Abstract
Nanotechnology manifests the progression in the arena of research and development, by increasing the efficacy of the product through delivery of innovative solutions. To overcome certain drawbacks associated with the traditional products, application of nanotechnology is escalating in the area of cosmeceuticals. Cosmeceuticals are regarded as the fastest growing segment of the personal care industry and the use has risen drastically over the years. Nanocosmeceuticals used for skin, hair, nail, and lip care, for conditions like wrinkles, photoaging, hyperpigmentation, dandruff, and hair damage, have come into widespread use. Novel nanocarriers like liposomes, niosomes, nanoemulsions, microemulsion, solid lipid nanoparticles, nanostructured lipid carrier, and nanospheres have replaced the usage of conventional delivery system. These novel nanocarriers have advantages of enhanced skin penetration, controlled and sustained drug release, higher stability, site specific targeting, and high entrapment efficiency. However, nanotoxicological researches have indicated concern regarding the impact of increased use of nanoparticles in cosmeceuticals as there are possibilities of nanoparticles to penetrate through skin and cause health hazards. This review on nanotechnology used in cosmeceuticals highlights the various novel carriers used for the delivery of cosmeceuticals, their positive and negative aspects, marketed formulations, toxicity, and regulations of nanocosmeceuticals.
1. Introduction
Nanotechnology is regarded as the most imminent technology of 21st century and is contemplated as a big boon in the cosmetic industry. The term nanotechnology is the combination of two words: namely, technology and the Greek numerical “nano” which means dwarf. Thus, nanotechnology is considered as the science and technology used to develop or manipulate the particles in the size range of 1 to 100 nm [1, 2]. Since 1959, nanotechnology has emerged in different fields like engineering, physics, chemistry, biology, and science and it has been virtually 40 years since nanotechnology has intruded into the field of cosmetics, health products, and dermal preparations. During the era of 4000BC, the use of nanotechnology has been recorded by the Egyptians, Greek, and Romans, with concept of hair dye preparation utilizing nanotechnology [3].
Founding member of US society of Cosmetic Chemists, Raymond Reed, coined the term “cosmetics” in the year 1961. Cosmetics can be defined as the products which amplify the appearance of the skin, intensify the cleansing, and promote the beauty of the skin [4]. As reported, the use of cosmetics was attributed to Egyptians around 4000BC and later Greeks, Romans, Chinese, Japanese, and Americans started using cosmetics. In the late 19th century, the use of cosmetics was secretly done by the women with household items in western countries and by 20th century the cosmetics were being done without concealment. By the 21th century, the cosmetics are being enormously used and with the development in technology, innovative cosmetic formulations are being developed by the incorporation of the latest technologies [5, 6].
Cosmeceuticals are the cosmetic products which incorporate biologically active ingredient having therapeutic benefits on the surface applied. These are utilized as cosmetics as they claim to enhance appearance [7]. Cosmeceuticals are chasm between pharmaceuticals and personal care products. Cosmeceutical products have measurable therapeutic efficacy on the skin, as drugs and formulations have diversified from skin to body to hair and they are used for the treatment of various conditions like hair damage, wrinkles, photoaging, skin dryness, dark spots, uneven complexion, hyperpigmentation, and so on [8].
Cosmeceuticals are contemplated as the fastest growing fragment of personal care industry and the market for personal care is increasing enormously [9]. Despite enormous benefits of nanoparticles, little is known about the short-term and long-term health effects in the environment and organisms. Safety concerns have been raised due to the reported toxicity and possible dangers of the nanomaterials. The present article reviews the diverse classes of nanocarriers like liposomes, niosomes, solid lipid nanoparticles, nanostructured lipid carriers, nanoemulsion, and so on which are being used for delivery of nanocosmeceuticals, marketed products, and positive and negative aspects.
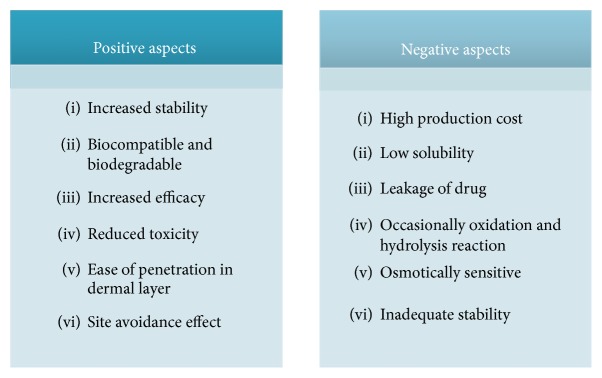
There are a number of advantages of nanocosmeceuticals. Namely, they provide the controlled release of active substances by controlling the drug release from carriers by several factors including physical or chemical interaction among the components, composition of drug, polymer and additives, ratio, and preparation method. They are used in hair care preparations, such as in treatment of hair loss and to prevent hair from turning grey such as Identik Masque Floral Repair, Origem hair recycling shampoo, and Nirvel hair-loss control shampoo. Nanocosmeceuticals make the fragrances last longer, for example, Allure Parfum and Allure Eau Parfum spray by Chanel. These make the skin care formulations more effective and increase the efficacy of sunscreens by improving UV protection in them. By having very small size of the particles, the surface area is increased which allows the active transport of the active ingredients into the skin. Occlusion provides the enhancement in the penetration and skin hydration is increased. Cosmeceuticals have high entrapment efficiency and good sensorial properties and are more stable than the conventional cosmetics. Most of the nanoparticles are suitable for both lipophilic and hydrophilic drug delivery. Nanomaterials are widely used in the preparation of antiwrinkle creams, moisturizing creams, skin whitening creams, hair repairing shampoos, conditioners, and hair serums [11, 12]. Several positive aspects of nanocosmeceuticals are discussed in Figure 1 [13].
Figure 1.
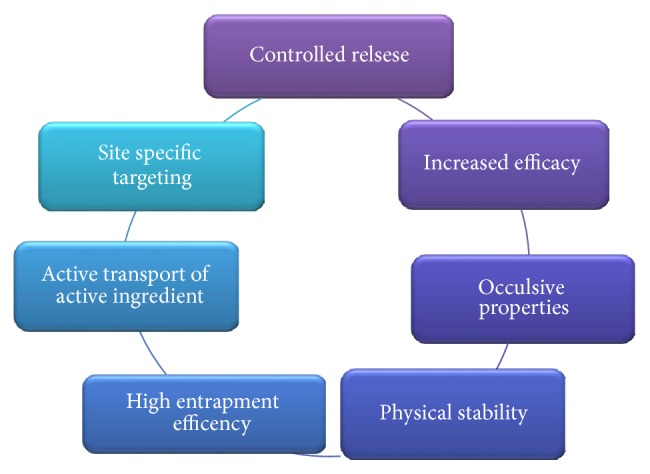
Pictorial presentation of positive aspects of nanocosmeceuticals.
As the rule of the nature, each and everything in this universe has some positive as well as negative aspects. Some of the drawbacks associated with nanocosmeceuticals are as follows. Due to production of large number of oxygen species, oxidation stress, inflammation, damage to DNA, proteins, and membranes may be caused by nanoparticles. Few ultrafine nanomaterials such as carbon nanotubes, carbon based fullerenes, TiO2, copper nanoparticles, and silver nanoparticles may be toxic to human tissues and cells. Titanium dioxide found in sunscreens has been demonstrated to cause damage to DNA, RNA, and fats within cells. No stringent scrutiny was imposed by the regulatory agencies for the approval and regulation of nanocosmeceuticals. Nanocosmeceuticals may be harmful to environment as well. No clinical trials are required for the approval of nanocosmeceuticals, thus raising a concern of toxicity after use [15, 16]. Negative aspects of nanocosmeceuticals are discussed in Figure 2 [17].
Figure 2.
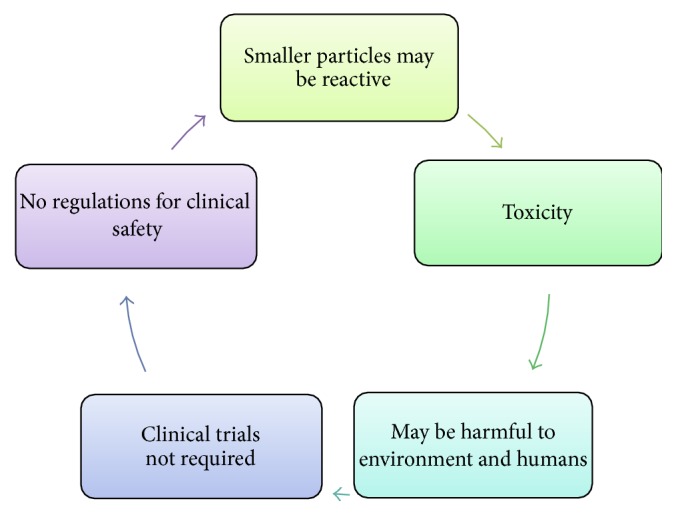
Pictorial presentation of negative aspects of nanocosmeceuticals.
2. Novel Nanocarriers for Cosmeceuticals
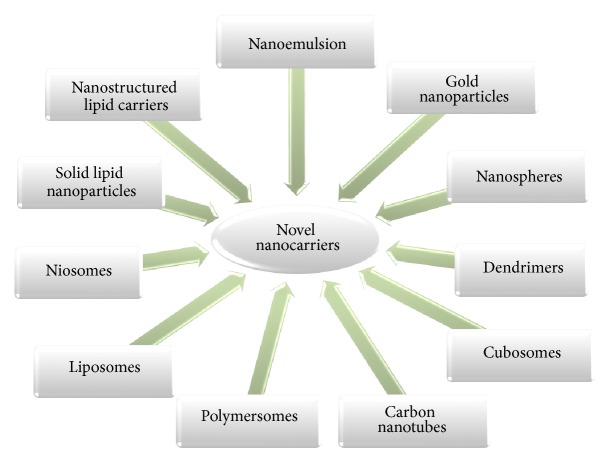
For the delivery of nanocosmeceuticals, carrier technology is employed which offers an intelligent approach for the delivery of active ingredients. Various novel nanocarriers for delivery of cosmeceuticals are depicted in Figure 3 [18, 19].
Figure 3.
Pictorial presentation of various nanocarriers for cosmeceuticals.
2.1. Liposomes
Liposomes are most widely used for the cosmeceutical preparations. They are the vesicular structures having an aqueous core which are enclosed by a hydrophobic lipid bilayer [20]. The main component of liposome lipid bilayer is phospholipids; these are GRAS (generally recognized as safe) ingredients, therefore minimizing the risk for adverse effects [21]. To protect the drug from metabolic degradation, liposome encapsulates the drug and releases active ingredients in a controlled manner [22]. Liposomes are suitable for delivery of both hydrophobic as well as hydrophilic compounds. Their size varies from 20 nm to several micrometers and can have either multilamellar or unilamellar structure [23].
Antioxidants such as carotenoids, CoQ10, and lycopene and active components like vitamins A, E, and K have been incorporated into liposomes, which are used to amplify their physical and chemical stability when dispersed in water [24].
Phosphatidylcholine is the key component of liposomes which has been used in various skin care formulations like moisturizer creams and so on and hair care products like shampoo and conditioner due to its softening and conditioning properties. Due to their biodegradable, nontoxic, and biocompatible nature, liposomes are used in variety of cosmeceuticals as they encapsulate active moiety [25]. Vegetable phospholipids are widely used for topical applications in cosmetics and dermatology because of their high content of esterified essential fatty acids. For topical applications in cosmetics and dermatology vegetable phospholipids are being widely used as they have high content of esterified essential fatty acids. After the application of linoleic acid, within a short period of time the barrier function of the skin is increased and water loss is decreased. Vegetable phospholipids and soya phospholipids are used because of their ability to form liposomes and their surface activity. The transport of linoleic acid into the skin is done by these phospholipids [26, 27]. In a clinical study, it was proven that flexible liposomes help in wrinkle reduction and show effects like decreasing of efflorescence in the acne treatment and an increase in skin smoothness [28].
