Abstract
BACKGROUND
Fatigue is prevalent and distressing among cancer survivors, but its subjective nature makes it difficult to identify. Fatigability, defined as task-specific fatigue, and endurance performance may be useful supplemental measures of functional status in cancer survivors.
METHODS
Fatigability, endurance performance, and cancer history were assessed every 2 years in Baltimore Longitudinal Study of Aging participants between 2007 and 2015. Fatigability was defined according to the Borg rating of perceived exertion scale after a 5-minute, slow treadmill walk; and endurance performance was calculated according to the ability and time to complete a fast-paced, 400-meter walk. The association between cancer history, fatigability, and endurance performance was evaluated using longitudinal analyses adjusted for age, sex, body mass index, and comorbidities.
RESULTS
Of 1665 participants, 334 (20%) reported a history of cancer. A combination of older age (>65 years) and a history of cancer was associated with 3.8 and 8.6 greater odds of high perceived fatigability and poor endurance, respectively (P < .01). Older adults with and without a history of cancer walked 42 and 23 seconds slower than younger adults without a history of cancer, respectively (P < .01). The median times to the development of high fatigability and poor endurance were shorter among those who had a history of cancer compared with those who had no history of cancer (P < .01).
CONCLUSIONS
The current findings suggest that a history of cancer is associated with fatigability and poor endurance and that this effect is significantly greater in older adults. Evaluating the effects of cancer and age on fatigability may illuminate potential pathways and targets for future interventions.
Keywords: aging, cancer-related fatigue, fatigability, functionality, mobility, survivorship
INTRODUCTION
Fatigue is a prevalent and distressing symptom among cancer survivors that often persists years after treatment completion.1–5 Defined as a lack of physical and/or mental energy that is subjectively perceived by the patient or caregiver as interfering with normal or desired activities, fatigue is negatively associated with quality of life, physical functioning, psychological status, and emotional well-being.6,7 Although as many as three-quarters of patients with cancer (undergoing active treatment) and cancer survivors (posttreatment) report fatigue, the experience of fatigue is difficult to define and quantify because of its subjective nature and situational dependence.6–9 Existing measures of fatigue used in cancer populations do not adequately reference symptoms to a certain level of physical effort, specific activities, or situational demands.8,9 Thus, fatigue may be underreported in cancer survivors, especially among those with low physical activity levels. Improved approaches to identify, quantify, and monitor issues related to fatigue in patients with cancer and survivors may increase our understanding of the nature and impact of fatigue and how best to manage it.
The construct of fatigability, a relatively new and emerging concept, assesses fatigue in relation to specified activities or situations and thus may provide an important and discriminating supplement to typical health and functional status evaluations in patients with cancer and survivors.6,10,11 Fatigability is well-validated in the gerontology literature and is commonly assessed using perceived effort and performance-based measures involving standardized walking tasks.11–13 Previous research has identified higher perceived fatigability as a risk factor for clinically meaningful decline in physical function (eg, gait speed, reported walking ability) in older adults, independent of generic (ie, nonactivity-anchored) reports of tiredness and low energy levels.8 There is currently a dearth of knowledge regarding the presence and impact of fatigability in older adults with a history of cancer diagnosis.
Routine assessment of fatigability in patients with cancer and cancer survivors may lead to a more complete and accurate picture of the psychological and physical effects of fatigue in this population and its threats to future function and quality of life. Given the increasing age and survivorship of the cancer population, a better understanding of the assessment, management, and treatment of cancer-related fatigue is imperative for improving quality of life and extending functional longevity among cancer survivors.
The current study examines baseline and longitudinal differences in perceived fatigability (the Borg rating of perceived exertion [RPE] after a 5-minute slow treadmill walk) and endurance walk performance in which failure to complete or slow performance can be interpreted as indicative of performance fatigability (ability and time to complete a fast 400-meter walk)11 by cancer history in participants from the Baltimore Longitudinal Study of Aging (BLSA), a well functioning cohort of middle-aged and older adults. We hypothesized that, after adjusting for meaningful confounders, those with a history of cancer would have higher perceived and performance fatigability at baseline and would experience a steeper increase in fatigability over subsequent follow-up.
MATERIALS AND METHODS
Study Population
The BLSA is a study of normative human aging that was established in 1958 and conducted by the National Institute on Aging Intramural Research Program. A general description of the sample and enrollment criteria has been previously reported.14 Briefly, the BLSA is a continuously enrolled cohort with some targeted recruitment (women, racial minorities) over its history. All participants are community-dwelling volunteers who pass a comprehensive health and functional screening evaluation and are free of all major chronic conditions and cognitive and functional impairments at the time of enrollment. Adults with a history of cancer may enroll provided they have been cancer-free for at least 10 years. However, once enrolled, participants are followed for life, regardless of disease development, and undergo extensive testing every 1 to 4 years, depending on age.
