Abstract
Skeletal muscle regeneration is a complex process orchestrated by multiple steps. Recent findings indicate that inflammatory responses could play central roles in bridging initial muscle injury responses and timely muscle injury reparation. The various types of immune cells and cytokines have crucial roles in muscle regeneration process. In this review, we briefly summarise the functions of acute inflammation in muscle regeneration.
The translational potential of this article
Immune system is closely relevant to the muscle regeneration. Understanding the mechanisms of inflammation in muscle regeneration is therefore critical for the development of effective regenerative, and therapeutic strategies in muscular disorders. This review provides information for muscle regeneration research regarding the effects of inflammation on muscle regeneration.
Keywords: Chronic muscle disorders, Cytokines, Immune cells, Inflammation, Muscle regeneration, Muscle stem cells
Muscle injury and muscle stem cells in muscle regeneration
Skeletal muscle is the most abundant tissue in human body, which accounts for about 40% of the body mass. Under normal conditions, the turnover rate of adult skeletal muscle is about 1–2% of myonuclei per week [1]. Muscle is susceptible for various injuries in daily life, such as the mechanical trauma, thermal stress, myotoxic agents, ischaemia, neurological damage and other pathogenic conditions. The most common cause of muscle injury is mechanical trauma [2]. It destroys the integrity of the myofibre plasma membrane and basal lamina, leading to the influx of extracellular calcium [3] which eventually leads to the degradation of muscle proteins and necrosis [4]. Then, the muscle degeneration was further promoted by the swelling and haematoma formation [5], as the consequence of the activation of acute inflammation. After the initial muscle degeneration, muscle regeneration mediated by muscle stem cells is switched on. The injured myofibres can be regenerated, and the muscle functions such as contraction force, metabolism can be restored.
Muscle stem cells (satellite cells) are the major contributor to muscle regeneration. They were discovered by Alexander Mauro in 1961 [6]. These cells are located in a membrane-enclosed niche between the sarcolemma (plasma membrane) and the basal lamina surrounding the myofibres. Muscle stem cells remain quiescent under normal conditions [7], [8]. In response to exercise, muscle growth, trauma or other stimuli, muscle stem cells are activated to enter the cell cycle, proliferated briefly and further differentiated to new myotubes or fuse to the damaged myofibres to repair muscle injury. After activation and proliferation, part of the muscle stem cells can return to quiescence and replenish the in vivo stem cell pool to prepare for the next regeneration process [9], [10].
The mechanism to activate muscle stem cells and promote muscle stem cell proliferation and differentiation in a timely manner remains to be explored. Understanding the mechanism will greatly facilitate the development of regenerative treatment for muscle injury and muscle degenerative diseases.
Acute inflammation bridges the conversion from muscle necrosis stage to regeneration stage
The process of muscle regeneration can be divided to several stages: necrosis of the injured muscle cell, activation of muscle stem cells, proliferation of the activated muscle stem cells, differentiation of the muscle stem cells, maturation of the newly formed muscle fibres and the remodelling of muscle fibres. Acute inflammation and immune cells play critical roles in almost all stages of muscle regeneration.
At the early stage of muscle regeneration, the injured muscle cells undergo necrosis in response to trauma. Upon muscle injury, the membranes of muscle fibres are damaged and the cellular contents and chemotactic factors are released to the extracellular space, which in turn induces the infiltration of many types of immune cells [11]. The infiltrated immune cells, such as mast cells and neutrophils, can help clearing the damaged myofibres at the injury site. Meanwhile, they can also secrete various types of cytokines to recruit more immune cells like macrophages. These immune cells can trigger on a cascade of cellular responses to regulate muscle stem cell activation, proliferation and differentiation. They serve as important mediators to orchestrate muscle regeneration.
The first wave of immune cells: complement system, mast cells and neutrophils
The major events of early stage of muscle regeneration after injury include muscle fibre necrosis, lesion enlargement and debris clearance. The activation and infiltration of the first wave of immune cells occur at the early stage of muscle regeneration. The early event of muscle repair is characterised by the necrosis of the damaged fibres after trauma. The immune system was activated by the cell debris and the cell content leakage from the damaged fibres at the muscle lesion site.
The complement system serves as the first sensor of the muscle injury. The complement system, which represents the first defence line of innate immunity, is activated immediately within seconds after injury [12]. It is made up of a collection of nine major complement proteins found in the bloodstream allowing a rapid immune response against an antigen [13], [14]. The activation of complement system in the injured muscle leads to infiltration of neutrophils and macrophages to the lesion site [15]. The complement C3 and C4 are two of complement proteins. Their cleavage products C3a and C4a are upregulated in the serum of population with prolonged exercises, revealing the involvement of the complement-mediated inflammation in the early stage of muscle injury [16].