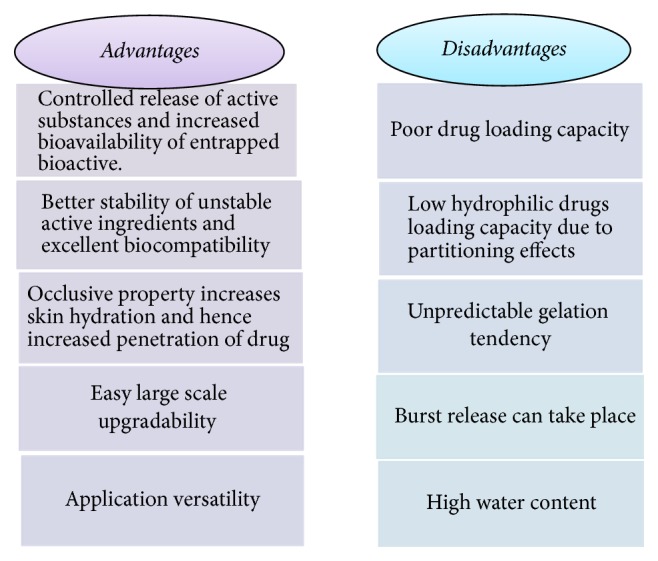
Liposomes are being developed for the delivery of fragrances, botanicals, and vitamins from anhydrous formulations, such as antiperspirants, body sprays, deodorants, and lipsticks. They are also being used in antiaging creams, deep moisturizing cream, sunscreen, beauty creams, and treatment of hair loss [29]. Several positive and negative aspects of liposomes are discussed in Figure 4 [30]. Various marketed formulations are given in Table 1 [31–33].
Figure 4.
Pictorial presentation of positive aspects of liposomes.
Table 1.
Marketed formulations of liposomes.
| Product name | Marketed by | Uses |
|---|---|---|
| Capture Totale | Dior | Removes wrinkles and dark spots and has radiance effect with sunscreen |
| Dermosome | Microfluidics | Moisturizer |
| Decorte Moisture Liposome Face Cream | Decorte | Moisturizer |
| Decorte Moisture Liposome Eye Cream | Decorte | Moisturizes, firms, and brightens the delicate skin around the eyes |
| Natural Progesterone Liposomal Skin Cream | NOW Solutions | Maintenance of healthy feminine balance |
| C-Vit Liposomal Serum | Sesderma | Hydration, boosts collagen synthesis, enhances skin's elasticity and firmness, and brightens the complexion |
| Advanced Night Repair Protective Recovery Complex | Estée Lauder | Skin repair |
| Fillderma Lips Lip Volumizer | Sesderma | Increases volume of lips, fills wrinkles contour, moisturizes the skin, and outlines the lips |
| Lumessence Eye Cream | Aubrey Organics | Antiwrinkle & firming |
| Russell Organics Liposome Concentrate | Russell Organics | Hydrating & rejuvenating. Makes skin firmer, softer, and smoother |
| Clinicians Complex Liposome Face & Neck Lotion | Clinicians Complex | Nourishes skin and prevents photoaging |
| Kerstin Florian Rehydrating Liposome Day Crème | Kerstin Florian | Moisturizer |
2.2. Niosomes
Niosomes are defined as vesicles having a bilayer structure that are made up by self-assembly of hydrated nonionic surfactants, with or without incorporation of cholesterol or their lipids [34].
Niosomes can be multilamellar or unilamellar vesicles in which an aqueous solution of solute and lipophilic components are entirely enclosed by a membrane which are formed when the surfactant macromolecules are organized as bilayer [35]. Size ranges from 100 nm to 2 μm in diameter. Size of small unilamellar vesicles, multilamellar vesicles, and large unilamellar vesicles ranges from 0.025–0.05 μm, =>0.05 μm, and 0.10 μm, respectively [36]. Major niosomes components include cholesterol and nonionic surfactants like spans, tweens, brijs, alkyl amides, sorbitan ester, crown ester, polyoxyethylene alkyl ether, and steroid-linked surfactants which are used for its preparation [37].
Niosomes are suitable for delivery of both hydrophobic as well as hydrophilic compounds. As a novel drug delivery system, niosomes can be used as vehicle for poorly absorbable drugs [38]. It provides encapsulation to the drug, due to which the drug in the systemic circulation is for prolonged period and penetration is enhanced into target tissue. Niosomes overcome the problems associated with liposomes, like stability problems, high price, and susceptibility to oxidation [39]. Niosomes are used in cosmetics and skin care applications since skin penetration of ingredients is enhanced because it possesses the property of reversibly reducing the barrier resistance of the horny layer, allowing the ingredient to reach the living tissues at greater rate. There is increased stability of entrapped ingredients and improvement in the bioavailability of poorly adsorbed ingredients. There are many factors affecting formation of niosomes, namely, nature and structure of surfactants, nature of encapsulated drug, membrane composition, and temperature of hydration which influences shape and size [40]. Specialized niosomes are called proniosomes; these are nonionic based surfactant vesicles, which are hydrated immediately before use to yield aqueous niosome dispersions. To enhance drug delivery in addition to conventional niosomes, proniosomes are also being used [41, 42].
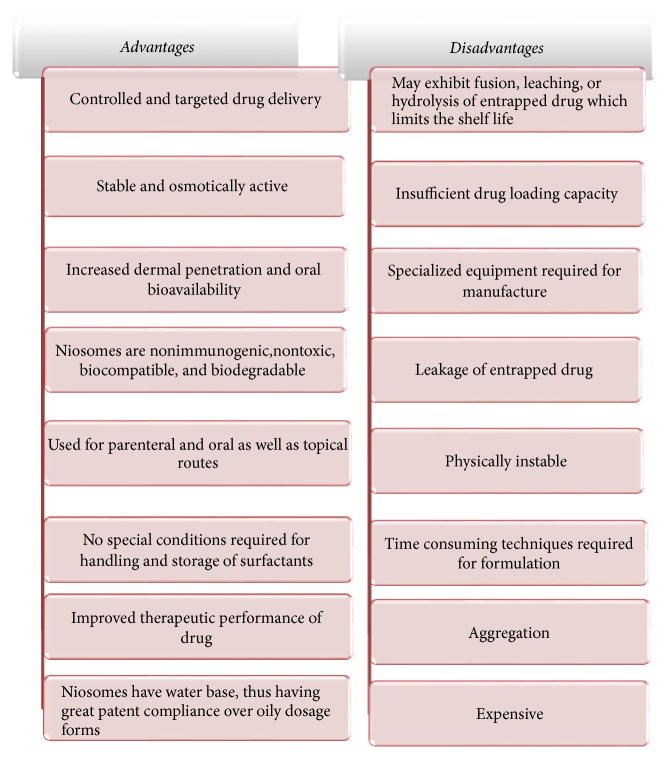
Niosomes were first developed by L'Oreal in the year 1970 by the research and development of synthetic liposomes. Niosomes were patented by L'Oreal in the year 1987 and were developed under the trade name of Lancome. Various niosomes cosmeceuticals preparations are available in market, antiwrinkle creams, skin whitening and moisturizing cream, hair repairing shampoo, and conditioner [43]. Several advantages and disadvantages of niosomes are discussed in Figure 5 [44–46]. Various marketed products and uses are discussed in Table 2 [47–49].
Figure 5.
Advantages and disadvantages of niosomes.
Table 2.
Marketed formulations and uses of niosomes.
| Product name | Marketed by | Uses |
|---|---|---|
| Niosome+ Perfected Age Treatment | Lancome | Removes wrinkles |
| Niosome+ | Lancome | Foundation cream, clear and white skin tone |
| Mayu Niosome Base Cream | Laon Cosmetics | Whitening and moisturizing |
| Anti-Age Response Cream | Simply Man Match | Treatment of wrinkles |
| Identik Masque Floral Repair | Identik | Hair repair masque |
| Identik Shampooing Floral Repair | Identik | Hair repair shampoo |
| Eusu Niosome Makam Pom Whitening Facial Cream | Eusu | Skin whitening |
2.3. Solid Lipid Nanoparticles
An unconventional carrier system, solid lipid nanoparticle (SLN), was developed at the beginning of the 1990s, over the conventional lipoidal carriers like emulsions and liposomes. 50 to 1000 nm is the size range of solid lipid nanoparticles [50].
They are composed of single layer of shells and the core is oily or lipoidal in nature. Solid lipids or mixtures of lipids are present in the matrix drug which is dispersed or dissolved in the solid core matrix. Phospholipids hydrophobic chains are embedded in the fat matrix. These are prepared from complex glycerides mixtures, purified triglycerides, and waxes; liquid lipid is replaced by solid lipid or blend of solid lipid which is solid at body and room temperature and is stabilized by surfactants or polymers [51]. Lipophilic, hydrophilic, and poorly water-soluble active ingredients can be incorporated into SLNs which consist of physiological and biocompatible lipids. With the use of biocompatible compounds for preparing SLN, toxicity problems are avoided [52]. Two principle methods for preparation of SLNs are high pressure homogenization method and precipitation method. Controlled release and sustained release of the active ingredients are possible; SLN which has drug enriched core leads to a sustained release and SLN having drug enriched shell shows burst release [53, 54].
SLNs are popular in cosmeceuticals and pharmaceuticals as they are composed of biodegradable and physiological lipids that exhibit low toxicity. Their small size ensures that they are in close contact with the stratum corneum which increases the penetration of active ingredients through the skin [55].
SLNs have UV resistant properties and act as physical sunscreens on their own, so improved photoprotection with reduced side effects can be achieved when they are combined with molecular sunscreen [56]. Solid lipid nanoparticles as carrier for 3,4,5-trimethoxybenzoylchitin and vitamin E sunscreen are developed to enhance UV protection [57]. SLNs have occlusive property which can be used to increase the skin hydration, that is, water content of the skin [58]. Perfume formulations also have SLNs as they delayed the release of perfume over longer period of time and are ideal for use in day creams as well [59, 60].
They have better stability coalescence when compared to liposomes because they are solid in nature and mobility is reduced of the active molecules, so the leakage from the carrier is prevented [61, 62]. Benefits and drawbacks of SLNs are depicted in Figure 6 [50, 63–65]. Different marketed products and their uses are given in Table 3 [66, 67].
Figure 6.
Benefits and drawbacks of SLNs.
Table 3.
Various marketed formulations of solid lipid nanoparticles.
| Product name | Marketed by | Uses |
|---|---|---|
| Allure Body Cream | Chanel | Body moisturizer |
| Allure Parfum Bottle | Chanel | Perfume |
| Allure Eau Parfum Spray | Chanel | Perfume |
| Soosion Facial Lifting Cream SLN Technology | Soosion | Antiwrinkle cream |
| Phyto NLC Active Cell Repair | Sireh Emas | Skin rejuvenation, overcomes hyperpigmentation, moisturizing & skin firming |
2.4. Nanostructured Lipid Carriers (NLC)
Nanostructured lipid carriers are considered as the second generation of the lipid nanoparticles. NLC have been developed so that the drawbacks associated with SLN can be overcome. NLC are prepared through blending by solid lipids along with spatially incompatible liquid lipid leading to amorphous solids in preferable ratio of 70 : 30 up to 99.9 : 0.1 being solid at body temperature [68, 69]. NLC are mainly of three types on the basis of which the structure is developed according to the formulation composition and production parameters, namely, imperfect type, amorphous type and multiple type. The particle size ranges from 10 to 1000 nm [70].
There is an increased scientific and commercial attention for NLC during the past few years because of the lower risk of systemic side effects. NLC when compared to SLN show higher drug-loading capacity for entrapped bioactive compound because of the distorted structure which helps in creating more space. Other limitations of SLN like reducing particle concentration and expulsion of drug during storage are solved by the formulation of NLC. They are formulated by biodegradable and physiological lipids that show very low toxicity [71]. NLC have modulated drug delivery profile, that is, a biphasic drug release pattern; in this the drug is released initially with a burst followed by a sustained release at a constant rate. They possess numerous advantageous features like increased skin hydration due to occlusive properties, and the small size ensures close contact to the stratum corneum leading to the increased amount of drug penetration into the skin. There are stable drug incorporation during storage and enhanced UV protection system with reduced side effects [72].
