Abstract
Background
Previous evidence linking diabetes to Alzheimer’s disease (AD) neuropathology is mixed and scant data are available from low- and middle-income countries.
Objective
To investigate the association between diabetes and AD neuropathology in a large autopsy study of older Brazilian adults.
Methods
In this cross-sectional study, diabetes was defined by diagnosis during life or use of antidiabetic medication. A standardized neuropathological examination was performed using immunohistochemistry. The associations of diabetes with Consortium to Establish and Registry for Alzheimer Disease (CERAD) scores for neuritic plaques and Braak-Braak (BB) scores for neurofibrillary tangles were investigated using multivariable ordinal logistic regression. We investigated effect modification of education, race, and APOE on these associations.
Results
Among 1,037 subjects (mean age = 74.4 ± 11.5 y; mean education = 4.0 3.7 y; 48% male, 61% White), diabetes was present in 279 subjects. Diabetes was not associated with BB (OR = 1.12, 95%±CI = 0.81–1.54, p = 0.48) or with CERAD (OR = 0.97, 95% CI = 0.68–1.38, p = 0.86) scores on analyses adjusted for sociodemographic and clinical variables. We observed effect modification by the APOE allele ε4 on the association between diabetes mellitus and BB scores.
Conclusion
No evidence of an association between diabetes and AD neuropathology was found in a large sample of Brazilians; however, certain subgroups, such as APOE allele ε4 carriers, had higher odds of accumulation of neurofibrillary tangles.
Keywords: Alzheimer’s disease, autopsy study, dementia, diabetes mellitus, neuropathology
INTRODUCTION
Dementia was estimated to affect 47 million people worldwide in 2015, and this number is projected to reach 131 million by 2050 [1]. This increase will be particularly important in low- and middle-income countries (LMIC), where 60% of people with dementia reside [2]. Similarly to dementia, people with diabetes are predominantly found in LMIC [3]. Diabetes has been associated with an increased risk of dementia, especially vascular dementia (VaD). However, the association between diabetes and AD, the most common single cause of dementia, remains controversial [1]. While some longitudinal clinical studies failed to find an association between diabetes and AD [4–8], other studies have shown that diabetes is associated with increased risk of AD [9–16]. These conflicting results might be attributed to differences in study designs, including diagnostic criteria applied to diabetes and subtypes of dementia, neuroimaging information, regional characteristics, and ethnicities of subjects.
Autopsy studies are extremely important because they are the gold standard for defining dementia etiology [17]. Most autopsy studies have failed to show an association between diabetes and AD neuropathology [18–23]. Interestingly, some autopsy studies have found fewer neuritic plaques (NP) and neurofibrillary tangles (NFT) in brains of diabetics [19, 21, 24]. However, all autopsy studies so far have been performed in high-income countries (HIC) [18–29]. Information from LMIC is important since these countries have a different sociodemographic profile (e.g., low education and admixed ethnicities) and a higher prevalence of cardiovascular risk factors when compared to HIC [30]. Therefore, we aimed to examine the association between diabetes and AD neuropathology in 1,037 Brazilian older adults.
METHODS
Subjects
Subjects were drawn from the Brain Bank of the Brazilian Aging Brain Study Group (BB-BABSG) of the University of São Paulo Medical School. Autopsies were performed at the São Paulo Autopsy Service (SPAS), which is the site of all autopsies for those who have died in the metropolitan area of São Paulo with no established cause of death during life. Inclusion and exclusion criteria for the BB-BABSG have been described elsewhere [31]. During the period spanning from 2004 to 2015, 1,083 subjects aged 50 years and older were eligible for this study. Forty-six subjects were excluded for having incomplete interview or neuropathological analyses. The final sample size was 1,037 subjects. The study was approved by the local ethics committee. Voluntarily signed informed consent was obtained from the deceased’s next-of-kin, who gave brain donation consent.
Clinical information and APOE genotyping
A structured interview was applied to the deceased’s next-of-kin by a team of gerontologists upon arrival at the SPAS. A knowledgeable next-of-kin was identified to provide reliable information about the deceased. This person had at least weekly contact with the deceased during the six months leading up to death. The interview collected information about demographics [age, sex, race (White, Black, Brown, and other), and education], previously diagnosed diseases (hypertension, dyslipidemia, heart failure, coronary artery disease, and stroke), lifestyle (alcoholism, smoking, sedentary lifestyle), medication intake, and cognitive and functional status [31]. Diabetes was defined by the next-of-kin report of diabetes diagnosis during life or by the use of antidiabetic medication. Socioeconomic status was determined using a Brazilian scale that classifies subjects into five strata [32]. Stratum A is the highest income and stratum E the lowest. Socioeconomic status was grouped into high (stratum A+B), middle (stratum C), and low (stratum D+E) socioeconomic status. The Clinical Dementia Rating scale (CDR) was used to evaluate cognitive status prior to death [33]. Presence of cognitive impairment was determined by a CDR ≥0.5.
