Summary
Lumbar laminectomy often results in failed back surgery syndrome. Most scholars support the three-dimensional theory of adhesion: Fibrosis surrounding the epidural tissues is based on the injured sacrospinalis behind, fibrous rings and posterior longitudinal ligaments. Approaches including using the minimally invasive technique, drugs, biomaterial and nonbiomaterial barriers to prevent the postoperative epidural adhesion were intensively investigated. Nevertheless, the results are far from satisfactory. Our review is based on various implant biomaterials that are used in clinical applications or are under study. We show the advantages and disadvantages of each method. The summary will help us to figure out ideas towards new techniques.
The translational potential of this article: This review summarises recent biomaterials-related clinical and basic research that focuses on prevention of epidural adhesion after lumbar laminectomy. We also propose a novel possible translational method where a soft scaffold acts as a physical barrier in the early stage, engineered adipose tissue acts as a biobarrier in the later stage in the application of biomaterials and adipose-derived mesenchymal stem cells are used for prevention of epidural adhesion.
Keywords: Adhesion, Biomaterials, Fibrosis, Implant, Laminectomy
Introduction
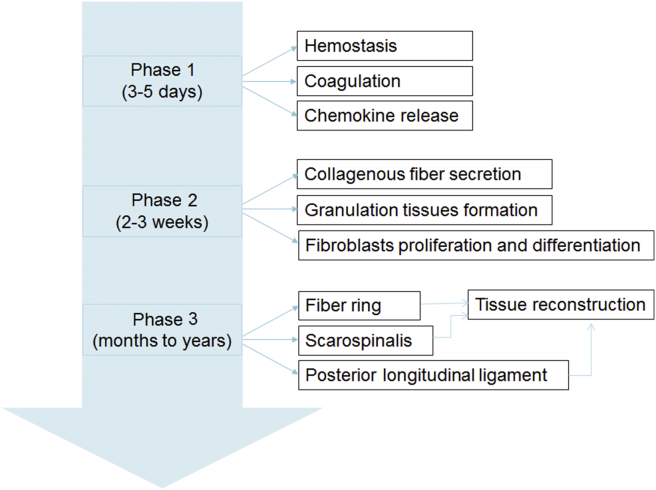
About 8–40% patients after lumbar laminectomy suffered from failed back surgery syndrome (FBSS), and 4–9% patients suffered from the second surgery [1]. Extension of scar tissue into the neural canal and adhesion to the dura mater are considered to be the major reason for leg and back pain. The formation and repair process of scar tissue can be classified into three phases (See Figure 1). The first phase is the local inflammatory reaction in the first 3–5 days after surgery, mainly including haemostasis and coagulation process and chemokine release such as phospholipase A2, which causes the aggregation of macrophagocytes, fibroblasts, mastocytes and endotheliocytes [2]. The second phase lasts 2–3 weeks. Fibroblasts proliferate and differentiate into fibrocytes, secrete collagenous fibres in the defect lesion and form granulation tissues gradually. Fibroblast proliferation, immigration and extracellular matrix synthesis are regulated by various cytokines, such as transforming growth factor (TGF)-β1, interleukin-6 (IL-6) and fibroblast growth factor (FGF). Fibroblasts could also secret TGF-β1, IL-6 and FGF-2 to improve fibroblast proliferation and extracellular matrix synthesis [3]. The third phase is tissue reconstruction which lasts months to years; fibrillar connective tissues deposit around the defect lesion and transform into scar tissues [4].
Figure 1.
Process of adhesion after lumbar laminectomy.
Origination of the epidural scar
People tried for a long period to find the origination of the epidural scar. Is the epidural scar originated from injured tissue due to surgical approach, including sacral spine muscle, lamina, ligamentum flavum, posterior longitudinal ligament or fibre ring? Key and Ford [5] considered injured intervertebral disc fibre is the major source of epidural adhesion. LaRocca and Macnab [6] performed the surgery in dogs and considered the rough surface of sacrospinalis in the surgical lesion behind the spinal canal to be the major source. Fibroblasts in the deep layer of sacrospinalis proliferate and form the laminectomy membrane from sacrospinalis to the side of the dura mater. However, so far, the most approved mechanism of adhesion is raised by Songer and Ghosh Spencer [7]. They proposed a three-dimensional theory. It is said that the scar tissue around the dura mater originates not only from sacrospinalis behind, but also from the fibre ring and posterior longitudinal ligament ahead. The hyperplasia of fibrous tissue around the ventrolateral nerve root caused epidural adhesion.
Evaluation of epidural adhesion
How to evaluate the degree of epidural adhesion? Generally, there are three main aspects to evaluate the adhesion: macroscopic analysis, histological analysis and magnetic resonance imaging (MRI) analysis. Macroscopic analysis is carried out in a space between the dura mater and surrounding soft tissues. It is based on the quality of wound healing, possible adverse effects and epidural adhesion. Adhesion tenacity is also evaluated using Rydell and Balazs’s standard score. Grade 0 shows no obvious adhesion between the dura mater and the scar; Grade 1 shows scattered and slight adhesion between the dura mater and the scar which is easily separable; Grade 2 shows extensive and compact adhesion between the dura mater and the scar, where it is difficult to separate adhesion surrounding the dura mater while keeping the dura mater complete; Grade 3 shows severe adhesion between the dura mater and the scar, and separation means destroying the dura mater [8].
Histological analysis is based on haematoxylin and eosin (H&E) staining and Masson's trichrome staining. H&E staining focuses on cell activity. Masson's trichrome staining shows inflammatory factor and fibrin. Modified Henderson's grading system is based on epidural fibrosis, abscess, acute inflammation and necrosis, dividing into grade 0 (no fibrosis, no inflammation, no abscess and no necrosis), grade 1 (mild interstitial fibrosis, mixed inflammation (25%), abscess area <2 and necrosis area <2), grade 2 (mild interstitial fibrosis, mixed inflammation (50%), abscess area >2 and necrosis area >2), grade 3 (marked fibrosis collagen formation, mixed inflammation (75%), marked abscess and marked necrosis) and grade 4 (massive fibrosis, massive inflammation, massive abscess and massive necrosis) [9], [10].
