Summary
Objective
This study aims to discover that the urinary C-terminal telopeptide of type II collagen (uCTX-II) levels differ between osteoarthritis (OA) patients and healthy individuals (controls). According to this difference, we may conclude that uCTX-II can be a biomarker for OA diagnosis.
Methods
We searched MEDLINE and EMBASE databases updated to 2014 to find literature on OA biomarkers. We retrieved the publications that met the required criterion. Literature quality was assessed according to the Newcastle–Ottawa Scale. Publication bias was assessed by Begg's test and Egger's test with the software STATA version 12.0. The weighted mean difference (WMD) was calculated, and the subgroup analysis was completed using STATA 12.0.
Results
Six publications were included in our analysis. The WMD for OA patients versus the controls was 83.05, which was within the 95% confidence interval. For subgroup analysis, the WMD of patients with severe OA was 119.92, whereas that of patients with mild OA was 28.07.
Conclusions
uCTX-II levels were higher in OA patients than in controls, subgroup analysis revealed that the uCTX-II levels rised with the OA severity, the heterogeneity originated from different levels of OA severity, These results showed that uCTX-II would be a promising clinical biomarker in OA diagnosis.
Keywords: biomarkers, osteoarthritis, urinary C-terminal telopeptide of type II collagen
Introduction
Osteoarthritis (OA) is a progressive disease with a long silent period showing signs of cartilage degradation, mild-to-moderate synovial inflammation, and altered bone structure, thereby resulting in severe destruction and impaired function of the affected joints [1]. To date, numerous people are suffering from OA which causes inconvenience in their daily life and work. If OA can be detected during the early stages, OA patients can be given early treatment. Therefore, the search for an early diagnostic biomarker of OA is an urgent task for OA diagnosis and treatment.
OA is commonly diagnosed by clinical symptoms and radiographic criteria. Clinical symptoms are identified by physical examination. Pain during movement and a limited range of motion are common to all forms of OA; each joint has unique findings during physical examination [2]. Plain radiography can also be helpful in confirming OA and ruling out other diseases, apart from the advanced imaging techniques, such as computed tomography or magnetic resonance imaging (MRI).
Physical examination lacks accuracy because it is highly dependent on the physician's technique and experience. Radiography depends much on technical support, including instrument's precision and sensitivity. The limitations of MRI include high cost, low availability, and absence of a validated international score [3]. Furthermore, physical examination and radiography are only effective when OA has reached an irreversible state, thereby delaying the treatment.
To detect OA during the early stages, accurate and reliable biomarkers are necessary. Several biomarkers have been previously studied, such as CTX-II, PIINP, COMP, IL-1β, IL-6, NTX-I, hyaluronic acid, and MMP [4]. The Osteoarthritis Biomarker Network, a consortium of five National Institute of Health-designated sites, has classified five categories of biomarkers: Burden of Disease, Investigative, Prognostic, Efficacy of Intervention, and Diagnostic (BIPED) [5]. Urinary C-terminal telopeptide of type II collagen (uCTX-II) has proven to be a promising biomarker in several researches [6], [7], [8]. It is produced and then excreted to the urine when type II collagen is degraded by cartilage-degrading enzymes as soon as OA occurs [9]. In human clinical trials, uCTX-II had good performance and met the primary clinical endpoints in three pharmacological trials conducted by the group of Christgau in 2004, Gineyts in 2004, and Manjcourt in 2006 [10]. Our study aims to collect as much literature as possible for a comprehensive analysis so that uCTX-II could be employed as a promising biomarker in OA diagnosis.
A biomarker used to predict disease should differ between the disease and non-disease control groups. Furthermore, the difference should be more significant when the disease becomes severe. In this study, we collected the means and standard deviations (SD) of uCTX-II levels for a meta-analysis. We compared the weighted mean difference (WMD) of uCTX-II in the OA and control groups to show that uCTX-II is much higher in the OA group. We performed subgroup analysis to further show that uCTX-II levels are higher in the severe OA group than in the mild OA group.