In a subsample of 524 subjects with DNA available, APOE genotypes (single-nucleotide polymorphisms rs429358 and rs7412) were determined by allele-specific amplification real-time PCR assays, performed in duplicate [34].
Neuropathological assessment
Autopsy was performed within an average of 20 (SD = 4) hours of death. One hemisphere was fixed in 4% buffered paraformaldehyde and the other hemisphere was coronally sectioned and snap frozen. Samples from the fixed hemisphere were embedded in paraffin and sectioned to produce sections of the following regions: middle frontal gyrus, middle and anterior temporal gyri, angular gyrus, superior frontal gyrus and anterior cingulate gyrus, visual cortex, hippocampus and parahippocampal gyrus at the level of the lateral geniculate body, amygdala at the level of mammillary bodies including the ambiens gyrus, basal ganglia at the level of the anterior commissure, thalamus, midbrain, pons, medulla oblongata, and cerebellum. All sampled regions were stained with hematoxylin & eosin (H&E). Selected sections were immunostained with antibodies against β-amyloid (4G8, dilution 1:10.000; Signet Pathology Systems, Dedham, MA), phosphorylated tau (PHF-1, dilution 1:2.000; gift of Prof. Peter Davies, NY), transactivation response DNA-binding protein of 43 kDA (TDP-43; 1:500, Proteintech, Chicago, IL), and α-synuclein (EQV, 1:10.000, gift of Kenji Ueda, Tokyo, Japan), as previously described [31].
Internationally accepted neuropathological criteria were used for diagnosing and staging. AD neuropathology was measured by semi-quantitative methods using the Braak and Braak (BB) staging system [35] for NFT and the Consortium to Establish and Registry for AD (CERAD) criteria for NP [36]. Neuropathological assessment was performed by a certified neuropathologist (LTG) who did not have access to information collected in the clinical interview.
Statistical analysis
Subjects with and without diabetes were compared using the unpaired t-test or Mann-Whitney test for quantitative variables, whereas the Chi-square test or Fisher’s exact test was employed for categorical variables. The dependent variables were the BB and CERAD scores (ordinal variables). BB score range from 0 to VI based on NFT severity and distribution, and was grouped into three commonly used categories: 0–II, III/IV, and V/VI [37–39]. The CERAD score is a semiquantitative estimation of NP density, ranging for 0 (absence of NP) to C (frequent NP). This score was grouped into three categories based on previous studies: 0/A, B, and C [37–39]. Simple and multivariable ordinal logistic regressions were used to evaluate the association between diabetes with BB and CERAD scores. Multivariable models were adjusted for sociodemographic variables (age, sex, race, and years of education) and cardiovascular risk factors (hypertension, dyslipidemia, physical inactivity, smoking, and alcohol use). The proportional odds assumption for the ordinal logistic models was tested using likelihood ratio tests.
To explore effect modification, we investigated whether the association of diabetes with BB and CERAD differed across different levels of education, adding a multiplicative interaction term between diabetes and years of education. We also tested whether the association of diabetes with BB and CERAD differed across different races by adding a multiplicative interaction term between diabetes and race (White versus Black and Brown). A total of 19 Asian participants were excluded for this analysis. We further investigated whether the association between AD neuropathology and cognitive impairment was modified by the presence of diabetes, using cognitive impairment defined by the CDR as the outcome and an interaction term of diabetes with BB and CERAD scores. The p-values for interaction terms were calculated using likelihood ratio tests. We considered an alpha level of 0.05 in two-tailed tests. Stata 13.0 (College Station, TX: StataCorp LP) was employed to perform the statistical analyses.
RESULTS
A total of 1,037 subjects were included, with a mean age at death of 74.4 ± 11.5 y (range = 50–105) and mean education of 4.0 ± 3.7 y, of which 496 (48%) were male and 628 (61%) were White. The majority of next-of-kin (68%) had daily contact with the deceased. Diabetes was present in 279 (27%) of the deceased. Cognitive impairment defined by CDR ≥0.5 was present in 42%. BB stage ≥III was found in 382 (37%) and CERAD ≥B in 258 (25%) individuals in the sample.
The associations between diabetes and sociodemographic and clinical variables are given in Table 1. Subjects with and without diabetes were similar for age, sex, education, race, and socioeconomic status. Hypertension, dyslipidemia, physical inactivity, coronary artery disease, and stroke were more frequent in those with diabetes. In addition, the frequency of cognitive impairment was similar in participants with and without diabetes (Table 1).
Table 1.