MRI observations of implant materials after lumbar laminectomy show the size of the material and that the shape changed along with the shape of the dura mater. The remodelling of the material occurs in relation to the postoperative transient shrinkage and expansion of the dura mater. MRI can monitor the state of implant to evaluate the function of implant based on the signal and diameter [11], [12].
Current strategies for prevention of epidural adhesion
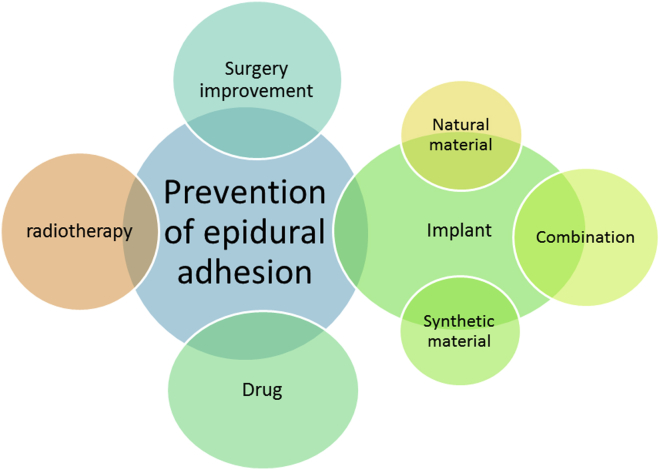
Various methods have been studied to prevent epidural fibrosis and to reduce the pain, such as developing drugs to reduce the inflammation, modifying surgical techniques, using roentgenotherapy and implanting a barrier between epidural space and its overlying muscles. The current methods used clinically include the minimally invasive technique, the usage of drugs such as mitomycin C [13], dexamethasone [14], hydroxycamptothecine [4], rosuvastatin [15] and non-steroidal anti-inflammatory drugs [16], [17], low dose radiation, traditional Chinese drugs and biomaterials such as autologous tissue [12], [18] and biodegradable polymeric materials(See Figure 2). Biomaterials have some important characteristics such as large molecular weight, complex structure, wide varieties and extensive biological function [19]. As for implants used as physical barriers, optimal biomaterials have progressively become a primary strategy to prevent epidural adhesion after lumbar laminectomy. Considering autologous tissue for physical barriers, autologous fat grafts are most commonly used to clinically prevent epidural adhesion after lumbar laminectomy compared with autologous bone grafts [12], [20]. Clinical research suggests that the isolated adipose tissue can reconstruct epidural adipose and reduce epidural adhesion and FBSS [21].
Figure 2.
Strategies for adhesion prevention.
Recently, with the rapid development of materials science and interdisciplinary communication, biodegradable polymeric materials have gained a wide theoretical interest and practical application in preventing epidural adhesion. On one hand, polymeric materials can be used as the physical barrier; on the other hand, polymeric materials can be used as the carrier in the controlled release of chemical drugs. It has become an important research area in the study of polymeric materials science. According to the property and constituent, we classify the polymeric materials into natural polymeric materials and synthetic polymeric materials.
Natural polymeric materials
Over the past decade, natural polymeric biomaterials such as chitosan, fibrin gel, hyaluronate and amniotic membrane were widely studied. Each of these materials has been proved to reduce the postoperative epidural adhesion, but success is limited; for example, gelatin sponge was effective in preventing postoperative epidural adhesion, but it was easy to form a haematoma after the expansion in blood absorption, and then it would transform into scar tissue with epidural adhesion and oppress the nerve root and dural sac [22]. Natural polymeric materials show uneven distribution and short persistent time and are easy to be hydrolised. At the same time, the foreign materials stimulate a reaction in the organism which causes injury [23] (See Table 1).
Table 1.
Natural polymeric materials used in prevention of adhesion.
| Material | Animal | Position | Follow-up (weeks) | Results | Reference |
|---|---|---|---|---|---|
| Cross-linked hyaluronic acid hydrogel | Rat | T11–T12,T12–L1, L3 | 8 | Lower grade of epidural fibrosis, thinner dura mater and larger epidural and subarachnoid spaces; formed a solid interpositional membrane barrier | [22] |
| Hybrid chitosan membrane | Rabbit | L1–L3 | 4 | Well-organised regenerating tissue integrated in the surrounding vertebral bone tissue with a regular and all-site interface on the chitosan sites | [26] |
| Amniotic membrane | Rat | Thoracolumbar junction | 1–8 | The amount of scar tissue and tenacity were reduced grossly; less inflammatory cell infiltration and fibroblast proliferation | [29], [30] |
| Dog | L1, L3, L5, L7 | 1–12 | Lower scar amount and adhesion tenacity; a white, slightly vascularised crossed-linked amniotic membrane layer without tenacious scar adhesion; reduced fibroblasts infiltration and epidural fibrosis (similar to autologous free fat) | [31] | |
| Silk-polyethylene glycol (PEG) hydrogels | Rabbit | 2–8 | No or mild adhesion is observed in silk-PEG hydrogel samples | [32] |
Cross-linked hyaluronate
High-molecular-weight hyaluronate (HA) is a hotspot in present studies for its semifluid condition, which meets the requirements of the ideal anti-adhesion materials. The hyaluronate can fit with the anomalous half ellipse dura mater adequately. Therefore, the proliferation of fibroblast and the sedimentation of collagen will be reduced. Cross-linked high-molecular-weight HA had positive effects on the prevention of epidural fibrosis and the reduction of fibrotic tissue density. The mechanism can be summarised into four parts: 1) The physical barrier prevents the connection between the local haematoma and epidural cicatricial tissue; 2) Reduce the release of inflammatory mediator; 3) Antifibrosis; 4) Suppress autophagy activity [24], [25]. Recently, oxidised HA/adipic acid dihydrazide hydrogel was reported to be a promising injectable and thermosensitive material for prevention of postoperative epidural fibrosis by reducing NIH/3T3 fibroblasts viability, downregulating S100a and P4hb expression in NIH/3T3 fibroblasts and reducing scar tissue formation in vivo [26]. However, the long-term application of hyaluronic acid is limited by tissue-mediated enzymatic degradation. To overcome its limitations, researchers developed a polygalacturonic acid and hyaluronate composite hydrogel by Schiff's base cross-linking reaction. It was not totally degraded in vivo after 4 weeks and prevented fibroblasts from adhesion and infiltration into the hydrogels. It can be easy to use due to its in situ cross-linkable property and potentially promising ability for adhesion prevention in spine surgeries [27]. The efficacy of this agent should also be verified in further experimental and clinical studies.