In October 2005, the first products containing lipid nanoparticles were introduced in the cosmetic market, namely, NanoRepair Q10® cream and NanoRepair Q10® serum, Dr. Rimpler GmbH, Germany, offering increased skin penetration. Currently there are more than 30 cosmetic products available in the market containing NLC [73, 74]. Depiction of various positive aspects of NLC is depicted in Figure 7 [75, 76]. List of marketed products, manufacturers, and their uses is given in Table 4 [77–80].
Figure 7.
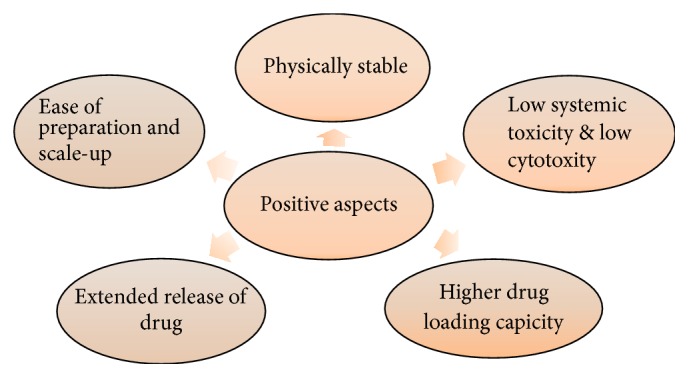
Various positive aspects depiction of NLC.
Table 4.
List of marketed products, manufacturers, and uses of NLC.
| Product name | Marketed by | Uses |
|---|---|---|
| Cutanova-Cream Nanorepair Q10 | Dr. Rimpler | Smoothing of fine lines, promotes restructuring of skin & aging |
| Intensive Serum Nanorepair Q10 | Dr. Rimpler | Antiwrinkle serum, fights sign of aging |
| Cutanova Cream Nanovital Q10 | Dr. Rimpler | Antiaging treatment with UV-protection, protective, |
| Iope Supervital Extra Moist Softner | Amore Pacific | Moisturizes dry and rough skin |
| Iope Supervital Extra Moist Eye Cream | Amore Pacific | Removes eye wrinkles, dullness, and poor elasticity |
| Surmer Masque Crème Nano-Hydratant | Isabelle Lancray | Restricting dry and dehydrated skin, reduction of wrinkles and fine lines |
| Olivenöl Augenpflegebalsam | Dr. Theiss/Medipharma cosmetics | Removes wrinkles, eye rings, and swelling of eyes |
| Olivenol Anti Falten Pflegekontrat | Dr. Theiss/Medipharma Cosmetics | Antiwrinkle and skin tightening |
| Regenerations Cream Intesive Ampoules | Scholl | Promotes cell regeneration and smoothes wrinkles |
| Swiss Cellular White Illuminating Eye Essence | La prairie | Removes under eye darkness and discoloration |
| Surmer Crème Legère Nano-Protection | Isabelle Lancray | Intensely hydrating |
2.5. Nanoemulsions
Nanoemulsions are considered as the kinetically or thermodynamically stable dispersion of liquid in which an oil phase and water phase are in combination with a surfactant. Their structure can be manipulated on the basis of method of preparation, so as to give different types of products. Depending on the composition different types of nanoemulsions are oil in water, water in oil, and bicontinuous nanoemulsion. They exhibit various sizes ranging from 50 nm to 200 nm. These are dispersed phase which comprises small particles or droplets, having very low oil/water interfacial tension [81]. They have lipophilic core, which is surrounded by a monomolecular layer of phospholipids, making it more suitable for delivery of lipophilic compounds.
Problems like sedimentation, coalescence, creaming, and flocculation are not associated with nanoemulsions like with macromolecules. Nanoemulsions are transparent or translucent and show properties like low viscosity, high kinetic stability, high interfacial area, and high solubilization capacity [82].
Nanoemulsions are widely used as medium for the controlled delivery of various cosmeceuticals like deodorants, sunscreens, shampoos, lotions, nail enamels, conditioners, and hair serums [83].
In cosmetics formulation, nanoemulsions provide rapid penetration and active transport of active ingredients and hydration to the skin. Merits of nanoemulsion are shown in Figure 8 [84–87]. Various marketed products name, manufacturers, and their uses are given in Table 5 [88–90].
Figure 8.
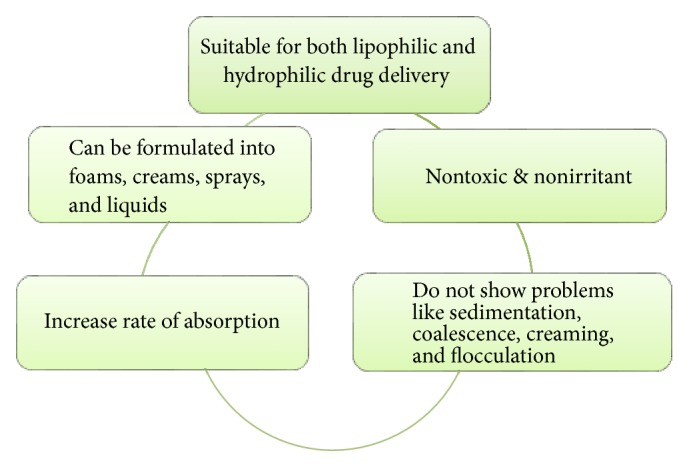
Merits of nanoemulsions.
Table 5.
Various marketed formulations of nanoemulsion.
| Product name | Marketed by | Uses |
|---|---|---|
| Korres Red Vine Hair Sun Protection | Korres | Prevents hair color from fading away |
|
| ||
| Nanocream | Sinerga | Wet wipes |
|
| ||
| Vital Nanoemulsion Α-VC | Marie Louise | Nutrition and miniaturization |
|
| ||
| Bepanthol-Protect Facial Cream Ultra | Bayer HealthCare | Moisturizing, antiaging, and antipollution |
|
| ||
| Coco Mademoiselle Fresh Moisture Mist | Chanel | Prolongs fragrance effect |
|
| ||
| Precision-Solution Déstressante Solution Nano Émulsion Peaux Sensitivity | Chanel | Moisturizer |
|
| ||
| Coni Hyaluronic Acid & Nanoemulsion Intensive Hydration Toner | Coni Beauty | Skin hydration |
|
| ||
| Phyto-Endorphin Hand Cream | Rhonda Allison | Softens and smoothes the skin |
|
| ||
| Nanovital Vitanics Crystal Moiture Cream | Vitacos Cosmetics | Skin moisturizing, elastic, and lightening effects |
|
| ||
| Vitacos Vita-Herb Nona-Vital Skin Toner | Vitacos Cosmetics | Moisturizer |
2.6. Gold Nanoparticles
Nanogold or gold nanoparticles exhibit various sizes ranging from 5 nm to 400 nm. Interparticle interactions and assembly of gold nanoparticles play an important role in determination of their properties [91]. They exhibit different shapes such as nanosphere, nanoshell, nanocluster, nanorod, nanostar, nanocube, branched, and nanotriangles. Shape, size, dielectric properties, and environmental conditions of gold nanoparticles strongly affect the resonance frequency. The color of nanogold ranges from red to purple, to blue and almost black due to aggregation [92]. Gold nanoparticles are inert in nature, highly stable, biocompatible, and noncytotoxic in nature. Nanogold is very stable in liquid or dried form and is nonbleaching after staining on membranes; they are also available in conjugated and unconjugated form [93]. They have high drug-loading capacity and can easily travel into the target cell due to their small size and large surface area, shape, and crystallinity [94].
Gold nanoparticles have been studied as a valuable material in cosmeceutical industries due to their strong antifungal and antibacterial properties. These nanoparticles are used in variety of cosmeceuticals products like cream, lotion, face pack, deodorant, antiaging creams, and so forth. Cosmetic giant companies like L'Oreal and L'Core Paris are using gold nanoparticles for manufacturing more effective creams and lotions [95]. Main properties of nanogold in beauty care consist of assets, namely, acceleration of blood circulation, anti-inflammatory property, antiseptic properties, improvising firmness and elasticity of skin, delaying aging process, and vitalizing skin metabolism [96]. Description of merits of gold nanoparticles is depicted in Figure 9 [97–99]. List of various marketed products name, manufacturers, and their uses is given in Table 6 [100–104].
Figure 9.
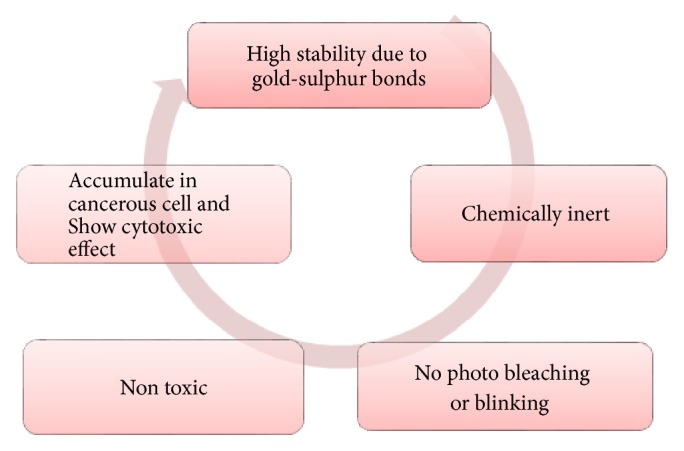
Description of merits of gold nanoparticles.
Table 6.
List of marketed formulation of gold nanoparticles.
| Product name | Marketed by | Uses |
|---|---|---|
| Chantecaille Nano Gold Energizing Cream | Chantecaille | Revitalizes, stimulates cell regeneration, collagen production, helps sun damage repair, and makes skin firm |
|
| ||
| Chantecaille Nano Gold Enerizing Eye Serum | Chantecaille | Prevents aging, promotes collagen, reduces inflammation, and cell growth repair |
|
| ||
| Ameizii Nano Gold Foil Liquid | Ameizii | Repairs skin damage, moisturizes, and promotes skin whitening |
|
| ||
| LR Nano Gold Day & Silk Day Cream | LR Zeitgard | Protects skin from UV rays and prevents light-induced premature skin aging |
|
| ||
| Nuvoderm Nano Gold Anti-Aging Lifting Serum | Nuvoderm | Reduces appearance of signs of aging including fine lines and wrinkles. Promotes collagen & elastin production |
|
| ||
| Orogold 24K Nano Ultra Silk Serum | Orogold | Restores loss of moisture, improves wrinkles and fine lines, and maintains healthy skin |
|
| ||
| Tony Moly Nano Gold BB Cream SPF 50 PA+++ | Tony Moly | Skin whitening, decreases wrinkles, and blocks UV rays |
|
| ||
| O3+ 24K Gold Gel Cream | O3+ | Makes skin glow and shine |
2.7. Nanospheres
Nanospheres are the spherical particles which exhibit a core-shell structure. The size ranges from 10 to 200 nm in diameter. In nanospheres, the drug is entrapped, dissolved, attached, or encapsulated to the matrix of polymer and drug is protected from the chemical and enzymatic degradation. The drug is physically and uniformly dispersed in the matrix system of polymer. The nature of the nanospheres can be crystalline or amorphous [105]. This system has great potential and is being able to convert poorly absorbed, labile biologically active substance and poorly soluble active substance into the propitious deliverable drug. The core of nanospheres can be enclosed with diverse enzymes, genes, and drugs [106].
Nanospheres can be divided into two categories: biodegradable nanospheres and nonbiodegradable nanospheres. Biodegradable nanospheres include gelatin nanospheres, modified starch nanospheres, and albumin nanospheres and nonbiodegradable nanospheres include polylactic acid, which is the only approved polymer.
In cosmetics, nanospheres are used in skin care products to deliver active ingredients into deep layer of the skin and deliver their beneficial effects to the affected area of the skin more precisely and efficiently. These microscopic fragments play a favorable role in protection against actinic aging. Use of nanospheres is increasing in the field of cosmetics especially in skin care products like antiwrinkle creams, moisturizing creams, and antiacne creams [107]. Pictorial presentation of favorable aspects of nanospheres is depicted in Figure 10 [14]. Marketed products name, manufacturers, and their uses are given in Table 7 [10].
