be targeted by HIV-1 since the existence of both viral latency and productive HIV-1 infection of human NK cells has never been demonstrated ex-vivo [22] but only in-vitro [62, 63].
Another strategy employed by HIV-1 to escape NK cell response is the Nef- and Vpu-induced down-modulation of poliovirus receptor (PVR or CD155) on infected CD4pos cells. PVR is the cognate ligand of the DNAM-1 (CD226), an aNKR constitutively expressed on all NK cells and whose engagement to activate NK cell killing is impaired by the HIV-1 induced decreased binding with CD155 [65]. Vpu accessory protein can also down-modulate NTB-A co-activation receptor ligands, thus further contributing to hamper NK-cell-mediated clearance of HIV-1 infected targets [66, 67]. Finally, the expansion of highly defective CD56neg NK cell has been also associated with the decreased expression of CD161, a aNKR receptor inducing proliferation and differentiation of NK cells [68].
NK cells also actively participate in the control of viral replication by releasing β-chemokines. In particular, they are an important source of the chemokines CCL3, CCL4 and CCL5 that represent the ligands for the co-receptor CCR5. Hence, the NK cell production of these β-chemokines could inhibit the entry of HIV-1 in the target cells by preventing the binding of CCR5 with viral envelope [21]. This effector function is highly impaired in active and chronic HIV-1 infection as NK cells from these viremic patients secrete low amount of these β-chemokines [69].
An additional mechanism by which NK cells eliminate virus-infected cell targets is antibody (ab)-dependent cell cytotoxicity (ADCC) [70, 71]. High levels of anti-HIV-1 Abs inducing ADCC are associated with slower disease progression [72–74] and with the control of HIV-1 infection in ECs [75]. Nevertheless, the role of NK cell-mediated ADCC in the pathogenesis of HIV-1 remains controversial. A few studies have shown that NK cells in HIV-1-infected patients remain capable of mediating ADCC [76, 77], an activity that have been also reported to be specifically directed against Env, Pol, Vpu and Tat proteins [78, 79]. This NK cell recognition of HIV-1 via ADCC might also lead to viral escape in the presence of particular epitopes associated with protein variants [80]. Other reports demonstrated that “exhausted” NK cells in chronic HIV-1 infection express lower levels of CD16 together with an impaired downstream signal pathway of this FcγRIII[49, 81]. This down-regulation of CD16 occurs through a mechanism mediated by matrix metallo-proteinases (MMPs) in an anti-HIV-1 antibody-dependent manner [82, 83]. Notably, studies performed in ECs and LTNPs have shown a degree of NK cell-mediated ADCC similar to that of healthy individuals [56, 84].
NK cell-mediated control of HIV-1 infection
NK cell tolerance toward autologous cells expressing “self” MHC-I molecules is regulated by a mechanism known as NK cell “licensing”. This process induces a proper terminal differentiation of NK cells from their precursor and requires the correct binding between iNKRs with specific self-HLA alleles representing their cognate ligands. The absence of these interactions affects the hypo-responsive state of NK cells and lead to the onset of auto-reactive clones [85–87]
Epidemiological studies showed that distinct genetic associations between KIRs expressed on NK cells and their specific HLA haplotypes on target cells influence the clinical outcomes of HIV-1 infection. More specifically, the presence of KIR3DS1 combined with HLA-Bw4-I80 allele in patients with chronic HIV-1 infection has a protective effect and is associated with lower viral load, slower decline of CD4+ T cell count and delayed progression to AIDS [61, 88–90]. This epidemiological evidence has been also confirmed in vitro with experiments showing that KIR3DS1pos NK cells strongly inhibit HIV-1 replication in target cells expressing the HLA-Bw4-I80 allele [91]. This KIR3DS1 recognition of HLA-Bw4 is peptide-dependent, thus further demonstrating that changes in the peptide repertoire associated with viral infection provide a trigger for the engagement of this activating KIR and for the subsequent NK cell activation [92]. Also, the KIR3DL1*h allotype, the inhibitory counterpart of KIR3DS1 characterized by an increased expression of this KIR, is associated with a reduced risk of HIV-1 infection in individuals expressing the HLA-B*57 allele [93]. However, other studies suggest that differences in the KIR/HLA combination may play a dual role by being either a positive or a negative prognostic factor in the clinical outcome of HIV-1 infection [94–97]. In any case, the binding of KIRs with their cognate ligands certainly impact the natural history of HIV-1 disease as confirmed by the natural resistance to infection of those HIV-1 exposed but seronegative sex workers showing KIR2DL2/KIR2DL3 heterozygosity in the absence of HLA-C1 and KIR3DL1 homozygosity in the absence of HLA-Bw4 [98].
MHC-I complex represents a natural target for HIV-1 proteins such as Nef, Vpu and Tat that induce the down-regulation of HLA-I molecules to avoid immune-surveillance [99–101]. On the other side, this mechanism of viral immune evasion might enhance the ability of NK cells to eliminate HIV-1 infected cells. In particular, the HIV-1-induced down-modulation of HLA-C, a MHC-I locus extensively interacting with the several inhibitory KIRs expressed by NK cells [102], can increase NK cell-mediated clearance of viral infected targets.
“Memory-like” NK cells in HIV-1 infection
A recent major advance in the field has been the identification of NK cells with adaptive immune traits. These long-living NK cells with specific “memory-like” features were first described in mice responding to a variety of antigens [103]. Only recently, a human CD57pos/KIRspos/NKG2Cpos “memory-like” NK cell subset showing increased effector functions when re-encountering viral antigens or following a proper activation with pro-inflammatory cytokines has been identified [44, 104–108]. The existence of antigen-specific NK cells has been recently reported also in rhesus macaques infected with simian or the simian/ human immunodeficiency virus (SIV/SHIV). These “memory-like” NK cells showed an antigen specificity towards Gag- and Env- viral proteins loaded on autologus DCs in an NKG2-dependent manner [109]. Notably, the recall of these adaptive traits of NK cells was associated with a high degree of cytotoxicity in animals vaccinated 5 years earlier with Ad26 (adenovirus 26 vectored SIV vaccine). Thus, this phenomenon likely lasts for quite a long time. Although very promising, this observation lacks direct evidence of NK cell memory responses in humans and, therefore, still remains an important and challenging aspect of NK cell biology in the context of viral infectioms.
NK cells and antiretroviral therapy
The main clinical milestone in the medical management of HIV-1 infection has been the introduction of therapeutic approaches using simultaneously different pharmaceutical compounds that dramatically suppress viral replication and reduce the plasma HIV-1 viral load to undetectable levels. Currently, even with salvage therapy, up to 90% of HIV-1-infected adults treated with these therapies become “aviremic”, meaning that their viral RNA plasma levels is below the limits of detection with the most sensitive clinical assays (<50 RNA copies/mL) [110]. To date, the armamentarium available to physicians for the treatment of HIV-1 includes six different type of antiviral drugs: (1) nucleoside-analog reverse transcriptase inhibitors (NNRTIs), (2) non–nucleoside reverse transcriptase inhibitors (NNRTIs), (3) integrase inhibitors, (4) protease inhibitors (PIs), (5) fusion inhibitors, and (6) co-receptor antagonists [111]. Although requiring a patients’ strong adherence to a life-long treatment with at least 3 of the above-mentioned anti-viral drugs. This anti-retroviral therapy (ART) is able to suppress viral replication for decades. It is also able to bring about the reconstitution of the immune system as measured by the increase in circulating CD4+ T lymphocytes [112, 113].
The control of HIV-1 viremia by ART also leads to recovery of NK cells with cytotoxicity against tumor cell targets (Table 1) [21, 40, 114]. These studies have shown that both ART and the in vivo administration of IL-2 partially restore NK cell distribution and function [23, 25, 40, 115, 116]. The positive effect of this therapy is also beneficial for DCs that acquire again the ability to undergo a proper maturation and to secrete those cytokines (i.e. IL-12, -15 and -18) required to prime NK cells and their production of IFN-γ [58]. In this regard, it has been reported that the “recontitutio ad integrum” of both NK cell phenotype and effector functions in HIV-1 infected patients requires not only viral suppression, but also the presence of properly functioning plasmacytoid DCs [117]. In addition, stimulation in vitro with IL-15 is able to rescue NK cells from apoptosis, enhanced proliferation and functional activity in HIV-1 patients not on ART [118, 119].