Hybrid chitosan membrane
A chitosan-silane membrane improved mechanical strength which makes it suitable to maintain a predefined shape to prevent adhesion [28]. Recently, a thermosensitive sol–gel antiadhesive agent (a main mixture of chitosan and gelatin) was developed. Histologic evaluation showed significant higher value than the negative control subgroup with regard to the ratio of adhesion less than 50%. The new thermosensitive agent showed superior efficacy at 1 week postoperatively but same efficacy as the hyaluronate-based agent at 4 weeks [29], [30].
Amniotic membrane
The amniotic membrane is a kind of natural membrane which has been used in surgical adhesion [31]. The amniotic membrane is the inner layer of foetal membrane, which acts as a barrier to reduce inflammation, inhibit vascularisation and limit postoperative adhesion. Hyu Jin et al found that the adhesion grade is lower than that in the control group in a rat model, which showed that the amniotic membrane can be helpful to reduce the adhesion [32]. Moreover, compared with fat graft, it shows better biocompatibility and capability of existing for a certain period in the body [33].
Silk-polyethylene glycol hydrogels
Biodegradable silk-polyethylene glycol (PEG) hydrogels are evaluated for adhesion prevention after laminectomies in New Zealand rabbits. Silk is fully degraded within 6 weeks, leaving a gap separating the scar tissue and the dura mater. No or mild adhesion is observed in silk-PEG hydrogel samples. The surface properties of the hydrogels and local and temporal release of PEG may account for its adhesion prevention effects [34].
Synthetic polymeric materials
Synthetic polymeric materials such as poly lactic-co-glycolic acid membrane (PLGA), expanded tetrafluoroethylenepolytetrafluoroethylene (e-PTFE) membrane and polyglycolic acid membrane were used in many areas and in the field of adhesion prevention. Their function is more like the physical barrier to isolate the dura mater from the scar tissue (See Table 2).
Table 2.
Synthetic polymeric materials used in prevention of adhesion.
| Material | Animal | Position | Follow-up | Results | Reference |
|---|---|---|---|---|---|
| PLGA | Rabbit | L5–L7 | 1, 12 and 24 weeks | Continuous linear adipose tissue regenerated; a distinct area of adipose tissue just overlaying the dura mater prevents formation of epidural fibrosis | [33] |
| ADCON-L | Patient | L1–L5 | 6 months | No operative or postoperative complications. No, mild or mild to moderate scarring in most patients; substantial reductions in pain and no significant differences between two groups. | [35] |
| Rat | L3–L5 | 6 weeks | Histopathological grades were improved; the mean values of the fibroblast count were not statistically significant | [36] | |
| e-PTFE | Patient | Laminectomy defect region | 3–24 months | Significantly lower rate of epidural fibrosis on MRI and of clinical manifestations of radiculalgia; epidural fibrosis was generally less extensive, but more seromas occurred | [37] |
| MAACP-nHA | Goat | C3-C5 | 4–24 weeks | Adhesion was significantly slighter; no dislocation of artificial lamina; no soft tissue projected into the spinal canal; artificial lamina had no obvious degradation with high integrity; some new bone formed at the interface between the synthetic material and bone | [38] |
| PLGA-PEG-PLGA | Rat | L2–L4 | 4 weeks | No cytotoxicity; The extent of epidural fibrosis, the area of epidural fibrosis and the density of fibroblasts and blood vessel were evaluated histologically. The efficiency of the PLGA-PEG-PLGA thermogel showed slightly improved comparing with the chitosan gel | [40], [41] |
e-PTFE = expanded polytetrafluoroethylene; MAACP-nHA = multi-amino acid copolymer nano-hydroxyapatite; MRI = magnetic resonance imaging ; PEG = polyethylene glycol; PLGA = poly lactic-co-glycolic acid.
Poly lactic-co-glycolic acid membrane
PLGA membrane has good biocompatibility and blood compatibility with slight organisation response, exact barrier function and moderate absorption cycle. Using PLGA membrane alone has a good barrier protection in the formation of scar tissue, which is similar to the implantation of free adipose tissue. Moreover, MRI examination, macroscopic analysis and histological examination showed that PLGA/chitosan barrier is effective in inhibiting epidural fibrosis and peridural adhesions in postlaminectomy rabbit model [35]. The drawback of PLGA is that they are absorbable materials. There will be a cavity after PLGA absorption.
ADCON-L
ADCON-L is a bioabsorbable carbohydrate polymer gel which is composed of a polyglycan ester and porcine-derived gelatin in phosphate-buffered saline [36]. ADCON-L was widely used clinically for the prevention of adhesion and pain after the surgery. The epidural application of the ADCON-L after lumbar microdiscectomy was found to be effective in reducing the epidural adhesion and controlling the postoperative pain [37]. However, a few case reports showed adverse events of ADCON-L after a period; acute complications such as inhibition of spontaneous posterior spinal fusion and delayed detected postoperative complication such as disturbance of muscle healing [38]. The acute and chronic complications remind us that the essential characteristic of the implant is nontoxicity. It has been banned in clinical use for many years because of the severe complications. We should focus on reducing the toxicity of ADCON-L and make full use of its advantages in future studies.
