Abstract
Suxiao jiuxin pill is considered an effective ancillary drug in patients with coronary heart disease. Although numerous small, single-center clinical trials have been conducted, the benefits and harms of suxiao jiuxin pill remain controversial. We performed a meta-analysis to clarify the efficacy of suxiao jiuxin pill on patients with coronary heart disease. Randomized controlled trials were identified by using the Cochrane Library, PubMed, Web of Science, Embase, Wanfang, Weipu, and China Knowledge Resource Integrated databases (until June 2016). Pooled relative risks (RR), weighted mean differences (WMD), and 95% confidence intervals (95% CIs) were estimated using random-effects models. Forty-one trials involving 6276 patients were included in our analysis. Administration of suxiao jiuxin pill significantly improved electrocardiogram (ECG) results when compared with other therapies (RR 1.32, 95% CI 1.26 to 1.38, and P < 0.001). Subgroup analyses revealed that suxiao jiuxin pills improve ECG results more than salvia tablets (RR 1.54, 95% CI 1.41 to 1.67, and P < 0.001), isosorbide dinitrate (RR 1.14, 95% CI 1.21 to 1.44, and P = 0.001), nitroglycerin (RR 1.35, 95% CI 1.16 to 1.56, and P < 0.001), and other drugs (RR 1.32, 95% CI 1.21 to 1.44, and P < 0.001). Available evidence additionally suggests that suxiao jiuxin pills could significantly reduce total cholesterol (WMD −0.62 mmol/L, 95% CI −1.06 to –0.18 mmol/L, and P = 0.005) and low-density lipoprotein (LDL) levels (WMD −1.12 mmol/L, 95% CI −1.42 to −0.82 mmol/L, and P < 0.001) and increase high-density lipoprotein (HDL) levels (WMD 0.32 mmol/L, 95% CI 0.07 to 0.58 mmol/L, and P = 0.014). However, no significant differences were observed in total triglyceride levels, plasma viscosity, hematocrit, and fibrinogen. No incidences of adverse reactions were observed after administration of suxiao jiuxin pill. Improvements in ECG results and lipid profiles were also observed after suxiao jiuxin administration compared to other therapies. It also decreased low-cut and high-cut whole blood viscosity without significant adverse reactions.
1. Introduction
Coronary heart disease (CHD) has become the leading cause of death in both men and women worldwide [1]. Most CHD-related deaths occur in individuals older than 65 years of age. The spectrum of CHD includes subclinical CHD, chronic stable angina pectoris, unstable angina, and acute myocardial infarction. A large number of elderly patients have asymptomatic heart disease; therefore, the prevalence of CHD may be underestimated [2]. Several large prospective clinical studies [3–7] have demonstrated that CHD is significantly associated with atrial fibrillation, congestive heart failure, stroke, and other serious diseases. Hence, it is important to develop effective therapies to mitigate the progression of this disease.
Suxiao jiuxin pills are one of the most commonly used Chinese medicines for cardiocerebral vascular conditions. They were first developed by Chinese medicine specialist Chenggui Zhang in the 1980s and manufactured by the Sixth Chinese Drugs Factory of Tianjin Zhongxin Pharmaceutical Co., Ltd. [8, 9]. Small doses of suxiao jiuxin pill have been shown to rapidly relieve angina pectoris and improve its symptoms without any obvious side effects. Several reports have suggested that suxiao jiuxin pill helps lower the patients' lipid profile and improve myocardial function [10]. The main components of suxiao jiuxin pills are borneol and Ligusticum chuanxiong Hort [9, 11–15]. These ingredients can effectively induce relaxation and inhibit artery contraction [9, 11]. In addition, several smaller clinical studies have been conducted to study the efficacy of suxiao jiuxin pill on CHD patients; however, these results have been inconsistent [16–19]. Cao and Zhang suggested that suxiao jiuxin pill was associated with symptom remission, reduced incidence of angina, and shorter duration of angina. Further, electrocardiogram results were significantly improved by nitroglycerin use compared with salvia [16]. Qiao et al. demonstrated that suxiao jiuxin pills plus trimetazidine therapy significantly reduced the effective rate of angina, but other relevant indices were not evaluated [17–19]. Clarifying the beneficial and harmful effects of suxiao jiuxin pill is particularly important for CHD patients, as they have not been distinctly determined with respect to ECG results, lipid profiles, hemorheology, and adverse reactions. Therefore, we performed a large-scale meta-analysis of the available randomized controlled trials to determine the benefits of suxiao jiuxin pill for CHD patients.
2. Materials and Methods
Ethical approval and written consent were not necessary for the meta-analysis, as the data was collected from published literature.
Our meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [20]. We searched the Cochrane Library, PubMed, Web of Science, Embase, Wanfang, Weipu, and China Knowledge Resource Integrated databases to identify relevant studies published in English or Chinese prior to June 2016. Our search terms included “coronary heart disease,” “suxiao jiuxin pill,” and “coronary artery disease.” We also searched for meta-analysis publications and bibliographies referenced in the selected publications. Gray literature was identified through related agencies and clinical trial registers. Clinical trials that compared the efficacy of suxiao jiuxin pill on coronary heart disease with those of placebo or standard therapy were included in this meta-analysis. Criteria for inclusion were as follows: (1) a randomized controlled study design, (2) the possibility of extracting accurate clinical data, (3) classifying coronary heart disease based on the updated guidelines of the American Heart Association and American College of Cardiology Foundation [21, 22], and (4) reporting ECG results, lipid profiles, and/or hemorheology changes as outcomes. Two reviewers (X. L. H. and J. Z. J.) independently reviewed the studies to determine whether they satisfied the eligibility criteria. Discrepancies between reviewers' opinions were resolved by consensus, and a third reviewer was consulted when necessary.
2.1. Data Extraction
Two independent reviewers using the same checklist evaluated the data from the included studies. Disagreements between the reviewers were resolved by discussion until consensus was reached. The following sets of data were extracted for each selected study when available: demographics and sample characteristics, definition of coronary heart disease, and usage of suxiao jiuxin pill. The primary outcome of the selected studies was the improvement in ECG results, including resting ECG returning to normal or negative submaximal exercise test. The secondary outcomes included changes in lipid profile (total cholesterol, total triglyceride, low-density lipoprotein, and high-density lipoprotein) and hemorheology (high-cut whole blood viscosity, low-cut whole blood viscosity, plasma viscosity, hematocrit, and fibrinogen) as well as any adverse reactions.
2.2. Quality Assessment
Two reviewers independently assessed the methodological quality of the studies using the Jadad scoring system [23]. Five aspects for each study were thoroughly evaluated: the statement of randomization, the method used for generating randomized sequences for treatment assignments, the use of double-blind design, the description of the double blinding method, and data on withdrawals and dropouts. Studies with a score less than 3 were considered as low quality studies with high bias risk. Studies that received a score of 3 or greater were considered as high-quality studies. Disagreements between the reviewers were resolved by consensus and consultation with a third reviewer when necessary.