Demographic, clinical, and neuropathological data of subjects with and without diabetes mellitus (n = 1,037)
| Variables | No Diabetes n = 758 |
Diabetes n = 279 |
p |
|---|---|---|---|
| Demographics, clinical variables, APOE genotyping | |||
| Age (y), mean (SD)* | 74.7 (11.7) | 73.5 (10.9) | 0.16 |
| Female, n (%)# | 388 (51.2) | 153 (54.8) | 0.30 |
| Education (y), mean (SD)* | 4.1 (3.6) | 4.2 (3.7) | 0.58 |
| Race, n (%)‡ | 0.67 | ||
| White | 456 (60.2) | 172 (61.7) | |
| Black | 157 (20.7) | 53 (19.0) | |
| Admixed | 129 (17.0) | 51 (18.3) | |
| Asian | 16 (2.1) | 3 (1.1) | |
| Socioeconomic class, n (%)# | 0.16 | ||
| High | 235 (31.0) | 83 (29.8) | |
| Middle | 346 (45.7) | 144 (51.6) | |
| Low | 176 (23.2) | 52 (18.6) | |
| Daily contact with the informant, n (%)# | 536 (70.7) | 194 (69.5) | 0.71 |
| Hypertension, n (%)# | 456 (60.2) | 224 (80.3) | <0.0001 |
| Dyslipidemia, n (%)# | 43 (5.7) | 50 (17.9) | <0.0001 |
| Physical inactivity, n (%)# | 379 (50.0) | 160 (57.3) | 0.04 |
| Smoking, n (%)# | 0.08 | ||
| Never | 384 (50.7) | 150 (53.8) | |
| Current | 208 (27.4) | 58 (20.8) | |
| Former | 164 (21.6) | 70 (25.1) | |
| Alcohol use, n (%)# | 0.42 | ||
| Never | 466 (61.5) | 182 (65.2) | |
| Current | 178 (23.5) | 55 (19.7) | |
| Former | 110 (14.5) | 41 (14.7) | |
| Coronary artery disease, n (%)# | 151 (19.9) | 74 (26.5) | 0.02 |
| Heart Failure, n (%)# | 121 (16.0) | 55 (19.7) | 0.15 |
| Stroke, n (%)# | 109 (14.4) | 58 (20.8) | 0.01 |
| CDR >0, n (%)# | 311 (41.0) | 121 (43.4) | 0.53 |
| APOE allele ε4, n (%)# § | 108 (30.2) | 37 (26.2) | 0.38 |
| Neuropathology | 0.31 | ||
| CERAD score, n (%)# | |||
| 0 | 452 (59.6) | 181 (64.9) | |
| A | 111 (14.7) | 35 (12.5) | |
| B | 106 (14.0) | 29 (10.4) | |
| C | 89 (11.7) | 34 (12.2) | |
| Braak-Braak stages, n (%)# | 0.81 | ||
| 0 | 146 (19.4) | 60 (21.6) | |
| I | 116 (15.4) | 46 (16.6) | |
| II | 214 (28.4) | 73 (26.3) | |
| III | 116 (15.4) | 49 (17.6) | |
| IV | 73 (9.7) | 21 (7.5) | |
| V | 41 (5.5) | 14 (5.0) | |
| VI | 47 (6.2) | 15 (5.4) |
CDR, Clinical Dementia Rating; APOE, apolipoprotein E; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; SD, standard deviation.
unpaired t-test;
Mann-Whitney test;
Chi-square test;
Fisher’s exact test.
Data available for 524 participants.
In multivariable regression models, diabetes was not associated with BB stage (OR = 1.12, 95% CI = 0.81–1.54, p = 0.48) or CERAD scores (OR = 0.97, 95% CI = 0.68–1.38, p = 0.86), after adjustment for age, sex, race, education, hypertension, dyslipidemia, physical inactivity, smoking, and alcohol use (Table 2). Neither of the ordinal logistic models violated the proportional odds assumption (for BB stage: χ2 = 0.06, p = 0.80; for CERAD: χ2 = 2.35, p = 0.31).
Table 2.
Association between neuropathological lesions and diabetes mellitus status (n = 1,037)
| Simple regression OR (95% CI) |
p | Multivariable regression*
|
||
|---|---|---|---|---|
| OR (95% CI) | p | |||
| Braak-Braak stage | 0.96 (0.72–1.26) | 0.75 | 1.12 (0.81–1.54) | 0.48 |
| CERAD score‡ | 0.88 (0.64–1.22) | 0.46 | 0.97 (0.68–1.38) | 0.86 |
Multivariable ordinal logistic regression adjusted for age, sex, race, education, hypertension, dyslipidemia, physical inactivity, smoking, and alcohol use.
CERAD, Consortium to Establish a Registry for Alzheimer’s Disease.
