Abstract
BACKGROUND
The improving efficacy of cancer treatment has resulted in an increasing array of treatment-related symptoms and associated burdens imposed on individuals undergoing aggressive treatment of their disease. Often, clinical trials compare therapies that have different types, and severities, of adverse effects. Whether rated by clinicians or patients themselves, it can be difficult to know which side effect profile is more disruptive or bothersome to patients. A simple summary index of bother can help to adjudicate the variability in adverse effects across treatments being compared with each other.
METHODS
Across 4 studies, a total of 5765 patients enrolled in cooperative group studies and industry-sponsored clinical trials were the subjects of the current study. Patients were diagnosed with a range of primary cancer sites, including bladder, brain, breast, colon/rectum, head/neck, hepatobiliary, kidney, lung, ovary, pancreas, and prostate as well as leukemia and lymphoma. All patients were administered the Functional Assessment of Cancer Therapy-General version (FACT-G). The single item “I am bothered by side effects of treatment” (GP5), rated on a 5-point Likert scale, is part of the FACT-G. To determine its validity as a useful summary measure from the patient perspective, it was correlated with individual and aggregated clinician-rated adverse events and patient reports of their general ability to enjoy life.
RESULTS
Analyses of pharmaceutical trials demonstrated that mean GP5 scores (“I am bothered by side effects of treatment”) significantly differed by maximum adverse event grade (P<.001) in all trials, with a clear trend toward increasing GP5 scores with level of increasing adverse event grade. Effect sizes ranged from 0.13 to 0.46. Analyses of cooperative group trials demonstrated a significant correlation between GP5 and item GF3 (“I am able to enjoy life”) in the predicted direction.
CONCLUSIONS
The single FACT-G item “I am bothered by side effects of treatment” is significantly associated with clinician-reported adverse events and with patients’ ability to enjoy their lives. It has promise as an overall summary measure of the burden of a given set of treatment toxicities compared with another. Future research can identify the contribution of individual side effects compared with one another in terms of how each may contribute to overall bother.
Keywords: cancer treatment, clinical trials, measurement science, oncology practice, outcomes, patient-reported outcomes (PROs), quality of life
INTRODUCTION
The number of cancer survivors continues to grow in the United States; it is estimated to be >15 million at the time of this writing,1 and is expected to grow to nearly 19 million by 2024.2 This is due primarily to improvements in treatment that have allowed patients diagnosed with cancer to live longer. This success has come with a cost, both monetary and personal. The increasing efficacy of cancer treatment has resulted in an increasing array of treatment-related symptoms and associated burdens imposed on individuals undergoing aggressive treatment of their disease. Patients treated aggressively with chemotherapy are at risk of a host of treatment-related side effects such as nausea, fatigue, depression, diarrhea, and declines in overall physical functioning.3 Some persistent side effects, such as peripheral neuropathy, have been found to increase health care costs and resource use.4 In addition, in cases in which treatment demands extend into years rather than months, avoiding toxicity may be a more relevant outcome than more traditional outcomes such as response rate or progression-free survival.5
To our knowledge, the extent to which treatment-related symptom burden is associated with outcomes such as treatment noncompliance, treatment discontinuation, and quality of life is largely unknown. Part of the reason for this is the lack of any clear way with which to summarize adverse treatment impact with a single summary or index of overall burden and tolerability. Instead, the safety profile of a given treatment consists of a broad array of adverse events (AEs) of varying prevalence and severity, with little available information regarding how to derive a “bottom line” treatment impact. To our knowledge, the concept of side effect bother is not well defined, but is believed to relate to whether or not treatment disrupts the normal activities of daily life, including social, emotional, and role functioning.
Recently, some oncologists have highlighted the importance of identifying individuals at high risk of side effects to attempt to minimize negative outcomes such as treatment discontinuation, dose reductions, and treatment delays.6 Clinician ratings of side effect severity tend to be unreliable, and underestimate the extent and severity. This highlights the need for patient self-report when assessing side effect burden.7 This is congruent with other similar phenomena, such as the stronger effect of self-reported health in comparison with physician-rated health in predicting mortality risk.8 In addition, at least one study to date has documented the superiority of self-rated health over physician-rated health specifically with respect to cancer mortality.9 The use of such self-reports may permit personalization of side effect management plans, tailored to individual patient needs. Recently, efforts have focused on developing patient-reported outcomes measures geared toward assessing when side effects are bothersome enough that a patient may wish to discontinue treatment,10 but to our knowledge to date there is no “gold standard” measure that is both concise and appropriately sensitive.