2.3. Data Analysis
Continuous variables, such as changes in lipid profiles and hemorheology, were expressed as mean ± standard deviation. Categorical data, such as ECG result improvement and adverse reaction incidence, were presented as frequencies and percentages. We computed the pooled relative risk (RR), weighted mean difference (WMD), and 95% confidence interval (CI), as well as the heterogeneity of the included studies by using random-effect (DerSimonian and Laird) models. Metaregression analysis was conducted based on sample size and mean age to explore the impact of sample size on the source of heterogeneity [24]. We also performed subgroup analyses to compare the efficacy of different drugs with those of suxiao jiuxin pill on ECG result improvement. Heterogeneity was quantified using the I2 statistic. We considered I2 values greater than 50% to indicate significant heterogeneity between the studies. Statistical heterogeneity between studies was also formally tested with the Cochran test (P < 0.10) [25, 26]. Publication bias was evaluated using the funnel plot and Egger's and Begg tests, with P values less than 0.05 considered significant publication bias. Two-tailed P values less than 0.05 were considered statistically significant. All statistical analyses were performed with STATA 12.0 (Stata Corporation, College Station, TX, USA).
3. Results
3.1. Search Results
The search strategy revealed 1515 potentially eligible publications. After duplicate removal, 1253 studies remained. Abstracts were evaluated based on the inclusion and exclusion criteria. 85 studies warranting further review were identified. Among these, 44 studies were excluded for the reasons listed in Figure 1. The remaining 41 studies were included in our meta-analysis. Journal articles and full manuscripts were obtained for all 41 studies.
Figure 1.
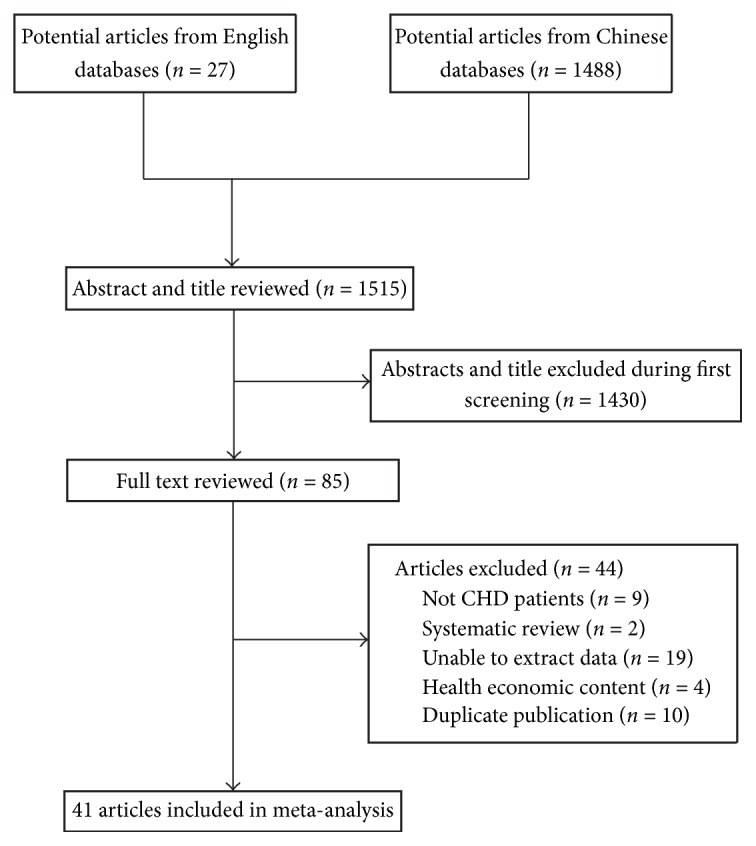
Process used to select relevant studies for inclusion in the meta-analysis.
3.2. Study Characteristics
The characteristics of the trials included in our meta-analysis are presented in Table 1. All of the included studies were conducted in China. The following studies were included as the control group: 2 studies involving standard treatment [27, 28], 7 studies involving nitroglycerin (using various formulations) [29–35], 12 studies involving isosorbide dinitrate [36–47], 11 studies involving salvia tablets [16, 17, 48–56], and 9 studies involving Chinese herbal pills other than suxiao jiuxin [18, 32, 57–64].
Table 1.
Characteristics of selected clinical trials included in the meta-analysis.
| Author name | Year | Sample size | Study design | Age | Age | Country | Comparators | Dosage/frequency/cycle | Outcomes | Follow-up period | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (treatment) | (control) | Suxiao jiuxin pills | Comparators | ||||||||
| He | 1995 | 48 | RCT | 42–60 | 40–57 | China | Salvia | 4# tid | 3# tid | Hemorheology | 4 weeks |
|
| |||||||||||
| Song | 1995 | 149 | RCT | 55 ± 7.2 | 56 ± 6.9 | China | Salvia | 5# tid | 3# tid | ECG, blood pressure, and heart rate | 4 weeks |
|
| |||||||||||
| Wang | 1996 | 60 | RCT | NR | NR | China | Shexiang Baoxin pills | 5# tid | 3# tid | ECG and symptom | 2 weeks |
|
| |||||||||||
| Ke | 1996 | 72 | RCT | 45–79 | 46–78 | China | Nitroglycerin | 6# tid | 0.5 mg Q8 h | ECG and symptom | 4 weeks |
|
| |||||||||||
| Gao et al. | 1996 | 147 | RCT | 36–85 | 35–84 | China | Nitroglycerin | 10# | 0.5 mg | ECG and symptom | Immediately |
|
| |||||||||||
| Yun | 1996 | 318 | RCT | 44–76 | 44–75 | China | Isosorbide dinitrate | 6–10# Q4–6 h |
10# tid | ECG and symptom | 2 weeks |
|
| |||||||||||
| Li | 1998 | 154 | RCT | 58.93 ± 10.91 | 59.25 ± 10.12 | China | Isosorbide dinitrate | 0.2 g tid | 10# tid | ECG and symptom | 8 weeks |
|
| |||||||||||
| Feng and Zhou | 2000 | 500 | RCT | 56 ± 4.24 | 55 ± 4.36 | China | Salvia | 10# tid | 10# tid | ECG and symptom, Lipid profile and hemorheology | 4 weeks |
|
| |||||||||||
| Mei | 2000 | 128 | RCT | NR | NR | China | Isosorbide dinitrate | 6# tid | 10# tid | ECG, symptom, and hemorheology | 4 weeks |
|
| |||||||||||
| Xiao | 2000 | 138 | RCT | NR | NR | China | Isosorbide dinitrate | 5# tid | 10# tid | ECG and symptom | 4 weeks |
|
| |||||||||||
| Ru | 2000 | 90 | RCT | 45–70 | NR | China | Salvia | 5# tid | 3# tid | ECG, symptom, lipid profile, and hemorheology | Unclear |
|
| |||||||||||
| Shu | 2000 | 80 | RCT | 58.3 ± 7.24 | 57.2 ± 8.27 | China | Isosorbide dinitrate | 10# tid | 10# tid | ECG and symptom | 4 weeks |
|
| |||||||||||
| Yong | 2000 | 148 | RCT | 45–72 | 47–70 | China | Nitroglycerin | 10# | 5 mg | ECG, Symptom, blood pressure, and heart rate | Immediately |
