Abstract
Rationale
Previous work indicated that progesterone (PRO) reduced impulsive choice for cocaine in female but not male rats (Smethells et al. 2016). Impulsive action, typically measured by responding for a reinforcer during a signaled period of nonavailability of natural reinforcers, predicts initiation and escalation of drug use in animals and humans. The present study examined impulsive action for cocaine using progesterone (PRO) in male and female rats trained on a Go/No-go task.
Objective
Rats were trained on a Go/No-go task to respond for cocaine infusions (0.4 mg/kg/inf). During the “Go” component, responding was reinforced on a VI 30-s schedule; whereas, during the “No-Go” component withholding a response was reinforced on a differential reinforcement of other behavior (DRO) 30-s schedule. A response during the No-go component reset the DRO timer and served as a measure of impulsive action. After baseline responding was established, rats were pretreated with vehicle (VEH) or PRO (0.5 mg/kg), and DRO resets and responding during the Go component for cocaine were compared in males vs. females.
Results
DRO resets were significantly lower following PRO treatment compared to VEH in female, but not male, rats. Response rates and overall infusions during the Go component were not significantly altered by PRO in either females or males.
Conclusion
Treatment with PRO resulted in a sex-specific reduction in impulsive action for cocaine, while not affecting cocaine self-administration.
Keywords: Drug addiction, Go/No-go, Impulsive action, Progesterone, Rats, Sex differences
Introduction
Cocaine and other stimulant addiction is a significant problem in the United States (Substance Abuse and Mental Health Services 2015), yet treatments are not effective in promoting long-term abstinence (Czoty et al. 2016, Somoza et al. 2013). Reducing maladaptive behaviors underlying drug addiction such as impulsivity (Bickel et al. 2007) and stress (Solinas et al. 2010) is a novel treatment approach to reduce drug seeking. Increased impulsivity is a factor linked to initiation and relapse of drug use (e.g., Anker et al., 2009; Perry et al. 2005, 2008), as well as escalation of drug intake, and at least two major forms of impulsivity have been implicated in drug abuse; impulsive choice and impulsive action (see reviews by Weafer and de Wit 2014; Carroll and Smethells 2016). Developing therapeutics that decrease impulsive action could prevent cocaine use and cocaine relapse. Recent work by Smethells et al. 2016 indicated that impulsive choice for cocaine was reduced by progesterone (PRO) in female but not male rats. Thus, the present study examined the effect of PRO on another major form of impulsivity, impulsive action for cocaine reward.
Impulsivity has been commonly conceptualized as impulsive action or impulsive choice for reinforcers (see Weafer and de Wit 2014), and it has been examined using similar methodologies in humans and non-human animals. Impulsive action is assessed by measuring the ability to inhibit inappropriate or nonproductive responses. Tasks most commonly used for this purpose in animals are the stop signal reaction time task (SSRT) and Go/No-go task (Grant and Chamberlain 2014). Both tasks signal times when responding is reinforced or when responding is punished by imposing delays to the reinforcer. Deficits in the inhibitory part of the task are considered a form of impulsive action. The focus of the present study is to examine treatments to decrease impulsive action as potential therapeutics to reduce drug dependence.
Much of the research examining the relationship between impulsivity and drug abuse has used food or monetary reinforcers. However, impulsive drug use and its treatment have been significantly understudied and examination of underlying behaviors such as impulsive action would afford a better understanding of the behaviors and how to target mechanisms underlying drug addiction to prevent relapse. In an impulsive choice (i.e., delay discounting) task in humans, drug users were more impulsive for drugs such as nicotine (Bickel et al. 1999), heroin (Madden et al. 1997; Giordano et al. 2002) and alcohol (Petry 2001) than for money. Furthermore, the degree of impulsivity increased during drug withdrawal (Madden et al., 1997), suggesting that impulsive drug use may be a target for novel treatments.
The effects of novel therapeutics such as PRO on impulsive action for drugs are unknown, but initial preclinical results on impulsive choice for drugs are promising. Smethells et al. (2016) reported that female and male rats differentially decreased impulsive choice for cocaine with a delay-discounting task, after treatment with PRO and ATO, respectively. The likely factor underlying this sex difference is that PRO, a female gonadal hormone, typically decreases drug-seeking behaviors more effectively in females vs. male animals (Anker et al. 2009; Evans and Foltin 2010; Zlebnik et al. 2014; Smethells et al. 2016) and humans (Evans and Foltin 2006; Fox et al. 2013; Saladin et al. 2015; Milivojevik et al. 2016; for reviews, see Evans and Foltin 2010; Quinones-Jenab and Jenab 2012; Carroll and Lynch 2016; Carroll and Smethells 2016, Wetherill et al. 2016). Interestingly, PRO decreased impulsive action for sucrose pellets in a Go/No-go procedure in both male and female rats (Swalve et al. 2016a). Impulsivity has been described in two major forms, impulsive choice and impulsive action. Recent work indicated that PRO reduced impulsive choice for cocaine in female but not in male rats (Smethells et al. 2016), yet the impact of PRO on impulsive action for drug reinforcers, such as cocaine, has not yet been studied.