Expanded polytetrafluoroethylene membrane
e-PTFE has excellent biocompatibility and stable structure so that fibroblasts cannot penetrate. It provides a separable safety interface for second operation, which is superior to free adipocytes. Because of that, it is considered as an ideal anti-adhesion material. e-PTFE is a kind of porous materials with better biocompatibility, which maintains a stable position [39]. Lladó et al conducted a clinical experiment with 66 patients, half of whom had an e-PTFE membrane implanted to cover the defect caused by laminectomy [39], and they proved the effectiveness and harmlessness of e-PTFE through clinical trials in which the MRI images show less epidural fibrosis and seromas in the defect lesion of patients treated with e-PTFE. e-PTFE acts as a barrier, but it cannot often cover the entire laminectomy defect so that the fibrosis can penetrate partial area. On the other hand, e-PTFE is also a foreign material which will improve the inflammatory reaction, eventually leading to scar proliferation and adhesion. Finding a kind of material combined with the e-PTFE to improve the antiadhesion effect is worth exploration.
Multi-amino acid copolymer (MAACP)/nano-hydroxyapatite (nHA)
MAACP-nHA is composed of MAACP and nHA. Compared with traditional biomaterials, MAACP shows better cell affinity and adjustable degradation rate. The degradative product is neutral so that they do not cause the aseptic inflammation. HA is the important part of osseous tissue. It has excellent biocompatibility and good bone conduction function. Studies have found that MAACP-nHA acts as cortical bone scaffolds with strong biomechanics of bone, good biological sluggishness and good biocompatibility. MAACP-nHA acts as a barrier to reduce epidural adhesion effectively [40].
PLGA-PEG-PLGA
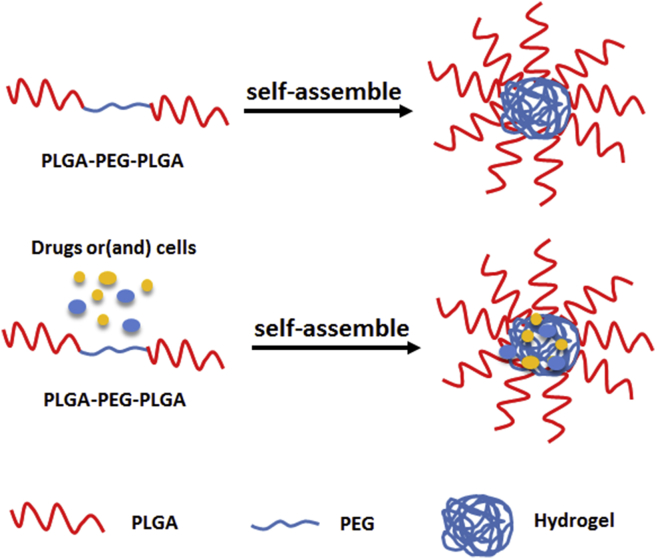
Poly (lactic-co-glycolic acid)-poly (ethylene glycol)-poly (lactic-co-glycolic acid) is composed of PLGA and PEG [41]. PEG is biodegradable and the results are highly repeatable. The thermo-gels composed of PEG and PLGA act as effective and well-modulated barrier devices to prevent postoperative abdominal adhesion [42], [43]. PLGA-PEG-PLGA can not only act as a barrier but also act as a support. PLGA-PEG-PLGA group showed less cytotoxicity, less epidural fibrosis and lower density of fibroblasts and blood vessel. We also can add some materials into the gel during the self-assembly of PLGA-PEG-PLGA to have the combined effect (See Figure 3).
Figure 3.
Formation of PLGA-PEG-PLGA. PEG = polyethylene glycol; PLGA = poly lactic-co-glycolic acid.
Combination strategies
A physical barrier combining the advantages of drugs such as dexamethasone, ibuprofen, mitomycin C, hydroxycamptothecine and Salvia miltiorrhiza shows the greater effect. Combining with immunomodulatory factors such as interferon also shows the preferable results (See Table 3).
Table 3.
Combination strategies.
| Material | Animal | Position | Follow-up | Results | Reference |
|---|---|---|---|---|---|
| Gelatin sponge + dexamethasone | Rat | L2–L4 | 4–12 weeks | Lower expressions of vascular endothelial growth factor and its receptor; no obvious adhesion formation | [14] |
| Fibrin glue + methyl prednisolone acetate |
Rat | L4–L5 | 1–6 | Lower necrosis grade, but no significant differences in epidural fibrosis, abscess and acute inflammatory | [42] |
| Mitomycin C–PEG film | Rat | L1–L2 | 4 weeks | Reduce adhesion by decreasing the concentration of hydroxyproline and increasing the apoptosis of fibroblasts | [45] |
| Mitomycin C–PLGA film | Rat | L1 | 4 weeks | The scar adhesion and scar area were significantly reduced; the deposition of collagen was significantly reduced, increased autophagy and altered expression of miRNAs in the scar tissue. | [46] |
| Ibuprofen-conjugated hyaluronate (HA)/polygalacturonic acid (PGA) hydrogel | Rat | L1 | 4 weeks | Suppressed both in vitro and in vivo inflammatory responses with ibuprofen-conjugated PGA and HA hydrogel and delayed condensation of scar tissue | [47] |
| PLGA-PIBU--IBU electrospun fibrous membrane | Rat | L2–5 | 4–8 weeks | Antiadhesion effect and associated neurological deficits were effectively reduced | [48] |
HA = hyaluronate; PEG = polyethylene glycol; PLGA = poly lactic-co-glycolic acid.