|
| |||||||||||
| Guang | 2000 | 248 | RCT | NR | NR | China | Isosorbide dinitrate | 5# tid | 10# tid | Blood pressure, heart rate, and lipid profile | 4 weeks |
|
| |||||||||||
| Li | 2000 | 166 | RCT | NR | NR | China | Nitroglycerin | 5# tid | 0.5 mg | ECG and symptom | 2 weeks |
|
| |||||||||||
| Yu | 2000 | 184 | RCT | 40–82 | NR | China | Isosorbide dinitrate | 5# tid | 10# tid | ECG and symptom | 15 days |
|
| |||||||||||
| Ya | 2000 | 60 | RCT | 60–84 | 61–84 | China | Nitroglycerin | 5# tid | 10# tid | ECG and symptom | 4 weeks |
|
| |||||||||||
| Yuan | 2000 | 102 | RCT | 61.68 ± 4.71 | 59.53 ± 5.62 | China | Huoxin pills | 5# tid | 1# tid | ECG, symptom, and lipid profile | 4 weeks |
|
| |||||||||||
| Duan and Yang | 2002 | 80 | RCT | 42–79 | 41–75 | China | Xinkeshu capsule | 5# tid | 4# tid | ECG, UCG, lipid profile, and hemorheology | 4 weeks |
|
| |||||||||||
| Hai | 2002 | 70 | RCT | 57 ± 7 | 59 ± 6 | China | Isosorbide dinitrate | 5# tid | 10# tid | Symptom and hemorheology | 4 weeks |
|
| |||||||||||
| Bu | 2002 | 100 | RCT | 32–72 | NR | China | Isosorbide dinitrate | 4–6# tid | 10–20# tid | ECG and symptom | Unclear |
|
| |||||||||||
| Pei | 2003 | 102 | RCT | 56.1 | 55.2 | China | Nitroglycerin | 5# tid | Unclear | ECG, lipid profile, and hemorheology | 8 weeks |
|
| |||||||||||
| Jin | 2003 | 178 | RCT | NR | NR | China | Isosorbide dinitrate | 5# tid | 20 mg qd | ECG, Symptom, lipid profile, and hemorheology | 6 weeks |
|
| |||||||||||
| Ma | 2004 | 116 | RCT | 63.4 ± 6.74 | 62.9 ± 7.84 | China | Salvia | 10# tid | 3# tid | ECG, symptom, and hemorheology | 4 weeks |
|
| |||||||||||
| Pei | 2004 | 100 | RCT | 57.5 ± 10.2 | 63.1 ± 7.9 | China | Xinkeshu capsule | 6# tid | 4# tid | ECG and symptom | 4 weeks |
|
| |||||||||||
| Yue | 2004 | 78 | RCT | 52–75 | 55–74 | China | Placebo | 5# tid | 10# tid | ECG, UCG, and symptom | 4 weeks |
|
| |||||||||||
| Hu | 2004 | 80 | RCT | 51.56 ± 11.69 | 50.89 ± 11.02 | China | Nitroglycerin | 10# tid | 0.5 mg tid | ECG and symptom | 24 weeks |
|
| |||||||||||
| Zhu | 2005 | 199 | RCT | 61.8 | 59.5 | China | Glucose-insulin-potassium therapy | 4–6# tid | Q2 d | ECG, symptom, and lipid profile | 15 days |
|
| |||||||||||
| Wan | 2005 | 64 | RCT | NR | NR | China | Hesu pills | 5# tid | 1# tid | ECG and symptom | 2 weeks |
|
| |||||||||||
| Shui | 2006 | 73 | RCT | 43–78 | 45–76 | China | Salvia | 10# tid | 10# tid | ECG and lipid profile | 8 weeks |
|
| |||||||||||
| Cao and Zhang | 2007 | 187 | RCT | 57.15 ± 5.38 | 58.77 ± 5.01 | China | Salvia | 6# tid | 6# tid | ECG and symptom | 2 weeks |
|
| |||||||||||
| Run | 2007 | 90 | RCT | NR | NR | China | Isosorbide dinitrate | 10–15# | 10 mg | ECG and symptom | Immediately |
|
| |||||||||||
| Wang | 2008 | 60 | RCT | 63.9 ± 12.1 | 64.1 ± 11.2 | China | Shexiang Baoxin pills | 5# tid | 1# tid | ECG and symptom | 2 weeks |
|
| |||||||||||
| L. Wen | 2009 | 88 | RCT | 56.7 | 56.8 | China | Isosorbide dinitrate | 5# tid | 10# tid | ECG and symptom | 8 weeks |
|
| |||||||||||
| Feng | 2009 | 900 | RCT | NR | NR | China | Salvia | 6# tid | 3# tid | ECG and symptom | 4 weeks |
|
| |||||||||||
| S. L. Wen | 2009 | 50 | RCT | NR | NR | China | Tongxinluo pills | 5# tid | 4# tid | ECG and symptom | 2 weeks |
|
| |||||||||||
| Guo | 2012 | 60 | RCT | 42–70 | 43–73 | China | Salvia | 5# tid | 3# tid | UCG and hemorheology | 2 weeks |
|
| |||||||||||
| Qiao | 2012 | 300 | RCT | 39–82 | 40–81 | China | Salvia | 5# tid | 3# tid | ECG and symptom | 4 weeks |
|
| |||||||||||
| Xin | 2013 | 289 | RCT | 58.86 ± 10.57 | 57.69 ± 9.93 | China | Standard therapy | 10# tid | Blank | ECG and symptom | 2 weeks |
|
| |||||||||||
| Long | 2013 | 120 | RCT | 43–68 | 42–67 | China | Standard therapy | 10# tid | Blank | ECG and symptom | 2 weeks |
|
| |||||||||||
| Li | 2015 | 100 | RCT | 44–75 | 45–76 | China | Salvia | 6# tid | 3# tid | ECG and symptom | 4 weeks |
NR: not reported; RCT: randomized controlled trial; tid: three times a day; Q4–6 h: every 4–6 h; Q8 h: every 8 h; ECG: electrocardiograph; UCG: ultrasound cardiogram; #: tablet.
The 41 studies consisted of 6276 patients with coronary heart disease. The mean age in the treatment group was 57.57 ± 8.15 years and 54.10% of the patients were male. The mean age in the control group was 57.80 ± 8.72 years and 45.96% of the patients were male. The baseline characteristics were balanced between the treatment and control groups. The majority of the included studies received low Jadad scores due to the lack of a double-blind design (Table 2).
Table 2.
Jadad quality scores of selected studies.