Methods
Search strategy
We searched the MEDLINE and EMBASE databases updated to 2014 to find literature that used uCTX-II as an OA biomarker, regardless of language, data, and design. The search keywords used were “osteoarthritis”, “uCTX-II”, and “biomarkers”. We extracted 40 studies that conformed to the above query. We further screened out studies by reviewing the full text according to the criteria for study selection and obtained the six included studies.
Criteria for study selection
-
A.
Studies that included only humans; studies involving animals were ruled out.
-
B.
Studies involving OA patients as well as healthy controls; studies involving only OA patients were ruled out.
-
C.
Studies that measured the uCTX-II value for comparison to differentiate between groups, c.
-
D.
Studies that diagnosed OA using X-ray and authorized criteria (for e.g., the American College of Rheumatology criteria). Studies that preferably used the Kellgren–Lawrence (KL) scale to distinguish the OA severity. Studies in which OA was not diagnosed by the two methods were ruled out.
-
E.
Studies that provided data including the means and SD of uCTX-II levels from each group; studies wherein the two kinds of data were not provided together were ruled out.
Data extraction
In our research, uCTX-II is the variable of interest; thus, we extracted the measured values of uCTX-II levels in both groups. Among these studies, the measured levels of uCTX-II are corrected by the urine creatinine concentration (uCTX-II: ng/mmol Cr). For the meta-analysis, we extracted the mean values and SDs for uCTX-II levels. For literature quality assessment, we gathered detailed information on patients and the healthy controls; the features for judging the literature quality included the age (mean ± SD), body mass index, and disease identification criteria.
Assessment of study quality
The study quality was assessed independently by two authors using the Newcastle–Ottawa Scale (NOS), which was applicable to case-control studies. The NOS scale consists of three aspects: patient selection standard of cases and controls (0–4 points); comparability between cases and controls based on the design or analysis (0–2 points); and ascertainment of exposure or non-response rate of exposure (0–3 points). The highest total number of points is 9 points. Each study was judged by these standards to obtain a score for quality assessment.
Meta-analysis
The publication bias was assessed by Egger's test and Begg's test. We compared the means and SDs of uCTX-II between OA group and non-OA control group to draw a forest plot by WMD. Subgroup analysis was completed according to sex and disease severity.
Results
Literature search and selection
We gathered 144 publications by searching the MEDLINE and EMBASE databases with the keyword “osteoarthritis biomarkers”. We screened 40 of these studies, which used uCTX-II as an OA biomarker, and finally selected 6 studies [8], [9], [11], [12], [13], [14] that met our selection criteria (Scheme 1). The study by Sowers et al [8] in 2009 contained two different experiments that were performed in 1997 and 2009. Merging these data might decrease the statistical significance; thus, experimental data was divided into two groups.
Scheme 1.
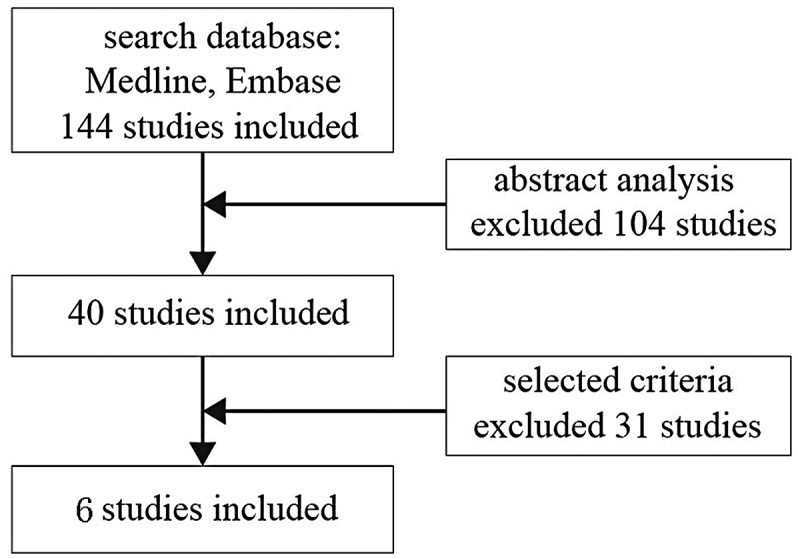
Flow diagram of literature search.