The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system has been in existence for 25 years, and was launched by publication of the flagship questionnaire, the Functional Assessment of Cancer Therapy-General.11 In its Physical Well-Being subscale, the FACT-G includes a question concerning side effect bother (item GP5: “I am bothered by side effects of treatment”), rated on a 5-point Likert scale from “not at all” to “very much.” This single question has the potential to be a useful summary index of side effect impact or burden to the individual patient. In prior research, it was found to differentiate imatinib from interferon/cytosine arabinoside treatment in patients with chronic myeloid leukemia12; to shed light, in the setting of metastatic kidney cancer, on the impact of axitinib versus sorafenib with regard to bother from similar treatments with different toxicity profiles5; and to predict which patients with breast cancer would prematurely discontinue aromatase inhibitor therapy.13 However, to the best of our knowledge, it has never been formally validated as a single question. The goal of the current study was to investigate the relationship between side effect burden, as measured by this single question, and relevant clinical outcome variables such as toxicity severity, on the Common Terminology Criteria for Adverse Events (CTCAE) as well as general life appreciation and patient quality of life.
MATERIALS AND METHODS
We analyzed a total of 4 data sets to assess the association between side effect bother, AEs, and quality of life. Two levels of analyses were conducted. Level 1 analyses examined the association between side effect bother and clinician-reported AEs within the context of 2 industry-sponsored clinical trials. Level 2 analyses examined the association between side effect bother and patient-reported ability to enjoy life in 2 cross-sectional studies. Wording of the single item was consistent across trials.
Level 1 Data Sets (Clinician-Reported Adverse Effects Correlated With GP5)
COMPARZ
COMPARZ (Pazopanib Versus Sunitinib in the Treatment of Locally Advanced and/or Metastatic Renal Cell Carcinoma) was a phase 3 randomized, open-label noninferiority trial of pazopanib compared with sunitinib in 1110 patients with advanced/metastatic renal cell carcinoma without prior systemic therapy (ClinicalTrials.gov identifiers NCT00720941 and NCT01147822).14,15 The study took place from 2008 to 2011. All AEs were graded according to version 3.0 of the National Cancer Institute CTCAE. The 19-item FACT Kidney Symptom Index,16 which includes FACT item GP5, was administered at baseline, on day 28 of cycles 1 through 9, and on day 42 of subsequent cycles.
ENESTnd
Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients (ENESTnd) was a phase 3, randomized, open-label, 3-arm trial conducted in adult patients with newly diagnosed Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase.17 From September 2007 to September 2008, a total of 846 patients were randomized to receive nilotinib or imatinib. AEs were graded according to version 3.0 of the National Cancer Institute CTCAE. The FACT-Leukemia (including the FACT-G and therefore item GP5) was completed at day 1 of cycle 1; at the end of cycles 3, 12, 24, 36, 48, and 60; and at early study discontinuation.
Level 2 Data Sets (Patient-Reported Quality of Life and Health Utility Correlated with GP5)
National Comprehensive Cancer Network
The National Comprehensive Cancer Network (NCCN) Symptom Index Study was a cross-sectional study that enrolled patients with bladder, brain, breast, colorectal, head/neck, hepatobiliary/pancreatic, kidney, lung, ovarian, and prostate cancers and lymphoma.18 A total of 533 patients were recruited from NCCN member institutions and 4 nonprofit social service organizations from 2005 through 2006. In addition to providing input regarding the most important symptom targets for advanced cancer treatment, study participants completed the FACT-G and the EuroQOL 5 Dimensions (EQ-5D), which is a widely used measure of health utility.