Figure 10.
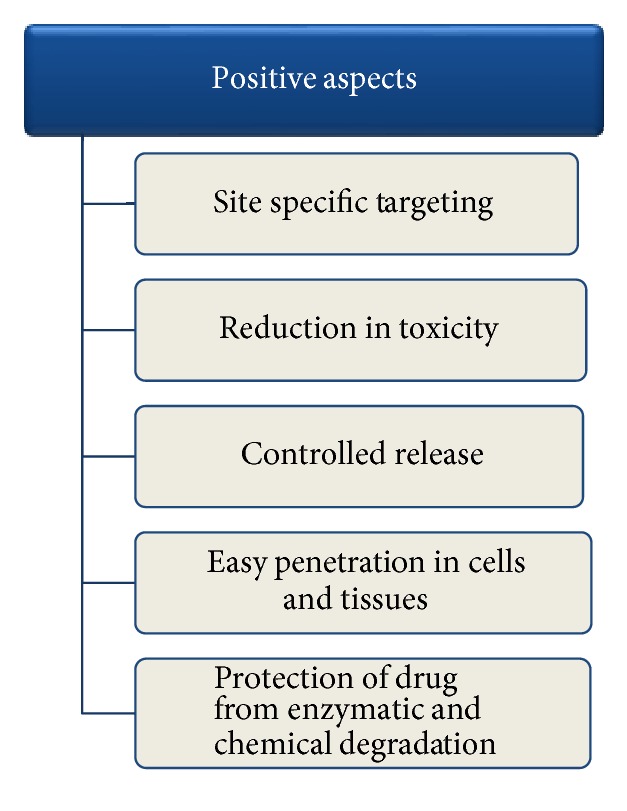
Favorable aspects of nanospheres [14].
Table 7.
Marketed formulation of nanospheres [10].
| Product name | Marketed by | Uses |
|---|---|---|
| Hydralane Ultra Moisturizing Day Cream | Hydralane Paris | Deep moisturizing and hydration |
|
| ||
| Fresh As A Daisy Body Lotion | Kara Vita | Moisturizing body lotion |
|
| ||
| Lip Tender | Kara Vita | Lip moisturizer |
|
| ||
| Nanosphere Plus | Dermaswiss | Antiaging and antiwrinkle |
|
| ||
| Coryse Salome Competence Hydration Ultra-Moisturizing Cream | Coryse Salome Paris | Moisturizing cream |
|
| ||
| Eye Tender | Kara Vita | Antiwrinkle |
|
| ||
| Nano Saltmmoisture Key | Salvona | Moisturizer |
|
| ||
| Clear It! Complex Mist | Kara Vita | Antiacne |
|
| ||
| Cell Act DNA Filler Intense Cream | CellAct Switzerland | Reduces wrinkles and firms skin |
2.8. Dendrimers
The term “dendrimer” arises from two Greek words: namely, “Dendron” that means tree and “Meros” meaning part. Dendrimers are highly branched, unimolecular, globular, micellar nanostructure, and multivalent nanoparticles whose synthesis theoretically affords monodisperse compounds. A dendrimer is typically built from a core on which one or a number of successive series of branches are engrafted in an arborescent way and often adopts a spherical three-dimensional morphology [108]. Generation of the dendrimer is determined by total number of series of branches: if it has one series of branches, then it is first-generation dendrimer; if it has two series, then it is second generation and so on. They are extremely small in size, having diameters in the range of 2–20 nm [109]. Its other properties like monodispersity, polyvalence, and stability make it an ideal carrier for drug delivery with precision and selectivity. To attach biologically active substances for targeting purpose terminal groups are modified. Dendrimers provide controlled release from the inner core and drugs are incorporated in interior as well as being attached on the surface [110].
Dendrimers are new class of macromolecular architecture and are also being used as nanotechnology based cosmeceuticals for various applications like in hair care, skin care, and nail care. Dendrimers have utility in various cosmetic products like shampoos, sunscreen, hair-styling gels, and antiacne products [111]. Companies like L'Oreal, The Dow Company, Wella, and Unilever have several patents for application of dendrimers in cosmeceuticals. Descriptions of advantages of dendrimers are represented in Figure 11.
Figure 11.
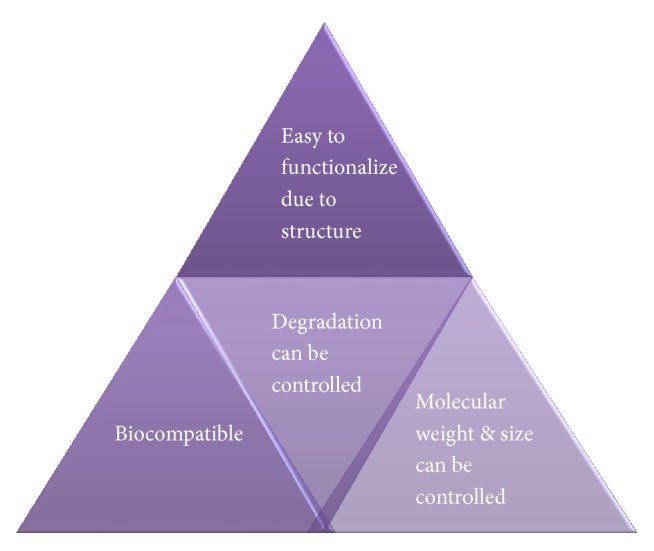
Advantages of dendrimers.
2.9. Carbon Nanotubes
In the field of nanotechnology, carbon nanotubes are represented as one of the most unique inventions. Carbon nanotubes (CNTs) can be described as the rolled graphene with SP2 hybridization. These are seamless cylindrical hollow fibers, comprised of walls formed by graphene as hexagonal lattice of carbon, which are rolled at specific and discrete “chiral” angles. Individual carbon nanotubes align themselves naturally into “ropes” held together by pi-stacking. The diameter ranges from 0.7 to 50 nm with lengths in the range of 10's of microns [112, 113]. Carbon nanotubes are extremely light in weight. These are further of 3 types: namely, single-walled CNTs, doubled-walled CNTs, and multiwalled CNTs. Single-walled CNTs are made up of single graphene sheet which is rolled upon itself with diameter of 1-2 nm, double-walled CNTs are made of two concentric carbon nanotubes, and multiwalled CNTs consist of multiple layers of graphene tubes having diameter ranging from 2 to 50 nm [114]. Major production methods of carbon nanotubes consist of arc discharge method, laser ablation, chemical vapor deposition method, flame synthesis method, and silane solution method [115]. Various patents of carbon nanoparticles have been filed in the field of cosmeceuticals like hair coloring and cosmetic compositions comprising carbon nanotubes and peptide-based carbon nanotube hair colorants and their use in hair colorant and cosmetic compositions [116, 117].
2.10. Polymersomes
Polymersomes are artificial vesicles which enclose a central aqueous cavity, composed of self-assembly of block copolymer amphiphiles. They have a hydrophilic inner core and lipophilic bilayer, so they can be used for both lipophilic and hydrophilic drugs and hydrophobic core provides a protein-affable environment [118]. Polymersomes are biologically stable and are highly versatile. Their drug encapsulation and release capabilities can be easily modulated by applying various block copolymers that are biodegradable or stimuli-responsive. There radius ranges from 50 nm to 5 μm or more [119]. Polymersomes are proficient for encapsulating and protecting sensitive molecules, namely, drugs, proteins, peptides, enzymes, DNA, and RNA fragments. For the preparation of polymersomes, generally synthetic block copolymers have been used. The composition and molecular weight of these polymers can be varied, which allows preparation of polymersomes with different properties, responsiveness to stimuli, different membrane thickness, and permeabilities [120, 121]. The flexibility in the membrane of polymersome makes them capable of targeting and controlling drug release. Due to the presence of a thick and rigid bilayer, they offer more stability than liposomes [122]. Polymersomes are being investigated in the cosmeceutical industry for their use and various patents have been filed for their use. Patents have been filed using polymersome for improving skin elasticity and in another patent polymersomes are used for skin cell activation energy enhancement [123, 124].
2.11. Cubosomes
Cubosomes are the advanced nanostructured particles which are discrete, submicron, and self-assembled liquid crystalline particles of surfactants with proper ratio of water that provides unique properties. Cubosomes are formed by self-assembled structures of aqueous lipid and surfactant systems when mixed with water and microstructure at a certain ratio [125]. Cubosomes are bicontinuous cubic liquid phase, which encloses two separate regions of water being divided by surfactant controlled bilayers and wrapped into a three dimension, periodic, and minimal surface, forming a strongly packed structure [126]. They consist of honeycombed (cavernous) structure and they appear like dots which are slightly spherical in structure. They exhibit size range from 10 to 500 nm in diameter. They have ability to encapsulate hydrophilic, hydrophobic, and amphiphilic substances. Cubosomes have relatively simple preparation methods; they render bioactive agents with controlled and targeted release, possess lipid biodegradability, and have high internal surface area with different drug-loading modalities [127, 128]. Cubosomes are an attractive choice for cosmeceuticals, so for this reason a number of cosmetic giants are investigating cubosomes. Various patents have been filed regarding the cosmetic applications of cubosomes.
3. Major Classes in Nanocosmeceuticals
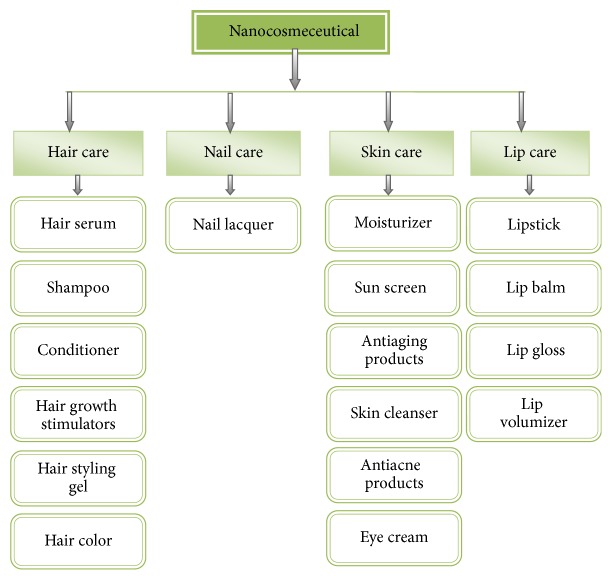
Cosmeceuticals are contemplated as the fastest growing segment of personal care industry. A plethora of nanocosmeceuticals are assimilated in nail, hair, lip, and skin care. Major classes in nanocosmeceuticals are depicted in Figure 12 [48].
Figure 12.
Major classes in nanocosmeceuticals.
3.1. Skin Care
Cosmeceuticals for skin care products ameliorate the skin texture and functioning by stimulating the growth of collagen by combating harmful effect of free radicals. They make the skin healthier by maintaining the structure of keratin in good condition. In sunscreen products zinc oxide and titanium dioxide nanoparticles are most effective minerals which protect the skin by penetrating into the deep layers of skin and make the product less greasy, less smelly, and transparent [129]. SLNs, nanoemulsions, liposomes, and niosomes are extensively used in moisturizing formulations as they form thin film of humectants and retain moisture for prolonged span. Marketed antiaging nanocosmeceutical products assimilating nanocapsules, liposomes, nanosomes, and nanospheres manifest benefits such as collagen renewal, skin rejuvenation, and firming and lifting the skin [130].
3.2. Hair Care
Hair nanocosmeceutical products include shampoos, conditioning agents, hair growth stimulants, coloring, and styling products. Hair follicle, shaft targeting, and increased quantity of active ingredient are achieved by intrinsic properties and unique size of nanoparticles. Nanoparticles subsuming in shampoos seals moisture within the cuticles by optimizing resident contact time with scalp and hair follicles by forming protective film [131]. Conditioning nanocosmeceuticals agents have purposive function of imparting softness, shine, silkiness, and gloss and enhance disentangling of hair. Novel carriers like niosomes, microemulsion, nanoemulsion, nanospheres, and liposomes have major function of repairing damaged cuticles, restoring texture and gloss, and making hair nongreasy, shiny, and less brittle [132].