Literature quality assessment
According to the NOS, the quality assessment (Table 1) showed that five studies were of high quality (> 6 points) and only 1 study was of low quality (< 4 points).
Table 1.
Characteristics of the studies involved in our meta-analysis.
| Study | Experiment year | No. of OA patients | No. of controls | Measurement of uCTX-II | Quality assessment of literature (NOS) | Criteria for OA confirmation |
|---|---|---|---|---|---|---|
| E.B.Dam 2009 | 2009 | Total = 36 Female = 16 Male = 20 Knee OA = 36 |
Total = 122 Female = 60 Male = 62 |
ELISA (Nordic Bioscience, Denmark) | 6 grades | X-ray assessment |
| Eya Kalai 2012 | 2012 | Total = 125 Female = 125 Knee OA = 125 |
Total = 57 Female = 57 |
ELISA (Nordic Bioscience, Denmark) | 6 grades | American College of Rheumatology criteria for primary knee OA |
| MaryFran Sowers 2009 | 1997 | Total = 18 (KL = 2) = 14 (KL = 3,4) = 4 Female = 18 Knee OA = 18 |
Total = 54 | ELISA (Nordic Bioscience, Denmark) | 7 grades | X-ray image |
| MaryFran Sowers 2009 | 2009 | Total = 36 (KL = 2) = 16 (KL = 3,4) = 20 Female = 36 Knee OA = 36 |
Total = 36 | ELISA (Nordic Bioscience, Denmark) | 7 grades | c image |
| P Garnero 2001 | 2001 | Total = 67 Knee OA = 67 |
Total = 67 | ELISA (manufacturer not mentioned) | 6 grades | X-ray evidence (Joint Space Narrowing) |
| P Garnero 2006 | 2006 | Total = 40 Hip OA = 40 |
Total = 75 | ELISA (Nordic Bioscience, Denmark) | 3 grades | X-ray image |
| Nobuchika Tanishi 2009 | 2009 | Total = 190 Male = 78 Female = 112 (KL = 2) = 126 (KL = 3,4) = 64 Knee OA = 190 |
Total = 106 Male = 47 Female = 59 |
ELISA (Nordic Bioscience, Denmark) | 8 grades | X-ray image |
ELISA = enzyme-linked immunosorbent assay; KL = Kellgren–Lawrence; NOS = Newcastle–Ottawa Scale; OA = osteoarthritis; uCTX-II = urinary C-terminal telopeptide of type II collagen.
Meta-analysis
-
1.
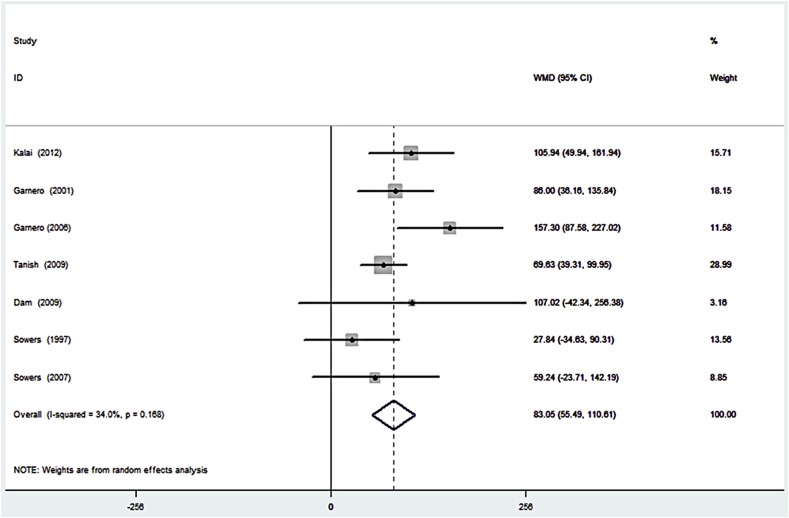
Forest plot shows that uCTX-II value in OA patients was higher than non-OA patients.