It is essential to study sex as a biological variable in both impulsive behaviors and drug addiction (see Weafer and DeWit 2014; Carroll and Lynch 2016; Carroll and Smethells 2016 for reviews), as in humans, women begin using cocaine at a younger age, have shorter times to relapse and use more frequently than men (Kosten et al. 1993; McCance-Katz et al. 1999; DeVito et al. 2014; for reviews, see Fattore et al. 2008; Fattore and Melis 2016). In most animal studies, females exceed males on acquisition (Lynch and Carroll 1999; Jackson et al. 2006; Lynch 2009; but see Caine et al. 2004; Swalve et al. 2016b; for review see Anker and Carroll 2011) and maintenance of cocaine self-administration (Lynch and Carroll 1999; Anker et al. 2011; Cummings et al. 2011; Peterson et al. 2014; Swalve et al. 2016c; but see Roberts et al. 1989; Cosgrove et al. 2002; Jackson et al. 2006; Kosten and Zhang 2008; Perry et al. 2013). Animal studies also indicate that females have a better response to PRO treatment for cocaine self-administration than males (Carroll and Lynch 2016; Carroll and Smethells 2016).
While there is relative consistency in the literature indicating greater sensitivity to the rewarding effects of cocaine in females vs. males, sex differences in impulsivity for drugs are mixed. In humans, males and females show significant differences in their response to motor impulsivity tasks. Women had more inhibitory errors and longer reaction times in an SSRT paradigm than men (Morgan et al. 2011; Crosbie et al. 2013), while men committed more inhibitory errors in continuous performance and Go/No-go tasks than women (Saunders et al. 2008; Liu et al. 2013). However, a few studies have shown contradictory results (Fields et al., 2009) or no sex differences (Reynolds et al. 2004; Townshend and Duka 2005; Fernie et al. 2010). Previous results in our laboratory are consistent with the preclinical literature, as female rats made more premature errors in a Go/No-go task for cocaine (Anker et al. 2008), and in a few other studies there were no sex differences in impulsive action with mice (Papaleo et al. 2012) and rats (Anker et al. 2008; Swalve et al., 2016a). In general, data are mixed on sex differences in impulsive action (see review by Fattore and Melis 2016), and little is known about the interaction between sex and impulsive action for cocaine use and its treatment.
Only a few studies have examined sex differences in impulsivity for drugs versus more common reinforcers such as food. They found that PRO decreased impulsive choice in females but not males (Smethells et al 2016). However, sex differences in impulsive action for a drug reinforcer and the effect of PRO on impulsive action for drugs in male and female animals is unknown. Given the discussed relationship between impulsive action for drugs and continued drug use as well as the sex-specific effects of drugs on impulsive choice, it is important to examine another major form of impulsivity, impulsive action. The rationale for studying the effect of PRO on impulsive action is based on our previous finding that PRO reduced impulsive choice for cocaine in female but not male rats (Smethells et al. 2016). Also, in another recent study PRO reduced impulsive action for sucrose pellets in both female and male rats (Swalve et al. 2016a). Thus there may be differences in the effect of PRO on drug- vs. natural-rewards such as sucrose (Swalve et al. 2016a). The goal of the present study was to examine sex differences in a Go/No-go task for a drug reinforcer (e.g. intravenous cocaine) and to examine PRO’s effects on impulsive action for cocaine. It was hypothesized that females would show greater impulsive responding for cocaine than males as measured by increased DRO resets during the No-go phase of the task. It was also expected that females would show a greater reduction in impulsive action for cocaine following PRO treatment than males.
Methods
Animals
Male (n = 16) and female (n = 14) Wistar rats (weighing 200–224 g for females and 250–274 g for males and matched for age at arrival) from Envigo Inc. (Madison, WI, United States) were used as subjects. Rats were initially housed in pairs in plastic home cages for a minimum of 3 days to allow for habituation to the facility. Food (Teklad 2018, Envigo Inc.) and water were available ad libitum during habituation. At the start of the experiment, rats were housed individually in experimental chambers. Rooms were maintained at 24° C and 40–50% humidity. All experiments occurred during the light cycle with a 12 h on/12 h off cycle (lights on at 600). Access to water was ad libitum during the experiments, but food was limited to 16 g for female rats and 20 g for male rats and was given 4 h after session conclusion. All experimental details were approved by the Institutional Animal Care and Use Committee at the University of Minnesota and performed in compliance with the Guide for the Care and Use of Animals (National Research Council, 2011). Research facilities were accredited by the American Association for the Accreditation of Laboratory Animal Care.
Apparatus
Customized octagonal chambers with alternating Plexiglas and stainless steel walls (Med Associates Inc, St. Albans, VT, USA) were encased in a sound-attenuating wooden box with a small ventilation fan. Each chamber contained an overhead white house light (4.6 W) and two retractable levers equipped with tri-colored stimulus lights (4.6 W) centered above each lever. During the drug self-administration portion of the experiment, a syringe pump (PHM-100, Med Associates) delivered infusions through a harness attached to a swivel-tether system (375/22PS, Instech, Plymouth Meeting, PA, USA; C313CS-MN, Plastics One, Roanoke, VA, USA). All experimental sessions were controlled by a computer running Med-PC IV® software (Med Associates).
Drugs
Cocaine HCl (National Institute of Drug Abuse, Research Triangle Institute, Research Triangle Park, NC, US) was dissolved in sterile saline to a final concentration of 1.6 mg cocaine/1 ml saline and given at a dose of 0.4 mg/kg/infusion. The duration of the infusion was set based on the weight of the rat (1s/100 g) and infused at 0.025 ml/s. Progesterone (PRO; Sigma Aldrich, St. Louis, MO) was dissolved in peanut oil (VEH; Sigma Aldrich) to a final dose of 0.5 mg/kg administered subcutaneously 30 minutes prior to experimental sessions. This dose has produced endogenously relevant levels of PRO (Jackson et al 2006), and it decreased cocaine-seeking and impulsivity for food and cocaine in our previous studies (see Anker et al. 2011 for a review; Smethells et al. 2016; Swalve et al. 2016a, b, d; Zlebnik et al. 2014).