Mast cells are large, ovoid cells of haematopoietic lineage that circulate in the blood and mature after entering peripheral tissues, with a centrally located nucleus and numerous large, intensely basophilic granules [17]. Mast cell degranulation is one of the earliest innate immune system responses involved in muscle damage and repair that leads to the consequent inflammatory events. Mast cell degranulation is often observed in areas surrounding injured myofibres. Upon muscle injury, the resident mast cells in skeletal muscle are rapidly activated. After activation, master cells degranulate and release proinflammatory cytokines, such as tumour necrosis factor-α (TNF-α), interleukin (IL)-1 and histamine to recruit more mast cells, neutrophils and other immune cells to the injury site [18], [19]. As the result, more mast cells and neutrophils infiltrated to the lesion to further promote inflammation [20].
Neutrophils are one of the most important immune cell types in the first wave of the proinflammatory phase following muscle injury. Like mast cells, the resident neutrophils in skeletal muscles can be activated immediately after the muscle injury and release the proinflammatory cytokines including TNF-α (tumor necrosis factor alpha), IFN-γ (Interferon-γ), and IL-1β (interleukin-1β) [21], [22]. The peripheral neutrophils can be further recruited by proinflammatory cytokines secreted by resident neutrophils and mast cells. This mechanism allows rapid infiltration of the large amount of neutrophils to the extracellular space around the damaged fibres within two hours. The number of the infiltrated neutrophils peaks in 6–24 hours after injury and declines rapidly 72–96 hours after injury [23].
Neutrophils can release a variety of factors such as cytokines, enzymes and oxidative factors to facilitate the clearance of the necrotic muscles [24], [25], [26]. The removal of the fibre debris facilitates the progress of muscle regeneration. The infiltrated neutrophils at the injury site produce IL-1 and IL-8 to induce the macrophage infiltration to the lesion [27]. The infiltration of macrophages can further improve the muscle injury repair as described in the following.
Neutrophils are the major source of reactive oxygen species after injury as well [28]. The neutrophil-derived reactive oxygen species has been shown to contribute to the muscle fibre degradation and vascular alterations induced by ischaemia–reperfusion injury [29]. Under this scenario, neutrophils temporarily worsen the muscle damage and delay the move to the next stage of the muscle regeneration that is contradictory to their functions of neutrophils to help muscle regeneration progress. Therefore, the infiltrated neutrophils play dual roles in the muscle injury repair process. How these two seemingly contradictory functions of neutrophils are unified to contribute to muscle injury repair remains to be explored.
The second wave of immune cells: macrophages and T cells
The second stage of muscle regeneration is marked by muscle stem cell activation and expansion. This stage is a companied by the activation of adaptive immunity and the infiltration of the second wave of immune cells.
Macrophages are originated in the bone marrow as monocytes [30]. They are derived from blood monocytes and recruited to the muscle injury sites by neutrophils shortly after injury [31], [32], [33], [34]. Macrophages started to be observed at the lesion 24 hours after injury. The number of macrophages increases significantly 2 days after injury along with the rapid decline of the number of neutrophils [30], [35]. Macrophages play central roles in the regulation of the skeletal muscle regeneration [30]. During the muscle regeneration process, these cells undergo two different stages of activation and are categorised to two major types: the classically activated macrophages M1 and the alternatively activated macrophages M2 [36]. The M1 macrophages are proinflammatory, whereas the M2 macrophages are antiinflammatory [37]. At the early stage of muscle regeneration, M1 macrophages are the most dominant macrophage type. In the blood, circulating monocytes can be classed into at least two populations that are distinguishable by their expression levels of Ly-6C (also known as GR1) and of chemokine receptors CCR2 and CX3CR1 [38], [39]. The interaction between CCR2 (C-C chemokine receptor type 2) and its ligand CCL2 (C-C Motif Chemokine Ligand 2) promotes the recruitment and differentiation of the Ly6C+ monocytes to M1 macrophages [40]. Then Ly6C monocytes enter damaged muscle in a CX3CR1 dependent manner after the onset of inflammation [41]. M1 macrophages initially function to remove the muscle debris generated by the trauma. M1 macrophages infiltrated to the lesion also secrete large amount of cytokines such as TNF-α, IL-6 and IL-1β.