Gelatin sponge + dexamethasone
Gelatin sponge separates the nervous tissue from the surrounding tissue, reducing the epidural scar tissue and nerve adhesion, which performs an interval barrier effect. On the basis of adhesion theory mentioned previously, researchers tried to use dexamethasone combined with gelatin sponge to stop the first and the second process of haematoma towards fibroblasts hyperplasia and then to reduce the scar tissue and epidural adhesion. Gelatin sponge–dexamethasone is placed between muscle and endorhachis as a barrier. Both have synergetic effects. Dexamethasone has anti-inflammatory effects, delaying the granulation formation to prevent adhesion, reducing scar formation and preventing adipocyte necrosis. In addition, gelatin sponge–dexamethasone can avoid the loss of dexamethasone. It takes a long time for gelatin sponge–dexamethasone complex to be absorbed, which forms a protective layer around the nerve root, reduces vertebral plate errhysis and blood seeping into the nerve root and around the endorhachis, and avoids spinal canal haematoma formation [14].
Fibrin glue
Fibrin glue is a macromolecular substance. On one hand, it can form the network structure to stop the bleeding of the multi-bioactivity protein. On the other hand, the fibrin glue has a faster degradation speed in vivo without bacterial infection. Injected regionally, the fibrin glue will adhere to the tissue, sealing the tissue. Some researchers consider that fibrin glue is similar to cell membrane in characteristics: nontoxic, nonreactive, biologically compatible material [44]. After the absorption of the fibrin glue, a space will be formed between the dura mater and sacrospinal muscle. Histology showed that the area with fibrin glue was less adhesive, and the fibroblast was fewer than in the control group.
Mitomycin C–polyethylene glycol controlled-release film
Mitomycin C is proved to be effective to reduce epidural fibrosis and adhesions after spinal laminectomy [13], [45]. However, high concentration of mitomycin C is cytotoxic, and the administrative pathway prevents its application [46]. Therefore, a mitomycin C–PEG film was developed. It was demonstrated that the treatment of postlaminectomy wounds with mitomycin C–PEG film could reduce the severity of adhesion by decreasing the concentration of hydroxyproline and increasing the apoptosis of fibroblasts [47]. Furthermore, controlled-release mitomycin C–PLGA film prevents epidural scar hyperplasia after laminectomy by inducing fibroblast autophagy and regulating the expression of miRNAs [48].
Ibuprofen-conjugated hyaluronate/polygalacturonic acid hydrogel
Local delivery of ibuprofen via a polygalacturonic acid–hyaluronic acid–based hydrogel reduces the possibility of epidural fibrosis [49]. Besides its low cytotoxicity, the conjugated ibuprofen decreased prostaglandin E2 production of the lipopolysaccharide-induced in RAW264.7 cells. Histological data indicated that the scar tissue adhesion of laminectomised male adult rats was reduced, that the population of giant cells was reduced and collagen deposition of scar tissue without inducing extensive cell recruitment. Incorporation of ibuprofen and its prodrug into PLGA electrospun fibrous membrane, with sustained release of ibuprofen, improved anti-adhesion effects and neurological outcomes in rats after lumbar laminectomy [26].
Conclusion and perspectives
Epidural adhesion is one of the most important causes of FBSS. Currently, there are various methods to prevent adhesions include lumbar minimally invasive resection, radiotherapy, drug treatment and implant treatment. These methods are based on the theory of mechanical barrier to avoid or at least decrease the friction between the dura mater and scar tissues and to stop fibroblast growth or reduce haematoma formation in a biochemical way.
Although autologous fat grafts have been most commonly used to prevent the epidural adhesion after lumbar laminectomy clinically, the clinical effect of isolated adipose tissue transplantation is still controversial. The time it works for is so short because it gets easily degraded due to atrophy and necrosis [50]. Autologous fat tissue transplantation with oppress dural sac occasionally causing cauda equina syndrome is reported [51]. In addition, a study with 2.6 years of follow-up found no effect of implanted adipose tissue in improving intervertebral disc herniation in patients with postoperative symptoms [51], [52].
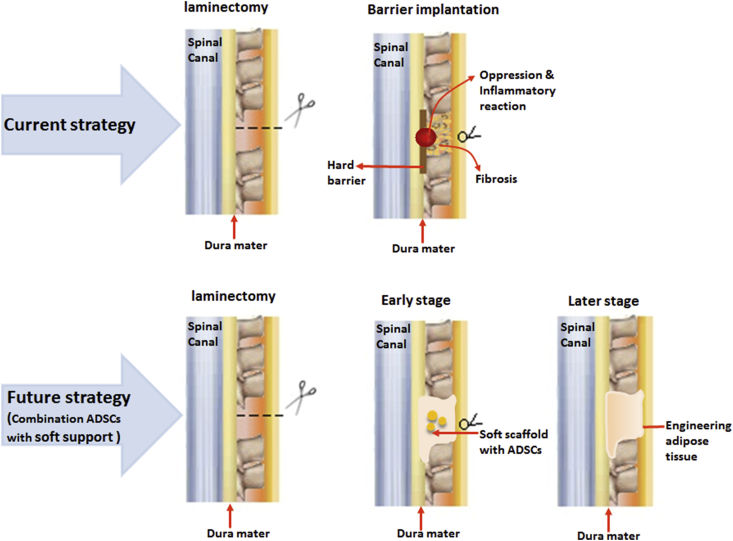
Therefore, novel materials and methods are becoming the focus of research on preventing epidural adhesion after laminectomy. For the bench studies and clinical trials today, the ideal material should have better biocompatibility, fully filled in the injury site, degradable absorption, capability of existing for a certain period in the body and naturally integrated into the host tissues. The clinical experiments or animal experiments show positive effect of natural polymeric materials and synthetic polymeric materials. Soft or viscous materials, such as gel and sponge, fully fill in the injury site and reach everywhere the adhesion may happen to block the invasive fibroblast, but they showed short persistent time and poor mechanical strength. Hard materials show poor variability and are removable. Therefore, the combination of various ways may reduce cicatrices formation of scar tissues to a lower degree, which cannot be reached only using one of the methods. Recent studies have found that the combination of two or more materials may have less complication and better prognosis. Interestingly, using tissue engineering techniques, Xu et al [53] used PLGA as an implantation scaffold with adipose-derived stem cells (ADSCs) which helps adipose tissue regeneration in situ to prevent epidural adhesion effectively after laminectomy. We believe that adipose tissue engineering will be a novel strategy in clinical postoperative adhesion prevention. Future studies may focus on the combination of autologous ADSCs with biomaterial scaffolds (See Figure 4).