Procedure
Autoshaping
Prior to examining impulsive action for cocaine, all animals were trained and tested in a Go/No-Go paradigm for food (see Swalve et al. 2016a). Briefly, rats were initially trained to lever-press on a randomly-assigned active lever for a sucrose pellet (Bioserv F0021) on an escalating variable-time and simultaneous fixed-ratio 1 (FR1) schedule. Rats were considered to have completed autoshaping after 3 consecutive days of 30 or more sucrose pellets earned per day.
Go/No-Go for Food
After criteria for autoshaping were met, rats were transferred to the Go/No-go phase. Briefly, the Go/No-go program consisted of alternating Go and No-go components. During the Go component, a lever press on the active lever produced a 45 mg sucrose pellet on a VI 30 sec schedule, and a press on the inactive lever produced no consequence. Alternatively, during the No-go component, withholding a response on the active lever produced a pellet on a Differential Reinforcement of Other Behavior (DRO) 30 sec schedule. Responding on the active lever during the No-go component reset the DRO timer, and a “DRO reset” was recorded as an index of impulsive action. The session began with the Go component and then moved to the No-go component, alternating back and forth throughout the session. Each component lasted 15 min with a 5 sec timeout between components for an approximately 2-h session. Thus, there were 4 Go alternating with 4 No-go components during the 2-hr session. Illumination of a stimulus light over the active lever marked the Go phase of the paradigm, while continuous flashing of the same stimulus light marked the No-go component. Once responding was stable (see Swalve et al. 2016a), rats were given either progesterone (0.5 mg/kg) or vehicle (peanut oil) 30 min prior to the beginning of the session, and they were tested to stability before proceeding to the Go/No-go for cocaine component. The data from several of these rats were reported previously in Swalve et al., (2016a) and will not be reported here.
Go/No-go for Cocaine
Rats initially underwent surgery according to the procedures described in Swalve et al. (2016a). Briefly, rats were anesthetized with a mixture of ketamine (60 mg/kg for females and 90 mg/kg for males) and xylazine (10 mg/kg) (Hsu et al. 1986). A single-beaded catheter (Plastics One, Roanoke, VA) was implanted into the jugular vein and attached to a harness and tether. Rats were given extended-release buprenorphine (0.05 mg/ml) post-surgery with ibuprofen (50 mg/kg) administered in the drinking water for pain relief. Patency was checked weekly with a mixture of ketamine (10 mg/kg) and midazolam (0.5 mg/kg) in saline. If a loss of righting reflex was observed within 10 seconds of the ketamine/midazolam infusion, the rat was considered patent; if patency was not observed, a second catheter was implanted in the opposite jugular vein using the surgical procedure described above.
Three days post-surgery, rats were started on the Go/No-go procedure for cocaine. This procedure is almost identical to the Go/No-go for food procedure with a few differences. First, the No-go phase was omitted during the initial training of cocaine self-administration. Ground food was initially placed on the active lever until rats completed 15 or more lever presses on the active lever for 3 days. After these criteria were met, rats were allowed to self-administer cocaine during the Go component in a 2-hour session until they reached the criteria of 25 or more infusions per session for 3 sessions to ensure that rats were reliably self-administering cocaine. The No-go phase was then added back to the session and responding continued until stability was reached. Identical to the food phase, each component lasted 15 min with a 5 sec timeout between components for a total of 2 hours with 4 phases of each component. Once stability was reached, rats were given treatment with either progesterone (0.5 mg/kg) or vehicle (peanut oil). The treatment received in the cocaine phase was identical to the treatment received in the food phase (e.g. if rats were treated with PRO in the food component, they were treated with PRO in the cocaine component). Stability was defined as the first 3 consecutive days of responding during treatment with 1 or more DRO resets and total infusions earned during the No-go component within 50% of the previous day’s level; stable sessions were used to examine group differences. Rats took between 10 and 62 days (mean = 32.2 ± 2.92) to reach baseline stability and between 3 and 26 days (mean = 7.7 ±0.68) to reach stability once treatment started.
Treatment with PRO vs. Vehicle (VEH)
A single dose of PRO (0.5 mg/kg) was used in this study, as it has preduced significant reductions in drug seeking in a comparison of males and females on drug-related behavior (see reviews, Carroll and Anker 2010; Anker and Carroll 2011). Previous studies indicated that there are relatively minor dose-effect differences of PRO, but significantly higher doses can trigger adverse hormonal effects (Larson et al. 2007; Anker et al. 2007, 2012; Smethells et al. 2016; Carroll and Lynch 2016; Carroll and Smethells 2016). Also, due to the relatively short catheter life, length of the training procedure used to maintain a stable Go/No-go baseline over a long period, and the primary comparison of sex differences, we were not able to test the Go/No-go behavior with a dose response effect of PRO.
Endocrine status of female rats
We did not monitor the endocrine status of the female rats in this study as it would have caused interference with catheter life and the delicate training procedures used to maintain a stable Go/No-go baseline over a long period. We attempted to limit potential direct effects of cycling hormones by including data over 3 consecutive days in females and males. Thus, the variability of the female rats’ 4-day estrous cycle was captured in the mean values used. However, when data were subsequently analyzed over the 3 individual test days, there was no day effect in females or males.
Data analysis
VI response rates during the Go component and DRO resets during the No-go component were recorded during the same 3 days of stability. Differences in stable responding during the VI and DRO components were normalized by transforming the raw data from rats into percent change from their baseline, as differences between rats on their VI responding and total DRO resets were occasionally high. Percent change was averaged over the 3 days of stable responding during baseline and treatment to represent each condition for analysis.