We used the collected data (means and standard error) of uCTX-II from the abovementioned studies to construct a forest plot. Results showed that uCTX-II levels were much higher in OA patients than in controls (Figure 1). As shown in the figure, these studies were homogenous (I2 = 34.0%, p = 0.168).
-
2.
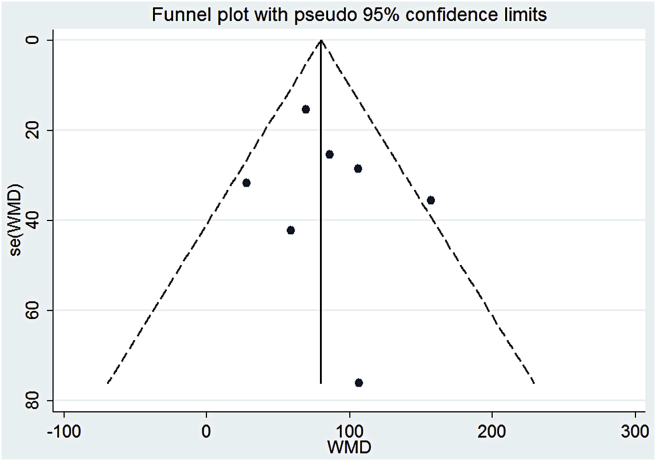
Funnel plot shows that no significant publication bias exists.
Figure 1.
Forest plot of the weighted mean difference of urinary C-terminal telopeptide of type II collagen in patients with radiographically diagnosed osteoarthritis compared with controls. CI = confidence interval; WMD = weighted mean difference.
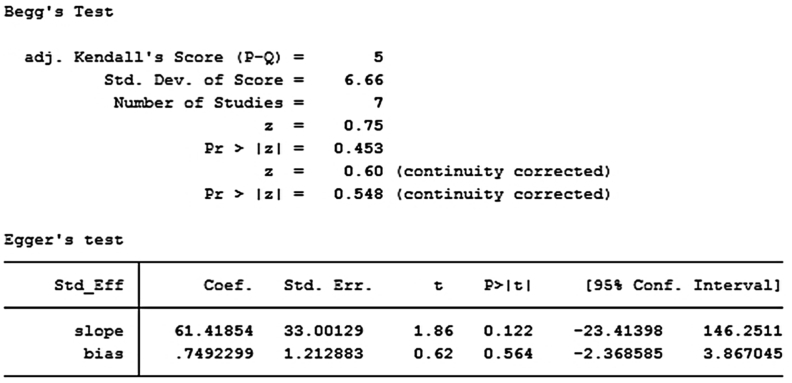
We drew a funnel plot to visually detect the publication bias (Figure 2). Each spot represented a study, and the spots were symmetrically distributed on both sides. Therefore, the publication bias was not significant. We also applied numerical calculation to show the publication bias more accurately. The Begg's test and Egger's test were both applied to detect the publication bias. For Begg's test, p = 0.548, whereas for Egger's test, p = 0.564 (Scheme 2). Both p values were > 0.05, indicating that the publication bias was not significant.
-
3.
Subgroup analysis shows that heterogeneity exists because of OA severity; uCTX-II increases with the OA severity.
Figure 2.
Funnel plot to assess the publication bias. WMD = weighted mean difference.
Scheme 2.
Begg's test and Egger's test to confirm the publication bias.
To explain why minimal heterogeneity existed, we performed subgroup analysis. According to the severity of OA and sex of patients, we conducted two subgroup analyses.