| Author name | Randomization | Generating randomized sequences | Blinding | Withdrawals and dropouts | Overall |
|---|---|---|---|---|---|
| Guan-hua He | 1 | 0 | 0 | 0 | 1 |
| Zhi-jin Song | 1 | 0 | 0 | 0 | 1 |
| Dong-ping Wang | 1 | 0 | 1 | 0 | 2 |
| Ke-fu Ji | 1 | 0 | 0 | 0 | 1 |
| Yu-chu Gao | 1 | 0 | 0 | 0 | 1 |
| Yun-yuan Guo | 1 | 0 | 0 | 0 | 1 |
| Li An | 1 | 0 | 0 | 0 | 1 |
| Ling Feng | 1 | 0 | 0 | 0 | 1 |
| Mei Hu | 1 | 0 | 0 | 1 | 2 |
| Xiao-chun Liu | 1 | 0 | 0 | 0 | 1 |
| Ru-bao Jia | 1 | 0 | 0 | 0 | 1 |
| Shu-dong Yang | 1 | 0 | 0 | 0 | 1 |
| Yong-jin Hou | 1 | 0 | 0 | 0 | 1 |
| Guang-yu Tang | 1 | 0 | 0 | 0 | 1 |
| Li-jun Zhou | 1 | 0 | 0 | 0 | 1 |
| Yu-ping Li | 1 | 0 | 0 | 0 | 1 |
| Ya-xiong Zhan | 1 | 0 | 1 | 0 | 2 |
| Jing-xian Yuan | 1 | 0 | 0 | 0 | 1 |
| Ke-jie Duan | 1 | 0 | 0 | 0 | 1 |
| Hai Shi | 1 | 0 | 0 | 0 | 1 |
| Bu-ce Sun | 1 | 0 | 0 | 0 | 1 |
| Pei-ying Wu | 1 | 0 | 0 | 0 | 1 |
| Jin Gao | 1 | 0 | 0 | 0 | 1 |
| Xian-zhen Ma | 1 | 0 | 0 | 0 | 1 |
| Pei-fen Chang | 1 | 0 | 0 | 0 | 1 |
| Yue-sheng Zhao | 1 | 0 | 0 | 0 | 1 |
| Gang Hu | 1 | 0 | 0 | 0 | 1 |
| Dong-you Zhu | 1 | 0 | 0 | 0 | 1 |
| Wei Wan | 1 | 0 | 0 | 0 | 1 |
| Shui-xiang Wan | 1 | 0 | 0 | 0 | 1 |
| Sheng-hai Cao | 1 | 0 | 0 | 0 | 1 |
| Run-lian Tang | 1 | 0 | 0 | 1 | 2 |
| Fei Wang | 1 | 0 | 0 | 0 | 1 |
| Wen Luo | 1 | 0 | 0 | 0 | 1 |
| Feng-hua Song | 1 | 0 | 0 | 0 | 1 |
| Wen-sheng Li | 1 | 0 | 1 | 0 | 2 |
| Wei-qin Guo | 1 | 0 | 0 | 0 | 1 |
| Qiao-kun Xu | 1 | 0 | 0 | 0 | 1 |
| Xin He | 1 | 0 | 0 | 0 | 1 |
| Long-jiang Qian | 1 | 0 | 0 | 0 | 1 |
| Li Xiao-jin | 1 | 0 | 0 | 1 | 2 |
3.3. ECG Result Improvement
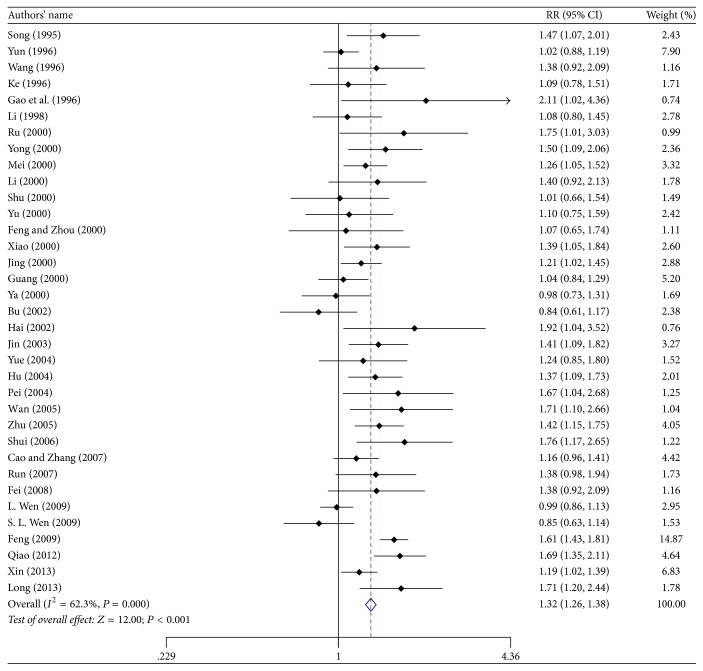
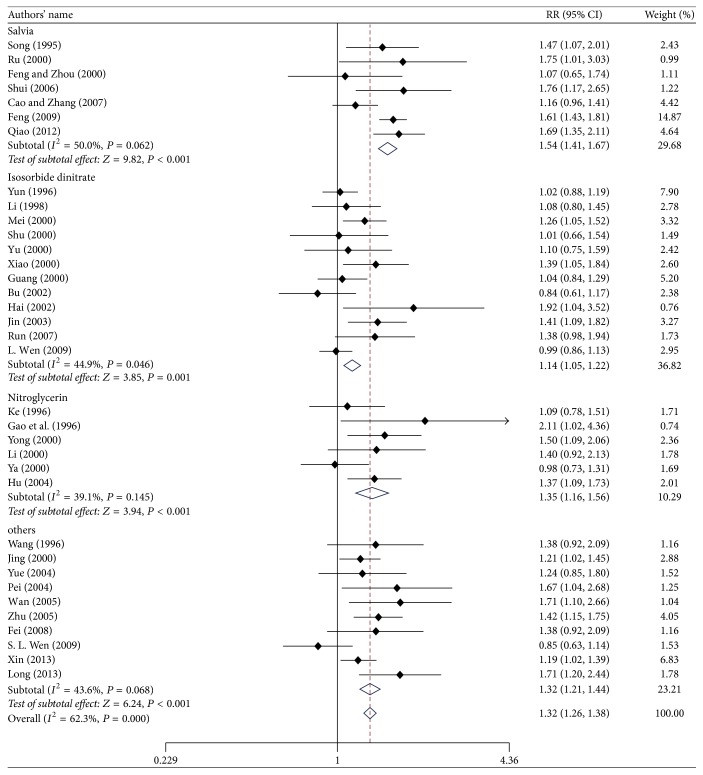
Figure 2 presents the results of the meta-analysis of ECG improvement following administration of suxiao jiuxin pills. ECG results were reported in 35 studies. Pooled analysis indicated significant benefits of suxiao jiuxin pill on ECG outcomes (RR 1.32; 95% CI 1.26 to 1.38, P < 0.001). However, there was significant heterogeneity between studies with respect to ECG results (I2 = 62.3%). Findings from the meta-regression analyses suggested that sample size and mean age of the patients were not significant factors contributing to the association between suxiao jiuxin pills and ECG outcomes (Table 3). Considering that the control group may be the source of the heterogeneity, we performed subgroup analyses to compare the effects of suxiao jiuxin pill with those of control treatment (Figure 3). The subgroup analyses indicated that suxiao jiuxin pill improved ECG results more than salvia tablets (RR 1.54; 95% CI 1.41 to 1.67, P < 0.001), isosorbide dinitrate (RR 1.14; 95% CI 1.05 to 1.22, P = 0.001), nitroglycerin (RR 1.35, 95% CI 1.16 to 1.56, P < 0.001), and other drugs (RR 1.32, 95% CI 1.21 to 1.44, P < 0.001). The I2 decreased to 39.1% in the nitroglycerin subgroup. However, moderate heterogeneity was still observed among the other three subgroups (I2 = 50.0%, 44.9%, and 43.6%, resp.). Sensitivity analysis was conducted by excluding individual studies one after another but did not reveal a substantial change in the overall trend of heterogeneity between studies. We also constructed a funnel plot to assess the degree of publication bias. The funnel plot was symmetrically distributed around the pooled effect size, which indicated the absence of significant publication bias in the included studies (Figure 4). In addition, examining the funnel plot asymmetry via Egger test (P = 0.067) and Begg test (P = 0.050) did not demonstrate publication bias.
Figure 2.
Relative risks for ECG improvement in the treatment and control groups.
Table 3.