BIOQOL and Q-Score
The Bilingual Intercultural Oncology Quality of Life (BIOQOL) study was conducted between 1994 and 1996.19 Participants were recruited from 7 public and private urban cancer care centers in Atlanta; Chicago; and San Juan, Puerto Rico. Patients had a diagnosis of breast, colon, head/neck, or lung cancer, or malignancies related to the acquired immunodeficiency syndrome. The FACT-G (version 3) was administered. Participants with high literacy were randomly assigned to interview- or self-administration of the questionnaire, and all low-literacy patients were assigned to interview-administration.
The Q-Score database20 contains data collected in 1995 and 1996 from medical centers in Chicago; Baltimore; Philadelphia; and Toledo, Ohio. These sites participated in a project to evaluate the comparability of 4 widely used cancer quality-of-life questionnaires, one of which was the FACT-G.
Both studies included individuals with human immunodeficiency virus, and these patients were excluded from the current analyses. Thus, the current analyses were performed on the pooled cancer sample for a total sample size of 2886.
Statistical Analyses
Level 1 analyses correlated clinician-reported AE severity with the single-item “I am bothered by side effects of treatment” item responses. GP5 responses were linked to simultaneous AEs, inclusive of the start and end dates of AEs. Unresolved AEs were given an end date after the last observed study date. Only those AEs corresponding to patient-relevant effects were included (ie, laboratory test-based AEs were excluded). For each GP5 assessment in a study, we calculated the maximum AE grade linked to that assessment. For consistency and to maximize response variability, we focused on visits with the highest mean AE grade. Chi-square tests for ordinal data were used to evaluate the statistical significance of associations. Effect sizes for the difference in mean GP5 scores between groups were calculated as the mean difference divided by the pooled standard deviation. An additional analysis was conducted in COMPARZ, examining the association between GP5 responses and the total number of AEs, regardless of grade. There were too few simultaneous AEs in the ENESTnd study to support this analysis in that data set. All analyses were conducted on the combined sample across treatment arms for each study. Pearson correlations were used throughout the study.
Level 2 analyses consisted of correlating patient-reported measures of overall quality of life and health use to GP5 responses. For all 3 data sets, the percentage of patients responding “quite a bit” or “very much” to FACT-G item GF3 (“I am able to enjoy life”) was estimated within each GP5 response category and the association evaluated using ordinal chi-square statistics. In the NCCN study only, mean EQ-5D health utility scores were compared across GP5 response categories using analysis of variance.
RESULTS
Characteristics of the samples included in the analyses are described in Table 1. There was a wide range of distributions with regard to cancer diagnoses, age, and sex represented.
TABLE 1.
Patient Characteristics for Each Data Set
| COMPARZ N51110 | ENESTnd N5846 | NCCN N5533 | BIOQOL and Q-Score N5286 | |
|---|---|---|---|---|
|
| ||||
| Randomized to Pazopanib or Sunitinib | Randomized to Nilotinib or Imatinib | Cross-Sectional, Prior Experience With Chemotherapy | Cross-Sectional | |
| Diagnosis | ||||
| Bladder | 31 (6%) | |||
| Brain | 50 (9%) | |||
| Breast | 52 (10%) | 804 (28%) | ||
| Colorectal | 50 (9%) | 439 (15%) | ||
| CML | 846 (100%) | |||
| Endometrial | ||||
| Head and neck | 49 (9%) | 411 (14%) | ||
| Hepatobiliary | 50 (9%) | |||
| Kidney | 1110 (100%) | 50 (9%) | ||
| Lung | 50 (9%) | 503 (17%) | ||
| Lymphoma | 50 (9%) | 199 (7%) | ||
| Ovarian | 51 (10%) | |||
| Prostate | 50 (9%) | 205 (7%) | ||
| Other/unknown | 323 (11%) | |||
| Age, y | ||||
| Range | 18–88 | 18–85 | 24–88 | 17–99 |
| Median | 61 | 46 | 59 | 58 |
| Female sex | 297 (27%) | 355 (42%) | 257 (48%) | 1552 (54%) |
| KPS | ||||
| 70 or 80 | 271 (24%) | |||
| 90 or 100 | 839 (76%) | |||
| ECOG PS | ||||
| 0 | 122 (23%) | 933 (32%) | ||
| 1 | 258 (48%) | 826 (29%) | ||
| 32 | 153 (29%) | 1119 (39%) | ||
Abbreviations: BIOQOL, Bilingual Intercultural Oncology Quality of Life; CML, chronic myelogenous leukemia; COMPARZ, Pazopanib Versus Sunitinib in the Treatment of Locally Advanced and/or Metastatic Renal Cell Carcinoma; ECOG PS, Eastern Cooperative Oncology Group performance status; ENESTnd, Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients; KPS, Karnofsky performance status; NCCN, National Comprehensive Cancer Network.