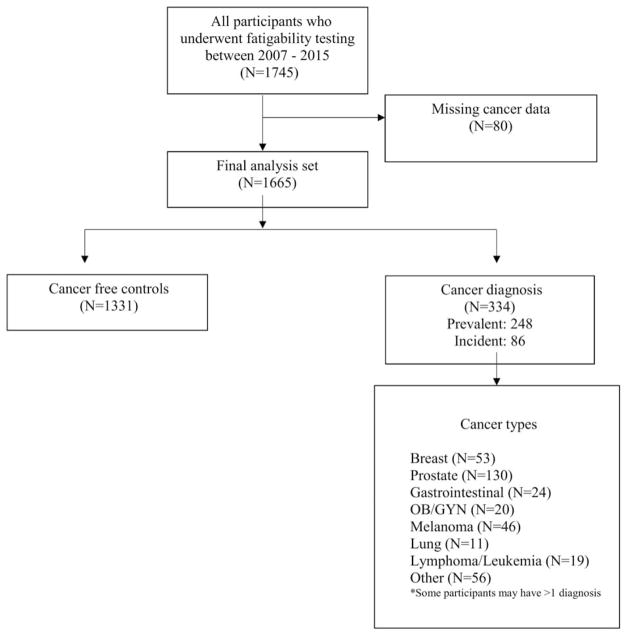
Among 1745 BLSA participants who underwent a comprehensive physical examination, health history assessment, and fatigability testing during their clinic visits between August 2007 and December 2015, 334 individuals reported a history of cancer and 1331 were cancer-free (Fig. 1). There were 80 patients who had unknown cancer status at baseline and were excluded from the analysis. The participants’ first clinic visit at which fatigability was assessed was termed the index visit for the purpose of this analysis. Participants with a history of cancer diagnosis either 1) enrolled 10 years after completion of treatment or 2) developed cancer during their time under study. Approximately 55% of BLSA participants are aged >65 years, and 20% of BLSA participants report a history of cancer, defined as ever having received a cancer diagnosis (excluding nonmelanoma skin and childhood cancers) and confirmed using International Classification of Diseases (9th edition) codes. The majority of individuals with a history of cancer were older than 65 years (79%). Trained and certified technicians administered all assessments according to standardized protocols. The Internal Review Board of the National Institute for Environmental Health Sciences approved the study protocol, and participants provided written informed consent at each visit.
Figure 1.
This is a participant flow diagram from 2007 (the time when fatigability testing was implemented into the Baltimore Longitudinal Study of Aging) through 2015. OB GYN indicates obstetrics and gynecology-related cancers.
Fatigability Assessment
Perceived fatigability
Perceived fatigability was assessed immediately after a slow-paced, 5-minute treadmill walk (1.5 miles per hour [0.67 miles per second] at 0% grade) by asking participants to rate their perceived exertion using the Borg RPE (range, 6–20; 6 indicates, no exertion at all; 9, very light exertion; 11, light exertion; 13, somewhat hard exertion; and 20, maximal exertion). The Borg RPE has previously been validated for the assessment of perceived fatigability in 605 community-dwelling adults within the BLSA.7,11 In the current analyses, perceived fatigability was treated as a continuous variable (range, 6–20) as well as a binary outcome, with high perceived fatigability defined as an RPE ≥10, a threshold that has been associated with decreased functional performance in previous validation work.11
Endurance walk performance
Endurance walk performance was evaluated as the ability and time to complete a fast 400-meter walk, over a 20-meter course in an uncarpeted corridor. Participants were instructed to walk “as quickly as possible at a pace that can be maintained” for 400 meters.15,16 Standard encouragement was provided with each lap, and individual lap times and total time to complete were recorded. Analyses examined endurance walk performance assessed as the time in seconds to walk 400 meters, with poor endurance walk performance as a binary outcome defined as either: 1) inability to complete the 400-meter walk or 2) taking more than 5 minutes to complete 400 meters. Previous work in the Health, Aging and Body Composition Study has determined that walk times longer than 5 minutes are indicative of substandard execution and thus can be considered evidence of performance deterioration.17 In addition, 80% of the study population at baseline could walk faster than 5 minutes at their index visit.18
Covariates
Covariates included age, sex, race, body mass index (BMI), smoking status, education, and comorbidities. Height and weight were assessed in light clothing using a stadiometer and calibrated scale, respectively, and BMI was calculated as mass in kilograms divided by height in meters squared. Age, education, and history of chronic conditions were derived from a health history interview conducted by a nurse practitioner. Age was dichotomized at 65 years to increase clinical interpretability. BMI was treated as a continuous variable, and sex, race, and smoking status were treated as categorical variables. Given the racial distribution of the BLSA cohort (70% white, 25% black, 5% other), race was dichotomized as white versus nonwhite race. Comorbidities included the presence of cardiovascular disease, pulmonary disease, liver disease, kidney disease, hypertension, diabetes, and depression. The total number of comorbidities was calculated for each individual and analyzed as a continuous variable.