ART also targets the defective NK cell-mediated ADCC in HIV-1 infection. In fact, although the ability of plasma anti-HIV-1 Abs to mediate ADCC decreases under ART, probably due to the lack of antigen stimulation, NK cell-mediated ADCC improves soon after the administration of the therapy. Indeed, the earlier ART is administered to HIV-1-infected patients, the more pronounced is the recovery of NK cell-mediated ADCC. This immune response reaches its highest peak if the antiviral treatment starts prior to sero-conversion and/or when the CD4+ T cell counts is above 350 cells/μl [120–122]. Importantly, the exposure of NK cells to IL-10 has been shown to reproduce an aberrant repertoire of NKRs that is similar to that observed during the course of HIV-1 infection. ART is able to lower the increased levels of IL-10 in active stages of HIV-1 infection and this response likely represents another mechanism of action by which this therapeutic approach normalizes NK cells in HIV-1 infected patients [123]. Other then recovering NK cell phenotypic and functional features, ART also enhances the terminal differentiation of CD56dim expressing CD57 [124]. The presence of this latter surface marker indicates the existence of a functionally stable and mature NK cell subset, whose frequency normally increases with age. High frequencies of circulating CD57pos/CD56pos NK cells have been also associated with a better clinical outcome in cancer and autoimmune diseases [125]. Thus, this subset represents a good prognostic factor in several models of human diseases.
Residual degrees of NK cell activation have been shown to persist even after the administration ART and this finding does not correlate with T cell activation, HIV-1 viremia and CD4+ T cell count [49, 126]. The pathogenic and clinical relevance of this persistent NK cell activation during the course of active therapy is still unclear. In this regard, a recent study claimed that these higher levels of NK cell activation are detectable predominantly in CD56dim subset of immunologic non-responders HIV-1 infected patients and is inversely correlates with CD4+ T cell recovery and viral suppression [127].
While several studies have addressed the impact of ART on circulating NK cells in HIV-1 infection, only a few reports investigated their mucosal levels. Chronic HIV-1 infection reduces NK cell frequency both in the intraepithelial and lamina propria gut compartments. ART substantially expands intestinal NK cell populations despite the incomplete recovery of circulating CD4+ T cells [128, 129]. A similar impact of ART has been confirmed as well in SIV-infected Rhesus monkeys in which the initial drop of NK cells in the gut is reversed by the administration of antiviral treatment [130, 131].
Targeting NK cells in HIV-1 therapy
The great advances in our understanding of NK cell physiology (i.e. ontogenesis, memory-like responses, editing of the adaptive immune system) and physiopathology (i.e. dysfunctions in immune-deficiency, autoimmune diseases, viral infections, etc.) gave us the opportunities to exploit novel therapeutic approaches that either manipulate or use NK cell antiviral immune-properties [4, 132]. Indeed, given the above-mentioned multifunctional effector-functions, NK cells can be efficiently employed to build protective vaccine responses and shape immune responses against HIV-1. Only recently, remarkable efforts targeting and optimizing NK cell functions in therapeutic and preventive interventions against HIV-1 have been put in place with encouraging results.
The natural development of therapeutically effective anti-HIV-1 broadly neutralizing Ab (bNAbs) is a rare observation even 2–4 years after the infection. Furthermore, those few patients able to produce bNAbs do not really benefit from their anti-viral activities because of low Ab titers in the plasma or the high variability and complexity of HIV-1 strains. Recently, a modern technology based on single cell cloning has made it possible to isolate, expand and characterize a 2nd generation of bNAbs [133–136]. These bNAbs show a wide range of affinities towards different sites on the HIV-1 envelope and are now being manufactured and tested in several clinical trials. In particular, we can now measure the ability of each of these monoclonal Abs (mAbs) obtained from millions of HIV-1-specific B cells purified from HIV-1 infected patients to inhibit viral replication. In regard to NK cell biology, bNAbs can be used to promote the killing of HIV-1 infected cells via ADCC [136–138] as the ability of these bNAbs to prevent HIV-1 infection has been extensively reported [139, 140]. Whether therapeutic approaches using ADCC will work in HIV-1 infection is still being debated and requires additional investigation and clinical trials. However, the protective effect of the RV144 human vaccine trial was associated, at least in part, with the increased ADCC activity [141–143].
Antibody Dependent Cell Cytotoxicity
The current working hypothesis postulates that ADCC employs two different mechanisms of action to boost the anti-viral potential of bNAbs: i) the direct clearance of free-cell HIV-1 via the binding of the viral envelope to the Fab fragment of these mAbs and ii) the lysis of HIV-1 infected targets via the recruitment of cellular effectors such as FcγRIIIpos NK lymphocytes. Several in vivo studies have shown that HIV-1 viremia is remarkably higher when the engagement of Fcγ receptors is reduced. This finding demonstrates that the therapeutic efficacy of bNAbs requires both the involvement of immune cellular effectors and the activation of their downstream pathway triggered by the binding of the Fc fragments of bNAbs to Fcγ receptors [138, 144]. In line with this finding, the single infusion of 3BNC117 bNAb in HIV-1-infected patients showed that its neutralizing effect is not only due to the elimination of free circulating viruses or to the prevention of new cellular infections, but also to the clearance of HIV-1-infected cells [145]. This latter mechanism has been confirmed also in a humanized mouse model where the administration of bNAbs induced a direct lysis of HIV-1-infected CD4+ T cells via the engagement of Fcγ receptors [146]. These different features of ADCC, in the context of new therapeutic approaches with the 2nd generation of bNAbs, can really make the difference by having a strong clinical efficacy even when the Ab neutralizing potentials are compromised by the high heterogeneity of HIV-1 strains. In this regard, several in vitro and in vivo studies have demonstrated that the binding of bNAbs to FcγRIIIa induces NK cell-mediated ADCC [147–150]. Structural and functional analyses of bNAbs also revealed that mutations in the variable region of these mAbs are able to increase the affinity and specificity of bNAbs to different viral epitopes and, subsequently, to enhance their neutralizing activity again HIV-1 via ADCC mediated by NK cells [134, 151, 152]. Since it is unlikely that a vaccine trial may specifically induce such changes in the Fab fragments of Abs, the generation of engineered bNAbs carrying mutations in their variable regions is an alternative strategy that is currently under development. Another approach for increasing NK cell-mediated ADCC against HIV-1 infected targets is to improve the ability of the Fc fragment of bNAbs to bind CD16. This second strategy has been already developed in cancer therapy [153–155]. Indeed, the engineered Fc variant of the S239D-I332E bNAb exhibits a stronger ability to bind FcγRIIa, FcγRIIb and FcγRIIIa, a phenomenon enhancing the ADCC-mediated clearance of cancer cell targets expressing specific tumor antigens [153].
Another factor regulating the NK cell-mediated ADCC against HIV-1 infected targets is the glycosylation of the Fc domain of bNAbs. Specifically, the shift from a global antibody-glycosylation profile toward an agalactosylated glycoform improves Fc-mediated binding of NK cells and is associated with higher NK cell anti-viral activity [156]. Moreover, the presence of one or another of the 4 different isoforms of the IgG subclasses (IgG1-4) has a deep impact on the ability of bNAbs to bind Fcγ receptors. Indeed, the spontaneous suppression of HIV-1 replication and progression to AIDS in ECs in the absence of ART is associated with virus-specific isotype IgG3 and IgG1 responses [157]. Notably, IgG3 Abs from polyclonal HIV-1 immune globulins are more potent then other subclasses in neutralizing the virus [158]. Interestingly, the protection of the RV144 vaccine trial is also associated with the occurrence of an IgG3 response in subjects who are more resistant to HIV-1 infection [159, 160]. The Fc-regions of IgG1 and IgG3 antibodies represent human isotopes able to regulate both ADCC and antibody-mediated complement-mediated lysis (ADCML). Both bNAbs non-bNAbs can eliminate HIV-1 virions as well as infected cells via ADCC and ADCML [161]. In particular, the binding of C3 complement protein on target cells in HIV-1 infection in vitro has been show to increase NK cell-mediated ADCC cytotoxicity [162]. However, the mechanism(s) regulating the complement system in NK cell-mediated ADCC activity in HIV-1 therapy have been not yet disclosed and could represent an important therapeutic target. Indeed, the deposit of C3 complement protein on opsonized target cells in tumor therapy induced the reduction of FcγRIII binding by NK cells, a phenomenon that can be reversed by C3 depletion [163, 164]. Whether a similar phenomenon occurs also in ADCC based-therapies against HIV-1 infection is unknown.