Metaregression based on sample size and mean age.
| Outcomes | Sample size | Mean age |
|---|---|---|
| Electrocardiogram improvement | 0.206 | 0.059 |
| Total cholesterol | 0.758 | 0.236 |
| Total triglycerides | 0.013 | 0.507 |
| Total low-density lipoprotein | 0.283 | 0.513 |
| Total high-density lipoprotein | 0.715 | 0.789 |
| Low-cutting whole blood Viscosity | 0.917 | 0.774 |
| High-cutting whole blood Viscosity | 0.412 | 0.621 |
| Plasma viscosity | 0.075 | 0.842 |
| Hematocrit | 0.049 | 0.490 |
| Fibrinogen | 0.890 | 0.345 |
| Adverse reactions | 0.554 | 0.772 |
Figure 3.
Relative risks for ECG improvement in the various subgroups.
Figure 4.
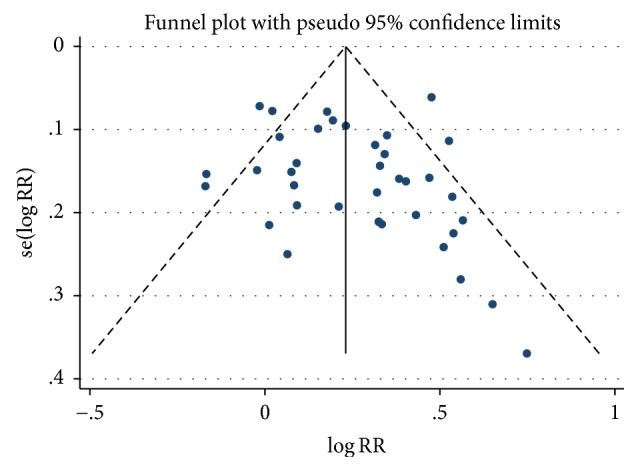
Funnel plot of studies included in the meta-analysis.
3.4. Lipid Profile
Only four of the studies reported the efficacy of suxiao jiuxin pill on patients' lipid profiles, and they all reported the total cholesterol in the patients' serum [33, 55, 57, 63]. Significant lower total cholesterol levels were reported in the suxiao jiuxin pill group compared with the control group (WMD −0.62 mmol/L, 95% CI −1.06 to –0.18 mmol/L, and P = 0.005). There was significant heterogeneity among the 4 studies with respect to total cholesterol levels (I2 = 77.1%) (Supplemental Figure S1). In addition, the four studies reported total triglyceride levels in the patients' serum [33, 55, 57, 63]. There was no significant difference in WMD between the treatment and control groups (WMD −0.59 mmol/L, 95% CI −1.72 to 0.54 mmol/L, and P = 0.303). We detected significant heterogeneity between studies among the 4 studies with respect to total triglyceride levels (I2 = 98.2%) (Supplemental Figure S2). Three of the studies reported LDL levels [33, 57, 63]. There were significantly lower LDL levels in the treatment group compared with the control group (WMD −1.12 mmol/L, 95% CI −1.42 to −0.82 mmol/L, and P < 0.001) and lower heterogeneity between the 3 trials (I2 = 56.5%) (Supplemental Figure S3). Furthermore, the four studies reported HDL levels [30, 52, 54, 60]. There were significantly higher HDL levels in the treatment group compared with the control group (WMD 0.32 mmol/L, 95% CI 0.07 to 0.58 mmol/L, and P = 0.014), however with significant heterogeneity with respect to HDL levels (I2 = 87.5%) (Supplemental Figure S4). Sensitivity analysis did not reveal any single study as the source of heterogeneity. We did detect sample size (P = 0.013) as a contribution to the association between suxiao jiuxin pill and total triglyceride level, with no other significant factors being observed (Table 3).
3.5. Hemorheology
Six studies reported the levels of low-cut whole blood viscosity after treatment [39, 41, 43, 53, 57, 63]. There were significantly lower low-cut whole blood viscosity levels in the treatment group compared to the control group (WMD −1.57 mpa·s, 95% CI −2.50 to −0.65 mpa·s, and P = 0.001). Significant heterogeneity was observed among these studies with respect to the level of low-cut whole blood viscosity (I2 = 86.8%) (Supplemental Figure S5). Six studies reported the levels of high-cut whole blood viscosity [39, 41, 43, 53, 57, 63], which were significantly lower in the treatment group (WMD −0.69 mpa·s, 95% CI −1.03 to −0.34 mpa·s, and P < 0.001). Significant heterogeneity was also observed among these studies with respect to the level of high-cut whole blood viscosity (I2 = 87.6%) (Supplemental Figure S6). Seven studies reported the levels of plasma viscosity [33, 39, 41, 43, 54, 57, 63]. There was no significant difference in WMD between the treatment and control groups (WMD −0.03 mpa·s, 95% CI −0.07 to 0.01 mpa·s, and P = 0.186) and no significant heterogeneity between studies among these studies with respect to the level of plasma viscosity (I2 = 4.9%) (Supplemental Figure S7). Four studies reported the levels of hematocrit [33, 54, 57, 63] and no significant differences were observed in WMD between the treatment and control groups (WMD −1.24%, 95% CI −3.26 to 0.77%, and P = 0.227). There was significant heterogeneity among three studies with respect to the level of hematocrit (I2 = 84.3%) (Supplemental Figure S8). Four studies reported the levels of fibrinogen [33, 53, 57, 63] with no significant difference in WMD between the treatment and control groups (WMD −0.76 g/L, 95% CI −1.32 to −0.20 g/L, and P = 0.008). There was significant heterogeneity among the three studies with respect to the fibrinogen level (I2 = 85%) (Supplemental Figure S9). Sensitivity analysis was conducted by excluding each study individually and showed no substantial change in the overall trend. Furthermore, sample size and mean age were not correlated with treatment efficacy of suxiao jiuxin pills on hemorheology (Table 3).
3.6. Adverse Reactions
Fourteen studies reported the incidence of adverse reactions. The most common symptoms were mild headache, dizziness, and facial flushing. Most of these symptoms resolved spontaneously. There was no significant difference in the adverse reaction rates between the treatment and the control groups (RR 1.12, 95% CI 0.50 to 2.51, and P = 0.785) and no significant heterogeneity with respect to adverse reaction rate (I2 = 49.1%) (Supplemental Figure S10). Additionally, meta-regression analyses suggested that both sample size and mean age were not associated with adverse reaction in the suxiao jiuxin pill treatment group (Table 3). The funnel plot was symmetrically distributed around the pooled effect size, which indicated the absence of significant publication bias in the included studies (Supplemental Figure S11). In addition, no publication bias was identified using the Egger test (P = 0.064) or Begg test (P = 0.274).
4. Discussion
Based on our meta-analysis, we found that suxiao jiuxin pills could significantly improve ECG results in CHD patients compared with other therapies used in the selected studies. Suxiao jiuxin pills decreased the levels of total cholesterol and LDL, increased the levels of HDL, and lowered low-cut and high-cut whole blood viscosity. Other hemorheology-related parameters, such as plasma viscosity, hematocrit, and fibrinogen, showed the same tendency, but these changes were not statistically significant.