Independent samples t-tests were used to examine differences in VI and DRO responding prior to treatment in males and females. Sex and treatment effects during the No-go component were used as a measure of impulsive action and analyzed using a two-way ANOVA with follow-up analyses using Tukey-Kramer post hoc tests. The number of infusions earned by males and females treated with PRO or VEH under the VI and DRO contingencies as well as total infusions during both components were also compared in two-way ANOVAs to examine underlying sex or treatment differences in the baseline levels of responding for cocaine. Statistical analyses were conducted with SPSS version 17 and GraphPad Prism version 7. The alpha value was set at p <0.05 for establishing statistical significance.
Results
Results of the t-tests indicated that there were no significant differences in VI response rates, DRO resets, or infusions during the Go or No-go components between males and females. There were also no significant differences in responding during the Go and No-go components during baseline in either males or females. There were also no significant differences in infusions during either component and no differences in VI response rates or DRO resets between treatment groups. Thus, males and females had similar levels of responding prior to the beginning of treatment, and PRO vs. VEH treatment groups were similar in responding as well.
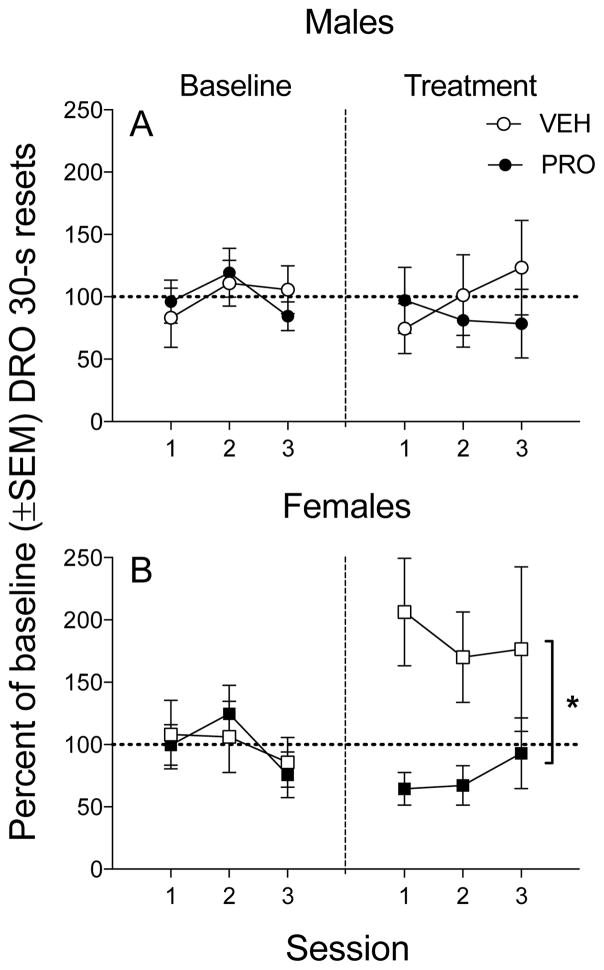
Figure 1 illustrates the percent change of DRO resets from baseline to treatment across stable sessions in males and females (each point indicates an individual subject and bars indicate group mean [+/− SEM]). According to a two-way ANOVA on the mean percent change of DRO resets across stable sessions of the No-go component, there was a main effect of treatment [F(1,26)=5.741, p<0.05], and an interaction between sex and treatment [F(1,26)=4.458, p<0.05], but there was no main effect of sex. Post hoc analyses showed that, compared to females treated with VEH, females treated with PRO had significantly lower percentage of baseline DRO resets. Table 1 shows the mean number of infusions, VI response rate and DRO resets (+/− SEM) by sex, schedule and treatment.
Fig 1.
Mean and individual DRO resets for cocaine expressed as a percentage of baseline (± SEM ) in males and females when pretreated with saline (open symbols) vs. PRO (filled symbols). The asterisks indicates a significant difference in the average percent change of baseline responding between the PRO- and VEH-treated groups *P<0.01
Table 1.