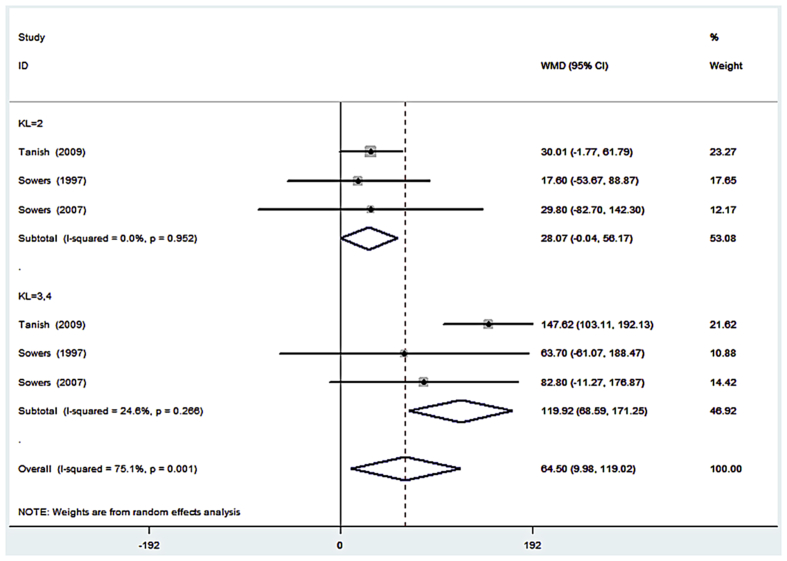
The first subgroup analysis was based on the OA severity, which was categorized by the KL grade. We classified OA with KL = 3, 4 as severe OA; OA with KL = 2 as mild OA; and OA with KL = 0, 1 as healthy controls.
We drew a forest plot according to our subgroup data (Figure 3). When OA was mild, the uCTX-II levels in patients were slightly higher than those in the controls (WMD = 28.07). When OA was severe, the uCTX-II levels in patients were much higher than those in the controls (WMD = 119.92). This result indicated that uCTX-II increased with the OA severity.
Figure 3.
Forest plot of the weighted mean difference of urinary C-terminal telopeptide of type II collagen in patients with different severity of osteoarthritis. CI = confidence interval; KL = Kellgren–Lawrence; WMD = weighted mean difference.
Furthermore, the overall heterogeneity was (I2 = 75.1%, p = 0.001) significant in the first subgroup; however, the subgroup heterogeneity was not significant for mild OA subgroup (I2 = 0.0%, p = 0.952) and severe subgroup (I2 = 24.6%, p = 0.266). Therefore, the uCTX-II levels in the same OA severity subgroup showed no significant heterogeneity, whereas the overall heterogeneity was caused by the different OA severity.
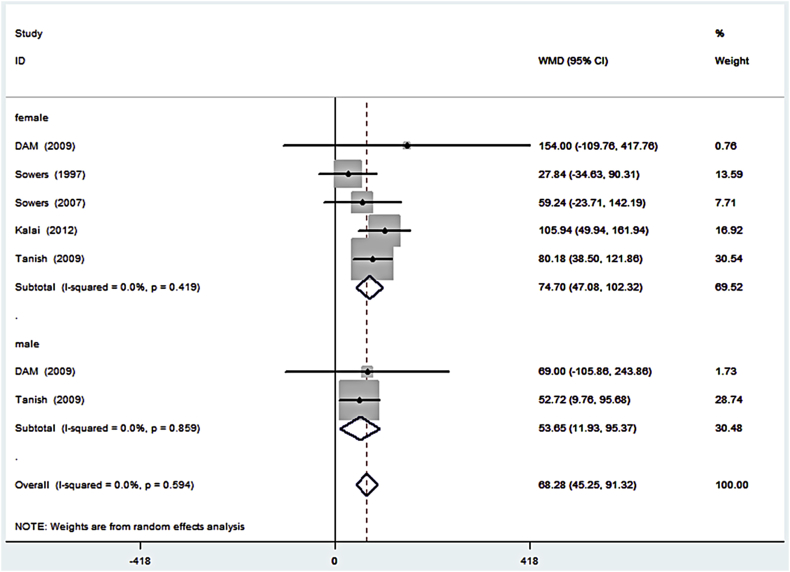
The second subgroup analysis was based on the sex of the OA patient. Previous studies reported that OA patients of different sex have different uCTX-II [9]. Therefore, to assess whether uCTX-II should be treated differently as an OA biomarker in male and female patients, we also drew a forest plot to analyse this subgroup (Figure 4). The result showed that there was no significant difference in the uCTX-II levels between sexes. The lack of significant differences in both overall and subgroup heterogeneity also indicated that the overall heterogeneity was caused by the OA severity rather than the different sex.