We also tested whether the presence of diabetes modified the association between cognitive symptoms and AD neuropathology. A significant interaction term was observed between diabetes and BB and CERAD scores on cognitive impairment (Table 3). Participants with diabetes had higher odds of cognitive impairment in the presence of larger BB and CERAD scores, when compared to participants without diabetes with the same degree of AD neuropathology (p < 0.0001 for both interaction terms).
Table 3.
Association of AD neuropathology with cognitive impairment, considering interaction terms between diabetes and AD neuropathology (ADNP) (n = 1,037)
| N with/without cognitive impairment | OR (95% CI) | p* | |
|---|---|---|---|
| Braak-Braak Score | <0.0001 | ||
| 0–II | 207/454 | 0.50 (0.35–0.72) | |
| III-IV | 128/131 | 0.86 (0.55–1.32) | |
| V-VI | 97/20 | 5.34 (2.72–10.49) | |
| CERAD | |||
| 0-A | 271/508 | 0.59 (0.41–0.83) | <0.0001 |
| B | 74/61 | 0.86 (0.51–1.44) | |
| C | 87/36 | 2.45 (1.39–4.30) |
p-value for the interaction between ADNP and diabetes. Multivariable ordinal logistic regression adjusted for age, sex, race, education, hypertension, dyslipidemia, physical inactivity, smoking, and alcohol use.
Moreover, a borderline effect modification by education on the association between BB score and diabetes mellitus was observed (Coefficient = 0.084, 95% CI = –0.002; 0.170; p = 0.06) (Fig. 1). For example, using the median education of the sample (4 y) as the cutoff, the OR for the association between diabetes and BB score was 0.99 (95% CI = 0.70–1.41) in individuals with ≤4 y of education and diabetes, while the OR was 1.80 (95% CI = 0.92–3.51) in individuals with >4 y. The interaction term between diabetes and education for the CERAD score was not significant (Coefficient = 0.035, 95% CI = –0.059; 0.130, p = 0.46) (Fig. 1). The interaction terms of diabetes with race for BB and CERAD scores were not significant (Table 4 and Supplementary Figure 1). In a subsample of 524 subjects with APOE genotyping, the interaction term between diabetes and APOE allele ε4 was significant for BB scores (p = 0.01), but not for CERAD scores (p = 0.12) (Table 4 and Supplementary Figure 2).
Fig. 1.
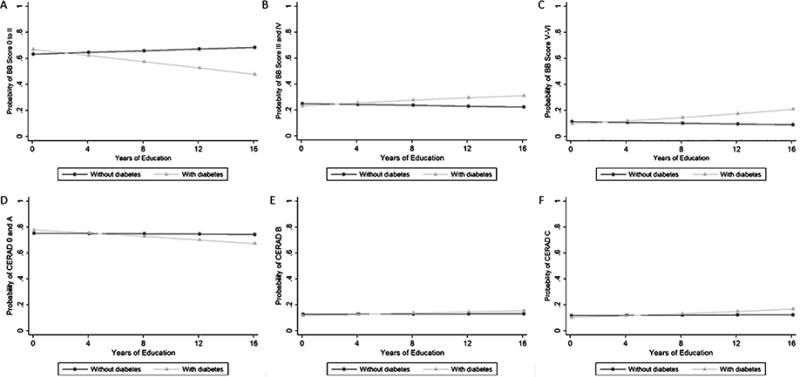
Probability of Braak-Braak (BB) score (A to C) and CERAD score (D to F) in subjects without diabetes (Black line with circle markers) and with diabetes (Grey line with triangle markers), using multivariable ordinal logistic regression with an interaction term between diabetes and education (continuous variable), adjusted for age, sex, race, hypertension, dyslipidemia, physical inactivity, smoking, and alcohol use (n = 1,037).
Table 4.
Association of diabetes with Braak-Braak and CERAD scores, considering interaction terms of diabetes with race and Apolipoprotein E allele ε4 (n = 1,037)
| Braak-Braak Score OR (95%CI) |
P* | CERAD Score‡ OR (95%CI) |
P* | |
|---|---|---|---|---|
| Interaction between diabetes and race | ||||
| White | 1.06 (0.71–1.58) | 0.85 | 0.93 (0.60–1.44) | 0.76 |
| Black+Brown | 1.14 (0.68–1.90) | 1.07 (0.60–1.90) | ||
| Interaction between diabetes and APOE ε4§ | ||||
| Absence of APOE ε4 | 0.76 (0.41–1.38) | 0.01 | 0.75 (0.38–1.50) | 0.12 |
| Presence of APOE ε4 | 2.82 (1.28–6.23) | 1.79 (0.78–4.11) |
p-value for the interaction term. Multivariable ordinal logistic regression adjusted for age, sex, race, education, hypertension, dyslipidemia, physical inactivity, smoking, and alcohol use.