Level 1 Results
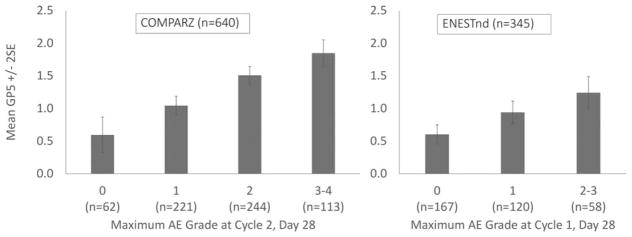
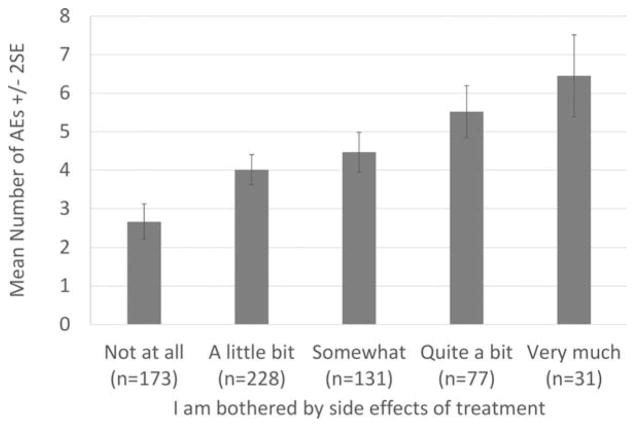
In COMPARZ, the highest mean AE grade occurred at day 28 of cycle 2. For ENESTnd, day 28 of cycle 1 was used. The correlation between GP5 scores and maximum AE grade at these selected visits was 0.34 in COMPARZ and 0.28 in ENESTnd. In both studies, GP5 scores significantly differed by maximum AE grade (P<.001), with a clear trend toward increasing scores with level of increasing maximum AE grade. The effect sizes (mean difference/pooled standard deviation) between adjacent groups ranged from 0.30 to 0.46 (Table 2) (Fig. 1). Patients in the COMPARZ study who reported more bother with side effects reported significantly more AEs of any grade (P<.001) (Fig. 2).
TABLE 2.
Mean GP5 Scores by Maximum AE Grade at Day 28 of Cycle 2
| Maximum AE Grade | No. | Mean GP5 | SD | ES |
|---|---|---|---|---|
| COMPARZ (N5640) | ||||
| 0 | 62 | 0.60 | 0.78 | - |
| 1 | 221 | 1.05 | 1.02 | 0.46 |
| 2 | 244 | 1.51 | 1.15 | 0.42 |
| 3–4 | 113 | 1.85 | 1.15 | 0.30 |
| ENESTnd (N5345) | ||||
| 0 | 167 | 0.60 | 0.90 | - |
| 1 | 120 | 0.94 | 0.96 | 0.36 |
| 2–3 | 58 | 1.24 | 1.01 | 0.30 |
Abbreviations: AE, adverse event; COMPARZ, Pazopanib Versus Sunitinib in the Treatment of Locally Advanced and/or Metastatic Renal Cell Carcinoma; ENESTnd, Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients; ES, effect size (mean difference between adjacent groups/pooled standard deviation); GP5, Functional Assessment of Cancer Therapy-General version (FACT-G) single item (“I am bothered by side effects of treatment”); SD, standard deviation.
Figure 1.