Other compounds affecting NK cell-mediated ADCC activity are metalloproteinases such as the cytoplasmic metalloproteinase ADAM17 that can prevent the shedding of CD16 following NK cells activation [165]. In this regard, the administration of metalloproteinase inhibitors in trials of anti-tumor immunotherapy based on the infusion of mAbs improved NK cell-mediated ADCC [81, 166]. Finally NKRs such as NKG2D have been recently reported to act as co-receptors of Fcγ for NK cell ADCC during the course of HIV-1 infection [167]. Moreover, both NKG2D and 2B4 synergize with Fcγ ligation to enhance NK cell calcium flux [168]. Yet, ligation of iKIRs with their specific alleles of MHC-I ligands can inhibit ADCC triggered by anti-HIV-1 and therapeutic monoclonal antibodies [169, 170].
TLR Ligands
The innate immune system, via TLR agonists, may enhance NK cell activity. TLRs belong to the family of pattern recognition receptors (PRR) and are able to activate an immune response against a given pathogen (eg. viral infections) following their direct interactions with different microbial compounds. TLRs are constitutively expressed on several immune cells from both innate and adaptive immune system, including NK cells [171]. Several studies have shown that TLRs agonists can directly activate NK cells and boost their anti-viral potentials [172–174]. One possible candidate is MGN1703, a novel TLR9 agonist currently under clinical testing for the treatment of metastatic colorectal cancer [175–178]. Indeed, MGN1703 is able to stimulate plasmacytoid DCs to produce IFN-α that, in turn, increase the expression of NKp46 on NK cells and induce the killing of HIV-1 infected T cell targets [22, 60, 174]. Recent clinical study confirmed that MGN1703 treatment in HIV-1 infected patients on ART has a dual potential by increasing HIV-1 transcription and enhancing cytotoxic NK cell activation [179]. Other agonists able to boost NK cell effector functions against cancer cells are the ones binding TLR7 [180, 181]. These latter compounds have also been reported to increase both CD8+ T cell- and NK cell-mediated lysis of HIV-1-infected cells [172].
Other Approaches
The currently available adoptive cell transfer therapies using NK cells to cure solid and hematologic cancers comprise several approaches that could be considered for HIV infection. These include the expansion ex vivo of autologous or allogeneic NK cells as well as the genetic engineering of NK cells to induce cytokine secretion, expression of Fc receptors and tumor-antigen receptors [182]. Recently NK cells obtained from human embryonic stem cells (hESCs), and induced pluripotent stem cells (iPSCs) are becoming an alternative promising source of NK cells for gene or immunotherapy. NK cells derived from hESCs and iPSCs have potent anti-HIV-1 activity against HIV-1 replication in CD4pos T cells both in vitro and in vivo [183]. Alternatively, a potent NK cell-mediates anti-HIV-1 activity can be achieve through recombinant chimeric antigen receptor (CAR) engineering. CAR T cells therapy are increasingly demonstrating success for the treatment of cancers and have been proposed for use in combating HIV-1 viral reservoirs [184, 185]. The efficacy of CAR-expressing NK cells in cancer treatment is currently being tested at the pre-clinical stage [186], In this regard, the possible use of CAR-NK cells to treat HIV-1 infection by implementing the technology of hESC- and/or iPSC-derided NK cells is currently being debated [183]. Hence, the development of the above-mentioned NK-cell immune therapeutic approaches will challenge the scientific community over the next decade.
Future perspectives
Regardless of the negative impact of HIV-1 on NK cell activity, the remarkable advances in biomedical technology make it now possible to use or modify these innate lymphocytes for the development of innovative and potentially effective antiviral strategies. Indeed, a therapy based on the administration of 2nd generation anti-HIV-1 bNAbs is one of the possible options to design a therapeutic vaccine boosting NK cell effector functions. Moreover, the acquisition of the best technologies to engineer NK cells is another frontier that is being currently considered to generate potent anti-HIV NK cells in the context of a customized and personal medicine.
All these approaches targeting NK cells for the treatment of HIV-1 infection are either in preliminary experimental and clinical trials or are currently being discussed among the scientific community. ART can restore in HIV-1 infected patients the frequency, phenotype and function of NK cells. Thus, these cells can be made available for therapy by employing different strategies under development.
Acknowledgments
J.M., F.O., E.Z., C.D.V. and D.M wrote the manuscript and approved the final version. This work was supported by the Italian Ministry of Health (GR-2013-02356522 to E.Z. and RF-ICH-2009-1304134 to J.M.), by the European Union (Marie Curie International Reintegration Grants 249249 to J.M.) and Humanitas Clinical and Research Center intramural program (Rozzano, Milan, Italy) to D.M. We are a part of the “BEAT-HIV Collaboratory” network and this work supported by UM1AI126620 co-funded by NIAID, NIMH, NINDS and NIDA.
Footnotes
Conflict of Interest
There are no conflicts of interest
References
- 1.Pahl J, Cerwenka A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiology. 2015;222:11–20. doi: 10.1016/j.imbio.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Waggoner SN, Reighard SD, Gyurova IE, Cranert SA, Mahl SE, Karmele EP, et al. Roles of natural killer cells in antiviral immunity. Curr Opin Virol. 2015;16:15–23. doi: 10.1016/j.coviro.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lugli E, Marcenaro E, Mavilio D. NK Cell Subset Redistribution during the Course of Viral Infections. Front Immunol. 2014;5:390. doi: 10.3389/fimmu.2014.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moretta A, Marcenaro E, Sivori S, Della Chiesa M, Vitale M, Moretta L. Early liaisons between cells of the innate immune system in inflamed peripheral tissues. Trends Immunol. 2005;26:668–675. doi: 10.1016/j.it.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattiola I, Pesant M, Tentorio PF, Molgora M, Marcenaro E, Lugli E, et al. Priming of Human Resting NK Cells by Autologous M1 Macrophages via the Engagement of IL-1beta, IFN-beta, and IL-15 Pathways. J Immunol. 2015;195:2818–2828. doi: 10.4049/jimmunol.1500325. [DOI] [PubMed] [Google Scholar]
- 8.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 9.De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proc Natl Acad Sci U S A. 2011;108:728–732. doi: 10.1073/pnas.1012356108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, et al. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrega P, Ferlazzo G. Natural killer cell distribution and trafficking in human tissues. Front Immunol. 2012;3:347. doi: 10.3389/fimmu.2012.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudspeth K, Donadon M, Cimino M, Pontarini E, Tentorio P, Preti M, et al. Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J Autoimmun. 2016;66:40–50. doi: 10.1016/j.jaut.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moretta A, Bottino C, Mingari MC, Biassoni R, Moretta L. What is a natural killer cell? Nat Immunol. 2002;3:6–8. doi: 10.1038/ni0102-6. [DOI] [PubMed] [Google Scholar]
- 16.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2008;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moretta A, Biassoni R, Bottino C, Mingari MC, Moretta L. Natural cytotoxicity receptors that trigger human NK-cell-mediated cytolysis. Immunol Today. 2000;21:228–234. doi: 10.1016/s0167-5699(00)01596-6. [DOI] [PubMed] [Google Scholar]
- 18.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 19.Karre K. Natural killer cell recognition of missing self. Nat Immunol. 2008;9:477–480. doi: 10.1038/ni0508-477. [DOI] [PubMed] [Google Scholar]