CERAD, Consortium to Establish a Registry for Alzheimer’s Disease.
Data available for 524 participants.
DISCUSSION
In this first large autopsy study from a LMIC, no association between diabetes and AD neuropathology was found. Our results are in agreement with several neuropathological studies reported in HIC [18–29]. One of the first retrospective autopsy studies on the association between diabetes and AD neuropathology was the investigation performed by Heitner and Dickson [18], which found no differences for senile plaque score or Braak stages in the entorhinal cortex and hippocampus in persons with diabetes compared to those without the disease. In a larger community-based study of older persons with and without diabetes, Arvanitakis et al. [20] showed that diabetes was not associated with NP, diffuse plaques, NFT, amyloid burden, or tangle density. In contrast, some studies have found fewer NP [19, 21] and NFT [19] in the brains of people with diabetes. Although Nelson et al. [21] showed lower NFT in the subiculum and NP in the temporal lobe in those with diabetes, a recent study found no association between diabetes and regional AD neuropathology [40]. Recently, Abner et al. studied AD and cerebrovascular neuropathology in 2,365 subjects from the Statistical Modeling of Aging and Risk of Transition (SMART) database [23]. The SMART database comprises data from 11 longitudinal studies of aging and cognition and used the modified version of the National Institute on Aging-Alzheimer’s Association criteria for AD neuropathology (AD-ABC score). The study found no associations of diabetes with CERAD score, BB stage, or the modified AD-ABC rating [23]. The controversies in the association between diabetes and AD may be partly due to methodological differences. Studies differ in sample size and cohort characteristics, follow-up time, criteria for diabetes diagnosis, and AD neuropathological assessment with different techniques, including silver staining [20–22], biochemical analysis [27], and immunohistochemistry [18–23, 25].
We found higher odds of cognitive impairment in the presence of larger BB and CERAD scores in participants with diabetes compared to those without diabetes. Abner et al. [23] found that subjects with diabetes and large amounts of AD neuropathology had significantly lower Mini-Mental State Examination scores than participants without diabetes yet with the same number of lesions [23].
Similar to previous studies from HIC [25, 26, 29], we found evidence that diabetes was related to a higher NFT burden when APOE ε4 was present. APOE ε4 has been associated with increased deposition of neurotoxic Aβ protein and decreased cortical plaque clearance [41]. Diabetes and APOE ε4 likely contribute synergistically to increased AD pathology, although the exact mechanism by which they interact remains unknown [29]. Contrary to previous findings [25, 26, 28, 29], the interaction term between diabetes and APOE ε4 for neuritic plaques burden evaluated by the CERAD score was not significant. The lack of significance for CERAD score may be related to low statistical power, since APOE genotyping was available for only about half of the sample.
Most studies investigating the association between diabetes and AD neuropathology were performed in Caucasian and Asian subjects with high education attainment (i.e., more than 10 y of formal education) [20, 23, 25]. The Brazilian population, akin to many other LMIC, has some particular features, such as low education and admixed ethnicities [30]. Therefore, we investigated whether the association between diabetes and AD neuropathology was modified by these factors. No previous studies investigating the effect modification of education or race on the association between diabetes and AD neuropathology were found. Surprisingly, we found a borderline effect modification by education and diabetes on BB score in a sample with a very low level of education (mean education = 4 y). In people with diabetes, higher education attainment was associated with higher odds of neurofibrillary tangle accumulation. Although the statistical analysis was adjusted for a comprehensive set of confounders (including age and sex), the possibility of selection bias cannot be ruled out [42]. In addition, further studies with larger samples from LMIC are needed to confirm this finding since the p-value for interaction term was borderline. Regarding race, we previously showed that African ancestry is associated with lower odds of NP accumulation [43]; therefore, a possible effect modification of race on the association between diabetes and AD neuropathology would be expected. However, we found no evidence of interaction between diabetes and race in our sample.
Autopsy studies provide reliable information on AD diagnosis [17]. Cardiovascular risk factors, including diabetes, have not been consistently associated with an increased burden of AD pathology, but were associated with cerebrovascular disease [44]. Although undiagnosed mild diabetes and well-controlled diabetes might be underreported by next-of-kin, the association of diabetes with dementia is probably mediated by an increased cerebrovascular lesion load [45]. There is strong evidence that diabetes increases the risk of cerebral infarcts, cerebral small vessel disease, cognitive decline, and VaD [17, 44]. The World Alzheimer Report concluded that diabetes is a much stronger risk factor for VaD than AD, and cerebrovascular disease is likely the main mechanism involved [1], while diabetes possibly decreases brain resilience, but does not directly cause AD neuropathology [1]. We did not include cerebrovascular data because these were not the main goal of this study.