Mean VI response rate, DRO resets and infusions (+/− SEM) under VI and DRO schedule for Males and Females treated with Progesterone (PRO) or Vehicle (VEH)
| VI 30-s (Go phase) | DRO 30-s (No-go phase) | |||||
|---|---|---|---|---|---|---|
| Sex | Treatment (n) | Condition | Resp. Rate (r/m) | Inf. | DRO resets | Inf. |
| Male | VEH (n = 8) | Baseline | 0.54 (0.15) | 17.79 (2.34) | 16.54 (6.16) | 21.83 (0.45) |
| Tx | 0.63 (0.21) | 18.58 (3.57) | 19.75 (11.87) | 22.13 (0.56) | ||
| PRO (n=8) | Baseline | 0.51 (0.09) | 22.33 (3.40) | 37.71 (23.27) | 20.13 (1.67) | |
| Tx | 0.51 (0.15) | 19.58 (4.05) | 29.87 (16.41) | 19.79 (1.71) | ||
| Female | VEH (n=7) | Baseline | 15.12 (13.81) | 46.19 (18.24) | 47.10 (34.60) | 20.67 (1.46) |
| Tx | 17.78 (16.72) | 49.14 (20.44) | 57.24 (30.10) | 18.86 (1.17) | ||
| PRO (n=7) | Baseline | 0.84 (0.28) | 35.76 (9.29) | 16.48 (5.30) | 19.29 (1.78) | |
| Tx | 0.75 (0.34) | 29.76 (9.67) | 9.19 (2.72) | 21.71 (0.87) | ||
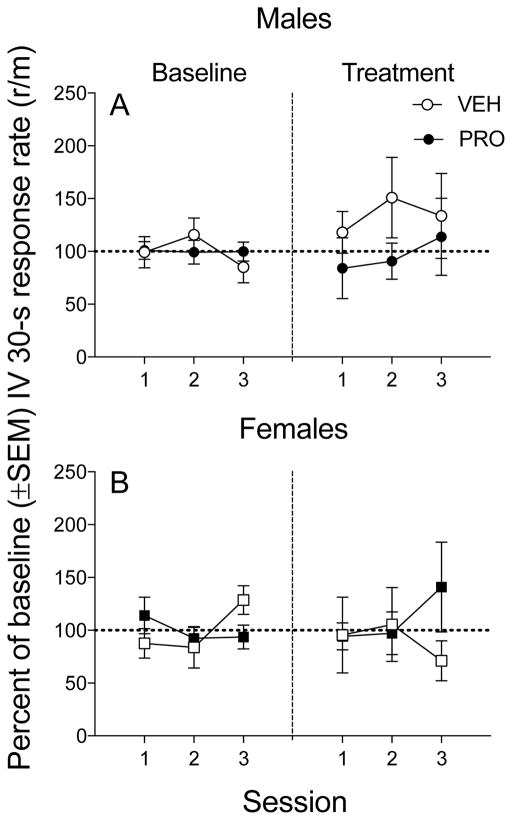
Figure 2 illustrates the percent change in VI response rates from baseline to treatment across stable sessions in males and females (each point indicates an individual subject and bars indicate group mean [+/− SEM]). A two-way ANOVA showed no significant effect of sex or treatment and no interaction between sex and treatment in the mean percent change of VI responding across stable sessions of the Go component. A separate two-way ANOVA showed no significant effect of sex or treatment and no interaction on VI response rates. Two-way ANOVA on infusions earned during the No-go component and total infusions over both components also indicated no significant main effects of sex and treatment and no interactions. Thus, treatment did not significantly affect responding for cocaine during the VI component or infusions earned during either component in either males or females.
Fig 2.
Mean and individual VI response rates for cocaine expressed as a percentage of baseline (± SEM ) in males and females when pretreated with saline (open symbols) vs. PRO (filled symbols).
Discussion
This study showed that PRO significantly reduced impulsive action for cocaine in females, as indicated by a relative decrease in percent change in DRO resets in the PRO group compared to the VEH group. Progesterone did not alter impulsive action in males, nor did it affect responding for cocaine during the Go component in either sex. This suggests that impulsive action for cocaine was attenuated by PRO compared to VEH in females, while activity and the overall reinforcement value of cocaine were not significantly altered by this dose. The effect of PRO in the present study was behaviorally specific to measures of impulsive action, as the attenuation of responding by PRO did not transfer to VI responding. The results also showed that impulsive action for cocaine was not significantly different prior to treatment in males vs. females. The present findings were consistent with recent previous results indicating that PRO reduced impulsive choice for cocaine infusions under a delay-discounting schedule in females but not in males (Smethells et al. 2016).
Impulsivity has emerged as a major factor contributing to drug addiction and its resistance to treatment (Perry and Carroll 2008; Weafer and de Wit 2014; Jentsch et al. 2014; Smethells et al. 2016). Reducing maladaptive behaviors such as impulsivity is a novel method for treating drug addiction (see Bickel et al., 2007). Results of the current study indicate that PRO, a female gonadal hormone, reduced impulsive action in female rats compared to VEH, but not in males. These results were consistent with previous findings from our laboratory showing that PRO attenuated impulsive choice for cocaine (Smethells et al. 2016) in females but not males. The findings that progesterone decreased both impulsive action (present study) and impulsive choice (Smethells et al. 2016) in female rats shows promise for treatment of behaviors such as impulsivity that underlie drug addiction in humans. In contrast, Smethells et al. (2016) reported that atomoxetine (ATO) reduced impulsivity choice for cocaine in males, but not in females. While it was beyond the scope of the present study, further studies are needed to examine ATO to determine whether male-specific treatments reduce impulsive action for cocaine.
The finding that PRO differentially alters impulsive action in female vs. male rats is consistent with previous reports on the effects of PRO on drug-taking and impulsivity. Progesterone was more effective in treating cocaine-seeking behavior in females than males in both human (Fox et al. 2013; Evans and Foltin 2006; for review, see Quinones-Jenab and Jenab 2010) and animal studies (Anker et al. 2009; Zlebnik et al. 2014; see Anker and Carroll, 2011; Smethells et al. 2016). Progesterone also significantly decreased impulsive choice for cocaine in females but not males in our previous study (Smethells et al., 2016).
While this is seemingly contrary to previous reports that PRO decreased cocaine self-administration and reinstatement, these findings are similar to that of Swalve et al. (2016d), that showed no effect of PRO on reinstatement. Both this study and previous work used a 2-h session length, and the present study had approximately 1 h of VI responding for cocaine. Session length has played a role in the lack of an effect of PRO, as sex differences are more often revealed during extended-access conditions (Roberts et al. 1989; Lynch and Carroll 1999; Lynch et al. 2000; Campbell et al. 2002; Carroll et al. 2002; Caine et al. 2004; Roth and Carroll 2004; Fuchs et al. 2005). In the present study, an advantage of short sessions was that the total amount of cocaine intake was limited, thus reducing the potential rate-altering effects of cocaine.