TNF-α has been reported to play an important role in muscle regeneration. TNF-α–deficient mice and TNF-α receptor knockout mice displayed severe muscle regeneration defects [42], [43]. TNF-α can attract muscle stem cells to the damaged site of the muscle and promote muscle stem cells proliferation by activating transcription factor nuclear factor-kappa B signalling [44]. Moreover, TNF-α activates p38 signalling pathway and stimulates the differentiation of muscle cells. Blocking TNF-α action using anti-TNF-α or inhibiting p38 kinase activity downregulates the expression of muscle differentiation markers such as MyoD, myogenin or myosin [45], [46].
IL-6 is produced by multiple cell types including macrophages, T cells and myofibres [47], [48], [49], [50]. It has long been suggested to regulate muscle regeneration and muscle homoeostatic maintenance [51]. IL-6 can stimulate the migration, proliferation and differentiation of myoblast [52]. In the IL-6−/− skeletal muscle cells, the myotube formation was impaired. Ablation of IL-6 expression led to a decrease in differentiation while overexpression of IL-6 increased differentiation of the muscle stem cells. This is consistent with the phenomenon in IL-6–deficient animal model, which the muscle stem cells activation and compensatory hypertrophy were impaired, revealing that IL-6 play a significant role in inducing proliferation and differentiation of muscle stem cells and the formation of myotubes.
IL-1β is mainly produced by macrophages [53]. T cells are the other source of IL-1β [54]. IL-1β can further recruit macrophages and T cells to the injury site [23]. IL-1β stimulates the production of IL-6 in skeletal muscle cells [55], suggesting that IL-1β can also target skeletal muscle cells.
M1 macrophages also highly express inducible nitric oxide synthase (iNOS), which is responsible for the generation of reactive free radical nitric oxide (NO) [56]. High concentration of NO can induce apoptosis of the damaged cells to help remove the cell debris after trauma [57], while low concentration of NO protects cells against oxidative damage [58], [59]. Attenuating the NO level by NOS inhibitor I-NAME leads to decreased number of muscle stem cells at the early stage of muscle injury and deposition of collagen (an indicator for fibrosis), suggesting that iNOS and NO promote the proliferation of muscle stem cells and prevents fibrosis after muscle injury [60]. There are peripheral evidences to suggest that M1 macrophages can attract muscle stem cells to the injury site and stimulate the proliferation of the muscle stem cells while repress their differentiation [61], [62]. The mechanism of how iNOS and NO regulates muscle regeneration remains to be explored.
T cells are the major cell population to be recruited to the lesion in the second wave of immune cell infiltration. M1 macrophages recruit T cells to infiltrate the injury site [63]. Both CD8+ and CD4+ T cells appear at the injury site about three days after injury and remains to be detected until 10 days after injury [64]. The sustained CD8+ and CD4+ T-cell presence throughout the regenerative process suggests the involvement of T cells in skeletal muscle repair [65]. Furthermore, the infiltration of the T cells also facilitates the subsequent recruitment of macrophage to the injured muscle [66].
Similar to macrophages, T cells also secrete a variety of growth factors and cytokines to modulate the microenvironment of the injury site. T cells express high amount of TNF-α, IFN-γ, IL-1β, IL-4, IL-12, IL-13 and other cytokines. Several cytokines such as TNF-α, IFN-γ and IL-1β are secreted by both macrophages and T cells that maintain the continuous presence and above-threshold concentration of these cytokines during the muscle regeneration process.
T-cell–deficient mice display delay of early growth in skeletal muscle [67]. The adult mice with T cell deficiency display impaired muscle regeneration abilities, while transplantation of CD3+ cells can fully rescue the muscle regeneration defects [68]. Application of the secretive products of human T cells accelerates the muscle wound healing [69], suggesting that cytokines and growth factors secreted by T cells facilitate muscle regeneration.
Recently, Fu et al. showed the direct link between T cells and muscle stem cells [68]. Fu et al. demonstrated that IL-1α, IL-13, TNF-α and IFN-γ secreted by T cells are sufficient to promote muscle stem cell expansion both in vivo and in vitro. Muscle stem cells can be serially expanded for over 20 passages when growing in medium containing IL-1α, IL-13, TNF-α and IFN-γ. The muscle stem cells expanded in vitro have been proved to be able to repair muscle injury in vivo efficiently after cell transplantation. The transplanted muscle stem cells are capable of homing to the right niche and replenish the in vivo muscle stem cell pool. They are also capable of repairing the secondary muscle injury, indicating that the muscle stem cells expanded in vitro meet the golden standard of stem cells. Constantly, these cells shared similar expression profiles to the endogenous muscle stem cells. These results suggest that IL-1α, IL-13, TNF-α and IFN-γ help maintain the stemness of muscle stem cells.