3.3. Lip Care
Lip care products in nanocosmeceuticals comprise lipstick, lip balm, lip gloss, and lip volumizer. Variety of nanoparticles can be coalesced into lip gloss and lipstick to soften the lips by impeding transepidermal water loss [20] and also prevent the pigments to migrate from the lips and maintain color for longer period of time. Lip volumizer containing liposomes increases lip volume, hydrates and outlines the lips, and fills wrinkles in the lip contour [133].
3.4. Nail Care
Nanocosmeceuticals based nail care products have greater superiority over the conventional products. The nail paints based on nanotechnology have merits such as improved toughness, fast dryness, durability, chip resistance, and ease of application due to elasticity [134]. New strategies such as amalgamating silver and metal oxide nanoparticles have antifungal properties in nail paints for the treatment of toe nails due to fungal infections [135].
4. Toxicity of Nanoparticles Used in Cosmeceuticals
Number of workforce and customers exposed to nanoparticles are escalating because of increasing production and application of the wide diversity of cosmeceuticals products that contain nanomaterials. Despite their huge potential benefit, little is known about the short-term and long-term health effects in the environment and organisms. Due to health hazards, product functionality, and environmental concerns, there may be possible constrains. Concerns have been raised on the possible dangers which may arise on the skin penetration of nanomaterials after their application to the skin [136].
Toxicity of nanoparticles immensely depends on variety of factors like surface properties, coating, structure, size, and ability to aggregate and these factors can be altered and manipulated in the manufacturing process. Nanoparticles having poor solubility have been shown to cause cancer and can exhibit more pronounced toxicity [137]. Health hazard may arise due to the surface area of nanoparticles when compared with the same mass concentration of large particles. Toxicity also depends on the chemical composition of nanoparticles which is absorbed on the skin [138]. There is a relationship between particle size and toxicity: the smaller the size of the nanoparticles, the greater the surface area to volume ratio, due to which there is higher chemical and biological reactivity.
Health hazard caused by nanoparticles to the humans depends on the degree of exposure and the route through which they access the body. Inhalation, ingestion, and dermal routes are the possible routes by which humans can get exposure to the nanoparticles [139]. Routes of exposure of nanoparticles are given in Figure 13.
Figure 13.
Routes of exposure of nanoparticles.
4.1. Inhalation
According to the National Institute of Occupational Health and Safety, the most common route for exposure of airborne nanoparticles is inhalation. Consumers may inhale the nanoparticles and may get exposure through respiratory route, while consuming the products, such as perfumes, powders, and aerosol and workers can get exposed to the nanoparticles during the production. Evidences from the studies conducted on animals suggest that vast majority of nanoparticles inhaled enter the pulmonary tract and some may travel via nasal nerves to the brain and gain access to other organs via blood [140]. Silicon dioxide inhalation toxicity study suggests that particle size of 1–5 nm produces more toxicological response than 10 nm equivalent dose. Experiments on carbon nanotubes have revealed that on chronic exposure interstitial inflammation and epithelioid granulomatous lesions are caused in lungs. Some carbon based fullerenes might oxidize cells or may be hazardous when inhaled [141]. Results of pulmonary administration of TiO2 ultrafine particles than TiO2 fine particles show that ultrafine particles resulted in more lung injury. Gold nanoparticles of sizes 2, 40, and 100 nm, when exposed to intratracheal route, were found in the liver and macrophages. It has been demonstrated that the exposure to TiO2 of particle size 20 nm even at low doses causes complete destruction of DNA, whereas 500 nm TiO2 have small ability for DNA strand breakage [142].
4.2. Ingestion
Nanomaterials may be ingested in the body from unintentional to intentional transfer from hand to mouth. Nanoparticles can be ingested from cosmeceuticals that are applied on lips or mouth like lipsticks, lip balms, lip gloss, and so on [143].
According to the studies, after ingestion, nanomaterials rapidly pass out of the body but sometimes some amount may be taken up that can migrate to the organs. Studies conducted on the layers of pig skin show that certain nanomaterials can penetrate in the layers of the skin within 24 hours of exposure [144]. When mice were orally ingested with zinc oxide nanoparticles with 20 nm and 120 nm at different doses, as a result spleen, heart, liver, bones, and pancreas became the target organs. Copper nanoparticles are found in variety of commercially available cosmeceuticals. Mice exhibited toxicological effects and heavy injuries to internal organs when exposed to copper nanoparticles [145]. Silver nanoparticles are widely used in wound dressing and antimicrobial formulations and now are being used in cosmeceuticals like soaps, face creams, and toothpaste. Silver nanoparticles are used for their antimicrobial activity in the cosmeceuticals. The silver concentration that is lethal for bacteria is the same concentration which is lethal for both fibroblasts and keratinocytes [146]. Various studies conducted on rats suggest that silver nanoparticles when exposed to rat neuronal cells led to size decrease and irregularities in shape and also demonstrated that mouse germline stem cell even at low concentrations of silver nanoparticles reduced drastically the function of mitochondria and cell viability. When mice were exposed to gold nanoparticles of 13.5 nm by ingestion, significant decrease in the RBCs, body weight, and spleen index was observed [147].
4.3. Dermal Routes
Intracellular, transcellular, and transfollicular are the three pathways by which infiltration across the skin occurs. The dermal exposure of lesser size particles <10 nm can penetrate more easily and are disastrous than greater ones >30 nm. There are possibilities that nanoparticle penetration could be affected by skin barrier alterations such as scrapes, wounds, and dermatitis conditions [148]. Prolonged erythema, eschar formation, and oedema were reported with nanoparticles less than 10 nm. Fullerenes are currently being used in cosmeceuticals like moisturizers and face creams but the toxicity related to them remains poorly understood. Report by Professor Robert F. has identified that face creams which have fullerenes incorporated are found to cause damage in the brain of the fish and have toxic effects in the liver cells in humans [149]. Some studies demonstrated that fullerene-based peptides had the capability of penetrating intact skin and their traversal into dermis could be easy due to mechanical stressor. Quantum dots taken intradermally could penetrate regional lymph nodes and lymphatics. There are proven studies that engineered nanoparticles like single or multiwall carbon nanotubes, quantum dots with surface coating, and nanoscale titania are able to alter gene or protein expression and have lethal effects on epidermal keratinocytes and fibroblast [150]. Currently there are few issues regarding the impact of nanoparticles of titanium dioxide and zinc oxide in sunscreens on health, safety, and environment. Increased production of reactive oxygen species (ROS), including free radicals, is due to greater surface area, greater chemical reactivity, and smaller size. Free radical and reactive oxygen species production is the primary mechanism for toxicity of nanoparticles. Titanium dioxide and zinc oxide generate ROS and free radical when exposed to ultraviolet (UV) radiations, which have potential in inflammation and oxidative stress and can significantly damage membranes, proteins, RNA, DNA, and fats within cells [151]. A research on TiO2 nanoparticles toxicity demonstrated that when these nanoparticles subcutaneously given to the pregnant mice, they were transferred to the offspring and there was a reduced sperm production in male offspring and brain damage as well. Nanoparticles of cobalt-chromium have potential that they can cross the skin barrier and damage fibroblast in humans [152].
5. Global Scenario of Nanocosmeceuticals
Drugs are subjected to the stringent scrutiny requirements imposed by FDA for their approval but there are no such requirements for cosmetics. Cosmeceuticals are the products which are on the borderline between cosmetics and pharmaceuticals. The Federal Food, Drug and Cosmetics Act and FDA do not recognize the term “cosmeceuticals” and the aesthetic and functional benefits are enjoyed by the products without crossing over into becoming over the counter drugs [153]. Many cosmeceuticals alter the physiological processes in the skin, but manufactures avoid holding clinical trials and making the specific claims to avoid subjecting their products to expensive and lengthy approval process by FDA. New and unfamiliar challenges are being faced by the cosmetic industry [154].
Extra category is created by some jurisdiction to accommodate cosmeceuticals or borderline products.
Japan. The products that fall between cosmetics and drugs are called “quasi-drugs.” Ingredients must be preapproved in the market before including them into the quasi-drugs and require preapproval before selling them in the market [155].
Korea. Cosmeceuticals are classified as “functional cosmetics” by Korea Food and Drug Administration (KFDA). For improving safety and evaluation of functional cosmetics KFDA is responsible [156].
Thailand. According to the ingredients used in cosmeceuticals, they are classified as “controlled cosmetics.” Before being marketed in Thailand, controlled cosmetics require controlled ingredients that require the notification from FDA for the use of products.
New Zealand. The category in which cosmeceuticals are accommodated is called “related products.”
Australia. In Australia goods can be categorized on the basis of claims about the product and product composition; the borderline products are classified as “therapeutic goods.” Only approved ingredients are used for the manufacturing of these products. Australian Register of Therapeutic Goods registers the therapeutic goods [157].
Canada. Cosmeceuticals are termed as “dermo-cosmetics” in Canada. Cosmeceuticals are not recognized as an independent cosmetic category; Canadian health authorities have identified Category V to accommodate products falling in category of both cosmetics and drugs. Less regulatory requirements are required for regulation of these products.
USA. There are 3 categories in US: namely, cosmetics, drugs, and OTC drugs, and there is no legal definition of cosmeceuticals according to USFDA. Classification in USFDA depends on the claims of the products [158].
European Union. In European Union, cosmetics are regulated under Cosmetic Directive 76/768/EEC. EU does not have category to be called cosmeceuticals, but it has stringent laws in which any claims made by the company are required to be submitted as a proof. According to new regulation by EU, manufacturers have to list the nanoparticles contained in the product which has to be marketed with European Union. Cosmetic regulation states that any product containing nanomaterials as ingredient should be clearly mentioned and has to insert word “nano” in brackets after ingredient listing [159, 160].
China. Cosmeceuticals are regarded as “cosmetics for special use.” According to China Food and Drug Administration (CFDA), all foreign cosmetic product manufacturers before selling the product in the Chinese market must complete a safety and health quality test and obtain a hygiene permit. Special use cosmetics have to undergo safety and health quality test such as microbiology, toxicology test, chronic toxicity, carcinogenic test, and conducting safe-for-human-use trials. Imported cosmetics are classified into two categories: ordinary cosmetics and special use cosmetics. Each category of cosmetics requires different type of license from State Food and Drug Administration (SFDA). For the marketing of cosmetics hygiene license or record-keeping certificate from Health Administration Department of the State Council-SFDA must be obtained [161].
If the FDA finds out that there is safety issue regarding use of any cosmetic or the ingredient including nanoparticles, FDA has authority to prohibit the sale and manufacturing of the product or various other options like ban on ingredients, seizing unsafe products, warning letters, and mandatory warning labels and even ban of the product worldwide. New research strategy has been issued by the US Environmental Protection Agency (EPA) so as to proactively examine the impact on environment and human health due to nanoparticles being used in cosmetics, sunscreens, paints, and so on [162]. Under this agency, focus is on the research on seven types of manufactured nanomaterials: titanium dioxide, silver nanoparticles, nanotubes, cerium oxide, fullerenes, and zero-valent [163].
Scientific committee on Consumer Products (SCCP) has raised concern over use of insoluble nanoparticles used in cosmetics that are applied topically because of the toxicity reasons. The royal society world's oldest scientific organization has also raised questions on nanoparticles whether it could enter the bloodstream, taken up by cells and impart its effect [164]. Simultaneously it has also expressed the desire for the conduction of more research in this field to address the chronic effects which may arise as a result of long-term use by people all over the globe [165].