Statistical Methods
Descriptive statistics were calculated as of the participant’s first clinic visit at which fatigability was assessed (the index visit). Differences in demographic characteristics and comorbidities were evaluated using Fisher exact and chi-square tests for categorical variables and the Student t test for continuous variables. Group differences in comorbidities were assessed using similar methods.
The association between fatigability and history of cancer was evaluated using longitudinal and time-to-event analyses. For the longitudinal analyses, the effect of cancer history on perceived fatigability and poor endurance walk performance was evaluated using generalized estimating equations with exchangeable correlations structures adjusted for age, BMI, sex, comorbidities, and race. The time origin (the index visit and the time since that visit in years) was the time variable. Two-by-2 contingency matrices were constructed to examine the independent and combined effects of aging and cancer on perceived fatigability, with the lowest risk group (those who were cancer-free and aged ≤65 years) used as the reference group and adjusting for sex, BMI, and the number of comorbidities.
Model fit statistics were compared to select the best model. Tests for interactions between age and cancer diagnoses were assessed. To understand differences in the time to high fatigability by cancer status, Kaplan-Meier survival plots for the time to high perceived fatigability (RPE >10) and poor endurance walk performance (400-meter walk time >5 minutes or failure) were created using the same time origin and metric used in the longitudinal analyses. Cox-regression analyses adjusted for sex, BMI, and the number of comorbidities were fit to calculate the hazard ratios associated with high perceived fatigability and poor endurance.
Subgroup analyses were conducted among participants who had been diagnosed with colorectal, breast, prostate, or lung cancers, because these represent the 4 most prevalent cancers among North Americans and are more homogeneous in treatments and long-term effects than less common cancers, such as leukemia. A sensitivity analysis was done using the same models as described above, but excluding participants diagnosed with cancer after the index visit. If the exclusion of incident cases did not alter the overall findings, it was decided to keep these cases in the analysis sample. All statistical analyses were performed using Stata version 14 (Stata Corporation, College Station, TX).
RESULTS
Study Population
In total, 1665 participants were included in the analysis; of these, 334 had a reported history of cancer, including 248 who had had a history of cancer at the time of their first fatigability visit, and an additional 86 developed cancer after the index visit (Fig. 1). The mean number of visits per participant was 4 (range, 1–11 visits), and 1166 participants (70%) had more than 1 fatigability assessment. The mean follow-up was 4.1 years (range, 0–10.7 years). The distribution of cancer types in this population is outlined in Figure 1. Among the participants who had a history of cancer, 213 were diagnosed with either breast (25%), lung (5%), prostate (61%), or colorectal (11%) cancer, and 44 were diagnosed with more than 1 primary cancer (excluding skin cancers). Demographic and medical characteristics of individuals with and without a history of cancer are presented in Table 1. Those with a history of cancer were more likely to be men, to be aged >65 years, and to have a history of smoking. The most common comorbidities included hypertension and cardiovascular disease. There were no differences between individuals with a history of cancer at the index visit (n = 248) and those who developed cancer after the index visit (n = 86). In individuals who had a history of cancer, the median age at cancer diagnosis was 66 years (interquartile range, 58–75 years), and the median time since diagnosis was 8 years.4–13
TABLE 1.