Figure 4.
Prospect of the development of strategy for adhesion prevention in future. ADSC = adipose-derived stem cell.
ADSCs are a kind of autologous stem cells. Compared with bone marrow stem cells, they show easy accessibility, relative abundance with proliferation ability and easy differentiation to fat tissue. Meanwhile, the scaffold should fulfil the items listed previously, and the degradable speed should match the proliferation and differentiation speed of ADSCs. In terms of the property that the material should reach everywhere the adhesion may happen to block the invasive fibroblast, soft scaffolds may be better than hard scaffolds. Soft scaffolds occupy every space in the defect area and act as the physical barrier in the early stage. After the stem cells differentiate into adipose tissue, the engineered adipose tissue will act as a biobarrier in the later stage. The engineered adipose tissue is still under study, and further work should figure out more suitable and effective materials and techniques.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Guyer R., Patterson M., Ohnmeiss D. Failed back surgery syndrome: diagnostic evaluation. J Am Acad Orthop Surg. 2006;14(9):534–543. doi: 10.5435/00124635-200609000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Laurent G., Chambers R., MR H Regulation of matrix turnover: fibroblasts, forces, factors and fibrosis. Biochem Soc Trans. 2007;35(4):647–651. doi: 10.1042/BST0350647. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J., Li Y., Shen W. Relationships between transforming growth factor.betal, myostatin, and decorin:implications for skeletal muscle fibrosis. Biol Chem. 2007;282(35):25852–25863. doi: 10.1074/jbc.M704146200. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y., Wang L., Sun S., Liu B., Wu N., Cao X. The effect of 10-hydroxycamptothecine in preventing fibroblast proliferation and epidural scar adhesion after laminectomy in rats. Eur J Pharmacol. 2008;593(1–3):44–48. doi: 10.1016/j.ejphar.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Key J., Ford L. Experimental intervertebral disc lesions. J Bone Joint Surg Am. 1948;30(3):621–630. [PubMed] [Google Scholar]
- 6.LaRocca H., Macnab I. The laminectomy membrane: studies in its evolution, characteristics, effects and prophylaxis in dogs. J Bone Joint Surg Br. 1974;56(3):545–550. [PubMed] [Google Scholar]
- 7.Songer M.N., Ghosh Spencer D.L. Effects of sodium hyaluronate on peridural fibrosis after lumbar laminotomy and discectomy. Spine. 1990;15(6):550–554. doi: 10.1097/00007632-199006000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Rydell N., Balazs E. Effect of intra-articular injection of hyaluronic acid on the clinical symptoms of osteoarthritis and on granulation tissue formation. Clin Orthop Relat Res. 1971;80:25–32. doi: 10.1097/00003086-197110000-00006. [DOI] [PubMed] [Google Scholar]
- 9.He Y., Revel M., Loty B. A quantitative model of post-laminectomy scar formation:Effects of a nonsteroidal anti-inflammatory drug. Spine. 1995;20(5):557–563. doi: 10.1097/00007632-199503010-00010. [DOI] [PubMed] [Google Scholar]
- 10.Sae-Jung S., Jirarattanaphochai K., Sumananont C., Wittayapairoj K., Sukhonthamarn K. Interrater reliability of the postoperative epidural fibrosis classification: a histopathologic study in the rat model. Asian Spine J. 2015;9(4):587–594. doi: 10.4184/asj.2015.9.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J., Chung C., Kim H. Decompression of the spinal subarachnoid space as a solution for syringomyelia without Chiari malformation. Spinal Cord. 2002;40(10):501–506. doi: 10.1038/sj.sc.3101322. [DOI] [PubMed] [Google Scholar]
- 12.Kanamori M., Kawaguchi Y., Ohmori K., Kimura T., Tsuji H., Matsui H. The fate of autogenous free-fat grafts after posterior lumbar surgery. Spine. 2001;26:2264–2270. doi: 10.1097/00007632-200110150-00019. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y., Wang L.X., Wang L., Sun S.X., Cao X.J., Wang P. A comparison of the effectiveness of mitomycin C and 5-fluorouracil in the prevention of peridural adhesion after laminectomy. J Neurosurg Spine. 2007;7(4):423–428. doi: 10.3171/SPI-07/10/423. [DOI] [PubMed] [Google Scholar]
- 14.Fuming T., Changwu D., Songtao Q., Liqun Z., Bo C., Haicheng Y. Preventive effect of dexamethasone gelatin sponge on the lumbosacral epidural ahesion. Int J Clin Exp Med. 2015:5478–5484. [PMC free article] [PubMed] [Google Scholar]
- 15.Gurer B., Kahveci R., Gokce E.C., Ozevren H., Turkoglu E., Gokce A. Evaluation of topical application and systemic administration of rosuvastatin in preventing epidural fibrosis in rats. Spine J. 2015;15(3):522–529. doi: 10.1016/j.spinee.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Emmez H., Borcek A., Durdag E. Immunomodulatory effectiveness of azithromycin in prevention of postlaminectomy epidural fibrosis. Neurol Res. 2011;33(4):344–348. doi: 10.1179/016164110X12767786356471. [DOI] [PubMed] [Google Scholar]