No sex differences were found in the present study between males on females on baseline levels of impulsive action in a Go/No-go task for cocaine. While previous reports showed that female rats responded more than males during a No-go component (Anker et al. 2008), there was no punishment contingency on responding during No-go in that study, which, combined with an increased session length, may have enhanced differences. The lack of sex differences in impulsivity prior to PRO treatment the present study is similar to results from studies in both humans (Townshend and Duka 2005, Reynolds et al. 2004; Fernie et al. 2010) and rodents (Papaleo et al. 2012). However, it is contrary to the results of tests of impulsive action in other models in humans and non-human animals (Jentsch and Taylor 2003; Anker et al. 2008; Saunders et al. 2008; Morgan et al. 2011; Bayless et al. 2012; Burton and Fletcher 2012; Papaleo et al. 2012; Crosbie et al. 2013; Liu et al. 2013). Further examination of the specificity of sex differences in different models of impulsive action is needed.
The present results support further examination of PRO in reducing impulsive action involved with cocaine seeking in humans, as results also suggest that a potential mechanism of PRO’s actions on drug use might be its ability to reduce impulsive drug seeking. In addition to the current Go/No-go task for impulsive action for cocaine, PRO has reduced several forms of drug seeking, ranging from initial acquisition of drug seeking after forced abstinence to maintenance of steady levels, escalation of drug intake, and relapse or reinstatement of drug seeking after a drug-free period (see reviews, Becker and Koob 2016; Carroll and Lynch 2016). Accumulating evidence suggests that the positive results in animal studies may apply to stimulant addiction in humans. For example, in one of the first studies of impulsivity and nicotine addiction in adolescent, Krishnan-Sarin et al. (2007) found that specific measures of impulsivity were associated with either the ability to initiate or to maintain abstinence from smoking. In another study, using a test of impulsive action (SSRT), Colzato et al (2010) found that women in the luteal phase of their menstrual cycle (when PRO is peaking) had less impulsive action than women in their follicular phase (when estrogen is peaking and PRO is low). Recently it was reported by Saladin et al. (2015) that higher endogenous PRO levels (luteal phase) were associated with higher odds of achieving smoking abstinence in women smokers treated with a nicotine patch or patch plus varenicline, compared to those who were administered PRO during the follicular phase, when the PRO to estrogen ratio is lower.
There are three main limitations to this study. First, acute cocaine has been shown to increase behavioral disinhibition (i.e., DRO resets) in the Go/No-go task (Paine and Olmstead, 2004) and increase impulsive choice in female rats (e.g., Smethells and Carroll 2015). Thus, the intake of cocaine could have significantly altered impulsivity throughout the session. However, there was no significant difference in total infusions, infusions during the Go components, or the discrimination index (e.g. the percentage of responding on the active vs. inactive lever). This suggests that measures of general responding and activity were not significantly altered by cocaine self-administration during the session, and only impulsive action was reduced by PRO treatment. Thus, potential rate-altering effects of cocaine as a reinforcer compared to sucrose or food may be limited.
Second, decreased in impulsive action by PRO, measured by a decrease in DRO resets, could lead to a higher overall amount of infusions during the No-go component. However, there were no effects of treatment on number of infusions earned during the No-go component. Only DRO resets were affected, suggesting that PRO was selectively limiting impulsive action, while having no effect on overall drug use and activity.
Third, decreased responding in the female PRO-treated group compared to the VEH group may have been driven by increased in responding in the VEH group. Female rats may have differentially shown increased impulsive action, as measured by DRO resets, over days of treatment that did not occur in male rats. This potentiation of impulsivity is what was reduced by PRO, but the potential behavioral and neurobiological mechanisms behind this increase in the VEH group and attenuation by PRO are unknown. It would be informative to examine the time-course of impulsive action for other drugs in male and female rats.
In summary, the present results revealed that PRO decreased impulsive action for cocaine compared to VEH in female but not male rats. Higher levels of impulsivity are associated with increased drug-seeking and initiation of drug use (see reviews by Weafer and de Wit 2014; Carroll and Smethells 2016), and treating these underlying behaviors may be a useful strategy for decreasing current or future drug use. Progesterone is a hormone that is already used in many contraceptives currently on the market, and it is commonly used among the female population; thus, future research on PRO’s ability to decrease impulsivity related to drug addiction, and other psychiatric disorders in humans, is warranted.
Acknowledgments
This study was supported by NIH/NIDA P50 DA033942 (MEC) and NIDA training grant T32 DA007097 (JRS: Thomas Molitor, PI).