In recent years, several randomized clinical trials have been performed to evaluate the efficacy of suxiao jiuxin pill on CHD patients [17, 27, 28, 56]. Long studied 120 patients with unstable angina and found that suxiao jiuxin pills significantly improved both ECG results and symptoms compared with standard treatment [28]. Bu evaluated the benefits of suxiao jiuxin pills with isosorbide dinitrate on one hundred coronary heart disease (CAD) patients [44]. However, they did not find any significant differences between the groups with respect to ECG result improvement and angina relief. This may be due to the small sample size, different inclusion criteria, and differences in treatment strategy.
Two previous meta-analyses that explored the efficacy of suxiao jiuxin pills on CHD [65, 66] found them to be effective in the treatment of angina pectoris, without any serious side effects. However, due to the limited sample sizes, low quality of the studies, and other potential confounding factors, the asymmetry funnel plot demonstrated the lack of reliability of these meta-analyses [65, 66]. As a result, we conducted this updated meta-analysis of randomized controlled trials to further clarify the effects of suxiao jiuxin pills on CHD patients.
Suxiao jiuxin pills have been widely used in China for many years in patients with angina. It has two main effective components, borneol and Ligusticum chuanxiong Hort, which can be found mainly in the Sichuan province of China. Ligusticum chuanxiong Hort was first described in the 'Divine Husbandman's Materia Medica.' L. chuanxiong has long been regarded as a traditional Chinese medicine and has been added to food for its health benefits. The main chemical components of L. chuanxiong include essential oils, phenolic acids, and phthalide lactones [67, 68]. Several researchers have demonstrated that L. chuanxiong could lower serum cholesterol and lipoprotein levels, reduce red blood cell deformability, and relieve angiotensin II–induced vascular smooth muscle cell proliferation. These unique roles may be due to the increase in nitric oxide and suppression of nuclear factor-κB activation [69, 70]. In addition, L. chuanxiong has a direct vasodilatory effect on isolated aortic rings in rats [71]. The mechanisms of this effect are related to the opening of SK (Ca) and ATPK channels, the reduction of ET-1, and the formation of reactive oxygen species (ROS) [72, 73]. Recent evidence has suggested that L. chuanxiong may exert antiplatelet effects by inhibiting the vWF-mediated process of platelet thrombus formation.
Borneol is a fragrant ingredient used in decorative cosmetics and is widely regarded as an adjuvant in Chinese herbs [74]. Several animal studies have demonstrated that borneol can dilate coronary arteries and improve coronary circulation. In addition, studies have demonstrated that borneol can inhibit the inflammatory response in animal models [75]. As a result, borneol is widely used in China for the treatment of CHD patients in clinical practice.
We found that the main side effects of suxiao jiuxin pills were mild headaches, dizziness, and facial flushing, all of which resolved spontaneously. The adverse reaction incidence associated with suxiao jiuxin pill was not significantly different from those associated with other treatments. Evidence suggests that suxiao jiuxin pills are safe with no adverse effects.
As in many meta-analyses, there were several limitations to our study. Most of the clinical studies included in our meta-analysis were of poor quality based on their Jadad scores. Only a few studies reported detailed research methodology, a factor that could decrease the reliability of this meta-analysis. In addition, all of the included studies were from Chinese publications, and this may be a source of bias. Significant heterogeneity between studies was noted in our meta-analysis even after subgroup analysis. Different study quality, sample size, usage of suxiao jiuxin pill, and control groups may contribute to this heterogeneity. In this study, we did not analyze cardiovascular death or drug-related complications.
In summary, our meta-analysis demonstrated that suxiao jiuxin pills improved ECG results and lipid profiles better compared with nitroglycerin, isosorbide dinitrate, salvia, and other Chinese herbal pills. They also decreased low-cut and high-cut whole blood viscosity.
5. Conclusions
Suxiao jiuxin pills can effectively decrease the lipid profiles and improve hemorheology parameters in CHD patients. This is due to the effects of their components (borneol and L. chuanxiong), which may improve coronary artery circulation and ECG results.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this article.
Supplementary Materials
Figure S1: mean difference in total cholesterol in the treatment and control groups. Figure S2: mean difference in total triglycerides in the treatment and control groups. Figure S3: mean difference in total low-density lipoprotein in the treatment and control groups. Figure S4: mean difference in total high-density lipoprotein in the treatment and control groups. Figure S5: mean difference in low-cut whole blood viscosity in the treatment and control groups. Figure S6: mean difference in high-cut whole blood viscosity in the treatment and control groups. Figure S7: mean difference in plasma viscosity in the treatment and control groups. Figure S8: mean difference in hematocrit in the treatment and control groups. Figure S9: mean difference in the fibrinogen in the treatment and control groups. Figure S10: relative risks for adverse reactions in the treatment and control groups. Figure S11: funnel plot of studies with reported adverse reactions included in the meta-analysis.
References
- 1.Yoon S. S., Dillon C. F., Illoh K., Carroll M. Trends in the Prevalence of Coronary Heart Disease in the U.S.: National Health and Nutrition Examination Survey, 2001–2012. American Journal of Preventive Medicine. 2016;51(4):437–445. doi: 10.1016/j.amepre.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeidan R. K., Farah R., Chahine M. N., et al. Prevalence and correlates of coronary heart disease: First population-based study in Lebanon. Vascular Health and Risk Management. 2016;12:75–84. doi: 10.2147/VHRM.S97252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haim M., Hoshen M., Reges O., Rabi Y., Balicer R., Leibowitz M. Prospective national study of the prevalence, incidence, management and outcome of a large contemporary cohort of patients with incident non-valvular atrial fibrillation. Journal of the American Heart Association. 2015;4(1, article e001486) doi: 10.1161/JAHA.114.001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannel W. B., Abbott R. D., Savage D. D., McNamara P. M. Coronary heart disease and atrial fibrillation: The Framingham Study. American Heart Journal. 1983;106(2):389–396. doi: 10.1016/0002-8703(83)90208-9. [DOI] [PubMed] [Google Scholar]
- 5.Lokshyn S., Mewis C., Kuhlkamp V. Atrial fibrillation in coronary artery disease. International Journal of Cardiology. 2000;72(2):133–136. doi: 10.1016/S0167-5273(99)00180-1. [DOI] [PubMed] [Google Scholar]