- 17.Sae-Jung S., Jirarattanaphochai K. Prevention of peridural fibrosis using a cyclooxygenase-2 inhibitor (nonsteroidal anti-inflammatory drug) soaked in absorbable gelatin sponge: an experimental comparative animal model. Spine. 2013;38(16):985–991. doi: 10.1097/BRS.0b013e318297c795. [DOI] [PubMed] [Google Scholar]
- 18.Alkalay R., Kim D., Urry D. Prevention of Postlaminectomye Pidural fibrosisusing bioelastic materials. Spine. 2003;28(15):1659–1665. doi: 10.1097/01.BRS.0000083161.67605.40. [DOI] [PubMed] [Google Scholar]
- 19.Chengxin L., Zixue Y., Baoan C. Signal improvement strategies for fluorescence detection of biomacromolecules. J Fluoresc. 2016;26(3):1131–1139. doi: 10.1007/s10895-016-1806-3. [DOI] [PubMed] [Google Scholar]
- 20.Magalon G., Daumas A., Sautereau N., Maqalon J., Sabatier F., Granel B. Regenerative approach to scleroderma with fat grafting. Clin Plast Surg. 2015;42(3):353–364. doi: 10.1016/j.cps.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Gambardella G., Gervasio O., Zaccone C. Prevention of recurrent radicular pain after lumbar disc surgery: a prospective study. Acta Neurochir Suppl. 2005;92:151–154. doi: 10.1007/3-211-27458-8_33. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs R., McClain O., Neff J. Control of postlaminectomy scar formation. An experimental and clinical study. Spine. 1980;5(3):223–229. doi: 10.1097/00007632-198005000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Keskin F., Esen H. Comparison of the effects of an adhesion barrier and chitin on experimental epidural fibrosis. Turk Neurosurg. 2010;20(4):457–463. doi: 10.5137/1019-5149.JTN.3205-10.2. [DOI] [PubMed] [Google Scholar]
- 24.Wu C.Y., Huang Y.H., Lee J.S., Tai T.W., Wu P.T., Jou I.M. Efficacy of topical cross-linked hyaluronic acid hydrogel in preventing post laminectomy/laminotomy fibrosis in a rat model. J Orthop Res. 2016;34(2):299–306. doi: 10.1002/jor.23001. [DOI] [PubMed] [Google Scholar]
- 25.Hsu D.Z., Jou I.M. 1,4-Butanediol diglycidyl ether-cross-linked hyaluronan inhibits fibrosis in rat primary tenocytes by down-regulating autophagy modulation. J Mater Sci Mater Med. 2016;27(5):84. doi: 10.1007/s10856-016-5689-2. [DOI] [PubMed] [Google Scholar]
- 26.Liu S., Pan G., Liu G., Neves J.D., Song S., Chen S. Electrospun fibrous membranes featuring sustained release of ibuprofen reduce adhesion and improve neurological function following lumbar laminectomy. J Control Release. 2017;264:1–13. doi: 10.1016/j.jconrel.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Lin C.Y., Peng H.H., Chen M.H., Sun J.S., Liu T.Y., Chen M.H. In situ forming hydrogel composed of hyaluronate and polygalacturonic acid for prevention of peridural fibrosis. J Mater Sci Mater Med. 2015;26(4):168. doi: 10.1007/s10856-015-5478-3. [DOI] [PubMed] [Google Scholar]
- 28.Carvalho M., Costa L.M., Pereira J.E., Shirosaki Y., Hayakawa S., Santos J.D. The role of hybrid chitosan membranes on scarring process following lumbar surgery: post-laminectomy experimental model. Neurol Res. 2015;37(1):23–29. doi: 10.1179/1743132814Y.0000000414. [DOI] [PubMed] [Google Scholar]
- 29.Shin S.J., Lee J.H., So J., Min K. Anti-adhesive effect of poloxamer-based thermo-sensitive sol-gel in rabbit laminectomy model. J Mater Sci Mater Med. 2016;27(11):162. doi: 10.1007/s10856-016-5773-7. [DOI] [PubMed] [Google Scholar]
- 30.Rajiv S., Drilling A., Bassiouni A., Harding M., James C., Robinson S. Chitosan Dextran gel as an anti adhesion agent in a postlaminectomy spinal sheep model. J Clin Neurosci. 2017;40:153–156. doi: 10.1016/j.jocn.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Young R., Cota J., Zund G., Mason B., Wheeler J. The use of an amniotic membrane graft to prevent postoperative adhesions. Fertil Steril. 1991;55:624–628. doi: 10.1016/s0015-0282(16)54197-1. [DOI] [PubMed] [Google Scholar]
- 32.Hyu Jin C., Kyong Beon K., Young Min K. Effect of amniotic membrane to reduce postlaminectomy epidural adhesion on a rat model. J Korean Neurosurg. 2011:323–328. doi: 10.3340/jkns.2011.49.6.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao H., Fan H. Implantation of amniotic membrane to reduce postlaminectomy epidural adhesions. Eur Spine J. 2009;18(8):1202–1212. doi: 10.1007/s00586-009-1013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Liang M., Zheng Z., Shi L., Su B., Liu J. Adhesion prevention after laminectomy using silk-polyethylene glycol hydrogels. Adv Healthc Mater. 2015;4(14):2120–2127. doi: 10.1002/adhm.201500392. [DOI] [PubMed] [Google Scholar]
- 35.Li C., Wang H., Liu H., Yin J., Cui L., Chen Z. The prevention effect of poly (L-glutamic acid)/chitosan on spinal epidural fibrosis and peridural adhesion in the post-laminectomy rabbit model. Eur Spine J. 2014;23(11):2423–2431. doi: 10.1007/s00586-014-3438-0. [DOI] [PubMed] [Google Scholar]