References
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and role of ovarian hormones. In: Neill JC, Kulkarni J, editors. Biological Basis of Sex Differences in Psychopharmacology: Current Topics in Behavioral Neurosciences. London, UK: Springer; 2011. pp. 73–96. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Gliddon LA, Carroll ME. Impulsivity on a Go/No-go task for intravenous cocaine and food in male and female rats selectively bred for high and low saccharin intake. Behav Pharmacol. 2008;19:615–629. doi: 10.1097/FBP.0b013e32830dc0ae. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Holtz NA, Carroll ME. Effects of progesterone on escalation of IV cocaine self-administration in rats selectively bred for high (HiS) or low (LoS) saccharin intake. Behav Pharmacol. 2012;23:205–2010. doi: 10.1097/FBP.0b013e32834f9e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp and Clin Psychopharm. 2007;15:472–480. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Zlebnik NE, Gliddon LA, Carroll ME. Performance under a Go/No-go task in rats selected for high and low impulsivity with a delay-discounting procedure. Behavioural Pharmacology. 2009;20:406–414. doi: 10.1097/FBP.0b013e3283305ea2. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Zlebnik NE, Navin SE, Carroll ME. Responding during signaled availability and nonavailability of iv cocaine and food in rats: age and sex differences. Psychopharmacology (Berl) 2011;215:785–799. doi: 10.1007/s00213-011-2181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless DW, Darling JS, Stout WJ, Daniel JM. Sex differences in attentional processes in adult rats as measured by performance on the 5-choice serial reaction time task. 2012 doi: 10.1016/j.bbr.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Becker JB, Koob GF. Sex differences in animal models: Focus on addiction. Pharmacol Rev. 2016;68:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, et al. Behavioral and neuroeconomics of drug addiction: Competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90:85–91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CL, Fletcher PJ. Age and sex differences in impulsive action in rats: the role of dopamine and glutamate. Behav Brain Res. 2012;230:21–33. doi: 10.1016/j.bbr.2012.01.046. [DOI] [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacol. 2004;29(5):929–42. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Morgan AD, Carroll ME. Sex differences in the effects of baclofen on the acquisition of intravenous cocaine self-administration in rats. Drug Alcohol Dep. 2002;66(1):61–69. doi: 10.1016/s0376-8716(01)00185-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian steroid hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ. How to study sex differences using animal models. Addic Biol. 2016;21(5):1007–29. doi: 10.1111/adb.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology. 2002;161(3):304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Smethells JR. Sex differences in behavioral dyscontrol: Role in drug addiction and novel treatments. Front Psychiatry. 2016 Feb 8;6:175. doi: 10.3389/fpsyt.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, Ruiz MJ, van den Wildenberg WP, Bajo MT, Hommel B. Estrogen modulates inhibitory control in healthy human females: evidence from the stop-signal paradigm. Neuroscience. 2010;167:709–715. doi: 10.1016/j.neuroscience.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter R, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav. 2002;73:663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Crosbie J, Arnold P, Paterson A, Swanson J, Dupuis A, Li X, Shan J, Goodale T, Tam C, Strug LJ, Schachar RJ. Response inhibition and ADHD traits: correlates and heritability in a sommunity sample. J Abnorm Child Psychol. 2013;41:497–507. doi: 10.1007/s10802-012-9693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB. Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol Sex Differ. 2011;2(3) doi: 10.1186/2042-6410-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Stoops WW, Rush Evaluation of the “pipeline” for development of medications for cocaine use disorder: A review of translational preclinical, human laboratory, and clinical trial research. Pharmacol Rev. 2016;68:533–562. doi: 10.1124/pr.115.011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Herman AI, Andrew JW, Valentine GW, Sofuoglu M. Subjective, physiological, and cognitive responses to intravenous nicotine: effects of sex and menstrual cycle phase. Neuropsychopharmacology. 2014;39:1413–1410. doi: 10.1038/npp.2013.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Hormones and Behav. 2010;58:13–21. doi: 10.1016/j.yhbeh.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous Progesterone Attenuates the Subjective Effects of Smoked Cocaine in Women, but not in Men. Neuropsychopharm. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Fattore L, Altea S, Fratta W. Sex differences in drug addiction: a review of animal and human studies. Womens Health (Lond) 2008;4:51–65. doi: 10.2217/17455057.4.1.51. [DOI] [PubMed] [Google Scholar]
- Fattore L, Melis M. Sex differences in impulsive and compulsive behaviors: a focus on drug addiction. Addiction Biology. 2016;21:1043–1051. doi: 10.1111/adb.12381. [DOI] [PubMed] [Google Scholar]
- Fernie G, Cole JC, Goudie AJ, Field M. Risk-taking but not response inhibition or delay discounting predict alcohol consumption in social drinkers. Drug Alcohol Depend. 2010;112:54–61. doi: 10.1016/j.drugalcdep.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Fields S, Collins C, Leraas K, Reynolds B. Dimensions of impulsive behavior in adolescent smokers and nonsmokers. Exp Clin Psychopharmacol. 2009;17:301–311. doi: 10.1037/a0017185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sofuoglu M, Morgan PT, et al. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: Impact of gender and cue type. Psychoneuroendocrinology. 2013;38:1532–1544. doi: 10.1016/j.psyneuen.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology. 2005;179(3):662–672. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Giordano LA, Bickel WK, Loewenstein G, et al. Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology. 2002;163:174–182. doi: 10.1007/s00213-002-1159-2. [DOI] [PubMed] [Google Scholar]
- Grant JE, Chamberlain SR. Impulsive action and impulsive choice across substance and behavioral addictions: cause or consequence? Addict Behav. 2014;39:1632–1639. doi: 10.1016/j.addbeh.2014.04.022. [DOI] [PubMed] [Google Scholar]