- 20.Alter G, Teigen N, Davis BT, Addo MM, Suscovich TJ, Waring MT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106:3366–3369. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 21.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5:835–843. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 22.Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci U S A. 2003;100:15011–15016. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunetta E, Hudspeth KL, Mavilio D. Pathologic natural killer cell subset redistribution in HIV-1 infection: new insights in pathophysiology and clinical outcomes. J Leukoc Biol. 2010;88:1119–1130. doi: 10.1189/jlb.0410225. [DOI] [PubMed] [Google Scholar]
- 24.Hu PF, Hultin LE, Hultin P, Hausner MA, Hirji K, Jewett A, et al. Natural killer cell immunodeficiency in HIV disease is manifest by profoundly decreased numbers of CD16+CD56+ cells and expansion of a population of CD16dimCD56− cells with low lytic activity. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:331–340. [PubMed] [Google Scholar]
- 25.Mavilio D, Lombardo G, Benjamin J, Kim D, Follman D, Marcenaro E, et al. Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A. 2005;102:2886–2891. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucia B, Jennings C, Cauda R, Ortona L, Landay AL. Evidence of a selective depletion of a CD16+ CD56+ CD8+ natural killer cell subset during HIV infection. Cytometry. 1995;22:10–15. doi: 10.1002/cyto.990220103. [DOI] [PubMed] [Google Scholar]
- 27.Tarazona R, Casado JG, Delarosa O, Torre-Cisneros J, Villanueva JL, Sanchez B, et al. Selective depletion of CD56(dim) NK cell subsets and maintenance of CD56(bright) NK cells in treatment-naive HIV-1-seropositive individuals. J Clin Immunol. 2002;22:176–183. doi: 10.1023/a:1015476114409. [DOI] [PubMed] [Google Scholar]
- 28.Scott-Algara D, Paul P. NK cells and HIV infection: lessons from other viruses. Curr Mol Med. 2002;2:757–768. doi: 10.2174/1566524023361781. [DOI] [PubMed] [Google Scholar]
- 29.Naluyima P, Eller MA, Laeyendecker O, Quinn TC, Serwadda D, Sewankambo NK, et al. Impaired natural killer cell responses are associated with loss of the highly activated NKG2A(+)CD57(+)CD56(dim) subset in HIV-1 subtype D infection in Uganda. AIDS. 2014;28:1273–1278. doi: 10.1097/QAD.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gasper MA, Kunwar P, Itaya G, Lejarcegui N, Bosire R, Maleche-Obimbo E, et al. Natural killer cell and T-cell subset distributions and activation influence susceptibility to perinatal HIV-1 infection. AIDS. 2014;28:1115–1124. doi: 10.1097/QAD.0000000000000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kottilil S, Shin K, Planta M, McLaughlin M, Hallahan CW, Ghany M, et al. Expression of chemokine and inhibitory receptors on natural killer cells: effect of immune activation and HIV viremia. J Infect Dis. 2004;189:1193–1198. doi: 10.1086/382090. [DOI] [PubMed] [Google Scholar]
- 32.Sirianni MC, Ensoli F, Alario C, Fiorelli V, Sacco G, Topino S, et al. Distribution of the natural killer-related receptor for HLA-C during highly active antiretroviral therapy for human immunodeficiency virus infection. Hum Immunol. 2001;62:1328–1334. doi: 10.1016/s0198-8859(01)00355-x. [DOI] [PubMed] [Google Scholar]
- 33.Ahmad R, Sindhu ST, Tran P, Toma E, Morisset R, Menezes J, et al. Modulation of expression of the MHC class I-binding natural killer cell receptors, and NK activity in relation to viral load in HIV-infected/AIDS patients. J Med Virol. 2001;65:431–440. [PubMed] [Google Scholar]
- 34.De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, et al. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur J Immunol. 2003;33:2410–2418. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 35.Korner C, Granoff ME, Amero MA, Sirignano MN, Vaidya SA, Jost S, et al. Increased frequency and function of KIR2DL1-3(+) NK cells in primary HIV-1 infection are determined by HLA-C group haplotypes. Eur J Immunol. 2014;44:2938–2948. doi: 10.1002/eji.201444751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jennes W, Verheyden S, Mertens JW, Camara M, Seydi M, Dieye TN, et al. Inhibitory KIR/HLA incompatibility between sexual partners confers protection against HIV-1 transmission. Blood. 2013;121:1157–1164. doi: 10.1182/blood-2012-09-455352. [DOI] [PubMed] [Google Scholar]
- 37.Hellmann I, Letvin NL, Schmitz JE. KIR2DL4 copy number variation is associated with CD4+ T-cell depletion and function of cytokine-producing NK cell subsets in SIV-infected Mamu-A*01-negative rhesus macaques. J Virol. 2013;87:5305–5310. doi: 10.1128/JVI.02949-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strauss-Albee DM, Fukuyama J, Liang EC, Yao Y, Jarrell JA, Drake AL, et al. Human NK cell repertoire diversity reflects immune experience and correlates with viral susceptibility. Sci Transl Med. 2015;7:297ra115. doi: 10.1126/scitranslmed.aac5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fogli M, Mavilio D, Brunetta E, Varchetta S, Ata K, Roby G, et al. Lysis of endogenously infected CD4+ T cell blasts by rIL-2 activated autologous natural killer cells from HIV-infected viremic individuals. PLoS Pathog. 2008;4:e1000101. doi: 10.1371/journal.ppat.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, et al. The decreased expression of Siglec-7 represents an early marker of dysfunctional natural killer-cell subsets associated with high levels of HIV-1 viremia. Blood. 2009;114:3822–3830. doi: 10.1182/blood-2009-06-226332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 42.Mikulak J, Di Vito C, Zaghi E, Mavilio D. Host Immune Responses in HIV-1 Infection: The Emerging Pathogenic Role of Siglecs and Their Clinical Correlates. Front Immunol. 2017;8:314. doi: 10.3389/fimmu.2017.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guma M, Budt M, Saez A, Brckalo T, Hengel H, Angulo A, et al. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107:3624–3631. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- 44.Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, et al. Chronic HIV-1 viremia reverses NKG2A/NKG2C ratio on natural killer cells in patients with human cytomegalovirus co-infection. AIDS. 2010;24:27–34. doi: 10.1097/QAD.0b013e3283328d1f. [DOI] [PubMed] [Google Scholar]
- 45.Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42:443–456. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim M, et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015;42:431–442. doi: 10.1016/j.immuni.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou J, Amran FS, Kramski M, Angelovich TA, Elliott J, Hearps AC, et al. An NK Cell Population Lacking FcRgamma Is Expanded in Chronically Infected HIV Patients. J Immunol. 2015;194:4688–4697. doi: 10.4049/jimmunol.1402448. [DOI] [PubMed] [Google Scholar]
- 48.Naranbhai V, Altfeld M, Karim SS, Ndung’u T, Karim QA, Carr WH. Changes in Natural Killer cell activation and function during primary HIV-1 Infection. PLoS One. 2013;8:e53251. doi: 10.1371/journal.pone.0053251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lichtfuss GF, Cheng WJ, Farsakoglu Y, Paukovics G, Rajasuriar R, Velayudham P, et al. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J Immunol. 2012;189:1491–1499. doi: 10.4049/jimmunol.1200458. [DOI] [PubMed] [Google Scholar]
- 50.Kuri-Cervantes L, de Oca GS, Avila-Rios S, Hernandez-Juan R, Reyes-Teran G. Activation of NK cells is associated with HIV-1 disease progression. J Leukoc Biol. 2014;96:7–16. doi: 10.1189/jlb.0913514. [DOI] [PubMed] [Google Scholar]