Moreover, injection of the combination of IL-1α, IL-13, TNF-α and IFN-γ to mice lacking T cells can fully rescue the muscle regeneration defects, further supporting the notion that IL-1α, IL-13, TNF-α and IFN-γ secreted by T cells are required for timely muscle regeneration. These discoveries showed that T cells and acute inflammation provide critical microenvironment for muscle stem cell proliferation. This also raise an interesting possibility that inflammatory environment can enable and enhance the functions of stem cells. The principle may be universally applied to injury repair in many tissues. Indeed, it has been reported that inflammation is required for proper neural injury repair [70] and cardiac muscle [71], suggesting that immune cells may be able to facilitate stem cell–mediated injury repair in multiple tissues.
The third wave of immune cells: the switch from proinflammatory to antiinflammatory immune cells
After the number of the muscle stem cells reaches the peak, muscle regeneration process enters the third stage. The major event at this stage is the differentiation of muscle stem cells and the maturation of the newly formed myofibres. The proinflammatory microenvironment at the muscle lesion has also been converted to the antiinflammatory microenvironment accordingly. The conversion to the antiinflammatory microenvironment at lesion is marked by the switch from M1 (proinflammatory) to M2 (antiinflammatory) macrophages [72]. As mentioned above, the Ly6C+ monocytes are recruited and differentiated to M1 macrophage in the second stage of muscle regeneration. In contrast, at the third stage of muscle regeneration, the Ly6C monocytes differentiate to M2 macrophages [61]. M2 macrophages produce antiinflammatory cytokines including IL-4, IL-10 and IL-13 to repress the local inflammatory response at injury site [62], [73]. Meanwhile, M2 macrophages have been indicated to promote muscle stem cells to differentiate to myotubes [61], thus promoting the late stage of myogenesis and regeneration [61], [74], [75]. The absence of M2 macrophages causes a delay in muscle growth and inhibits muscle differentiation and regeneration [76]. Thus, this transition in macrophage phenotype is an essential component of muscle regeneration in vivo following acute or chronic muscle damage [77].
Regulatory T cells (Tregs) are denoted as the CD4+CD25+Foxp3+ subpopulation of T cells [78]. Treg has potent immune repression abilities [79]. The number of Tregs is low at the early stage of muscle regeneration [68], while the number increases dramatically at the late stage of muscle regeneration accompanied with the decrease of cell number of other subtypes of T cells [80]. Tregs secrete IL-10 and other cytokines to facilitate the conversion of M1 to M2 macrophages, therefore promote myoblast differentiation [77], [80]. Tregs can also reduce the number of conventional T cells, especially CD8+ T cells. The reduction of CD8+ T cells slows down muscle stem cell proliferation and promotes myoblast differentiation [81]. Depletion of Tregs in mice results in muscle regeneration defects, suggesting that Tregs are required for the timely muscle regeneration [80].
A special subpopulation of Tregs characterised by the special complementarity determining region 3 sequence in T-cell receptors have been identified in injured skeletal muscle [80]. Muscle Treg express amphiregulin, the ligand for epidermal growth factor receptor. Amphiregulin can promote the proliferation and differentiation of myoblasts [80].
The number of muscle Tregs decreases in aged mice and leads to delayed muscle regeneration after injury. Injection of IL-33, which stimulates the accumulation of Tregs, into the old mice improves the ability of skeletal muscle injury repair further supporting the notion that Tregs facilitate muscle regeneration [82].