Our study has some strengths that should be considered: 1) the large sample size; 2) the well-structured autopsy study; 3) neuropathologists blinded to clinical information; and 4) the first investigation to address this issue in a LMIC, where education attainment is lower than in high income countries (HIC) [23, 25, 26, 28] and the population more ethnically admixed [43, 46]. Nevertheless, this study has some limitations. First, this was a cross-sectional study, limiting determination of causality. Second, all clinical data, including the diabetes diagnosis and cognitive evaluation, were obtained post-mortem by interview with a next-of-kin. To minimize this limitation, only reliable informants that had at least weekly contact with the deceased were included. Moreover, our clinical interview was evaluated previously and showed good reliability [47]. Indeed, diabetes frequency in our sample was similar to the rate found in the Brazilian population [48] and comparable to other neuropathological studies [21–23]. Third, previous objective measures of diabetes (e.g., glycated hemoglobin test) allowing more reliable diabetes diagnosis, including diabetes severity, were not available.
In conclusion, in a large autopsy series from a LMIC country, diabetes was not associated with higher AD neuropathology burden. Subjects with diabetes and carriers of the APOE-ε4 had higher odds of NFT accumulation.
Supplementary Material
Acknowledgments
We are grateful to the families of the brain donors and to the SPAS physicians and staff for their unconditional support.
This work was supported by Fundação de Apoio à Pesquisa do Estado de São Paulo—FAPESP (grant 2009/01934-4 and scholarship to R.E.P.L.), LIM22 of the Department of Pathology of the University of São Paulo Medical School, Albert Einstein Education and Research Institute, São Paulo (grant 240/07), and the Coordenadoria de Apoio ao Pessoal de Nivel Superior—CAPES, Brazil (scholarship to students of the BABSG). LTG is supported by NIH R01AG040311 and institutional NIH grants P50AG023501 and P01AG019724. ZA is a Principal Investigator of NIH grants R01 NS084965 and R01 AG040039.
Footnotes
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/17-0179r2).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-170179.
References
- 1.Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M. World Alzheimer report 2016: Improving healthcare for people living with dementia: Coverage, quality and costs now and in the future. Alzheimer’s Disease International; 2016. [Google Scholar]
- 2.World Health Organization. World report on ageing and health. Luxembourg. 2015:1–260. [Google Scholar]
- 3.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 5.MacKnight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer’s disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2002;14:77–83. doi: 10.1159/000064928. [DOI] [PubMed] [Google Scholar]
- 6.Hassing LB, Johansson B, Nilsson SE, Berg S, Pedersen NL, Gatz M, McClearn G. Diabetes mellitus is a risk factor for vascular dementia, but not for Alzheimer’s disease: A population-based study of the oldest old. Int Psychogeriatr. 2002;14:239–248. doi: 10.1017/s104161020200844x. [DOI] [PubMed] [Google Scholar]
- 7.Raffaitin C, Féart C, Le Goff M, Amieva H, Helmer C, Akbaraly TN, Tzourio C, Gin H, Barberger-Gateau P. Metabolic syndrome and cognitive decline in French elders The Three-City Study. Neurology. 2011;76:518–525. doi: 10.1212/WNL.0b013e31820b7656. [DOI] [PubMed] [Google Scholar]
- 8.Xu WL, von Strauss E, Qiu CX, Winblad B, Fratiglioni L. Uncontrolled diabetes increases the risk of Alzheimer’s disease: A population-based cohort study. Diabetologia. 2009;52:1031–1039. doi: 10.1007/s00125-009-1323-x. [DOI] [PubMed] [Google Scholar]
- 9.Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O’Brien PC, Palumbo PJ. Risk of dementia among persons with diabetes mellitus: A population-based cohort study. Am J Epidemiol. 1997;145:301–308. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- 10.Brayne C, Gill C, Huppert FA, Barkley C, Gehlhaar E, Girling DM, O’Connor DW, Paykel ES. Vascular risks and incident dementia: Results from a cohort study of the very old. Dement Geriatr Cogn Disord. 1998;9:175–180. doi: 10.1159/000017043. [DOI] [PubMed] [Google Scholar]