- 51.Thobakgale CF, Fadda L, Lane K, Toth I, Pereyra F, Bazner S, et al. Frequent and strong antibody-mediated natural killer cell activation in response to HIV-1 Env in individuals with chronic HIV-1 infection. J Virol. 2012;86:6986–6993. doi: 10.1128/JVI.00569-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 53.Nolting A, Dugast AS, Rihn S, Luteijn R, Carrington MF, Kane K, et al. MHC class I chain-related protein A shedding in chronic HIV-1 infection is associated with profound NK cell dysfunction. Virology. 2010;406:12–20. doi: 10.1016/j.virol.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ward J, Bonaparte M, Sacks J, Guterman J, Fogli M, Mavilio D, et al. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood. 2007;110:1207–1214. doi: 10.1182/blood-2006-06-028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vieillard V, Strominger JL, Debre P. NK cytotoxicity against CD4+ T cells during HIV-1 infection: a gp41 peptide induces the expression of an NKp44 ligand. Proc Natl Acad Sci U S A. 2005;102:10981–10986. doi: 10.1073/pnas.0504315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marras F, Nicco E, Bozzano F, Di Biagio A, Dentone C, Pontali E, et al. Natural killer cells in HIV controller patients express an activated effector phenotype and do not up-regulate NKp44 on IL-2 stimulation. Proc Natl Acad Sci U S A. 2013;110:11970–11975. doi: 10.1073/pnas.1302090110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sennepin A, Baychelier F, Guihot A, Nel I, Ho Tsong Fang R, Calin R, et al. NKp44L expression on CD4+ T cells is associated with impaired immunological recovery in HIV-infected patients under highly active antiretroviral therapy. AIDS. 2013;27:1857–1866. doi: 10.1097/qad.0b013e328361a3fe. [DOI] [PubMed] [Google Scholar]
- 58.Mavilio D, Lombardo G, Kinter A, Fogli M, La Sala A, Ortolano S, et al. Characterization of the defective interaction between a subset of natural killer cells and dendritic cells in HIV-1 infection. J Exp Med. 2006;203:2339–2350. doi: 10.1084/jem.20060894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tasca S, Tambussi G, Nozza S, Capiluppi B, Zocchi MR, Soldini L, et al. Escape of monocyte-derived dendritic cells of HIV-1 infected individuals from natural killer cell-mediated lysis. AIDS. 2003;17:2291–2298. doi: 10.1097/00002030-200311070-00003. [DOI] [PubMed] [Google Scholar]
- 60.Tomescu C, Mavilio D, Montaner LJ. Lysis of HIV-1-infected autologous CD4+ primary T cells by interferon-alpha-activated NK cells requires NKp46 and NKG2D. AIDS. 2015;29:1767–1773. doi: 10.1097/QAD.0000000000000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 62.Valentin A, Rosati M, Patenaude DJ, Hatzakis A, Kostrikis LG, Lazanas M, et al. Persistent HIV-1 infection of natural killer cells in patients receiving highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2002;99:7015–7020. doi: 10.1073/pnas.102672999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bernstein HB, Wang G, Plasterer MC, Zack JA, Ramasastry P, Mumenthaler SM, et al. CD4+ NK cells can be productively infected with HIV, leading to downregulation of CD4 expression and changes in function. Virology. 2009;387:59–66. doi: 10.1016/j.virol.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varchetta S, Lusso P, Hudspeth K, Mikulak J, Mele D, Paolucci S, et al. Sialic acid-binding Ig-like lectin-7 interacts with HIV-1 gp120 and facilitates infection of CD4pos T cells and macrophages. Retrovirology. 2013;10:154. doi: 10.1186/1742-4690-10-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matusali G, Potesta M, Santoni A, Cerboni C, Doria M. The human immunodeficiency virus type 1 Nef and Vpu proteins downregulate the natural killer cell-activating ligand PVR. J Virol. 2012;86:4496–4504. doi: 10.1128/JVI.05788-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Richard J, Cohen EA. HIV-1 Vpu disarms natural killer cells. Cell Host Microbe. 2010;8:389–391. doi: 10.1016/j.chom.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shah AH, Sowrirajan B, Davis ZB, Ward JP, Campbell EM, Planelles V, et al. Degranulation of natural killer cells following interaction with HIV-1-infected cells is hindered by downmodulation of NTB-A by Vpu. Cell Host Microbe. 2010;8:397–409. doi: 10.1016/j.chom.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kulkarni A, Paranjape R, Thakar M. Expansion of defective NK cells in early HIV type 1C infection: a consequence of reduced CD161 expression. AIDS Res Hum Retroviruses. 2012;28:100–105. doi: 10.1089/aid.2011.0110. [DOI] [PubMed] [Google Scholar]
- 69.Kottilil S, Chun TW, Moir S, Liu S, McLaughlin M, Hallahan CW, et al. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J Infect Dis. 2003;187:1038–1045. doi: 10.1086/368222. [DOI] [PubMed] [Google Scholar]
- 70.Smalls-Mantey A, Connors M, Sattentau QJ. Comparative efficiency of HIV-1-infected T cell killing by NK cells, monocytes and neutrophils. PLoS One. 2013;8:e74858. doi: 10.1371/journal.pone.0074858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 72.Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J, Rinaldo C, et al. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157:2168–2173. [PubMed] [Google Scholar]
- 73.Chung AW, Navis M, Isitman G, Wren L, Silvers J, Amin J, et al. Activation of NK cells by ADCC antibodies and HIV disease progression. J Acquir Immune Defic Syndr. 2011;58:127–131. doi: 10.1097/QAI.0b013e31822c62b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wren LH, Chung AW, Isitman G, Kelleher AD, Parsons MS, Amin J, et al. Specific antibody-dependent cellular cytotoxicity responses associated with slow progression of HIV infection. Immunology. 2013;138:116–123. doi: 10.1111/imm.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lambotte O, Ferrari G, Moog C, Yates NL, Liao HX, Parks RJ, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. 2009;23:897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katz JD, Mitsuyasu R, Gottlieb MS, Lebow LT, Bonavida B. Mechanism of defective NK cell activity in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. II. Normal antibody-dependent cellular cytotoxicity (ADCC) mediated by effector cells defective in natural killer (NK) cytotoxicity. J Immunol. 1987;139:55–60. [PubMed] [Google Scholar]
- 77.Johansson SE, Rollman E, Chung AW, Center RJ, Hejdeman B, Stratov I, et al. NK cell function and antibodies mediating ADCC in HIV-1-infected viremic and controller patients. Viral Immunol. 2011;24:359–368. doi: 10.1089/vim.2011.0025. [DOI] [PubMed] [Google Scholar]
- 78.Stratov I, Chung A, Kent SJ. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J Virol. 2008;82:5450–5459. doi: 10.1128/JVI.01952-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Madhavi V, Navis M, Chung AW, Isitman G, Wren LH, De Rose R, et al. Activation of NK cells by HIV-specific ADCC antibodies: role for granulocytes in expressing HIV-1 peptide epitopes. Hum Vaccin Immunother. 2013;9:1011–1018. doi: 10.4161/hv.23446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chung AW, Isitman G, Navis M, Kramski M, Center RJ, Kent SJ, et al. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc Natl Acad Sci U S A. 2011;108:7505–7510. doi: 10.1073/pnas.1016048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Q, Sun Y, Rihn S, Nolting A, Tsoukas PN, Jost S, et al. Matrix metalloprotease inhibitors restore impaired NK cell-mediated antibody-dependent cellular cytotoxicity in human immunodeficiency virus type 1 infection. J Virol. 2009;83:8705–8712. doi: 10.1128/JVI.02666-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang CC, Isitman G, Bruneau J, Tremblay C, Bernard NF, Kent SJ, et al. Phenotypical and functional profiles of natural killer cells exhibiting matrix metalloproteinase-mediated CD16 cleavage after anti-HIV antibody-dependent activation. Clin Exp Immunol. 2015;181:275–285. doi: 10.1111/cei.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parsons MS, Tang CC, Jegaskanda S, Center RJ, Brooks AG, Stratov I, et al. Anti-HIV antibody-dependent activation of NK cells impairs NKp46 expression. J Immunol. 2014;192:308–315. doi: 10.4049/jimmunol.1301247. [DOI] [PubMed] [Google Scholar]