Figure 4.
Forest plot of the weighted mean difference of urinary C-terminal telopeptide of type II collagen in osteoarthritis patients of different sexes. CI = confidence interval; WMD = weighted mean difference.
Discussion
OA is a common heterogeneous syndrome with different clinical phenotypes that continuously evolve and eventually lead to common clinical manifestations [15]. Generally, X-ray is used to confirm the diagnosis of OA because it can reveal clinical changes at the joint margin, such as bony outgrowths and joint space narrowing. However, these radiographic evidences are only found after a substantial cartilage loss has already occurred. Therefore, radiographic evidence cannot prevent OA disease exacerbation. Thus, biomarkers might be a potential alternative for the earlier diagnosis of asymptomatic OA to prevent exacerbation [10].
The best candidates for biomarkers in OA are most likely structural molecules or fragments linked to cartilage, bone, or synovial fluid; these markers may be specific to one type of joint tissue or common to all [3]. Levels of uCTX-II, urinary Glc-Gal-Pyd, and the serum N-propeptide II of type II collagen have been found to increase in OA patients. These compounds are noted as potentially useful biomarkers for the presence of OA because they are also correlated with the joint surface area [13]. A systematic review applied the BIPED classification and indicated that uCTX-II and serum cartilage oligomeric matrix protein had the best performance among all the commercially available biomarkers [4]. uCTX-II originates from type II collagen, degraded by cartilage-degrading enzyme and then secreted in the urine, which has been found to associate with cartilage degeneration and OA [9]. Several studies have tested that uCTX-II levels differed between the OA and non-OA groups [6], [9], [11], [12], [13], [14], [16], [17], [18], [19].
Several conditions can affect the levels of biomarkers. Some evidence suggests that normal physical activity, hormone levels in the body, medication, and different sex all can lead to fluctuations in biomarkers levels. For example, uCTX-II levels were reported to increase after 3 months in a 1-year study [20]. The uCTX-II levels differed significantly between premenopausal and postmenopausal women [9], [21] and also differed between sexes [9]. The uCTX-II levels were found to decrease after the use of bisphosphonates [9]. Furthermore, evidence suggested differences of some biomarkers in different joints or tissues [22], [23], [24]. Because the levels of biomarkers have fluctuating properties, the measured data of one independent test has high data error. And in order to decrease the data error, in our study, we analyzed and summarized the measured data of several independent tests. Meanwhile, differences exist in biomarkers levels in different groups; therefore, we conducted subgroup analysis to discover the significance of this difference.
According to the patients' conditions from the literature, we performed two subgroup analyses in our research. For sex subgroup analysis, the WMD of female patients was slightly higher than that of male patients, indicating that uCTX-II levels were not significantly different between female and male patients. However, in the OA severity subgroup analysis, we found an increasing trend in the elevated uCTX-II levels as the OA severity increased.
Nevertheless, our meta-analysis has some limitations. First, the literature available related to our research is limited. A large amount of studies focused on OA biomarkers and uCTX-II, but most of them could not provide detailed measurement data that we needed. Based on our selection criteria, we had to exclude most of them. Second, the seven comparisons are not from the same cohort, including the Asian, European, and Caucasian populations. This difference may cause variance resulting from ethnic differences. Finally, the amounts are not well-proportioned in sex subgroup analysis. The sex subgroup analysis had two male experiments and five female experiments. Therefore, sampling may have biased the overall effect in the OA sex subgroup. The results in our study need to be verified in subsequent experiments.