6. Conclusion
Nanotechnology is considered to be the most promising and revolutionizing field. Over the last dozen of years, nanotechnology is widely being used and is beneficial in the field of dermatology, cosmetics, and biomedical applications as well. New technologies and novel delivery systems have been invented by scientists, which are currently being used in the manufacture of cosmeceuticals. By the increase in use of cosmeceuticals, the conventional delivery systems are being replaced by the novel delivery systems. Novel nanocarriers which are currently being used are liposomes, niosomes, NLC, SLNs, gold nanoparticles, nanoemulsion, and nanosomes in various cosmeceuticals. These novel delivery systems have remarkable potential in achieving various aspects like controlled and targeted drug delivery, site specificity, better stability, biocompatibility, prolonged action, and higher drug-loading capacity. There is lack of convincing evidences for the claims of effectiveness, so industries are required to provide them. There are huge controversies regarding the toxicity and safety of the nanomaterials; various researches are being carried out to determine the possible health hazard and toxicity. Meticulous studies on the safety profile of the nanomaterials are required. Nanoproducts should be fabricated in such a way that their value and health of the customers are improved. Clinical trials are not required for the approval of cosmeceuticals so the manufacturers enjoy the benefit and avoid holding clinical trials and lengthy procedures. Lastly, stringent laws should be imposed on the regulation and safety of cosmeceuticals and nanoparticles used in them.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Logothetidis S. Nanostructured materials and their applications. NanoScience and Technology. 2012;12:p. 220. [Google Scholar]
- 2.Maynard A. D. Nanotechnology: A Research Strategy for Addressing Risk. PEN. 2006. [Google Scholar]
- 3.Bangale M. S., Mitkare S. S., Gattani S. G., Sakarkar D. M. Recent nanotechnological aspects in cosmetics and dermatological preparations. International Journal of Pharmacy and Pharmaceutical Sciences. 2012;4(2):88–97. [Google Scholar]
- 4.Gautam A., Singh Vijayaraghavan R. Dermal exposure of nanoparticles: an understanding. Journal of Cell and Tissue Research. 2011;11(1):2703–2708. [Google Scholar]
- 5.Sharma R. Cosmeceuticals and herbal drugs: practical uses. International Journal of Pharmaceutical Sciences and Research. 2012;3(1):59–65. [Google Scholar]
- 6.Dureja H., Kaushik D., Gupta M., Kumar V., Lather V. Cosmeceuticals: an emerging concept. Indian Journal of Pharmacology. 2005;37(3):155–159. doi: 10.4103/0253-7613.16211. [DOI] [Google Scholar]
- 7.Mukta S., Adam F. Cosmeceuticals in day-to-day clinical practice. Journal of Drugs in Dermatology. 2010;9:62–69. [PubMed] [Google Scholar]
- 8.Srinivas K. The current role of nanomaterials in cosmetics. Journal of Chemical and Pharmaceutical Research. 2016;8(5):906–914. [Google Scholar]
- 9.Brandt F. S., Cazzaniga A., Hann M. Cosmeceuticals: current trends and market analysis. Seminars in Cutaneous Medicine and Surgery. 2011;30(3):141–143. doi: 10.1016/j.sder.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Nano-materials: prevalence in personal care products. https://www.ewg.org/skindeep/2007/08/25/hundreds-of-personal-care-products-contain-poorly-studied-nano-materials/#.Wka44VWWbIU.
- 11.Mu L., Sprando R. L. Application of nanotechnology in cosmetics. Pharmaceutical Research. 2010;27(8):1746–1749. doi: 10.1007/s11095-010-0139-1. [DOI] [PubMed] [Google Scholar]
- 12.Nohynek G. J., Lademann J., Ribaud C., Roberts M. S. Grey Goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Critical Reviews in Toxicology. 2007;37(3):251–277. doi: 10.1080/10408440601177780. [DOI] [PubMed] [Google Scholar]
- 13.Antonio J. R., Antônio C. R., Soares-Cardeal I. L., Ballavenuto J. M. A., Oliveira J. R. Nanotechnology in dermatology. Anais Brasileiros de Dermatologia. 2014;89(1):126–136. doi: 10.1590/abd1806-4841.20142228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gref R., Gref R., Minamitake Y., et al. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263(5153):1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 15.Dahiya A., Romano J. F. Cosmeceuticals: a review of their use for aging and photoaged skin. Cosmetic Dermatology. 2006;19(7):479–484. [Google Scholar]
- 16.Starzyk E., Frydrych A., Solyga A. Nanotechnology: does it have a future in cosmetics? SÖFW Journal. 2008;134(6):42–52. [Google Scholar]
- 17.Mukta S., Adam F. Cosmeceuticals in day-to-day clinical practice. Journal of Drugs in Dermatology. 2010;9:62–66. [PubMed] [Google Scholar]
- 18.Nasir A. Nanotechnology and dermatology: Part II-risks of nanotechnology. Clinics in Dermatology. 2010;28(5):581–588. doi: 10.1016/j.clindermatol.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Fox C. Cosmetic and Pharmaceutical Vehicles: Skin Care, Hair Care, Makeup and Sunscreens. Vol. 113. Cosmetics & Toiletries; 1998. [Google Scholar]
- 20.Tripura P., Anushree H. Anushree novel delivery systems: current trend in cosmetic industry. European Journal of Pharmaceutical and Medical Research. 2017;4(8):617–627. [Google Scholar]
- 21.Arora N., Agarwal S., Murthy R. S. R. Latest technology advances in cosmaceuticals. International Journal of Pharmaceutical Sciences and Drug Research. 2012;4(3):168–182. [Google Scholar]
- 22.Hope M. J., Kitson C. N. Liposomes: a perspective for dermatologists. Dermatologic Clinics. 1993;11(1):143–154. [PubMed] [Google Scholar]
- 23.Bhupendra G., Prajapati Niklesh K., Manan M., Rakesh P. P. Topical Liposomes in Drug Delivery: A Review. International journal of pavement research and technology. 2012;4(1):39–44. [Google Scholar]
- 24.Tasleem A., Nuzhatun N., Syed S. A., Sheikh S., Raheel M., Muzafar R. S. herapeutic and Diagnostic Applications of Nanotechnology in Dermatology and Cosmetics Nanomedicine & Biotherapeutic. Discovery Journal of Nanomedicine & Biotherapeutic Discovery. 2015;5(3):1–10. [Google Scholar]
- 25.Egbaria K., Weiner N. Liposomes as a topical drug delivery system. Advanced Drug Delivery Reviews. 1990;5(3):287–300. doi: 10.1016/0169-409X(90)90021-J. [DOI] [Google Scholar]
- 26.Rieger M. M. Skin Lipids and Their Importance to Cosmetic Science. Vol. 102. Cosmetics Toiletries; 1987. [Google Scholar]
- 27.Blume G., Teichmüller E., Teichmüller E. New evidence of the penetration of actives by liposomal carrier system. Cosmetics & Toiletries Manufacture Worldwide. 1997:135–139. [Google Scholar]
- 28.Ghyczy M., Nissen H.-P., Biltz H. The treatment of acne vulgaris by phosphatidylcholine from soybeans, with a high content of linoleic acid. Journal of Applied Cosmetology. 1996;14(4):137–145. [Google Scholar]
- 29. Flexible Liposomes for topical Applications in Cosmetics Dr. Gabriele Blume.
- 30.Lasic D. D. Novel applications of liposomes. Trends in Biotechnology. 1998;16(7):307–321. doi: 10.1016/S0167-7799(98)01220-7. [DOI] [PubMed] [Google Scholar]
- 31.Akbarzadeh A., Rezaei-Sadabady R., Davaran S., et al. Liposome: classification, preparation, and applications. Nanoscale Research Letters. 2013;8, article 102 doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dermosome. https://www.ulprospector.com/en/la/PersonalCare/Detail/1832/44044/Dermosome.
- 33.Decorte. https://www.decortecosmetics.com/skincare/liposome.
- 34.Karim K., Mandal A., Biswas N., et al. Niosome: a future of targeted drug delivery systems. Journal of Advanced Pharmaceutical Technology & Research. 2010;1(4):374–380. doi: 10.4103/0110-5558.76435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duarah S., Pujari K., Durai R. D., Narayanan V. H. B. Nanotechnology-based cosmeceuticals: A review. International Journal of Applied Pharmaceutics. 2016;8(1):8–12. [Google Scholar]
- 36.Gandhi A. Suma oomen sen, abhijit paul. current trends in niosome as vesicular drug delivery system. Asian Journal of Pharmacy and Life Science. 2012;2(2):339–352. [Google Scholar]
- 37.Karim et al. K. M. A future of targeted drug delivery systems. Journal of Advanced Pharmaceutical Technology & Research. 2010;1(4):374–380. doi: 10.4103/0110-5558.76435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pola Chandu V., Arunachalam A., Jeganath S., Yamini K., Tharangini K. Niosomes: a novel drug delivery system. International Journal of Novel Trends in Pharmaceutical Sciences. 2012;2(1):2277–2782. [Google Scholar]
- 39.Kumar G. P., Rajeshwarrao P. Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta Pharmaceutica Sinica B (APSB) 2011;1(4):208–219. doi: 10.1016/j.apsb.2011.09.002. [DOI] [Google Scholar]
- 40.Biswa S., Murthy P. N., Sahu J., Amir F. Vesicles of non-ionic surfactants (niosomes) and drug delivery potential. International journal of pharmaceutical sciences and nanotechnology. 2008;1(1):1–8. [Google Scholar]
- 41.Sudheer P., Kaushik K. Review on Niosomes - a Novel Approach for Drug Targeting. Journal of Pharmaceutical Research. 2015;14(1):20–25. doi: 10.18579/jpcrkc/2015/14/1/78376. [DOI] [Google Scholar]
- 42.Tripura Sundari P., Anushree H. Novel delivery systems: current trend in cosmetic industry. European Journal of Pharmaceutical and Medical Research. 2017;4(8):617–627. [Google Scholar]
- 43.Nasir A., Harikumar S., Amanpreet K. Niosomes: an excellent tool for drug delivery. International Journal of Research in Pharmacy and Chemistry. 2012;2(2):479–487. [Google Scholar]
- 44.Madhav N. V. S., Saini A. Niosomes: a novel drug delivery system. International Journal of Research in Pharmacy and Chemistry. 2011;1(3):498–511. [Google Scholar]
- 45.Gandhi M., Sanket P., Mahendra S., et al. Niosomes: Novel Drug Delivery System. International Journal of Pure & Applied Bioscience. 2014;2(2):267–274. [Google Scholar]
- 46.Navneet S., Pooja M., Vinay V. Nanoparticle vesicular systems: A versatile tool for drug delivery. Journal of Chemical and Pharmaceutical Research. 2010;2(2):496–509. [Google Scholar]
- 47.Thakur V., Arora S., Prashar B., Vishal P. Niosomes and liposomes-vesicular approach towards transdermal drug delivery. International Journal of Pharmaceutical and Chemical Sciences. 2012;1(3):981–993. [Google Scholar]
- 48.Lohani A., Verma A., Joshi H., Yadav N., Karki N. Nanotechnology-based cosmeceuticals. ISRN Dermatology. 2014;2014:14. doi: 10.1155/2014/843687.843687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta A., Prajapati S. K., Balamurugan M., Singh M., Bhatia D. Design and Development of a Proniosomal Transdermal Drug Delivery System for Captopril. Tropical Journal of Pharmaceutical Research. 2007;6(2) doi: 10.4314/tjpr.v6i2.14647. [DOI] [Google Scholar]
- 50.Puri D., Bhandari A., Sharma P., Choudhary D. Lipid nanoparticles (SLN, NLC): A novel approach for cosmetic and dermal pharmaceutical. Journal of Global Pharma Technology. 2010;2(9):1–15. [Google Scholar]
- 51.Bangale M. S., Mitkare S. S., Gattani S. G., Sakarkar D. M. Recent nanotechnological aspects in cosmetics and dermatological preparations Dm2. International Journal of Pharmacy and Pharmaceutical Sciences. 2012;4(2):149–165. [Google Scholar]
- 52.Mohamed H. H., Omaima N. G. Rice Bran Solid Lipid Nanoparticles: Preparation and Characterization. 2. Vol. 1. Universal Research Publications; 2011. [Google Scholar]
- 53.Kaur I. P., Agrawal R. Nanotechnology: a new paradigm in cosmeceuticals. Recent patents on drug delivery & formulation. 2007;1(2):171–182. doi: 10.2174/187221107780831888. [DOI] [PubMed] [Google Scholar]
- 54.zur Mühlen A., Schwarz C., Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery—drug release and release mechanism. European Journal of Pharmaceutics and Biopharmaceutics. 1998;45(2):149–155. doi: 10.1016/s0939-6411(97)00150-1. [DOI] [PubMed] [Google Scholar]
- 55.Pardeike J., Hommoss A., Müller R. H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. International Journal of Pharmaceutics. 2009;366(1-2):170–184. doi: 10.1016/j.ijpharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Arora S. A., Murthy R. S. R. Latest Technology Advances in Cosmaceuticals. International Journal of Pharmaceutical Sciences and Drug Research. 2012;4(3):168–182. [Google Scholar]
- 57.Patidar A., Devendra S. T., Peeyush K., Jhageshwar V. A review on novel lipid based nanocarriers. International Journal of Pharmacy and Pharmaceutical Sciences. 2010;2(4):234–257. [Google Scholar]
- 58.Song C., Liu S. A new healthy sunscreen system for human: Solid lipid nannoparticles as carrier for 3,4,5-trimethoxybenzoylchitin and the improvement by adding Vitamin E. International Journal of Biological Macromolecules. 2005;36(1-2):116–119. doi: 10.1016/j.ijbiomac.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Müller R. H., Radtke M., Wissing S. A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Advanced Drug Delivery Reviews. 2002;54, supplement 1:S131–S155. doi: 10.1016/S0169-409X(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 60.Hassan H., Omaima N. Rice bran solid lipid nanoparticles: Preparation and characterization Mohamed. International Journal of Research in Drug Delivery. 2011;1(2):6–9. [Google Scholar]
- 61.López-García R., Ganem-Rondero A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): occlusive effect and penetration enhancement ability. Journal of Cosmetics, Dermatological Sciences and Applications. 2015;5(2):62–72. doi: 10.4236/jcdsa.2015.52008. [DOI] [Google Scholar]
- 62.Souto E. B., Müller R. H. Cosmetic features and applications of lipid nanoparticles (SLN, NLC) International Journal of Cosmetic Science. 2008;30(3):157–165. doi: 10.1111/j.1468-2494.2008.00433.x. [DOI] [PubMed] [Google Scholar]
- 63.Ekambaram P., Abdul Hasan A., Sathali., Priyanka K. Solid lipid nanoparticles: a review. Scientific Reviews & Chemical Communication. 2012;2(1):80–102. [Google Scholar]
- 64.Ramteke J., Dhole. Solid lipid nanoparticle: a review. IOSR Journal of Pharmac. 2012;2(6):34–44. [Google Scholar]
- 65.Lasoń E., Ogonowski J. Solid Lipid nanoparticles - Characteristics, application and obtaining. Chemik. 2011;65(10):960–967. [Google Scholar]
- 66.Herbal massage cream. http://herbalmassagecream.buy.peerflix.com/pz6cc822b-facial-lifting-cream-anti-aging-face-cream-sln-technology-3d-tightener.html.