- 84.Vieillard V, Fausther-Bovendo H, Samri A, Debre P. Specific phenotypic and functional features of natural killer cells from HIV-infected long-term nonprogressors and HIV controllers. J Acquir Immune Defic Syndr. 2010;53:564–573. doi: 10.1097/QAI.0b013e3181d0c5b4. [DOI] [PubMed] [Google Scholar]
- 85.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 86.Kim S, Sunwoo JB, Yang L, Choi T, Song YJ, French AR, et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci U S A. 2008;105:3053–3058. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fauriat C, Ivarsson MA, Ljunggren HG, Malmberg KJ, Michaelsson J. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood. 2010;115:1166–1174. doi: 10.1182/blood-2009-09-245746. [DOI] [PubMed] [Google Scholar]
- 88.Korner C, Altfeld M. Role of KIR3DS1 in human diseases. Front Immunol. 2012;3:326. doi: 10.3389/fimmu.2012.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qi Y, Martin MP, Gao X, Jacobson L, Goedert JJ, Buchbinder S, et al. KIR/HLA pleiotropism: protection against both HIV and opportunistic infections. PLoS Pathog. 2006;2:e79. doi: 10.1371/journal.ppat.0020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pelak K, Need AC, Fellay J, Shianna KV, Feng S, Urban TJ, et al. Copy number variation of KIR genes influences HIV-1 control. PLoS Biol. 2011;9:e1001208. doi: 10.1371/journal.pbio.1001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alter G, Martin MP, Teigen N, Carr WH, Suscovich TJ, Schneidewind A, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 2007;204:3027–3036. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O’Connor GM, Vivian JP, Gostick E, Pymm P, Lafont BA, Price DA, et al. Peptide-Dependent Recognition of HLA-B*57:01 by KIR3DS1. J Virol. 2015;89:5213–5221. doi: 10.1128/JVI.03586-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boulet S, Kleyman M, Kim JY, Kamya P, Sharafi S, Simic N, et al. A combined genotype of KIR3DL1 high expressing alleles and HLA-B*57 is associated with a reduced risk of HIV infection. AIDS. 2008;22:1487–1491. doi: 10.1097/QAD.0b013e3282ffde7e. [DOI] [PubMed] [Google Scholar]
- 94.Hens J, Jennes W, Kestens L. The role of NK cells in HIV-1 protection: autologous, allogeneic or both? AIDS Res Ther. 2016;13:15. doi: 10.1186/s12981-016-0099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parsons MS, Wren L, Isitman G, Navis M, Stratov I, Bernard NF, et al. HIV infection abrogates the functional advantage of natural killer cells educated through KIR3DL1/HLA-Bw4 interactions to mediate anti-HIV antibody-dependent cellular cytotoxicity. J Virol. 2012;86:4488–4495. doi: 10.1128/JVI.06112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tomescu C, Duh FM, Hoh R, Viviani A, Harvill K, Martin MP, et al. Impact of protective killer inhibitory receptor/human leukocyte antigen genotypes on natural killer cell and T-cell function in HIV-1-infected controllers. AIDS. 2012;26:1869–1878. doi: 10.1097/QAD.0b013e32835861b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Teijlingen NH, Holzemer A, Korner C, Garcia-Beltran WF, Schafer JL, Fadda L, et al. Sequence variations in HIV-1 p24 Gag-derived epitopes can alter binding of KIR2DL2 to HLA-C*03:04 and modulate primary natural killer cell function. AIDS. 2014;28:1399–1408. doi: 10.1097/QAD.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jennes W, Verheyden S, Demanet C, Adje-Toure CA, Vuylsteke B, Nkengasong JN, et al. Cutting edge: resistance to HIV-1 infection among African female sex workers is associated with inhibitory KIR in the absence of their HLA ligands. J Immunol. 2006;177:6588–6592. doi: 10.4049/jimmunol.177.10.6588. [DOI] [PubMed] [Google Scholar]
- 99.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 100.Apps R, Del Prete GQ, Chatterjee P, Lara A, Brumme ZL, Brockman MA, et al. HIV-1 Vpu Mediates HLA-C Downregulation. Cell Host Microbe. 2016;19:686–695. doi: 10.1016/j.chom.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carroll IR, Wang J, Howcroft TK, Singer DS. HIV Tat represses transcription of the beta 2-microglobulin promoter. Mol Immunol. 1998;35:1171–1178. doi: 10.1016/s0161-5890(98)00107-2. [DOI] [PubMed] [Google Scholar]
- 102.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–214. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 103.Paust S, von Andrian UH. Natural killer cell memory. Nat Immunol. 2011;12:500–508. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
- 104.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, et al. Cytokine activation induces human memory-like NK cells. Blood. 2012;120:4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Della Chiesa M, Sivori S, Carlomagno S, Moretta L, Moretta A. Activating KIRs and NKG2C in Viral Infections: Toward NK Cell Memory? Front Immunol. 2015;6:573. doi: 10.3389/fimmu.2015.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu Z, Sinzger C, Frascaroli G, Reichel J, Bayer C, Wang L, et al. Human cytomegalovirus-induced NKG2C(hi) CD57(hi) natural killer cells are effectors dependent on humoral antiviral immunity. J Virol. 2013;87:7717–7725. doi: 10.1128/JVI.01096-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Djaoud Z, David G, Bressollette C, Willem C, Rettman P, Gagne K, et al. Amplified NKG2C+ NK cells in cytomegalovirus (CMV) infection preferentially express killer cell Ig-like receptor 2DL: functional impact in controlling CMV-infected dendritic cells. J Immunol. 2013;191:2708–2716. doi: 10.4049/jimmunol.1301138. [DOI] [PubMed] [Google Scholar]
- 109.Reeves RK, Li H, Jost S, Blass E, Schafer JL, Varner V, et al. Antigen-specific NK cell memory in rhesus macaques. Nat Immunol. 2015;16:927–932. doi: 10.1038/ni.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yazdanpanah Y, Fagard C, Descamps D, Taburet AM, Colin C, Roquebert B, et al. High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: results of the ANRS 139 TRIO trial. Clin Infect Dis. 2009;49:1441–1449. doi: 10.1086/630210. [DOI] [PubMed] [Google Scholar]
- 111.Arts EJ, Hazuda DJ. HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med. 2012;2:a007161. doi: 10.1101/cshperspect.a007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lederman MM, Connick E, Landay A, Kuritzkes DR, Spritzler J, St Clair M, et al. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS Clinical Trials Group Protocol 315. J Infect Dis. 1998;178:70–79. doi: 10.1086/515591. [DOI] [PubMed] [Google Scholar]
- 113.Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 114.Weber K, Meyer D, Grosse V, Stoll M, Schmidt RE, Heiken H. Reconstitution of NK cell activity in HIV-1 infected individuals receiving antiretroviral therapy. Immunobiology. 2000;202:172–178. doi: 10.1016/S0171-2985(00)80063-7. [DOI] [PubMed] [Google Scholar]
- 115.Michaelsson J, Long BR, Loo CP, Lanier LL, Spotts G, Hecht FM, et al. Immune reconstitution of CD56(dim) NK cells in individuals with primary HIV-1 infection treated with interleukin-2. J Infect Dis. 2008;197:117–125. doi: 10.1086/524141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kuylenstierna C, Snyder-Cappione JE, Loo CP, Long BR, Gonzalez VD, Michaelsson J, et al. NK cells and CD1d-restricted NKT cells respond in different ways with divergent kinetics to IL-2 treatment in primary HIV-1 infection. Scand J Immunol. 2011;73:141–146. doi: 10.1111/j.1365-3083.2010.02484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chehimi J, Azzoni L, Farabaugh M, Creer SA, Tomescu C, Hancock A, et al. Baseline viral load and immune activation determine the extent of reconstitution of innate immune effectors in HIV-1-infected subjects undergoing antiretroviral treatment. J Immunol. 2007;179:2642–2650. doi: 10.4049/jimmunol.179.4.2642. [DOI] [PubMed] [Google Scholar]