Conclusion
We obtained six publications by screening the literature according to abstract analysis and selection criteria. Our study showed that the uCTX-II levels were higher in OA patients than in the healthy controls. Subgroup analysis revealed that uCTX-II level increased with OA severity. We thought that uCTX-II could be a promising biomarker in the diagnosis of the future.
Authors' contributions
MJH and JYZ searched literature related to this project, selected our desired studies, extracted useful data, processed these data, and drafted the manuscript. YH and LMD provided software 12.0 analysis support. XLZ conceived this project, and helped with manuscript writing.
Conflict of interest
None of the authors has any financial or personal relationships with other people or organisations that could potentially and inappropriately influence (bias) their work and conclusions.
Acknowledgement of funding sources
This work was supported by grants from The Ministry of Science and Technology of China (No. 2015DFG32200), National Natural Science Foundation of China (No. 81572123) and Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (No. 20161314).
Contributor Information
Mingjian Huang, Email: mingjian77@gmail.com.
Jingyu Zhao, Email: zhaojingyu@sjtu.edu.cn.
Yan Huang, Email: huangyan1113@gmail.com.
Liming Dai, Email: lmdai@sibs.ac.cn.
Xiaoling Zhang, Email: xlzhang@shsmu.edu.cn.
References
- 1.Goldring M.B., Goldring S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 2.Sinusas K. Osteoarthritis: diagnosis and treatment. Am Fam Physician. 2012;85:49–56. [PubMed] [Google Scholar]
- 3.Lotz M., Martel-Pelletier J., Christiansen C., Brandi M.L., Bruyere O., Chapurlat R. Value of biomarkers in osteoarthritis: current status and perspectives. Ann Rheum Dis. 2013;72:1756–1763. doi: 10.1136/annrheumdis-2013-203726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Spil W.E., DeGroot J., Lems W.F., Oostveen J.C., Lafeber F.P. Serum and urinary biochemical markers for knee and hip-osteoarthritis: a systematic review applying the consensus BIPED criteria. Osteoarthritis Cartilage. 2010;18:605–612. doi: 10.1016/j.joca.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Bauer D.C., Hunter D.J., Abramson S.B., Attur M., Corr M., Felson D. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. 2006;14:723–727. doi: 10.1016/j.joca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Dam E.B., Loog M., Christiansen C., Byrjalsen I., Folkesson J., Nielsen M. Identification of progressors in osteoarthritis by combining biochemical and MRI-based markers. Arthritis Res Ther. 2009;11:R115. doi: 10.1186/ar2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reijman M., Hazes J.M., Bierma-Zeinstra S.M., Koes B.W., Christgau S., Christiansen C. A new marker for osteoarthritis: cross-sectional and longitudinal approach. Arthritis Rheum. 2004;50:2471–2478. doi: 10.1002/art.20332. [DOI] [PubMed] [Google Scholar]
- 8.Sowers M.F., Karvonen-Gutierrez C.A., Yosef M., Jannausch M., Jiang Y., Garnero P. Longitudinal changes of serum COMP and urinary CTX-II predict X-ray defined knee osteoarthritis severity and stiffness in women. Osteoarthritis Cartilage. 2009;17:1609–1614. doi: 10.1016/j.joca.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanishi N., Yamagiwa H., Hayami T., Mera H., Koga Y., Omori G. Relationship between radiological knee osteoarthritis and biochemical markers of cartilage and bone degradation (urine CTX-II and NTX-I): the Matsudai Knee Osteoarthritis Survey. J Bone Miner Metab. 2009;27:605–612. doi: 10.1007/s00774-009-0077-3. [DOI] [PubMed] [Google Scholar]