- 67.Chanel Fragrance. https://www.chanel.com/en_US/fragrance-beauty/allure-138049.
- 68.Dilip K., Surendra T., Suresh K. N., Roohi K. Nanostructured lipid carrier (Nlc) a modern approach for topical delivery: a review. World Journal of Pharmacy and Pharmaceutical Sciences. 2013;2(3):921–938. [Google Scholar]
- 69.Saupe A., Wissing S. A., Lenk A., Schmidt C., Müller R. H. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) - Structural investigations on two different carrier systems. Bio-Medical Materials and Engineering. 2005;15(5):393–402. [PubMed] [Google Scholar]
- 70.Shailesh L., Patwekar Snehal R., Ashwini P., Manoj S., Arvind B. Nanostructured lipid carriers in stability improvement forcosmetic nanoparticles. International Journal of Pharmacy & Pharmaceutical Resarch. 2016;6(1):168–180. [Google Scholar]
- 71.Purohit D. K., Nandgude T. D., Poddar S. S. Nano-lipid carriers for topical application: Current scenario. Asian Journal of Pharmaceutics. 2016;9(5):544–553. doi: 10.22377/ajp.v10i03.757. [DOI] [Google Scholar]
- 72.Westesen K., Bunjes H., Koch M. H. J. Physicochemical characterization of lipid nanoparticles and evaluation of their drug loading capacity and sustained release potential. Journal of Controlled Release. 1997;48(2-3):223–236. doi: 10.1016/S0168-3659(97)00046-1. [DOI] [Google Scholar]
- 73.Khan S., Baboota S., Ali J., Khan S., Narang R., Narang J. Nanostructured lipid carriers: An emerging platform for improving oral bioavailability of lipophilic drugs. International Journal of Pharmaceutical Investigation. 2015;5(4):p. 182. doi: 10.4103/2230-973X.167661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Müller R. H., Radtke M., Wissing S. A. Nanostructured lipid matrices for improved microencapsulation of drugs. International Journal of Pharmaceutics. 2002;242(1-2):121–128. doi: 10.1016/S0378-5173(02)00180-1. [DOI] [PubMed] [Google Scholar]
- 75.Sharma B., Sharma A. Future prospect of nanotechnology in development of anti-ageing formulations. International Journal of Pharmacy and Pharmaceutical Sciences. 2012;4(3):57–66. [Google Scholar]
- 76.Naseri N., Valizadeh H., Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: Structure preparation and application. Advanced Pharmaceutical Bulletin (APB) 2015;5(3):305–313. doi: 10.15171/apb.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarabjot K., Ujjwal N., Ramandeep S., et al. Nanostructure lipid carrier (NLC): the new generation of lipid nanoparticles. Asian Pacific Journal of Health Sciences. 2015;2(2):76–93. [Google Scholar]
- 78.Fang C. L., Al-Suwayeh S. A., Fang J. Y. Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent Patents on Nanotechnology. 2013;7(1):41–55. doi: 10.2174/187221013804484827. [DOI] [PubMed] [Google Scholar]
- 79.Dragicevic N., Maibach H. I. Howard I. Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement. [DOI]
- 80. Http://Drrimpler.Com/Dr-Rimpler-Professional-Treatments/Nanocare-Q10-Treatment/?V=920f83e594a1.
- 81.Shah P., Bhalodia D., Shelat P. Nanoemulsion: A pharmaceutical review. Systematic Reviews in Pharmacy. 2010;1(1):24–32. doi: 10.4103/0975-8453.59509. [DOI] [Google Scholar]
- 82.Ronak P. P., Jay R. J. An overview on nanoemulsion: a novel approach. International Journal of Pharmaceutical Sciences and Research. 2012;3(12):4640–4650. [Google Scholar]
- 83.Ozgun S. Nanoemulsions in cosmetics. Nanomaterials and Nanotechnology.
- 84.Maali A., Mosavian M. T. H. Preparation and Application of Nanoemulsions in the Last Decade (2000-2010) Journal of Dispersion Science and Technology. 2013;34(1):92–105. doi: 10.1080/01932691.2011.648498. [DOI] [Google Scholar]
- 85.Barnett J. M., Scher R. K. Nail cosmetics. International Journal of Dermatology. 1992;31(10):675–681. doi: 10.1111/j.1365-4362.1992.tb01368.x. [DOI] [PubMed] [Google Scholar]
- 86.Gupta A., Eral H. B., Hatton T. A., Doyle P. S. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12(11):2826–2841. doi: 10.1039/C5SM02958A. [DOI] [PubMed] [Google Scholar]
- 87.Sharma. Nanoemulsions for Cosmetics. International Journal of Advanced Resarech In Pharmaceutical & Bio Sciences. 2012;2(3):408–415. [Google Scholar]
- 88.Padmadevi C., Diana Ariffin F., Ahmad M., Asaad A., Nagib A. Nanoemulsion for Cosmetic Application. European Journal of Biomedical and Pharmaceutical Sciences. 2016;3(7):8–11. [Google Scholar]
- 89.Patel. An overview on nanoemulsion: a novel approach. International Journal of Pharmaceutical Sciences and Research. 2012;3(12):4640–4650. [Google Scholar]
- 90. https://www.chanel.com/en_WW/fragrance-beauty/fragrance/p/women/coco-mademoiselle/coco-mademoiselle-fresh-moisture-mist-p116850.html#skuid-0116850.
- 91.Lata K., Arvind K. J., Laxmana N., Rajan S. Gold nanoparticles: preparation, characterization and its stability in buffer. A Journal of Nanotechnology and Its Applications. 2014;17(1):1–10. [Google Scholar]
- 92.Khan A. K., Rashid R., Murtaza G., Zahra A. Gold nanoparticles: Synthesis and applications in drug delivery. Tropical Journal of Pharmaceutical Research. 2014;13(7):1169–1177. doi: 10.4314/tjpr.v13i7.23. [DOI] [Google Scholar]
- 93.Verma H. N., Singh P., Chavan R. M. Gold nanoparticle: synthesis and characterization. Veterinary World. 2014;7(2):72–77. doi: 10.14202/vetworld.2014.72-77. [DOI] [Google Scholar]
- 94.Yeh Y.-C., Creran B., Rotello V. M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale. 2012;4(6):1871–1880. doi: 10.1039/c1nr11188d. doi: 10.1039/c1nr11188d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khalid A., Tawfik A. S. Gold and Silver Nanoparticles: Synthesis Methods, Characterization Routes and Applications towards Drugs. Journal of Environmental & Analytical Toxicology. 2016;6(4):1–10. [Google Scholar]
- 96.Thakor A. S., Jokerst J., Zavaleta C., Massoud T. F., Gambhir S. S. Gold nanoparticles: a revival in precious metal administration to patients. Nano Letters. 2011;11(10):4029–4036. doi: 10.1021/nl202559p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alanazi F. K., Radwan A. A., Alsarra I. A. Biopharmaceutical applications of nanogold. Saudi Pharmaceutical Journal. 2010;18(4):179–193. doi: 10.1016/j.jsps.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen P. C., Mwakwari S. C., Oyelere A. K. Gold nanoparticles: from nanomedicine to nanosensing. Nanotechnology Science Application. 2008;1:45–65. doi: 10.2147/nsa.s3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arvizo R., Bhattacharya R., Mukherjee P. Gold nanoparticles: opportunities and challenges in nanomedicine. Expert Opinion on Drug Delivery. 2010;7(6):753–763. doi: 10.1517/17425241003777010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. http://www.totalbeauty.com/reviews/product/6184661/chantecaille-nano-gold-energizing-cream.
- 101. URL https, https://www.aliexpress.com/item/AMEIZII-Nano-Gold-Original-Liquid-Skin-Care-Face-Day-creams-Facial-Whitening-Moisturizing-Anti-Aging, Serum/32823859689.html.
- 102. https://www.amazon.com/Nanogold-Silk-Skincare-Set-Cleansing/dp/B011HDPFCG.
- 103. https://www.consumerhealthdigest.com/wrinkle-cream-reviews/nuvoderm-nano-gold.html.
- 104. https://www.orogoldcosmetics.com/24k-nano-ultra-silk-serum.html.