- 6.Otterstad J. E., Kirwan B.-A., Lubsen J., et al. Incidence and outcome of atrial fibrillation in stable symptomatic coronary disease. Scandinavian Cardiovascular Journal. 2006;40(3):152–159. doi: 10.1080/14017430600746268. [DOI] [PubMed] [Google Scholar]
- 7.Patel N. J., Patel A., Agnihotri K., et al. Prognostic impact of atrial fibrillation on clinical outcomes of acute coronary syndromes, heart failure and chronic kidney disease. World Journal of Cardiology. 2015;7(7):397–403. doi: 10.4330/wjc.v7.i7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang P., Qiu R., Shen R., Liang J. Clinical observation in 40 cases of postmenopausal coronary heart disease treated with Yanghuo Sanzi Tang. Journal of Traditional Chinese Medicine. 2003;23(3):182–184. [PubMed] [Google Scholar]
- 9.Lu Z., Zhang Y., Zhuang P., et al. Protective effect of Suxiao jiuxin pill, a traditional Chinese medicine, against acute myocardial ischemia in dogs. BMC Complementary and Alternative Medicine. 2015;15(1, article no. 373) doi: 10.1186/s12906-015-0908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J., Zhuang P., Lu Z., et al. Suxiaojiuxin pill enhances atherosclerotic plaque stability by modulating the MMPs/TIMPs balance in ApoE-deficient Mice. Journal of Cardiovascular Pharmacology. 2014;64(2):120–126. doi: 10.1097/FJC.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 11.Bai X.-Y., Zhang P., Yang Q., et al. Suxiao Jiuxin Pill induces potent relaxation and inhibition on contraction in human artery and the mechanism. Evidence-Based Complementary and Alternative Medicine. 2014;2014:11. doi: 10.1155/2014/956924.956924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C.-S., Qu Z.-Q., Wang S.-S., et al. Effects of suxiao Jiuxin Pill on oxidative stress and inflammatory response in rats with experimental atherosclerosis. Journal of Traditional Chinese Medicine. 2011;31(2):107–111. doi: 10.1016/S0254-6272(11)60022-8. [DOI] [PubMed] [Google Scholar]
- 13.Dong S. F., Zhu Z. G. Determination of the contents of Ca, Mg, Fe, Cu and Zn in suxiao jiuxin pill and the analysis of Ca/Mg and Cu/Zn values. Guang Pu Xue Yu Guang Pu Fen Xi. 2002;22(3):478–479. [PubMed] [Google Scholar]
- 14.Guo Q., Zhang J., Li Y., Zhang G. Study on anti-atherosclerotic effect of suxiao jiuxin pill and its mechanism. African Journal of Traditional, Complementary and Alternative Medicines. 2014;11(1):97–102. doi: 10.4314/ajtcam.v11i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X.-L., Liu Y.-M., Zhu G.-J. Effects of suxiao jiuxin pill on patients with acute coronary syndrome undergoing early percutaneous coronary intervention. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32(11):1483–1487. [PubMed] [Google Scholar]
- 16.Cao S. H., Zhang J. Y. Effects of suxiao jiuxin pill on 105 coronary heart diseases. Chinese Medicine. 2007;29(4):486–488. [Google Scholar]
- 17.Qiao K. X. Effect of suxiao jiuxin pill combined with Trimetazidine on patients with Angina. Journal of North Pharmacy. 2012;10, article 38 [Google Scholar]
- 18.Pei F. C. Effects of continuous suxiao jiuxin pill on 60 patients with coronary heart diseases. Chinese Journal of Geriatrics. 2004;2(3):24–26. [Google Scholar]
- 19.Duan X., Zhou L., Wu T., et al. Chinese herbal medicine suxiao jiuxin wan for angina pectoris. Cochrane Database of Systematic Reviews (Online) 2008;(1):p. CD004473. doi: 10.1002/14651858.CD004473.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D. G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Medicine. 2009;3(3):e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 21.Smith Jr. S. C., Benjamin E. J., Bonow R. O., et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. Journal of the American College of Cardiology. 2011;58(23):2432–2446. doi: 10.1016/j.jacc.2011.10.824. [DOI] [PubMed] [Google Scholar]
- 22.Smith Jr. S. C., Benjamin E. J., Bonow R. O., et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: American College of Cardiology Foundation. Circulation. 2011;124(22):2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 23.Jadad A. R., Moore R. A., Carroll D., et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clinical Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 24.Thompson S. G., Higgins J. P. T. How should meta-regression analyses be undertaken and interpreted? Statistics in Medicine. 2002;21(11):1559–1574. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 25.Deeks J. J., Higgins J. P. T., Altman D. G. Analyzing data and undertaking meta-analyses. In: Higgins J., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1. Oxford, UK: The Cochrane Collaboration: Chap 9; 2008. [Google Scholar]
- 26.Higgins J. P. T., Thompson S. G., Deeks J. J., Altman D. G. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xin H. Effect of suxiao jiuxin pill on unstable angina. Chinses Community Docotors. 2013;41:47–48. [Google Scholar]
- 28.Long J. Q. Effect of suxiao jiuxin pill on unstable angina. Chinses Community Docotors. 2013;7, article 23 [Google Scholar]
- 29.Gao Y. C., Liu A. X., Yin D. F., Chen C. H., Gao R. X. Effect of suxiao jiuxin pill on 105 patients with angina. Journal of Emergency in Traditional Chinese Medicine. 1996;2:74–75. [Google Scholar]
- 30.Yong J. H. Effect of suxiao jiuxin pill on 84 patients with angina. Tianjin Pharmacy. 2000;12(B11):42–43. [Google Scholar]
- 31.Hu G. Effect of suxiao jiuxin pill on patients with coronary heart disease. Clinical Journal of Traditional Chinese Medicine. 2004;16(1):53–54. [Google Scholar]
- 32.Ke F. J. Effect of suxiao jiuxin pill on 36 patients with angina. Journal of Emergency in Traditional Chinese Medicine. 1996;5(3, article 118) [Google Scholar]
- 33.Pei Y. W. Comparison between suxiao jiuxin pill and Isosorbide Mononitrate on angina. Modern Medicine and Health. 2003;19(3, article 274) [Google Scholar]
- 34.Ya X. Z. Effect of suxiao jiuxin pill on eldly patients with angina. Tianjin Pharmacy. 2000;12(B11):46–47. [Google Scholar]
- 35.Li J. Z. Effect of suxiao jiuxin pill on patients with angina. Tianjin Pharmacy. 2000;12(B11, article 62) [Google Scholar]
- 36.Li A. Effect of suxiao jiuxin pill on 103 patients with angina. Qingdao Medical Journal. 1998;6:39–40. [Google Scholar]
- 37.Jin G. Effect of suxiao jiuxin pill on 98 patients with angina. Journal of Chinese General Practice. 2003;6(3):250–251. [Google Scholar]
- 38.Yun Y. G. Effect of suxiao jiuxin pill on patients with angina. Journal of Emergency in Traditional Chinese Medicine. 1996;5(3):115–116. [Google Scholar]
- 39.Mei H. Effect of suxiao jiuxin pill on 68 patients with angina. Tianjin Pharmacy. 2000;12(B11):36–37. [Google Scholar]
- 40.Yu P. L. Effect of suxiao jiuxin pill on patients with angina. Tianjin Pharmacy. 2000;12(B11, article 47) [Google Scholar]
- 41.Xiao C. L. Effect of suxiao jiuxin pill on 78 patients with angina. Tianjin Pharmacy. 2000;12(B11):811–812. [Google Scholar]
- 42.Wen L. Effect of suxiao jiuxin pill combined with compound danshen dripping pill on patients with angina. Shanxi Journal of Traditional Chinese Medicine. 2009;25(8):12–13. [Google Scholar]