- Hsu WH, Bellin SI, Dellmann HD, Hanson CE. Xylazine-ketamine-induced anesthesia in rats and its antagonism by yohimbine. J Am Vet Med Assoc. 1986;189:1040–3. [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharm. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Ashenhurst JR, Cervantes M, James AS, Groman SM, Pennington ZT. Dissecting impulsivity and its relationships to drug addictions. Ann N Y Acad Sci. 2014;1327:1–26. doi: 10.1111/nyas.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Sex-related differences in spatial divided attention and motor impulsivity in rats. Behav Neurosci. 2003;117:76–83. doi: 10.1037//0735-7044.117.1.76. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat. 1993;10:63–66. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY. Sex differences in non-reinforced responding for cocaine. Am j Drug Alcohol Abuse. 2008;34:473–488. doi: 10.1080/00952990802082206. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss, McFetridge TA, Cavallo DA, Carroll KM, Potenza MN. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Dr Alc Dep. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 2007;15:461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Liu T, Xiao T, Shi J. Response inhibition preattentive processing, and sex difference in young children: an event-related potential study. Neuroreport. 2013;24:126–130. doi: 10.1097/WNR.0b013e32835d846b. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol. 2009;14:34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152:132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control patients: Drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers—implications for treatment and prognosis. Am J Addict. 1999;8:300–311. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- Millivojevic V, Fox HC, Sofuoglu M, Covault J, Sinha R. Effects of progesterone stimulated allopregnanolone on craving and stress response in cocaine dependent men and women. Psychoneuroendocrinology. 2016;65:44–53. doi: 10.1016/j.psyneuen.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JE, Gray NS, Snowden RJ. The relationship between psychopathy and impulsivity: A multi-impulsivity measurement approach. Personality and Individual Differences. 2011;51:429–434. [Google Scholar]
- National Research Council. Guidelines for the Care and Use of Laboratory Animals. 8. The National Academic Press; Washington, DC: 2011. [Google Scholar]
- Paine TA, Olmstead MC. Cocaine disrupts both behavioural inhibition and conditional discrimination in rats. Psychopharmacology (Berl) 2004;175:443–450. doi: 10.1007/s00213-004-1845-3. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Erickson L, Liu G, Chen J, Weinberger DR. COMT genetic reduction produces sexually divergent effects on cortical analomy and working memory in mice and humans. Creeb Cortex. 2012;25:2529–2541. doi: 10.1093/cercor/bhu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AB, Aivick DP, Lynch WJ. Dose-dependent effectiveness of wheel running to attenuate cocaine-seeking: impact of sex and estrous cycle in rats. Psychopharmacology (Berl) 2014;231:2661–2670. doi: 10.1007/s00213-014-3437-1. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB. The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors. PloS One. 2013;8(11):946–5. doi: 10.1371/journal.pone.0079465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1026. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of i.v. cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Jenab S. Influence of sex differences and gonadal hormones on cocaine addiction. ILAR J. 2012;53:14–22. doi: 10.1093/ilar.53.1.14. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav processes. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self- administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology. 2004;172(4):443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Saladin ME, McClure EA, Baker MS, Carpenter MJ, Viswanathan R, Hartwell, Gray K. Increasing progesterone levels are associated with smoking abstinence among free-cycling women smokers who receive brief pharmacotherapy. Nic Tobacco Res. 2015;17:398–406. doi: 10.1093/ntr/ntu262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders B, Farag N, Vincent AS, Collins FL, Jr, Sorocco KH, Lovallo WR. Impulsive errors on a Go-NoGo reaction time task. Alcohol Clin Exp Res. 2008;32:888–894. doi: 10.1111/j.1530-0277.2008.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smethells JR, Carroll ME. Discrepant effects of acute cocaine on impulsive choice (delay discounting) in female rats during an increasing-and adjusting-delay procedure. Psychopharmacol. 2015;232:2455–2462E. doi: 10.1007/s00213-015-3874-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smethells JR, Swalve NL, Eberly LE, Carroll ME. Sex differences in the reduction of impulsive choice (delay discounting) for cocaine in rats with atomoxetine and progesterone. Psychopharmacology. 2016;233:2999–3008. doi: 10.1007/s00213-016-4345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Thiriet C, Jaber M. Prevention and treatment of drug addiction by environmental enrichment. Neurobiol. 2010;96:572–592. doi: 10.1016/j.pneurobio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Somoza EC, Winship D, Gorodetzky CW, Lewis D, Ciraulo DA, Galloway GP, et al. A multisite, double-blind, placebo-controlled clinical trial to evaluate the safety and efficacy of vigabatrin for treating cocaine dependence. JAMA Psychiatry. 2013;70:630–637. doi: 10.1001/jamapsychiatry.2013.872. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2014 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-50, HHS Publication No. (SMA) 15-4927. NSDUH Series H-50). Retrieved from http://www.samhsa.gov/data/
- Swalve N, Smethells JR, Carroll ME. Progesterone attenuates impulsive action for sucrose in a Go/No-go task for sucrose pellets in female and male rats. Horm Behav. 2016a;85:43–47. doi: 10.1016/j.yhbeh.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swalve N, Smethells JR, Carroll ME. Sex differences in attenuation of nicotine reinstatement after individual and combined treatments of progesterone and varenicline. Behav Brain Res. 2016b;308:46–52. doi: 10.1016/j.bbr.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swalve N, Smethells JR, Carroll ME. Sex differences in the acquisition and maintenance of cocaine and nicotine self-administration in rats. Psychopharmacology. 2016c;233:1005–1013. doi: 10.1007/s00213-015-4183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swalve N, Smethells JR, Zlebnik NE, Carroll ME. Sex differences in reinstatement of cocaine-seeking behavior with combination treatments of progesterone and atomoxetine. Pharmacol Biochem Behav. 2016d;145:17–23. doi: 10.1016/j.pbb.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Binge drinking, cognitive performance and mood in a population of young social drinkers. Alcohol Clin Exp Res. 2005;29:317–325. doi: 10.1097/01.alc.0000156453.05028.f5. [DOI] [PubMed] [Google Scholar]
- Weafer J, de Wit H. Sex differences in impulsive action and impulsive choice. Addictive Behaviors. 2014;39:1573–1579. doi: 10.1016/j.addbeh.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Franklin TR, Allen SS. Ovarian hormones, menstrual cycle phase, and smoking: a review with recommendations for future studies. Curr Addict Rep. 2016;3:1–8. doi: 10.1007/s40429-016-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Saykao AT, Carroll ME. Effects of combined exercise and progesterone treatments on cocaine seeking in male and female rats. Psychopharmacology. 2014;231:3787–3798. doi: 10.1007/s00213-014-3513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]