Characteristics of Included Participants by Cancer Status at the Time of the First Fatigability Assessment
| Characteristic | No. of Participants (%) | Pc | ||
|---|---|---|---|---|
|
| ||||
| History of Cancer | No History of Cancer, N = 1331 | |||
|
| ||||
| Prevalent, N = 248a | Incident, N = 86b | |||
| Age, y | ||||
| Mean ± SD | 73.6 ± 9.9 | 74.1 ± 12.1 | 69.3 ± 15.6 | |
| ≥65 | 52 (21.0) | 20 (23.3) | 486 (36.5) | < .001 |
| >65 | 196 (79.0) | 66 (76.7) | 845 (63.5) | |
| Sex | ||||
| Men | 152 (61.3) | 56 (65.1) | 597 (44.9) | < .001 |
| Women | 96 (38.7) | 30 (34.9) | 734 (55.2) | |
| BMI: Mean ± SD, kg/m2 | 26.9 ± 4.7 | 27.4 ± 4.3 | 27.4 ± 5.3 | |
| Race | .5 | |||
| Caucasian | 206 (83.1) | 59 (68.6) | 922 (69.3) | |
| Other | 42 (16.9) | 27 (31.4) | 409 (30.7) | .002 |
| Smoking statusd | ||||
| Ever smoker | 127 (51.4) | 45 (53.6) | 542 (41.1) | |
| Never smoker | 120 (48.6) | 39 (46.4) | 776 (58.9) | < .001 |
| Comorbidities | ||||
| Mean no. ± SD | 1.4 ± 1.2 | 1.4 ± 1.3 | 1.2 ± 1.3 | |
| Cardiovascular disease | 50 (20.2) | 13 (15.1) | 174 (13.1) | < .001 |
| Hypertension | 117 (47.2) | 41 (47.7) | 541 (40.7) | < .001 |
| Pulmonary disease | 14 (5.6) | 3 (3.5) | 68 (5.1) | .02 |
| Diabetes | 23 (9.3) | 9 (10.5) | 103 (7.7) | .67 |
| Stroke | 12 (4.8) | 6 (7.0) | 64 (4.8) | .27 |
| Liver disease | 2 (0.81) | 4 (4.6) | 13 (0.9) | .02 |
| Kidney disease | 15 (6.0) | 8 (9.3) | 64 (4.8) | .72 |
| Peripheral neuropathy | 28 (11.3) | 6 (7.0) | 149 (11.2) | .02 |
| Arthritis | 86 (34.7) | 32 (37.2) | 3 (0.7) | .03 |
| Depression | 44 (17.9) | 17 (20.7) | 247 (18.7) | .32 |
Abbreviations: BMI, body mass index; SD, standard deviation.
These were participants who had a diagnosis of cancer at the time of the index visit.
These were participants who had a diagnosis of cancer after the index visit (incident).
Differences are between patients who had any history of cancer versus those without a history of cancer.
Numbers shown for participants who reported smoking status- missing smoking status in 3 participants with a history of cancer and 13 cancer-free participants.
Longitudinal Analyses
In the overall study population, fatigability increased over time. At the index visit, the mean ± standard deviation (SD) Borg RPE was 8.5 ± 2.36, and 20% of participants (n = 115) reported high fatigability (Borg RPE >10). At the most recent visit, 287 adults (27%) reported high perceived fatigability after the standardized treadmill test with a Borg RPE >10 and a mean ± SD RPE of 8.7 ± 2.4. When assessing endurance walk performance at baseline, the mean ± SD 400-meter walk time was 246 ± 51 seconds, which increased to 265 ± 48 seconds as of the most recent visit (t test; P < .0001). The percentage of individuals who took longer than 5 minutes to complete the 400-meter walk or were unable to complete the walk increased from 17% to 32% (chi-square test; P < .0001). Participants who had a history of cancer were more likely to report high perceived fatigability (35% vs 25%; P = .005) and to take longer than 5 minutes or be unable to complete the 400-meter walk (29% vs 14%; P < .001) compared with those who had no history of cancer as of their most recent visit.
After adjusting for sex, BMI, and comorbidities in the longitudinal model, a history of cancer diagnosis was associated with a 1.6 increased odds of high perceived fatigability (95% confidence interval [CI], 1.04–2.36; P = .03), and age >65 years was associated with a 5.7 increased odds (95% CI, 3.7–8.6; P < .001). A history of cancer diagnosis was associated with a 400-meter walk time that was 14 seconds slower on average (95% CI, 7.9–20.0 seconds slower; P < .001) than the time among those without a history of cancer, and older age (>65 years) was associated with a 36-second increase in walking time for the 400-meter walk (95% CI, 31.2–41.1 seconds slower; P < .001) compared with younger adults (≤65 years).
When evaluating the combined effects of cancer history and age, the increase in perceived fatigability over time was greater in cancer survivors aged >65 years for both the continuous and categorical analyses compared with cancer-free adults aged ≤65 years (β, RPE 1.26; P < .05; odds ratio [OR], 3.8; 95% CI, 2.6–14.4) (Table 2). These results indicate that perceived fatigability in older adults (aged >65 years) with a history of cancer increased an average of 1.26 units on the Borg scale over time (years since index visit) compared with the reference group. Furthermore, cancer survivors aged >65 years had 3.8 greater odds of reporting high fatigability (Borg RPE >10) after a 5-minute, slow treadmill walk compared with cancer-free adults aged ≤65 years.
TABLE 2.