- 43.Hai S. Effect of suxiao jiuxin pill on 40 patients with angina. Chinese Medincie. 2002;24(11):852–854. [Google Scholar]
- 44.Bu C. S. Comparison between suxiao jiuxin pill and Isosorbide dinitrate on angina. Chinese Journal of Natural Medicine. 2002;4(3):185–186. [Google Scholar]
- 45.Guang Y. T. Effect of suxiao jiuxin pill on 124 patients with angina. Tianjin Pharmacy. 2000;12(1):40–41. [Google Scholar]
- 46.Run L. T. Comparison between suxiao jiuxin pill and Isosorbide dinitrate on angina. Journal of Snake. 2007;19(1, article 37) [Google Scholar]
- 47.Shu D. Y. Effect of suxiao jiuxin pill on patients with unstable angina. Tianjin Pharmacy. 2000;12(B11):28–29. [Google Scholar]
- 48.Feng L., Zhou Y. P. Effect of suxiao jiuxin pill on patients with angina. Journal of Emergency in Traditional Chinese Medicine. 2000;9(1):4–6. [Google Scholar]
- 49.Feng H. S. Effect of suxiao jiuxin pill on patients with angina. Beijing Journal of Traditional Chinese Medicine. 2009;28(9):25–26. [Google Scholar]
- 50.Shui X. W. Effect of suxiao jiuxin pill and compound danshen dripping pill on patients with angina. Jiangxi Medicine. 2006;S1:1073–1074. [Google Scholar]
- 51.Song Z. J. Effect of suxiao jiuxin pill on patients with angina. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care. 1995;2:83–84. [Google Scholar]
- 52.He G. H. Effect of suxiao jiuxin pill on patients with angina. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care. 1995;5:214–215. [Google Scholar]
- 53.Ma X. Z. Effect of suxiao jiuxin pill on 83 patients with angina. Modern Medicine and Health. 2004;20(21):2211–2212. [Google Scholar]
- 54.Guo W. Q. Effect of suxiao jiuxin pill on patients with coronary heart disease. Chinese Community Doctors. 2012;14, article 11 [Google Scholar]
- 55.Ru B. J. Effect of suxiao jiuxin pill on patients with angina. Tianjin Pharmacy. 2000;12(B11, article 46) [Google Scholar]
- 56.Li X. J. Clinical observation on effect of suxiao jiuxin pill in treating angina pectoris of coronary heart disease. Strait Pharmacy. 2015;2:98–99. [Google Scholar]
- 57.Duan K. J., Yang X. Y. Effect of suxiao jiuxin pill on 40 patients with angina. Tianjin Journal of Traditional Chinese Medicine. 2002;19(1):20–21. [Google Scholar]
- 58.Wen S. L. Comparison between suxiao jiuxin pill and Tongxinluo pill on angina. Chinese Community Docotor. 2009;6, article 62 [Google Scholar]
- 59.Wan W. Comparison between suxiao jiuxin pill and Guanxinsuhe pill on angina. Medicine Industry Information. 2005;2(17, article 59) [Google Scholar]
- 60.Wang D. P. Comparison between suxiao jiuxin pill and shexiang baoxin pill on angina. Shanghai Medicine. 1996;12:25–26. [Google Scholar]
- 61.Fei W. Comparison between suxiao jiuxin pill and shexiang baoxin pill on angina. Medicine Review. 2008;14(19):3022–3024. [Google Scholar]
- 62.Yue S. Z. Effect of suxiao jiuxin pill on 78 patients with angina and heart failure. Journal of Practical Medical Techniques. 2004;6:31–32. [Google Scholar]
- 63.Zhu D. Y. Effect of suxiao jiuxin pill on 118 patients with angina. Henan Chinese Medicine. 2005;25(9):76–77. [Google Scholar]
- 64.Jing X. Y. Effect of suxiao jiuxin pill on 102 patients with coronary heart disease. Tianjin Pharmacy. 2000;12(B11):37–38. [Google Scholar]
- 65.Wei W. W. Efficacy of Suxiaojiuxinwan vs. Isosorbide Dinitratein patients with angina pectoris: a meta-analysis. Chinese Journal of Evidence-Based Cardiovascular Medicine. 2015;3:298–303. [Google Scholar]
- 66.Xiao F. R. Meta-analysis of Suxiao Jiuxin Pill on Acute Coronary Syndrome. Journal of Emergency in Traditional Chinese Medicine. 2016;3:380–383+425. [Google Scholar]
- 67.Li W., Tang Y., Chen Y., Duan J.-A. Advances in the chemical analysis and biological activities of chuanxiong. Molecules. 2012;17(9):10614–10651. doi: 10.3390/molecules170910614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ran X., Ma L., Peng C., Zhang H., Qin L.-P. Ligusticum chuanxiong Hort: a review of chemistry and pharmacology. Pharmaceutical Biology. 2011;49(11):1180–1189. doi: 10.3109/13880209.2011.576346. [DOI] [PubMed] [Google Scholar]
- 69.Ren D. C., Yang N. Y., Qian S. H., Xie N., Zhou X. M., Duan J. A. Chemical study on aerial parts of Ligusticum chuanxiong. Zhongguo Zhong Yao Za Zhi. 2007;32(14):1418–1420. [PubMed] [Google Scholar]
- 70.Ren X.-Y., Ruan Q.-R., Zhu D.-H., Zhu M., Qu Z.-L., Lu J. Tetramethylpyrazine inhibits agiontensin II-induced nuclear factor-kappaB activation and bone morphogenetic protein-2 downregulation in rat vascular smooth muscle cells. Sheng Li Xue Bao. 2007;59(3):339–344. [PubMed] [Google Scholar]
- 71.Tsai C.-C., Lai T.-Y., Huang W.-C., Liu I.-M., Cheng J.-T. Inhibitory effects of potassium channel blockers on tetramethylpyrazine-induced relaxation of rat aortic strip in vitro. Life Sciences. 2002;71(11, article no. 8922):1321–1330. doi: 10.1016/S0024-3205(02)01852-0. [DOI] [PubMed] [Google Scholar]
- 72.Hou Y. Z., Zhao G. R., Yang J., Yuan Y. J., Zhu G. G., Hiltunen R. Protective effect of Ligusticum chuanxiong and Angelica sinensis on endothelial cell damage induced by hydrogen peroxide. Life Sciences. 2004;75(14):1775–1786. doi: 10.1016/j.lfs.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 73.Wong K.-L., Wu K.-C., Wu R. S.-C., Chou Y.-H., Cheng T.-H., Hong H.-J. Tetramethylpyrazine inhibits angiotensin II-increased NAD(P)H oxidase activity and subsequent proliferation in rat aortic smooth muscle cells. American Journal of Chinese Medicine. 2007;35(6):1021–1035. doi: 10.1142/s0192415x0700548x. [DOI] [PubMed] [Google Scholar]
- 74.Chan E. W. E. C., Wong S. K. U. Phytochemistry and pharmacology of ornamental gingers, Hedychium coronarium and Alpinia purpurata: a review. Journal of Integrative Medicine. 2015;13(6):368–379. doi: 10.1016/S2095-4964(15)60208-4. [DOI] [PubMed] [Google Scholar]
- 75.Bhatia S. P., Letizia C. S., Api A. M. Fragrance material review on borneol. Food and Chemical Toxicology. 2008;46(11):S77–S80. doi: 10.1016/j.fct.2008.06.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: mean difference in total cholesterol in the treatment and control groups. Figure S2: mean difference in total triglycerides in the treatment and control groups. Figure S3: mean difference in total low-density lipoprotein in the treatment and control groups. Figure S4: mean difference in total high-density lipoprotein in the treatment and control groups. Figure S5: mean difference in low-cut whole blood viscosity in the treatment and control groups. Figure S6: mean difference in high-cut whole blood viscosity in the treatment and control groups. Figure S7: mean difference in plasma viscosity in the treatment and control groups. Figure S8: mean difference in hematocrit in the treatment and control groups. Figure S9: mean difference in the fibrinogen in the treatment and control groups. Figure S10: relative risks for adverse reactions in the treatment and control groups. Figure S11: funnel plot of studies with reported adverse reactions included in the meta-analysis.