- 36.Ivanic G.M., Pink P.T., Schneider F., Stuecker M., Homann N.C., Preidler K.W. Prevention of epidural scarring after microdiscectomy: a randomized clinical trial comparing gel and expanded polytetrafluoroethylene membrane. Eur Spine J. 2006;15(9):1360–1366. doi: 10.1007/s00586-006-0120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasimcan M.O., Bakar B., Aktas S., Alhan A., Yilmaz M. Effectiveness of the biophysical barriers on the peridural fibrosis of a postlaminectomy rat model: an experimental research. Injury. 2011;42(8):778–781. doi: 10.1016/j.injury.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Sung Bum K., Youngjin L. Delayed detected unexpected complication of ADCON-L gel in lumbar surgery. J Korean Neurosurg. 2010:268–271. doi: 10.3340/jkns.2010.48.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Llado A., Sologaistua E., Guimera J., Marin M. Expanded polytetrafluoroethylene membrane for the prevention of epidural fibrosis after spinal surgery:a clinical study. Eur Spine J. 1999:144–150. doi: 10.1007/s005860050145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lü C., Song Y., Liu H., Liu L., Gong Q., Li T. Novel artificial lamina for prevention of epidural adhesions after posterior cervical laminectomy. Chin J Reparative Reconstr Surg. 2013;27(7):829–835. [PubMed] [Google Scholar]
- 41.Mohammadian F., Abhari A., Dariushnejad H., Zarghami F., Nikanfar A., Pilehvar-Soltanahmadi Y. Upregulation of Mir-34a in AGS gastric cancer cells by a PLGA-PEG-PLGA chrysin nano formulation. Asian Pac J Cancer Prev APJCP. 2016;16(18):8259–8263. doi: 10.7314/apjcp.2015.16.18.8259. [DOI] [PubMed] [Google Scholar]
- 42.Chaoliang H., Hecheng M., Yilong C., Dongsong L., Yabao G., Jianguo L. PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for localized and combined treatment of human osteosarcoma. J Contr Release. 2015:e8–e152. doi: 10.1016/j.jconrel.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 43.Li X., Chen L., Lin H., Cao L., Cheng J., Dong J. Efficacy of poly(D,L-lactic acid-co-glycolic acid)-poly(Ethylene glycol)-poly(D,L-lactic acid-co-glycolic acid) thermogel as a barrier to prevent spinal epidural fibrosis in a postlaminectomy rat model. Clin Spine Surg. 2017;30(3):E283–E290. doi: 10.1097/BSD.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 44.Cekinmez M., Sen O., Atalay B., Erdogan B., Bavbek M., Caner H. Effects of methyl prednisolone acetate, fibrin glue and combination of methyl prednisolone acetate and fibrin glue in prevention of epidural fibrosis in a rat model. Neurol Res. 2010;32(7):700–705. doi: 10.1179/016164110X12556180206239. [DOI] [PubMed] [Google Scholar]
- 45.Kurt G., Aytar M.H., Dogulu F., Cemil B., Erdem O., Baykaner M.K. A comparison of the local effectiveness of mitomycin C, aprotinin, and Adcon-L in experimental peridural fibrosis. Surg Neurol. 2008;70(6):608–613. doi: 10.1016/j.surneu.2007.07.071. discussion 13. [DOI] [PubMed] [Google Scholar]
- 46.Sui T., Zhang J., Du S., Su C., Que J., Cao X. Potential risk of mitomycin C at high concentrations on peripheral nerve structure. Neural Regen Res. 2014;9(8):821–827. doi: 10.4103/1673-5374.131598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J., Ni B., Zhu L., Yang J., Cao X., Zhou W. Mitomycin C-polyethylene glycol controlled-release film inhibits collagen secretion and induces apoptosis of fibroblasts in the early wound of a postlaminectomy rat model. Spine J. 2010;10(5):441–447. doi: 10.1016/j.spinee.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 48.Wang B.B., Xie H., Wu T., Xie N., Wu J., Gu Y. Controlled-release mitomycin C-polylactic acid film prevents epidural scar hyperplasia after laminectomy by inducing fibroblast autophagy and regulating the expression of miRNAs. Eur Rev Med Pharmacol Sci. 2017;21(10):2526–2537. [PubMed] [Google Scholar]
- 49.Lin C.Y., Peng H.H., Chen M.H., Sun J.S., Chang C.J., Liu T.Y. Ibuprofen-conjugated hyaluronate/polygalacturonic acid hydrogel for the prevention of epidural fibrosis. J Biomater Appl. 2016;30(10):1589–1600. doi: 10.1177/0885328216635838. [DOI] [PubMed] [Google Scholar]
- 50.Mayer P., Jacobsen F. Cauda. Equina syndrome after surgical treatment of lumbar spinal stenosis with application of free autogenous fat graft-A report of two cases. J Bone Joint Surgery. 1989;71:1090–1093. [PubMed] [Google Scholar]
- 51.Imran Y., Halim Y. Acute cauda equina syndrome secondary to free fat graft following spinal decompression. Singapore Med J. 2005;46(1):25–27. [PubMed] [Google Scholar]
- 52.Gurgle A., Simsek O., Cobanoglu S., Imer M., Parsak T. The effect of epidural free fat graft on the outcome of lumbar disc surgery. Neurosurg Rev. 2004;27(3):181–184. doi: 10.1007/s10143-003-0310-9. [DOI] [PubMed] [Google Scholar]
- 53.Xu J., Chen Y., Yue Y., Sun J., Cui L. Reconstruction of epidural fat with engineered adipose tissue from adipose derived stem cells and PLGA in the rabbit dorsal laminectomy model. Biomaterials. 2012;33(29):6965–6973. doi: 10.1016/j.biomaterials.2012.06.010. [DOI] [PubMed] [Google Scholar]