Continuous and Categorical Longitudinal Associations Among Perceived Fatigability, Endurance Walk Performance, and Cancer History Stratified by Agea
| Continuous Measuresc | Regression Coefficient (95% CI) | |
|---|---|---|
|
| ||
| Age ≤65 Years | Age >65 Years | |
| Perceived fatigability: RPE, continuous | ||
| Cancer | 0.37 (−0.14, 0.88) | 1.26 (0.95–1.59)b |
| No cancer | Ref | 0.98 (0.76–1.19)b |
| Endurance walking performance: Seconds, continuous | ||
| Cancer | 10.1 (−0.52, 20.7) | 42.3 (35.1–49.5)b |
| No cancer | Ref | 23.3 (18.9–27.7) |
|
| ||
| Categorical Measuresc | Odds Ratio (95% CI) | |
|
| ||
| Age ≤65 Years | Age >65 Years | |
|
| ||
| Perceived fatigability: RPE >10 | ||
| Cancer | 1.73 (0.95–2.56) | 3.8 (2.6–14.4)b |
| No cancer | Ref | 2.9 (2.2–9.3)b |
| Poor endurance walk performance: Time >5 min or inability to complete | ||
| Cancer | 1.71 (0.96–3.05) | 8.6 (6.1–12.2)b |
| No cancer | Ref | 4.6 (3.6–6.0)b |
Abbreviations: CI, confidence interval; OR, odds ratio; Ref, reference category; RPE, the Borg rating of perceived exertion.
Analyses were adjusted for sex, race, body mass index, and the number of comorbidities.
P <.001.
Regression coefficients and odds ratios comparing participants who had ever been diagnosed with cancer compared to those without a history of cancer stratified by age. In total, 850 participants were evaluable for the perceived fatigability analysis and 965 were evaluable for the endurance walk performance test.
Similarly, among cancer survivors aged >65 years, endurance walk performance deteriorated significantly over time (P < .05), and deterioration was greater among those who had a history of cancer (β, 42.3 seconds; P < .05; OR, 8.6; 95% CI, 6.1–12.2) (Table 2). These results indicate that older adults (aged >65 years) with a history of cancer slowed by an average of 42.3 seconds more over follow-up than younger adults (aged ≤65 years) without a history of cancer. In addition, older adults with a history of cancer had 8.6 greater odds of taking more than 5 minutes or being unable to walk 400 meters than younger adults without a history of cancer, indicating worse endurance performance. Tests for interactions between age and cancer were statistically significant for the endurance walk performance (P < .0001), but not for perceived fatigability (P= .11).
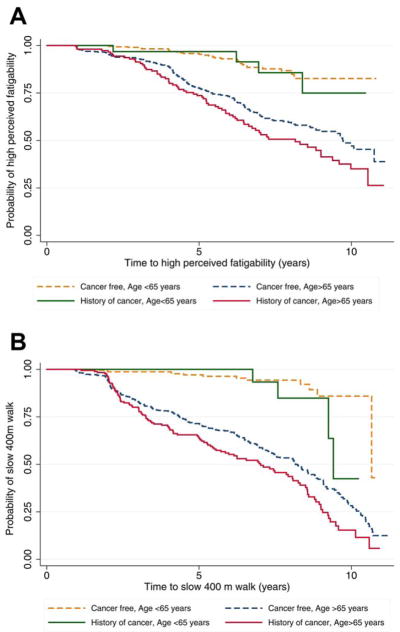
Survival Analyses
To provide clinical perspective, we examined the association between cancer history and time to high perceived fatigability and poor endurance walk performance. Figure 2 indicates that the trajectories of time to high fatigability differed (log-rank P <.001) by cancer history. Specifically, when stratified by cancer history and age, older adults with a history of cancer showed the shortest time to high perceived fatigability (Fig. 2A) and poor endurance walk performance (400 meter time >5 minutes) (Fig. 2B). Cox proportional-hazards models adjusted for age, sex, BMI, race, and number of comorbidities, participants who had a history of cancer had a 34% increased hazard of high perceived fatigability and a 42% increased hazard of poor endurance compared with those who had no history of cancer (Table 3). Thus, cancer survivors had a significantly greater risk of having a perceived fatigability rating on the Borg scale >10 or taking longer than 5 minutes to complete the 400-meter walk compared with those who had no history of cancer. No statistically significant interactions were observed between age and cancer history in Cox regression models.
Figure 2.
Charts illustrated (A) the time to high perceived fatigability and (B) the time to poor endurance walk performance.
TABLE 3.