- 11.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 12.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 13.Borenstein AR, Wu Y, Mortimer JA, Schellenberg GD, McCormick WC, Bowen JD, McCurry S, Larson EB. Developmental and vascular risk factors for Alzheimer’s disease. Neurobiol Aging. 2005;26:325–334. doi: 10.1016/j.neurobiolaging.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Cheng D, Noble J, Tang MX, Schupf N, Mayeux R, Luchsinger JA. Type 2 diabetes and late-onset Alzheimer’s disease. Dement Geriatr Cogn Disord. 2011;31:424–430. doi: 10.1159/000324134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang KC, Woung LC, Tsai MT, Liu CC, Su YH, Li CY. Risk of Alzheimer’s disease in relation to diabetes: A population-based cohort study. Neuroepidemiology. 2012;38:237–244. doi: 10.1159/000337428. [DOI] [PubMed] [Google Scholar]
- 16.Ohara T, Doi Y, Ninomiya T, Hirakawa Y, Hata J, Iwaki T, Kanba S, Kiyohara Y. Glucose tolerance status and risk of dementia in the community: The Hisayama study. Neurology. 2011;77:1126–1134. doi: 10.1212/WNL.0b013e31822f0435. [DOI] [PubMed] [Google Scholar]
- 17.Chui HC, Zheng L, Reed BR, Vinters HV, Mack WJ. Vascular risk factors and Alzheimer’s disease: Are these risk factors for plaques and tangles or for concomitant vascular pathology that increases the likelihood of dementia? An evidence-based review. Alzheimers Res Ther. 2012;4:1. doi: 10.1186/alzrt98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heitner J, Dickson D. Diabetics do not have increased Alzheimer-type pathology compared with age-matched control subjects. A retrospective postmortem immunocytochemical and histofluorescent study. Neurology. 1997;49:1306–1311. doi: 10.1212/wnl.49.5.1306. [DOI] [PubMed] [Google Scholar]
- 19.Beeri MS, Silverman JM, Davis KL, Marin D, Grossman HZ, Schmeidler J, Purohit DP, Perl DP, Davidson M, Mohs RC, Haroutunian V. Type 2 diabetes is negatively associated with Alzheimer’s disease neuropathology. J Gerontol A Biol Sci Med Sci. 2005;60:471–475. doi: 10.1093/gerona/60.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arvanitakis Z, Schneider JA, Wilson RS, Li Y, Arnold SE, Wang Z, Bennett DA. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology. 2006;67:1960–1965. doi: 10.1212/01.wnl.0000247053.45483.4e. [DOI] [PubMed] [Google Scholar]
- 21.Nelson PT, Smith CD, Abner EA, Schmitt FA, Scheff SW, Davis GJ, Keller JN, Jicha GA, Davis D, Wang-Xia W, Hartman A, Katz DG, Markesbery WR. Human cerebral neuropathology of Type 2 diabetes mellitus. Biochim Biophys Acta. 2009;1792:454–469. doi: 10.1016/j.bbadis.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, Sulkava R, Kivipelto M. Diabetes, Alzheimer disease, and vascular dementia: A population-based neuropathologic study. Neurology. 2010;75:1195–1202. doi: 10.1212/WNL.0b013e3181f4d7f8. [DOI] [PubMed] [Google Scholar]
- 23.Abner EL, Nelson PT, Kryscio RJ, Schmitt FA, Fardo DW, Woltjer RL, Cairns NJ, Yu L, Dodge HH, Xiong C, Masaki K, Tyas SL, Bennett DA, Schneider JA, Arvanitakis Z. Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimers Dement. 2016;12:882–889. doi: 10.1016/j.jalz.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beeri MS, Schmeidler J, Silverman JM, Gandy S, Wysocki M, Hannigan CM, Purohit DP, Lesser G, Grossman HT, Haroutunian V. Insulin in combination with other diabetes medication is associated with less Alzheimer neuropathology. Neurology. 2008;71:750–757. doi: 10.1212/01.wnl.0000324925.95210.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peila R, Rodriguez BL, Launer LJ, Honolulu-Asia Aging Study Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 26.Alafuzoff I, Aho L, Helisalmi S, Mannermaa A, Soininen H. Beta-amyloid deposition in brains of subjects with diabetes. Neuropathol Appl Neurobiol. 2009;35:60–68. doi: 10.1111/j.1365-2990.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 27.Sonnen JA, Larson EB, Brickell K, Crane PK, Woltjer R, Montine TJ, Craft S. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol. 2009;66:315–322. doi: 10.1001/archneurol.2008.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y, Sekita A, Suzuki SO, Kanba S, Kiyohara Y, Iwaki T. Insulin resistance is associated with the pathology of Alzheimer disease: The Hisayama study. Neurology. 2010;75:764–770. doi: 10.1212/WNL.0b013e3181eee25f. [DOI] [PubMed] [Google Scholar]