Inflammation in chronic muscle disorders
In the acute muscle injury, the self-limiting physiological acute inflammatory responses are involved, while the persistent chronic inflammations are observed in chronic muscle disorders which are a heterogeneous group of diseases characterised by progressive muscle wasting and include muscular dystrophies [83]. Although there are many similarities of inflammation between acute and chronic muscle injury, the kinetics of the immune cells diversify in many aspects. In acute injury, M1 macrophages accumulate and produce proinflammatory cytokines at the early stage of muscle regeneration. M2 macrophages only appear in the later stage of muscle regeneration [56]. Similar to what is observed in acute damage, the muscle inflammation by an infiltrate of inflammatory neutrophils and M1 macrophages is also a prominent feature of chronic muscular dystrophies [84]. For example, neutrophils and activated M1 macrophages invading the muscle are observed around 4 weeks of age in the mdx mice, a genetic mouse model for Duchenne muscular dystrophy (DMD) [84]. In contrast, the infiltration of M2 macrophages also occurs at the early stages of inflammation in DMD [85]. This differs from acute muscle injury, which M2 macrophages usually predominate at later stages of inflammation. The invasion of M2 macrophages at the early stages of inflammation inhibits production of NO by M1 macrophages and greatly decreases M1 macrophage lysis of muscle cells. The repression of fibrosis by NO is therefore attenuated. It leads to pathological fibrosis occurs at the late stage of muscle regeneration in chronic muscle disorders [86]. M2 macrophages also participate in activation of cytotoxic T cells that are then able to promote muscle damage through perforin-mediated processes [87]. In addition, M2 macrophages induce an increase of antiinflammatory cytokines such as IL-4, IL-10 and IL-13, which can induce the activation of eosinophils that promotes muscle fibrosis through major basic protein-1–mediated processes [73], [88]. Besides the innate immune response, some degree of adaptive immune response is also involved in mdx mice and DMD patients. T cells are infiltrated into affected muscles of mdx mice aged 4–8 weeks [89]. Although many studies aimed to characterise T-cell populations and their role in muscle dystrophies, the results were not as exhaustive and were sometimes contradictory. Other chronic disorders such as facioscapulohumeral muscular dystrophy and the limb girdle muscular dystrophies also have to been shown to present clear hallmarks of inflammation although these disorders are caused by different genetic alterations [90], [91]. However, the relevance to the onset and progression of the pathology remains ambiguous.
Dysregulation of inflammation and orderly infiltration of immune cells to the lesion disrupts normal muscle regeneration by inhibiting muscle stem cell proliferation, differentiation and increasing fibrosis. The mechanism needs further exploration.
Conclusion
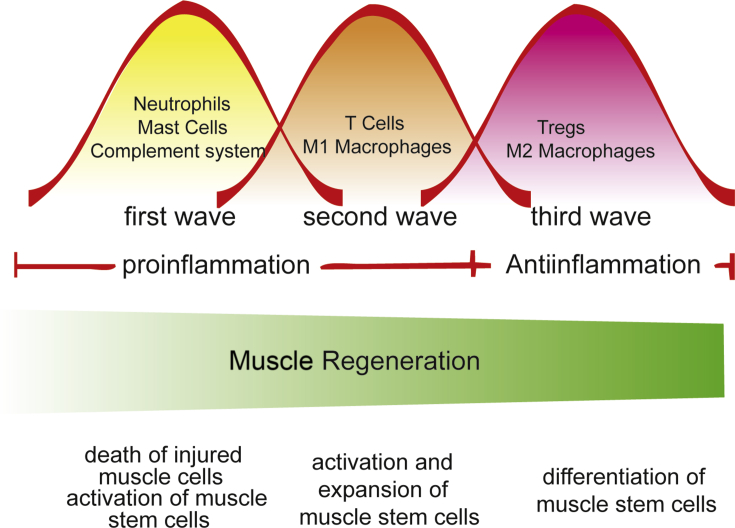
Here we summarise the current knowledge on the functions of inflammation and immune cells in muscle regeneration. Both innate immune system and adaptive immune system are actived after muscle injury. Immune cells are recruited to the lesion in an orderly manner after trauma to facilitate switch from the proinflammatory environment to the antiinflammatory environment and orchestrate the activation, expansion and differentiation of muscle stem cells during muscle regeneration (Figure 1). They are responsible for debris clearance and microenvironment modification by secreting various types of cytokines, growth factors, enzymes and other factors. These functions are indispensable for proper muscle regeneration.
Figure 1.
Inflammation and muscle regeneration. Three waves of immune cells were recruited to the muscle injury site orderly. The recruitment of various types of immune cells regulates the activation, expansion and differentiation of muscle stem cells to facilitate muscle regeneration.
How are immune cells recruited to the muscle injury site in an orderly manner? What leads to the specificity of immune cell–muscle cell interaction? Are there any special types of immune cells present at the injury site in each organ? How can multi types of cytokines and growth factor crosstalk with each other and form the hierarchical network of the signal transductions in the muscle regeneration process? These are interesting questions to pursue in the future. Finding the answers to these questions will greatly enhance our knowledge on muscle regeneration and help us develop more efficient treatment for muscle diseases.
Conflict of interest
All authors declare no conflicts of interest.