- 10.Poonpet T., Honsawek S. Adipokines: biomarkers for osteoarthritis? World J Orthop. 2014;5:319–327. doi: 10.5312/wjo.v5.i3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dam E.B., Byrjalsen I., Karsdal M.A., Qvist P., Christiansen C. Increased urinary excretion of C-telopeptides of type II collagen (CTX-II) predicts cartilage loss over 21 months by MRI. Osteoarthritis Cartilage. 2009;17:384–389. doi: 10.1016/j.joca.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Garnero P., Charni N., Juillet F., Conrozier T., Vignon E. Increased urinary type II collagen helical and C telopeptide levels are independently associated with a rapidly destructive hip osteoarthritis. Ann Rheum Dis. 2006;65:1639–1644. doi: 10.1136/ard.2006.052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garnero P., Piperno M., Gineyts E., Christgau S., Delmas P.D., Vignon E. Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: relations with disease activity and joint damage. Ann Rheum Dis. 2001;60:619–626. doi: 10.1136/ard.60.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalai E., Bahlous A., Charni N., Bouzid K., Sahli H., Laadhar L. Association of serum levels of aggrecan ARGS, NITEGE fragments and radiologic knee osteoarthritis in Tunisian patients. Joint Bone Spine. 2012;79:610–615. doi: 10.1016/j.jbspin.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Castaneda S., Roman-Blas J.A., Largo R., Herrero-Beaumont G. Osteoarthritis: a progressive disease with changing phenotypes. Rheumatology. 2014;53:1–3. doi: 10.1093/rheumatology/ket247. [DOI] [PubMed] [Google Scholar]
- 16.Garnero P., Ayral X., Rousseau J.C., Christgau S., Sandell L.J., Dougados M. Uncoupling of type II collagen synthesis and degradation predicts progression of joint damage in patients with knee osteoarthritis. Arthritis Rheum. 2002;46:2613–2624. doi: 10.1002/art.10576. [DOI] [PubMed] [Google Scholar]
- 17.Garnero P., Conrozier T., Christgau S., Mathieu P., Delmas P.D., Vignon E. Urinary type II collagen C-telopeptide levels are increased in patients with rapidly destructive hip osteoarthritis. Ann Rheum Dis. 2003;62:939–943. doi: 10.1136/ard.62.10.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung M., Christgau S., Lukoschek M., Henriksen D., Richter W. Increased urinary concentration of collagen type II C-telopeptide fragments in patients with osteoarthritis. Pathobiology. 2004;71:70–76. doi: 10.1159/000074419. [DOI] [PubMed] [Google Scholar]
- 19.Rousseau J.C., Chevrel G., Schott A.M., Garnero P. Increased cartilage type II collagen degradation in patients with osteogenesis imperfecta used as a human model of bone type I collagen alterations. Bone. 2010;46:897–900. doi: 10.1016/j.bone.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Bruyere O., Collette J., Kothari M., Zaim S., White D., Genant H. Osteoarthritis, magnetic resonance imaging, and biochemical markers: a one year prospective study. Ann Rheum Dis. 2006;65:1050–1054. doi: 10.1136/ard.2005.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bay-Jensen A.C., Tabassi N.C., Sondergaard L.V., Andersen T.L., Dagnaes-Hansen F., Garnero P. The response to oestrogen deprivation of the cartilage collagen degradation marker, CTX-II, is unique compared with other markers of collagen turnover. Arthritis Res Ther. 2009;11:R9. doi: 10.1186/ar2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meulenbelt I., Kloppenburg M., Kroon H.M., Houwing-Duistermaat J.J., Garnero P., Hellio-Le Graverand M.P. Clusters of biochemical markers are associated with radiographic subtypes of osteoarthritis (OA) in subject with familial OA at multiple sites. The GARP study. Osteoarthritis Cartilage. 2007;15:379–385. doi: 10.1016/j.joca.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Catterall J.B., Hsueh M.F., Stabler T.V., McCudden C.R., Bolognesi M., Zura R. Protein modification by deamidation indicates variations in joint extracellular matrix turnover. J Biol Chem. 2012;287:4640–4651. doi: 10.1074/jbc.M111.249649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onnerfjord P., Khabut A., Reinholt F.P., Svensson O., Heinegard D. Quantitative proteomic analysis of eight cartilaginous tissues reveals characteristic differences as well as similarities between subgroups. J Biol Chem. 2012;287:18913–18924. doi: 10.1074/jbc.M111.298968. [DOI] [PMC free article] [PubMed] [Google Scholar]