Multivariable Cox Regression Hazard Ratios and 95% Confidence Intervals for the Risk of Developing High Fatigability in Adults With a History of Cancer Versus Those Without a History of Cancera
| Variable | Risk of High Perceived Fatigabilityb | Risk of Poor Endurance Performancec | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Ever diagnosed with cancer | 1.34 (1.04–1.72) | .02 | 1.42 (1.13–1.78) | .003 |
| Age >65 y | 2.89 (2.05–4.09) | < .001 | 8.3 (5.1–13.6) | < .001 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Participants were analyzed from the time of the index visit to the time of high fatigability (perceived or endurance performance) among participants who had ever been diagnosed with cancer compared to those without a history of cancer. In total, 850 participants were evaluable for the perceived fatigability analysis, and 965 were available for the endurance performance test.
High perceived fatigability was defined as first time that patient rated their perceived fatigability as >10 on the Borg rating of perceived exertion (RPE) scale after a slow 5-minute treadmill walk.
Poor endurance performance was defined as the first time a patient took longer than 5 minutes to complete a fast 400-meter walk.
Analyses were adjusted for sex, race, body mass index, and the number of comorbidities.
Sensitivity Analysis
Findings were similar when we excluded participants who developed cancer after the index visit (n = 86) for perceived fatigability and 400-meter walk endurance performance. Furthermore, no significant differences were observed across patient characteristics between incident and prevalent cases, and the change in perceived and performance fatigability were similar. Thus, patients with both incident and prevalent cancer were included in the analyses.
Subgroup Analysis
Analyses were repeated in a subgroup of participants who had a history of lung, prostate, breast, or colorectal cancer diagnosis to verify findings among more prevalent types of cancer.
Perceived fatigability
A history of 1 of these types of cancer was associated with 1.55 increased odds (95% CI, 1.13–2.14; P = .006) of high perceived fatigability compared with cancer-free individuals after adjusting for demographic factors and comorbidities. A significant interaction between age and cancer diagnosis further indicated that individuals aged >65 who had a history of 1 of these types of cancer had an increased odds of high perceived fatigability compared with individuals aged ≤65 years who had no history of cancer (P < .05).
Endurance walk performance
Individuals with a history of 1 of these types of cancer were less likely to complete the 400-meter walk test (14% vs 8%; P < .001) than those without a history of cancer. Among individuals who completed the 400-meter walk, those who had a history of 1 of these 4 types of cancer declined, on average, 21 seconds more than those who had no history of cancer (β, 20.7 seconds; 95% CI, 12.9–28.5 seconds; P < .001). An interaction between age and cancer diagnosis was significantly associated with worse endurance walk performance among those aged >65 years (OR, 2.8; 95% CI, 1.9–4.3; P < .001) compared with those aged ≤65 years who had no history of cancer.
DISCUSSION
Fatigue is a serious challenge for patients with cancer and for cancer survivors, and it poses a considerable threat to quality of life with aging.4,8 The construct of fatigability is a validated method of gauging the impact of fatigue by anchoring it to a standardized task.6,14 To our knowledge, this is the first study to evaluate fatigability prospectively in a large cohort of middle-aged and older adults and to compare these observations among those with and without a history of cancer. We observed that, even in this well functioning population, a history of cancer was associated with higher perceived fatigability and poorer endurance walk performance. Furthermore, the combination of older age and history of cancer appears to accelerate the onset and progression of fatigability. These findings support the hypothesis that a history of cancer is associated with higher fatigability and that this effect worsens with advancing age.
Both aging and cancer have been independently associated with declines in objective measures of physical function, including gait speed and loss of mobility.14,19–23 Our results suggest a possible additive effect of cancer and aging, because the median times to high perceived fatigability and poor endurance walk performance were 2.5 and 2.0 years faster, respectively, for those who had a history of cancer among older adults. Combined with our previous work defining an increased risk of poor functional outcomes among those with higher fatigability6,11 independent of reported tiredness and energy level, the current analysis underscores the increased risk of functional decline in older adults with a history of cancer and links this risk to fatigability status and poor endurance.
A typical individual aged 65 years is living with 2 or more comorbid conditions.24 In the current study, an increasing number of comorbidities also was identified as a strong risk factor for high perceived fatigability and poor endurance. This is consistent with findings in the gerontology literature, in which multiple chronic conditions have been associated with energy dysregulation, which subsequently may contribute to worsening fatigue and reduced daily physical activity.25,26 The addition of cancer and associated symptoms, active cancer treatment, and recovery from treatment to this burden increases the stress on an aging system, creating a need for more attention to effects that may persist long after the completion of treatment and may significantly impact quality of life.