- 29.Malek-Ahmadi M, Beach T, Obradov A, Sue L, Belden C, Davis K, Walker DG, Lue L, Adem A, Sabbagh MN. Increased Alzheimer’s disease neuropathology is associated with type 2 diabetes and ApoE ε4 carrier status. Curr Alzheimer Res. 2013;10:654–659. doi: 10.2174/15672050113109990006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt MI, Duncan BB, Azevedo e Silva G, Menezes AM, Monteiro CA, Barreto SM, Chor D, Menezes PR. Chronic non-communicable diseases in Brazil: Burden and current challenges. Lancet. 2011;377:1949–1961. doi: 10.1016/S0140-6736(11)60135-9. [DOI] [PubMed] [Google Scholar]
- 31.Grinberg LT, Ferretti RE, Farfel JM, Leite R, Pasqualucci CA, Rosemberg S, Nitrini R, Saldiva PH, Filho WJ, Brazilian Aging Brain Study Group Brain bank of the Brazilian Aging Brain Study Group – a milestone reached and more than 1,600 collected brains. Cell Tissue Bank. 2007;8:151–162. doi: 10.1007/s10561-006-9022-z. [DOI] [PubMed] [Google Scholar]
- 32.Almeida P, Wickerhauser H. O critério ABA/ ABIPEME – em busca de uma atualização: Um estudo e uma proposta submetidos à ABA e à ABIPEME Brazil 1991 [Google Scholar]
- 33.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 34.Calero O, Hortigüela R, Bullido MJ, Calero M. Apolipoprotein E genotyping method by real time PCR, a fast and cost-effective alternative to the TaqMan and FRET assays. J Neurosci Methods. 2009;183:238–240. doi: 10.1016/j.jneumeth.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 35.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 36.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 37.Sinka L, Kövari E, Gold G, Hof PR, Herrmann FR, Bouras C, Giannakopoulos P. Small vascular and Alzheimer disease-related pathologic determinants of dementia in the oldest-old. J Neuropathol Exp Neurol. 2010;69:1247–1255. doi: 10.1097/NEN.0b013e3181ffc3b9. [DOI] [PubMed] [Google Scholar]
- 38.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 39.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease from the National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Pruzin JJ, Schneider JA, Capuano AW, Leurgans SE, Barnes LL, Ahima RS, Arnold SE, Bennett DA, Arvanitakis Z. Diabetes, hemoglobin A1C, and regional Alzheimer Disease and infarct pathology. Alzheimer Dis Assoc Disord. 2017;31:41–47. doi: 10.1097/WAD.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB. Diabetes mellitus and Alzheimer’s disease: Shared pathology and treatment? Br J Clin Pharmacol. 2011;71:365–376. doi: 10.1111/j.1365-2125.2010.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernán MA, Alonso A, Logroscino G. Cigarette smoking and dementia: Potential selection bias in the elderly. Epidemiology. 2008;19:448–450. doi: 10.1097/EDE.0b013e31816bbe14. [DOI] [PubMed] [Google Scholar]
- 43.Schlesinger D, Grinberg LT, Alba JG, Naslavsky MS, Licinio L, Farfel JM, Suemoto CK, de Lucena Ferretti RE, Leite RE, de Andrade MP, dos Santos AC, Brentani H, Pasqualucci CA, Nitrini R, Jacob-Filho W, Zatz M, Brazilian Aging Brain Study Group African ancestry protects against Alzheimer’s disease-related neuropathology. Mol Psychiatry. 2013;18:79–85. doi: 10.1038/mp.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson K, Stephan BC, Ince PG, Brayne C, Matthews FE, Esiri MM. The neuropathology of vascular disease in the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Curr Alzheimer Res. 2012;9:687–696. doi: 10.2174/156720512801322654. [DOI] [PubMed] [Google Scholar]
- 45.Vagelatos NT, Eslick GD. Type 2 diabetes as a risk factor for Alzheimer’s disease: The confounders, interactions, and neuropathology associated with this relationship. Epidemiol Rev. 2013;35:152–160. doi: 10.1093/epirev/mxs012. [DOI] [PubMed] [Google Scholar]
- 46.Nascimento C, Suemoto CK, Rodriguez RD, Alho AT, Leite RP, Farfel JM, Pasqualucci CA, Jacob-Filho W, Grinberg LT. Higher prevalence of TDP-43 proteinopathy in cognitively normal Asians: A clinicopathological study on a multiethnic sample. Brain Pathol. 2016;26:177–185. doi: 10.1111/bpa.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferretti RE, Damin AE, Brucki SM, Morillo LS, Perroco TR, Campora F, Moreira EG, Balbino ES, Lima MC, Battela C, Ruiz L, Grimberg LT, Farfel JM, Leite RE, Suemoto CK, Pasqualucci CA, Rosemberg S, Saldiva PH, Jacob-Filho W, Nitrini R. Post-mortem diagnosis of dementia by informant interview. Dement Neuropsychol. 2010;4:138–144. doi: 10.1590/S1980-57642010DN40200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iser BP, Vigo A, Duncan BB, Schmidt MI. Trends in the prevalence of self-reported diabetes in Brazilian capital cities and the Federal District, 2006-2014. Diabetol Metab Syndr. 2016;8:70. doi: 10.1186/s13098-016-0185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