Mean Functional Assessment of Cancer Therapy-General version (FACT-G) single-item GP5 (“I am bothered by side effects of treatment”) scores by maximum adverse event (AE) grade. COMPARZ indicates Pazopanib Versus Sunitinib in the Treatment of Locally Advanced and/or Metastatic Renal Cell Carcinoma; ENESTnd, Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients; SE, standard error.
Figure 2.
Mean number of adverse events (AEs) of any grade by Functional Assessment of Cancer Therapy-General version (FACT-G) single-item GP5 (“I am bothered by side effects of treatment”) response on day 28 of cycle 2 of the COMPARZ (Pazopanib Versus Sunitinib in the Treatment of Locally Advanced and/or Metastatic Renal Cell Carcinoma) study. SE, standard error.
Level 2 Results
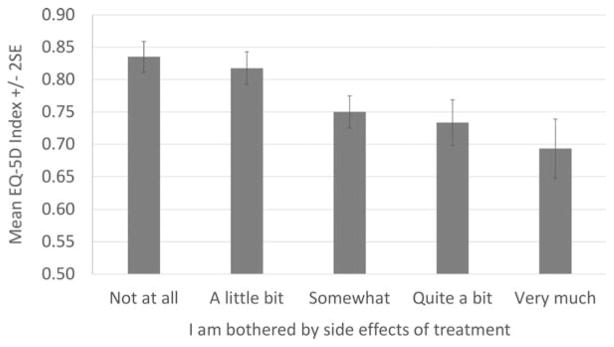
In the NCCN Symptom Index Study, the correlation between GP5 (“I am bothered by side effects of treatment”) and GF3 (“I am able to enjoy life”) was 0.28. This correlation also was 0.28 in the BIOQOL and Q-Score data set at 4 weeks after the initiation of treatment. GP5 response levels significantly differed in NCCN patients reporting that they were able to enjoy life “quite a bit” or “very much” (P<.001), ranging from 76% for those not at all bothered by side effects to 39% for those very much bothered (Table 3). A very similar response pattern was observed for the other data set. Patients in the NCCN study who reported more bother with side effects reported significantly worse EQ-5D health utility scores (P<.001) (Fig. 3).
TABLE 3.
Percentage of Patients Able to Enjoy Life “Quite a Bit” or “Very Much” Based on GP5 Response
| I Am Bothered by Side Effects Of Treatment | NCCN N5533 |
BIOQOL and Q-Score N52886 |
|---|---|---|
| Not at all | 76% | 78% |
| A little bit | 65% | 72% |
| Somewhat | 61% | 51% |
| Quite a bit | 43% | 48% |
| Very much | 39% | 42% |
| Chi-square P | <.0001 | <.0001 |
Abbreviations: BIOQOL, Bilingual Intercultural Oncology Quality of Life; GP5, Functional Assessment of Cancer Therapy-General version (FACT-G) single item (“I am bothered by side effects of treatment”); NCCN, National Comprehensive Cancer Network.
Figure 3.
Mean EuroQOL 5 Dimensions (EQ-5D) index scores by Functional Assessment of Cancer Therapy-General version (FACT-G) single-item GP5 (“I am bothered by side effects of treatment”) response in the National Comprehensive Cancer Network study (533 patients). SE indicates standard error.
DISCUSSION
The score on item GP5 of the FACT-G (“I am bothered by side effects of treatment”) was consistently associated with severity of AEs, such that patients reporting the highest side effect bother also had the highest maximum AE grade. This was true across disease and treatment types in the 2 industry-sponsored trials analyzed. This finding is striking in that there appears to be a high degree of concordance between patient-reported outcomes (GP5 score) and clinician-rated AEs. In addition, in the COMPARZ trial, higher scores on GP5 also were found to be highly correlated with a higher total number of AEs. Nevertheless, the correspondence is not so high that one could reasonably simply sum up the clinician-rated AEs and understand the patient perspective. The patient perspective, although correlated with overall AE burden and thereby lending validity data, is necessary to fully understand the impact of AEs on patients and their ability to enjoy life while receiving cancer therapy.