- 105.Singh A., Garg G., Sharma P. K. Nanospheres: A novel approach for targeted drug delivery system. International Journal of Pharmaceutical Sciences Review and Research. 2010;5(3):84–88. [Google Scholar]
- 106.Mamo B. Literature review on Biodegradable Nanospheres for Oral and Targeted Drug Delivery. Asian Journal of Biomedical and Pharmaceutical Sciences. 2015;05(51):01–12. doi: 10.15272/ajbps.v5i51.761. [DOI] [Google Scholar]
- 107.Guterres S. S., Alves M. P., Pohlmann A. R. Polymeric Nanoparticles, Nanospheres and Nanocapsules, for Cutaneous Applications. Drug Target Insights. 2017;2:p. 117739280700200. doi: 10.1177/117739280700200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fruchon S., Poupot R. Pro-inflammatory versus anti-inflammatory effects of dendrimers: The two faces of immuno-modulatory nanoparticles. Nanomaterials. 2017;7(9, article no. 251) doi: 10.3390/nano7090251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klajnert B., Bryszewska M. Dendrimers: Properties and applications. Acta Biochimica Polonica. 2001;48(1):199–208. [PubMed] [Google Scholar]
- 110.Awani K. R., Ruchi T., Priyanka M., Pooja Y. Dendrimers: a potential carrier for targeted drug delivery system. Pharmaceutical and Biological Evaluations June. 2016;3(3):275–287. [Google Scholar]
- 111.Yapar E. A., Inal Ö. Nanomaterials and cosmetics. Journal of Pharmacy of Istanbul University. 2012;42(1):43–70. [Google Scholar]
- 112.Ibrahim K. S. Carbon nanotubes-properties and applications: a review. Carbon Letters. 2013;14(3):131–144. doi: 10.5714/CL.2013.14.3.131. [DOI] [Google Scholar]
- 113.Kaushik B. K., Majumder M. K. Carbon nanotube: Properties and applications. SpringerBriefs in Applied Sciences and Technology. 2015;(9788132220466):17–37. doi: 10.1007/978-81-322-2047-3_2. [DOI] [Google Scholar]
- 114.Hirlekar R., Yamagar M., Garse H., Mohit V., Kadam V. Carbon nanotubes and its applications: A review. Asian Journal of Pharmaceutical and Clinical Research. 2009;2(4):17–27. [Google Scholar]
- 115.Zhang M., Li J. Carbon nanotube in different shapes. Materials Today. 2009;12(6):12–18. doi: 10.1016/s1369-7021(09)70176-2. [DOI] [Google Scholar]
- 116. https://www.google.com/patents/WO2006052276A2.
- 117. https://www.google.com/patents/WO2005117537A3.
- 118.Ambikanandan M. Challenges in Delivery of Therapeutic Genomics and Proteomics. Elsevier; 2011. [Google Scholar]
- 119.Kim S.-H., Shum H. C., Kim J. W., Cho J.-C., Weitz D. A. Multiple polymersomes for programmed release of multiple components. Journal of the American Chemical Society. 2011;133(38):15165–15171. doi: 10.1021/ja205687k. [DOI] [PubMed] [Google Scholar]
- 120.Discher D. E., Eisenberg A. Polymer vesicles. Science. 2002;297(5583):967–973. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 121.Bermudez H., Brannan A. K., Hammer D. A., Bates F. S., Discher D. E. Molecular weight dependence of polymersome membrane structure, elasticity, and stability. Macromolecules . 2002;35(21):8203–8208. doi: 10.1021/ma020669l. [DOI] [Google Scholar]
- 122.Zhang X.-Y., Zhang P.-Y. Polymersomes in nanomedicine - A review. Current Molecular Pharmacology. 2017;13(2):124–129. doi: 10.2174/1573413712666161018144519. [DOI] [Google Scholar]
- 123. https://patents.google.com/patent/US20130171274.
- 124. https://patents.google.com/patent/KR101659314B1/en.
- 125.Tilekar K., Prashant K., Sujit K., Sachin K., Ravindra P. Cubosomes- a drug delivery system. International Journal of Pharmaceutical, Chemical and Biological Sciences. 2014;4(4):812–824. [Google Scholar]
- 126.Rohit R. B., Riyaz A. O., Bhargav R. H., Prasanna P. G. Cubosomes: the inimitable nanoparticulate drug carriers. Scholars Academic Journal of Pharmacy. 2013;2(6):481–486. [Google Scholar]
- 127.Madhurilatha T., Paruchuri S. K., Suria Prabha K. Overview of cubosomes: a nano particle. International Journal of Research in Pharmacy and Chemistry. 2011;1:535–541. [Google Scholar]
- 128.Bhosale R. R., Osmani R. A., Harkare B. R., Ghodake P. P. Cubosomes: the inimitable nanoparticulate drug carriers. Scholars Academic Journal of Pharmacy. 2013;2(6):481–486. [Google Scholar]
- 129.Smijs T. G., Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnology, Science and Applications. 2011;4(1):95–112. doi: 10.2147/NSA.S19419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Glaser D. A. Anti-aging products and cosmeceuticals. Facial Plastic Surgery Clinics of North America. 2004;12(3):363–372. doi: 10.1016/j.fsc.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 131.Rosen J., Landriscina A., Friedman A. Nanotechnology-Based Cosmetics for Hair Care. Cosmetics. 2015;2(4):211–224. doi: 10.3390/cosmetics2030211. [DOI] [Google Scholar]
- 132.Hu Z., Liao M., Chen Y., et al. A novel preparation method for silicone oil nanoemulsions and its application for coating hair with silicone. International Journal of Nanomedicine. 2012;7:5719–5724. doi: 10.2147/IJN.S37277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sesderma fillderma lips lip volumizer. https://www.dermacaredirect.co.uk/sesderma-fillderma-lip.html.
- 134.Bethany H. Zapping nanoparticles into nail polish. Laser Ablation Method Makes Cosmetic and Biomedical Coatings in a Flash. 2017;95(12):p. 9. [Google Scholar]
- 135.Pereira L., Dias N., Carvalho J., Fernandes S., Santos C., Lima N. Synthesis, characterization and antifungal activity of chemically and fungal-produced silver nanoparticles against Trichophyton rubrum. Journal of Applied Microbiology. 2014;117(6):1601–1613. doi: 10.1111/jam.12652. [DOI] [PubMed] [Google Scholar]
- 136.Oberdörster G., Oberdörster E., Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environmental Health Perspectives. 2005;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Buzea C., Pacheco I. I., Robble K. Nanomaterial and nanoparticles: sources and toxicity. Biointerphases. 2007;2(4):17–71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 138.Yah C. S., Iyuke S. E., Simate G. S. A review of nanoparticles toxicity and their routes of exposures. Iranian Journal of Pharmaceutical Sciences. 2012;8(1):299–314. [PubMed] [Google Scholar]
- 139.Zhu M.-T., Feng W.-Y., Wang Y., et al. Particokinetics and extrapulmonary translocation of intratracheally instilled ferric oxide nanoparticles in rats and the potential health risk assessment. Toxicological Sciences. 2009;107(2):342–351. doi: 10.1093/toxsci/kfn245. [DOI] [PubMed] [Google Scholar]
- 140.Tsuji J. S., Maynard A. D., Howard P. C., et al. Research strategies for safety evaluation of nanomaterials, part IV: risk assessment of nanoparticles. Toxicological Sciences. 2006;89(1):42–50. doi: 10.1093/toxsci/kfi339. [DOI] [PubMed] [Google Scholar]
- 141.Paul J., Roel P. F. S. Toxicological characterization of engineered nanoparticles. Nanoparticle Technology for Drug Delivery. 2006:161–170. [Google Scholar]
- 142.Wakefield G., Lipscomb S., Holland E., Knowland J. The effects of manganese doping on UVA absorption and free radical generation of micronised titanium dioxide and its consequences for the photostability of UVA absorbing organic sunscreen components. Photochemical & Photobiological Sciences. 2004;3(7):648–652. doi: 10.1039/b403697b. [DOI] [PubMed] [Google Scholar]
- 143.Hoet P. H. M., Brüske-Hohlfeld I., Salata O. V. Nanoparticles—known and unknown health risks. Journal of Nanobiotechnology. 2004;2, article 12 doi: 10.1186/1477-3155-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Toll R., Jacobi U., Richter H., Lademann J., Schaefer H., Blume-Peytavi U. Penetration profile of microspheres in follicular targeting of terminal hair follicles. Journal of Investigative Dermatology. 2004;123(1):168–176. doi: 10.1111/j.0022-202X.2004.22717.x. [DOI] [PubMed] [Google Scholar]
- 145.Nel A., Xia T., Mädler L., Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 146.Poon V. K. M., Burd A. In vitro cytotoxity of silver: implication for clinical wound care. Burns. 2004;30(2):140–147. doi: 10.1016/j.burns.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 147.Zhang X.-D., Wu H.-Y., Wu D., et al. Toxicologic effects of gold nanoparticles in vivo by different administration routes. International Journal of Nanomedicine. 2010;5(1):771–781. doi: 10.2147/IJN.S8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mavon A., Miquel C., Lejeune O., Payre B., Moretto P. In vitro percutaneous absorption and in vivo stratum corneum distribution of an organic and a mineral sunscreen. Skin Pharmacology and Physiology. 2006;20(1):10–20. doi: 10.1159/000096167. [DOI] [PubMed] [Google Scholar]
- 149.Sayes C. M., Fortner J. D., Guo W., et al. The differential cytotoxicity of water-soluble fullerenes. Nano Letters. 2004;4(10):1881–1887. doi: 10.1021/nl0489586. [DOI] [Google Scholar]
- 150.Arvidson B. A review of axonal transport of metals. Toxicology. 1994;88(1-3):1–14. doi: 10.1016/0300-483X(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 151.Shi H., Magaye R., Castranova V., Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Particle and Fibre Toxicology. 2013;10(1, article 15) doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Posada O. M., Tate R. J., Grant M. H. Toxicity of cobalt-chromium nanoparticles released from a resurfacing hip implant and cobalt ions on primary human lymphocytes in vitro. Journal of Applied Toxicology. 2015;35(6):614–622. doi: 10.1002/jat.3100. [DOI] [PubMed] [Google Scholar]
- 153.Abbott K. W., Gopalan S., Marchant G. E., Sylvester D. J. International regulatory regimes for nanotechnology. Social Science Research Network. 2006;2(5) doi: 10.2139/ssrn.907353. [DOI] [Google Scholar]
- 154.Sandoval B. M. Perspectives on FDA's Regulation of Nanotechnology: Emerging Challenges and Potential Solutions. 4. Vol. 8. Institute of Food Technologist; 2009. [Google Scholar]
- 155.Khaiat A. Regulations in Asia from China to Japan, Korea, ASEAN. http://asia.incosmetics.com/RXUK/RXUK_InCosmeticsAsia/2014/Documents/AlainKhaiatCosmeticRegulationsFromchinaToJapanKoreaASEAN.pdf?v=635524377936486453.
- 156.Marchant G. E., Sylvester D. J. Transnational models for regulation of nanotechnology. Journal of Law Medicine & Ethics. 2006;34(4):2–13. doi: 10.1111/j.1748-720X.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 157.David N. B. Review of the regulation of products at the interface between cosmetics and therapeutic goods. 2005.
- 158.Raj C. R. K., Kaushal K. C. Regulatory Requirements for Cosmetics in Relation with Regulatory Authorities in India against US, Europe, Australia and Asean. International Journal of Pharma Research and Health Science. 2016;4(5):1332–1341. [Google Scholar]
- 159.Dhull. Swagat Tripathy and Harish Dureja. Cosmetics: Regulatory Scenario in USA, EU and India. Journal of Pharmaceutical Technology Research and Management. 2015;3(2):127–139. [Google Scholar]
- 160.Nanomaterials and the EU Cosmetics Regulation: Implications for Your Company. http://www.gcimagazine.com/business/management/regulation/143553126.html?pa.
- 161.Asia Personal Care & Cosmetics Market Guide. 2016.
- 162.Hammes C. Cosmeceuticals, The cosmetic-drug borderline, Drug discovery approaches for developing Cosmeceuticals: advanced skin care and cosmetic products. IBC Library Series; 1997. [Google Scholar]
- 163.SCCP/1147/07: Opinion on Safety of Nanomaterials in Cosmetic Products, Scientific Committee on Consumer Products, Health & Consumer Protection Directorate-General, European Commission. Adopted by the SCCP after the public consultation on the 14th plenary of 18 December 2007.
- 164.Millikan L. E. Cosmetology, cosmetics, cosmeceuticals: Definitions and regulations. Clinics in Dermatology. 2001;19(4):371–374. doi: 10.1016/S0738-081X(01)00195-X. [DOI] [PubMed] [Google Scholar]
- 165.Liang B. A., Hartman K. M. It's only skin deep: FDA regulation of skin care cosmetics claims. Cornell journal of law and public policy. 1999;8(2):249–280. [PubMed] [Google Scholar]