Although the underlying mechanisms driving this association remain to be defined, there are several plausible explanations that warrant further investigation, including: 1) biologic precursors associated with both aging and cancer, such as sarcopenia, telomere shortening, impaired mitochondrial function, altered metabolism/energy expenditure, and cellular senescence2,15,16,23,27–29; 2) chronic inflammation,30–33 including markers such as interleukin-6, C-reactive protein, and tumor necrosis factor, which have been associated with fatigue after physical exertion, poor physical performance, and perceived health34–36; 3) age-related and cancer-related comorbid conditions, such as chronic obstructive pulmonary disease or chronic kidney disease; and 4) long-term side effects of cancer treatment(s) that are not well understood.
There are limitations associated with this analysis. First, the BLSA is a longitudinal cohort study that was designed to investigate the process of aging and is not specific to cancer. Therefore, additional information regarding cancer stage, treatment details, and pathology was not available. Some cancers may result in higher levels of fatigue than others,37 but the differential effects of cancer type could not be determined given the relatively small sample size. For instance, we observed a higher proportion of prostate cancer survivors (61%) compared with survivors of other cancer types, which may have influenced the overall findings. Although treatment details were not provided in the BLSA questionnaires, the majority of cancer survivors would have received some form of treatment for their respective cancers. Thus, the ability to detect a difference regardless of disease stage and treatment effects strengthens our confidence in these findings. In the majority of patients, the index visit (time of the first fatigability assessment) occurred after a cancer diagnosis; therefore, the time since diagnosis of cancer varied across participants and could introduce survivor bias in our findings, especially in the survival analysis. Finally, the BLSA study participants are generally healthier than the general population because of enrollment criteria. Therefore, lower rates of cancer were observed than expected, and survivors with a history of more aggressive cancers were less likely to return for their visits or to perform in the 400-meter walk test, resulting in potential underestimation of the true effects. Our findings should be explored and validated in clinical populations with known pathology and treatment history given the known associations between fatigue and certain treatment regimens, such as chemotherapy and/or radiation. It would also be of interest to study the impact of different cancer regimens in patients with advanced cancer and advanced cancer survivors.
In conclusion, our findings suggest that the risk of both perceived fatigability and poor endurance is high among older adults with a history of cancer. More research on fatigability and the interactions with cancer treatments, as well as its impact on quality of life, will help elucidate how best to incorporate this construct into cancer symptom and survivorship research. Currently, there are no screening interventions or tools for fatigability in cancer survivors. Given that the additional burden of cancer on an inevitable aging process is complex, more research is needed to disentangle their combined effects on fatigability. The use of this methodology among those with a history of cancer may help illuminate potential causal and treatment pathways for managing fatigue in this population.
Acknowledgments
FUNDING SUPPORT
This work was supported by grants from the National Institutes of Health/National Cancer Institute (R21AG053198) and the National Institutes of Health/National Institute on Aging (P30AG021334). These data were obtained from the Baltimore Longitudinal Study of Aging, part of the Intramural Research Program of the National Institute on Aging.
Footnotes
CONFLICTS OF INTEREST DISCLOSURES
The authors made no disclosures.
AUTHOR CONTRIBUTIONS
Gillian Gresham: Conceptualization, methodology, validation, formal analysis, data curation, writing–original draft, writing–review and editing, and visualization. Sydney M. Dy: Methodology, investigation, writing–review and editing, and supervision. Vadim Zipunnikov: Methodology and validation, writing–review and editing. Ilene S. Browner: Writing–review and editing. Stephanie A. Studenski: Investigation and writing–review and editing. Eleanor M. Simonsick: Conceptualization, methodology, investigation, data curation, and writing–review and editing. Luigi Ferrucci: Methodology, investigation, resources, writing–review and editing, supervision, and funding acquisition (National Institute on Aging, Intramural). Jennifer A. Schrack: Conceptualization (principal investigator), methodology, validation, investigation, data curation, resources, writing–review and editing, supervision, project administration, and funding acquisition.