- 118.Meier UC, Owen RE, Taylor E, Worth A, Naoumov N, Willberg C, et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005;79:12365–12374. doi: 10.1128/JVI.79.19.12365-12374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Naora H, Gougeon ML. Enhanced survival and potent expansion of the natural killer cell population of HIV-infected individuals by exogenous interleukin-15. Immunol Lett. 1999;68:359–367. doi: 10.1016/s0165-2478(99)00077-2. [DOI] [PubMed] [Google Scholar]
- 120.Jensen SS, Hartling HJ, Tingstedt JL, Larsen TK, Nielsen SD, Pedersen C, et al. HIV-specific ADCC improves after antiretroviral therapy and correlates with normalization of the NK cell phenotype. J Acquir Immune Defic Syndr. 2015;68:103–111. doi: 10.1097/QAI.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 121.Jensen SS, Fomsgaard A, Borggren M, Tingstedt JL, Gerstoft J, Kronborg G, et al. HIV-Specific Antibody-Dependent Cellular Cytotoxicity (ADCC) -Mediating Antibodies Decline while NK Cell Function Increases during Antiretroviral Therapy (ART) PLoS One. 2015;10:e0145249. doi: 10.1371/journal.pone.0145249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Madhavi V, Ana-Sosa-Batiz FE, Jegaskanda S, Center RJ, Winnall WR, Parsons MS, et al. Antibody-dependent effector functions against HIV decline in subjects receiving antiretroviral therapy. J Infect Dis. 2015;211:529–538. doi: 10.1093/infdis/jiu486. [DOI] [PubMed] [Google Scholar]
- 123.Parato KG, Kumar A, Badley AD, Sanchez-Dardon JL, Chambers KA, Young CD, et al. Normalization of natural killer cell function and phenotype with effective anti-HIV therapy and the role of IL-10. AIDS. 2002;16:1251–1256. doi: 10.1097/00002030-200206140-00007. [DOI] [PubMed] [Google Scholar]
- 124.Ahmad F, Tufa DM, Mishra N, Jacobs R, Schmidt RE. Terminal Differentiation of CD56(dim)CD16(+) Natural Killer Cells Is Associated with Increase in Natural Killer Cell Frequencies After Antiretroviral Treatment in HIV-1 Infection. AIDS Res Hum Retroviruses. 2015;31:1206–1212. doi: 10.1089/aid.2015.0115. [DOI] [PubMed] [Google Scholar]
- 125.Nielsen CM, White MJ, Goodier MR, Riley EM. Functional Significance of CD57 Expression on Human NK Cells and Relevance to Disease. Front Immunol. 2013;4:422. doi: 10.3389/fimmu.2013.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Leeansyah E, Zhou J, Paukovics G, Lewin SR, Crowe SM, Jaworowski A. Decreased NK Cell FcRgamma in HIV-1 infected individuals receiving combination antiretroviral therapy: a cross sectional study. PLoS One. 2012;5:e9643. doi: 10.1371/journal.pone.0009643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Luo Z, Li Z, Martin L, Hu Z, Wu H, Wan Z, et al. Increased Natural Killer Cell Activation in HIV-Infected Immunologic Non-Responders Correlates with CD4+ T Cell Recovery after Antiretroviral Therapy and Viral Suppression. PLoS One. 2017;12:e0167640. doi: 10.1371/journal.pone.0167640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sips M, Sciaranghella G, Diefenbach T, Dugast AS, Berger CT, Liu Q, et al. Altered distribution of mucosal NK cells during HIV infection. Mucosal Immunol. 2012;5:30–40. doi: 10.1038/mi.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mela CM, Steel A, Lindsay J, Gazzard BG, Gotch FM, Goodier MR. Depletion of natural killer cells in the colonic lamina propria of viraemic HIV-1-infected individuals. AIDS. 2007;21:2177–2182. doi: 10.1097/QAD.0b013e3282f08b72. [DOI] [PubMed] [Google Scholar]
- 130.Liyanage NP, Gordon SN, Doster MN, Pegu P, Vaccari M, Shukur N, et al. Antiretroviral therapy partly reverses the systemic and mucosal distribution of NK cell subsets that is altered by SIVmac(2)(5)(1) infection of macaques. Virology. 2014;450–451:359–368. doi: 10.1016/j.virol.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hong HS, Rajakumar PA, Billingsley JM, Reeves RK, Johnson RP. No monkey business: why studying NK cells in non-human primates pays off. Front Immunol. 2013;4:32. doi: 10.3389/fimmu.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Scully E, Alter G. NK Cells in HIV Disease. Curr HIV/AIDS Rep. 2016;13:85–94. doi: 10.1007/s11904-016-0310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Burton DR, Mascola JR. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat Immunol. 2015;16:571–576. doi: 10.1038/ni.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. Antibodies in HIV-1 vaccine development and therapy. Science. 2013;341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mouquet H. Antibody B cell responses in HIV-1 infection. Trends Immunol. 2014;35:549–561. doi: 10.1016/j.it.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 136.Euler Z, Alter G. Exploring the potential of monoclonal antibody therapeutics for HIV-1 eradication. AIDS Res Hum Retroviruses. 2015;31:13–24. doi: 10.1089/aid.2014.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee WS, Parsons MS, Kent SJ, Lichtfuss M. Can HIV-1-Specific ADCC Assist the Clearance of Reactivated Latently Infected Cells? Front Immunol. 2015;6:265. doi: 10.3389/fimmu.2015.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reh L, Magnus C, Schanz M, Weber J, Uhr T, Rusert P, et al. Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent. PLoS Pathog. 2015;11:e1004966. doi: 10.1371/journal.ppat.1004966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Malbec M, Porrot F, Rua R, Horwitz J, Klein F, Halper-Stromberg A, et al. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. J Exp Med. 2013;210:2813–2821. doi: 10.1084/jem.20131244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, et al. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J Infect Dis. 2012;206:431–441. doi: 10.1093/infdis/jis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 144.Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514:642–645. doi: 10.1038/nature13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP, Jr, Buckley N, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lu CL, Murakowski DK, Bournazos S, Schoofs T, Sarkar D, Halper-Stromberg A, et al. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science. 2016;352:1001–1004. doi: 10.1126/science.aaf1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kramski M, Stratov I, Kent SJ. The role of HIV-specific antibody-dependent cellular cytotoxicity in HIV prevention and the influence of the HIV-1 Vpu protein. AIDS. 2015;29:137–144. doi: 10.1097/QAD.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 148.Alvarez RA, Hamlin RE, Monroe A, Moldt B, Hotta MT, Rodriguez Caprio G, et al. HIV-1 Vpu antagonism of tetherin inhibits antibody-dependent cellular cytotoxic responses by natural killer cells. J Virol. 2014;88:6031–6046. doi: 10.1128/JVI.00449-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gooneratne SL, Richard J, Lee WS, Finzi A, Kent SJ, Parsons MS. Slaying the Trojan horse: natural killer cells exhibit robust anti-HIV-1 antibody-dependent activation and cytolysis against allogeneic T cells. J Virol. 2015;89:97–109. doi: 10.1128/JVI.02461-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bruel T, Guivel-Benhassine F, Amraoui S, Malbec M, Richard L, Bourdic K, et al. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun. 2016;7:10844. doi: 10.1038/ncomms10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Klein F, Diskin R, Scheid JF, Gaebler C, Mouquet H, Georgiev IS, et al. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell. 2013;153:126–138. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kepler TB, Liao HX, Alam SM, Bhaskarabhatla R, Zhang R, Yandava C, et al. Immunoglobulin gene insertions and deletions in the affinity maturation of HIV-1 broadly reactive neutralizing antibodies. Cell Host Microbe. 2014;16:304–313. doi: 10.1016/j.chom.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bowles JA, Wang SY, Link BK, Allan B, Beuerlein G, Campbell MA, et al. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood. 2006;108:2648–2654. doi: 10.1182/blood-2006-04-020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu Z, Gunasekaran K, Wang W, Razinkov V, Sekirov L, Leng E, et al. Asymmetrical Fc engineering greatly enhances antibody-dependent cellular cytotoxicity (ADCC) effector function and stability of the modified antibodies. J Biol Chem. 2014;289:3571–3590. doi: 10.1074/jbc.M113.513366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest. 2013;123:2183–2192. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ackerman ME, Mikhailova A, Brown EP, Dowell KG, Walker BD, Bailey-Kellogg C, et al. Polyfunctional HIV-Specific Antibody Responses Are Associated with Spontaneous HIV Control. PLoS Pathog. 2016;12:e1005315. doi: 10.1371/journal.ppat.1005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Scharf O, Golding H, King LR, Eller N, Frazier D, Golding B, et al. Immunoglobulin G3 from polyclonal human immunodeficiency virus (HIV) immune globulin is more potent than other subclasses in neutralizing HIV type 1. J Virol. 2001;75:6558–6565. doi: 10.1128/JVI.75.14.6558-6565.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med. 2014;6:228ra239. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med. 2014;6:228ra238. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 161.Yang K, Lan J, Shepherd N, Hu N, Xing Y, Byrd D, et al. Blockage of CD59 Function Restores Activities of Neutralizing and Nonneutralizing Antibodies in Triggering Antibody-Dependent Complement-Mediated Lysis of HIV-1 Virions and Provirus-Activated Latently Infected Cells. J Virol. 2015;89:9393–9406. doi: 10.1128/JVI.01614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Parker SJ, Sadlon TA, Gordon DL. Enhancement of NK cell-mediated antibody-dependent lysis of recombinant gp120-coated CD4 cells by complement. J Infect Dis. 1995;171:186–189. doi: 10.1093/infdis/171.1.186. [DOI] [PubMed] [Google Scholar]
- 163.Wang SY, Racila E, Taylor RP, Weiner GJ. NK-cell activation and antibody-dependent cellular cytotoxicity induced by rituximab-coated target cells is inhibited by the C3b component of complement. Blood. 2008;111:1456–1463. doi: 10.1182/blood-2007-02-074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang SY, Veeramani S, Racila E, Cagley J, Fritzinger DC, Vogel CW, et al. Depletion of the C3 component of complement enhances the ability of rituximab-coated target cells to activate human NK cells and improves the efficacy of monoclonal antibody therapy in an in vivo model. Blood. 2009;114:5322–5330. doi: 10.1182/blood-2009-01-200469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Romee R, Foley B, Lenvik T, Wang Y, Zhang B, Ankarlo D, et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17) Blood. 2013;121:3599–3608. doi: 10.1182/blood-2012-04-425397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhou Q, Gil-Krzewska A, Peruzzi G, Borrego F. Matrix metalloproteinases inhibition promotes the polyfunctionality of human natural killer cells in therapeutic antibody-based anti-tumour immunotherapy. Clin Exp Immunol. 2013;173:131–139. doi: 10.1111/cei.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Parsons MS, Richard J, Lee WS, Vanderven H, Grant MD, Finzi A, et al. NKG2D Acts as a Co-Receptor for Natural Killer Cell-Mediated Anti-HIV-1 Antibody-Dependent Cellular Cytotoxicity. AIDS Res Hum Retroviruses. 2016;32:1089–1096. doi: 10.1089/AID.2016.0099. [DOI] [PubMed] [Google Scholar]
- 168.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Terszowski G, Klein C, Stern M. KIR/HLA interactions negatively affect rituximab-but not GA101 (obinutuzumab)-induced antibody-dependent cellular cytotoxicity. J Immunol. 2014;192:5618–5624. doi: 10.4049/jimmunol.1400288. [DOI] [PubMed] [Google Scholar]
- 170.Ward JP, Bonaparte MI, Barker E. HLA-C and HLA-E reduce antibody-dependent natural killer cell-mediated cytotoxicity of HIV-infected primary T cell blasts. AIDS. 2004;18:1769–1779. doi: 10.1097/00002030-200409030-00005. [DOI] [PubMed] [Google Scholar]
- 171.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 172.Schlaepfer E, Speck RF. Anti-HIV activity mediated by natural killer and CD8+ cells after toll-like receptor 7/8 triggering. PLoS One. 2008;3:e1999. doi: 10.1371/journal.pone.0001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Winckelmann AA, Munk-Petersen LV, Rasmussen TA, Melchjorsen J, Hjelholt TJ, Montefiori D, et al. Administration of a Toll-like receptor 9 agonist decreases the proviral reservoir in virologically suppressed HIV-infected patients. PLoS One. 2013;8:e62074. doi: 10.1371/journal.pone.0062074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Offersen R, Nissen SK, Rasmussen TA, Ostergaard L, Denton PW, Sogaard OS, et al. A Novel Toll-Like Receptor 9 Agonist, MGN1703, Enhances HIV-1 Transcription and NK Cell-Mediated Inhibition of HIV-1-Infected Autologous CD4+ T Cells. J Virol. 2016;90:4441–4453. doi: 10.1128/JVI.00222-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schmidt M, Hagner N, Marco A, Konig-Merediz SA, Schroff M, Wittig B. Design and Structural Requirements of the Potent and Safe TLR-9 Agonistic Immunomodulator MGN1703. Nucleic Acid Ther. 2015;25:130–140. doi: 10.1089/nat.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wittig B, Schmidt M, Scheithauer W, Schmoll HJ. MGN1703, an immunomodulator and toll-like receptor 9 (TLR-9) agonist: from bench to bedside. Crit Rev Oncol Hematol. 2015;94:31–44. doi: 10.1016/j.critrevonc.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 177.Weihrauch MR, Richly H, von Bergwelt-Baildon MS, Becker HJ, Schmidt M, Hacker UT, et al. Phase I clinical study of the toll-like receptor 9 agonist MGN1703 in patients with metastatic solid tumours. Eur J Cancer. 2015;51:146–156. doi: 10.1016/j.ejca.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 178.Schmoll HJ, Wittig B, Arnold D, Riera-Knorrenschild J, Nitsche D, Kroening H, et al. Maintenance treatment with the immunomodulator MGN1703, a Toll-like receptor 9 (TLR9) agonist, in patients with metastatic colorectal carcinoma and disease control after chemotherapy: a randomised, double-blind, placebo-controlled trial. J Cancer Res Clin Oncol. 2014;140:1615–1624. doi: 10.1007/s00432-014-1682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vibholm L, Schleimann MH, Hojen JF, Benfield T, Offersen R, Rasmussen K, et al. Short-Course Toll-Like Receptor 9 Agonist Treatment Impacts Innate Immunity and Plasma Viremia in Individuals With Human Immunodeficiency Virus Infection. Clin Infect Dis. 2017;64:1686–1695. doi: 10.1093/cid/cix201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wiedemann GM, Jacobi SJ, Chaloupka M, Krachan A, Hamm S, Strobl S, et al. A novel TLR7 agonist reverses NK cell anergy and cures RMA-S lymphoma-bearing mice. Oncoimmunology. 2016;5:e1189051. doi: 10.1080/2162402X.2016.1189051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhou Z, Yu X, Zhang J, Tian Z, Zhang C. TLR7/8 agonists promote NK-DC cross-talk to enhance NK cell anti-tumor effects in hepatocellular carcinoma. Cancer Lett. 2015;369:298–306. doi: 10.1016/j.canlet.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 182.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10:230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ni Z, Knorr DA, Bendzick L, Allred J, Kaufman DS. Expression of chimeric receptor CD4zeta by natural killer cells derived from human pluripotent stem cells improves in vitro activity but does not enhance suppression of HIV infection in vivo. Stem Cells. 2014;32:1021–1031. doi: 10.1002/stem.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lam S, Sung J, Cruz C, Castillo-Caro P, Ngo M, Garrido C, et al. Broadly-specific cytotoxic T cells targeting multiple HIV antigens are expanded from HIV+ patients: implications for immunotherapy. Mol Ther. 2015;23:387–395. doi: 10.1038/mt.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhen A, Kitchen S. Stem-cell-based gene therapy for HIV infection. Viruses. 2013;6:1–12. doi: 10.3390/v6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17:1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]