In the cross-sectional studies, the GP5 score was found to be inversely correlated with patients’ responses to the question “I am able to enjoy life ‘quite a bit’ or ‘very much’.” This means that patients who were more bothered by the side effects of treatment were less likely to report that they were able to enjoy life “quite a bit” or “very much.” This was true across all disease types and treatments. In addition, in the NCCN study, the GP5 response was found to be inversely correlated with scores on the EQ-5D, a measure of health utility. This indicates that patients who report the lowest level of side effect bother are those reporting the highest levels of overall life enjoyment.
The results of the current study support the use of a single, patient-reported outcome item, “I am bothered by side effects of treatment,” as a summary measure of the overall impact of treatment toxicity based on its association with the number and degree of AEs in clinical trials. In addition, the single item has demonstrated a significant relationship with overall quality of life as indicated by the ability to enjoy life. This result was found across cancer types and among varying treatment regimens in >5700 patients with cancer in 4 studies. One criticism of patient-reported outcomes measures has been the length of time that it takes patients to complete them, which results in patient burden but also burden on the staff working clinically or in a research setting with patients. This is a large part of the reason why patient-reported outcomes measures are not widely used in clinical practice and are only now becoming more widespread in cancer clinical trials. The current study addresses this concern regarding patient and clinician burden and allowed this single item to be used in pharmaceutical trials, as well as inpatient and out-patient clinical settings. It is our sincere hope that the current study will be useful to clinicians hoping to quantify the impact of patient-reported symptom burden on outcome variables such as treatment discontinuation.
The current research is aligned with the current US Food and Drug Administration interest in evaluating the patient’s perspective regarding AEs of treatment. This single item allows for a very brief summary assessment of the aggregate burden of side effects from the perspective of patients. Future research should investigate the use of this single-item scale to identify the contribution of individual side effects in relation to one another and within the side effect profile for a given drug. In addition, future studies may wish to explore the validity and usefulness of custom assessments drawn from the FACIT library. As an example, 11 cancer site-specific symptom indexes all include this single side effect burden question.18 Finally, assessing change over time is an intended use of this single item, and several of the data sets have longitudinal data that will support future explorations of responsiveness.
There are some limitations to the current study. The study analyzed the results of 2 pharmaceutical trials and 2 observational studies. As such, the outcome variables (other than the FACT-G) were not the same across trials. However, this also could be considered a strength in that the outcome variables were not limited just to those rated by clinicians (AEs) or patients (patient-reported outcome measures) but included both. In addition, the same limitation/strength argument could be made for the lack of standardization of disease types and treatments. We chose to include the wide range of subjects, believing that including such diverse groups would demonstrate the generalizability of the results to patients with a range of cancer diagnoses undergoing various treatment regimens.
One final limitation of the current study is the possibility that patients would underreport the severity of side effects for fear of treatment discontinuation or a “chemotherapy holiday” when the patient perceives the treatment as their best/last hope of prolonging life. Although to the best of our knowledge there is no literature to suggest that this is the case, it is our hope that by embedding the single item within a larger bank of patient-reported outcome measures we have reduced the possibility of this occurrence. Applications of this single-item rating across clinical trials of varying diagnoses and treatments will help to define the place of this summary measure over time.
Acknowledgments
FUNDING SUPPORT
No specific funding was disclosed.
Footnotes
AUTHOR CONTRIBUTIONS
All listed authors have contributed to the planning, conduct, and reporting of the work described in the article. Timothy P. Pearman is responsible for the overall content as guarantor.
CONFLICT OF INTEREST DISCLOSURES
Jennifer L. Beaumont has acted as a paid consultant for Novartis and received honorarium from GlaxoSmithKline for work performed outside of the current study. Daniel Mroczek was supported by National Institutes of Health/National Institute on Aging grant R01-AG018436 for work performed as part of the current study. David Cella was supported by a grant from Novartis for work performed as part of the current study and has had a patent issued for FACIT.org.