References
- 1.Bower JE. Prevalence and causes of fatigue after cancer treatment: the next generation of research. J Clin Oncol. 2005;23:8280–8282. doi: 10.1200/JCO.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Bower JE. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Cancer Netw. 2015;13:1012–1039. doi: 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones JM, Olson K, Catton P, et al. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J Cancer Surviv. 2016;10:51–61. doi: 10.1007/s11764-015-0450-2. [DOI] [PubMed] [Google Scholar]
- 5.Matias M, Baciarello G, Neji M, et al. Fatigue and health behaviors in cancer survivors: a cross-sectional population based study [abstract] J Clin Oncol. 2017;35(15 suppl):10069. [Google Scholar]
- 6.Simonsick EM, Glynn NW, Jerome GJ, Shardell M, Schrack JA, Ferrucci L. Fatigued, but not frail: perceived fatigability as a marker of impending decline in mobility-intact older adults. J Am Geriatr Soc. 2016;64:1287–1292. doi: 10.1111/jgs.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 8.Mortimer JE, Barsevick AM, Bennett CL, et al. Studying cancer-related fatigue: report of the NCCN scientific research committee. J Natl Compr Cancer Netw. 2010;8:1331–1339. doi: 10.6004/jnccn.2010.0101. [DOI] [PubMed] [Google Scholar]
- 9.Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21:1525–1550. doi: 10.1007/s00520-012-1688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eldadah BA. Fatigue and fatigability in older adults. PM R. 2010;2:406–413. doi: 10.1016/j.pmrj.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Simonsick EM, Schrack JA, Glynn NW, Ferrucci L. Assessing fatigability in mobility-intact older adults. J Am Geriatr Soc. 2014;62:347–351. doi: 10.1111/jgs.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94–104. doi: 10.1016/j.jpainsymman.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Jensen RE, Potosky AL, Moinpour CM, et al. United States population-based estimates of patient-reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. J Clin Oncol. 2017;35:1913–1920. doi: 10.1200/JCO.2016.71.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter CS, Sonntag WE, Onder G, Pahor M. Physical performance and longevity in aged rats. J Gerontol A Biol Sci Med Sci. 2002;57:B193–B197. doi: 10.1093/gerona/57.5.b193. [DOI] [PubMed] [Google Scholar]
- 15.Cohen HJ. Biology of aging as related to cancer. Cancer. 1994;74(7 suppl):2092–2100. doi: 10.1002/1097-0142(19941001)74:7+<2092::aid-cncr2820741717>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 16.Cavanagh PR, Kram R. Mechanical and muscular factors affecting the efficiency of human movement. Med Sci Sports Exerc. 1985;17:326–331. [PubMed] [Google Scholar]
- 17.Simonsick EM, Newman AB, Visser M, et al. Mobility limitation in self-described well functioning older adults: importance of endurance walk testing. J Gerontol A Biol Sci Med Sci. 2008;63:841–847. doi: 10.1093/gerona/63.8.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010;60:120–132. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]
- 19.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–562. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 22.Anisimov VN. Biology of aging and cancer. Cancer Control. 2007;14:23–31. doi: 10.1177/107327480701400104. [DOI] [PubMed] [Google Scholar]
- 23.Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 24.Federal Interagency Forum on Aging-Related Statistics. Older Americans 2016: Key Indicators of Well-Being. Washington, DC: US Government Printing Office; 2016. [Google Scholar]
- 25.Ruggiero C, Metter EJ, Melenovsky V, et al. High basal metabolic rate is a risk factor for mortality: the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2008;63:698–706. doi: 10.1093/gerona/63.7.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrack JA, Gresham G, Wanigatunga AA. Understanding physical activity in cancer patients and survivors: new methodology, new challenges, and new opportunities [serial online] Cold Spring Harb Mol Case Stud. 2017;3:a001933. doi: 10.1101/mcs.a001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bronstein AM, Brandt T, Woollacott MH, editors. Clinical Disorders of Balance, Posture and Gait. London, UK: Edward Arnold; 1996. [Google Scholar]
- 28.Wright WE, Shay JW. Telomere biology in aging and cancer. J Am Geriatr Soc. 2005;53(9 suppl):S292–S294. doi: 10.1111/j.1532-5415.2005.53492.x. [DOI] [PubMed] [Google Scholar]
- 29.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Il’yasova D, Colbert LH, Harris TB, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 31.Caruso C, Lio D, Cavallone L, Franceschi C. Aging, longevity, inflammation, and cancer. Ann N Y Acad Sci. 2004;1028:1–13. doi: 10.1196/annals.1322.001. [DOI] [PubMed] [Google Scholar]
- 32.Lisanti MP, Martinez-Outschoorn UE, Pavlides S, et al. Accelerated aging in the tumor microenvironment: connecting aging, inflammation and cancer metabolism with personalized medicine. Cell Cycle. 2011;10:2059–2063. doi: 10.4161/cc.10.13.16233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 34.Brinkley TE, Leng X, Miller ME, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455–461. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raison CL, Lin J-MS, Reeves WC. Association of peripheral inflammatory markers with chronic fatigue in a population-based sample. Brain Behav Immun. 2009;23:327–337. doi: 10.1016/j.bbi.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Christian LM, Glaser R, Porter K, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Poorer self-rated health is associated with elevated inflammatory markers among older adults. Psychoneuroendocrinology. 2011;36:1495–1504. doi: 10.1016/j.psyneuen.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:123–133. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]