References
- 1.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivor-ship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 3.Pearman TP, Garcia S, Penedo F, Yanez B, Wagner LI, Cella D. Implementation of distress screening in an oncology setting. J Community Support Oncol. 2015;13:423–428. doi: 10.12788/jcso.0198. [DOI] [PubMed] [Google Scholar]
- 4.Pike CT, Birnbaum HG, Muehlenbein CE, Pohl GM, Natale RB. Healthcare costs and workloss burden of patients with chemotherapy-associated peripheral neuropathy in breast, ovarian, head and neck, and nonsmall cell lung cancer. Chemotherapy Res Pract. 2012;2012:913848. doi: 10.1155/2012/913848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cella D, Escudier B, Rini B, et al. Patient-reported outcomes for axitinib vs sorafenib in metastatic renal cell carcinoma: phase III (AXIS) trial. Br J Cancer. 2013;108:1571–1578. doi: 10.1038/bjc.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aprile G, Rihawi K, DeCarlo E, Sonis ST. Treatment-related gastrointestinal toxicities and advanced colorectal or pancreatic cancer: a critical update. World J Gastroenterol. 2015;21:11793–11803. doi: 10.3748/wjg.v21.i41.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett BK, Park SB, Lin CS, Friedlander ML, Kiernan MC, Goldstein D. Impact of oxaliplatin-induced neuropathy: a patient perspective. Support Care Cancer. 2012;20:2959–2967. doi: 10.1007/s00520-012-1428-5. [DOI] [PubMed] [Google Scholar]
- 8.Desalvo KB, Muntner P. Discordance between physician and patient self-rated health and all-cause mortality. Ochsner J. 2011;11:232–240. [PMC free article] [PubMed] [Google Scholar]
- 9.Giltay EJ, Vollaard AM, Kromhout D. Self-rated health and physician-rated health as independent predictors of mortality in elderly men. Age Ageing. 2012;41:165–171. doi: 10.1093/ageing/afr161. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser K, Beaumont JL, Webster K, et al. Development and validation of the Functional Assessment of Cancer Therapy-antiangiogenesis subscale. Cancer Med. 2015;4:690–698. doi: 10.1002/cam4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 12.Hahn EA, Glendenning GA, Sorensen MV, et al. IRIS Investigators. Quality of life in patients with newly diagnosed chronic phase chronic myeloid leukemia on imatinib versus interferon alfa plus low-dose cytarabine: results from the IRIS Study. J Clin Oncol. 2003;21:2138–2146. doi: 10.1200/JCO.2003.12.154. [DOI] [PubMed] [Google Scholar]
- 13.Wagner L, Zhao F, Chapman J, et al. Patient-reported predictors of early treatment discontinuation: NCIC JMA.27/E1Z03 quality of life study of postmenopausal women with primary breast cancer randomized to exemestane or anastrozole [abstract] Cancer Res. 2011;71(suppl 24):S2–S6. doi: 10.1007/s10549-018-4713-2. Abstract S6–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus suniti-nib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Hutson TE, McCann L, Deen K, Choueiri TK. Overall survival in renal-cell carcinoma with pazopanib versus sunitinib. N Engl J Med. 2014;370:1769–1770. doi: 10.1056/NEJMc1400731. [DOI] [PubMed] [Google Scholar]
- 16.Rao D, Butt Z, Rosenbloom S, et al. A comparison of the Renal Cell Carcinoma-Symptom Index (RCC-SI) and the Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI) J Pain Symptom Manage. 2009;38:291–298. doi: 10.1016/j.jpainsymman.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saglio G, Kim DW, Issaragrisil S, et al. ENESTnd Investigators. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 18.Cella D, Rosenbloom SK, Beaumont JL, et al. Development and validation of 11 symptom indexes to evaluate response to chemotherapy for advanced cancer. J Natl Compr Canc Netw. 2011;9:268–278. doi: 10.6004/jnccn.2011.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahn EA, Rao D, Cella D, Choi SW. Comparability of interview-and self-administration of the Functional Assessment of Cancer Therapy-General (FACT-G) in English- and Spanish-speaking ambulatory cancer patients. Med Care. 2008;46:423–431. doi: 10.1097/MLR.0b013e3181648e6e. [DOI] [PubMed] [Google Scholar]
- 20.Chang CH, Cella D. Equating health-related quality of life instruments in applied oncology settings. Phys Med Rehabil. 1997;11:397–406. [Google Scholar]