Funding
This work was sponsored by grants from the Ministry of Science and Technology of China (2017YFA0102700 and 2014CB964700 to P.H.), the “Strategic Priority Research Program” of Chinese Academy of Sciences (XDA01010204 to P. H.), the National Natural Science Foundation of China (31671536 and 91649104 to P. H., 81200355 to W. Y., and 81530071 to L. C.), NN-CAS Foundation (to P.H.), CAS-CSIRO cooperative Research Program (GJHZ1504 to P. H.), CAS-Youth Innovation Pro- gram Association (2016246 to W.Y.) and Science and Technology Commission of Shanghai Municipality (Y753S11802 to P. H.).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jot.2018.01.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Schmalbruch H., Lewis D.M. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve. 2000;23(4):617–626. doi: 10.1002/(sici)1097-4598(200004)23:4<617::aid-mus22>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.Huard J., Li Y., Fu F.H. Muscle injuries and repair: current trends in research. J Bone Jt Surg Am Vol. 2002;84-A(5):822–832. [PubMed] [Google Scholar]
- 3.Jarvinen T.A., Kaariainen M., Jarvinen M., Kalimo H. Muscle strain injuries. Curr Opin Rheumatol. 2000;12(2):155–161. doi: 10.1097/00002281-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 4.St Pierre B.A., Tidball J.G. Differential response of macrophage subpopulations to soleus muscle reloading after rat hindlimb suspension. J Appl Physiol. 1994;77(1):290–297. doi: 10.1152/jappl.1994.77.1.290. [DOI] [PubMed] [Google Scholar]
- 5.Hurme T., Kalimo H., Lehto M., Jarvinen M. Healing of skeletal muscle injury: an ultrastructural and immunohistochemical study. Med Sci Sports Exerc. 1991;23(7):801–810. [PubMed] [Google Scholar]
- 6.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang N.C., Rudnicki M.A. Satellite cells: the architects of skeletal muscle. Curr Top Dev Biol. 2014;107:161–181. doi: 10.1016/B978-0-12-416022-4.00006-8. [DOI] [PubMed] [Google Scholar]
- 8.Comai G., Tajbakhsh S. vol. 110. 2014. Molecular and cellular regulation of skeletal myogenesis; pp. 1–73. (BHLH Transcription Factors in development and disease). [DOI] [PubMed] [Google Scholar]
- 9.Bischoff R. Regeneration of single skeletal muscle fibers in vitro. Anat Rec. 1975;182(2):215–235. doi: 10.1002/ar.1091820207. [DOI] [PubMed] [Google Scholar]
- 10.Konigsberg U.R., Lipton B.H., Konigsberg I.R. The regenerative response of single mature muscle fibers isolated in vitro. Dev Biol. 1975;45(2):260–275. doi: 10.1016/0012-1606(75)90065-2. [DOI] [PubMed] [Google Scholar]
- 11.Chazaud B., Sonnet C., Lafuste P., Bassez G., Rimaniol A.C., Poron F. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol. 2003;163(5):1133–1143. doi: 10.1083/jcb.200212046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zipfel P.F., Reuter M. Complement activation products C3a and C4a as endogenous antimicrobial peptides. Int J Pept Res Therapeut. 2009;15(2):87–95. [Google Scholar]
- 13.Ricklin D., Reis E.S., Lambris J.D. Complement in disease: a defence system turning offensive. Nat Rev Nephrol. 2016;12(7):383–401. doi: 10.1038/nrneph.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lubbers R., van Essen M.F., van Kooten C., Trouw L.A. Production of complement components by cells of the immune system. Clin Exp Immunol. 2017;188(2):183–194. doi: 10.1111/cei.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frenette J., Cai B., Tidball J.G. Complement activation promotes muscle inflammation during modified muscle use. Am J Pathol. 2000;156(6):2103–2110. doi: 10.1016/S0002-9440(10)65081-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dufaux B., Order U. Complement activation after prolonged exercise. Clin Chim Acta; Int J Clin Chem. 1989;179(1):45–49. doi: 10.1016/0009-8981(89)90021-1. [DOI] [PubMed] [Google Scholar]
- 17.Abraham S.N., St John A.L. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10(6):440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radley H.G., Grounds M.D. Cromolyn administration (to block mast cell degranulation) reduces necrosis of dystrophic muscle in mdx mice. Neurobiol Dis. 2006;23(2):387–397. doi: 10.1016/j.nbd.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Gordon J.R., Galli S.J. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346(6281):274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 20.Gorospe J.R., Nishikawa B.K., Hoffman E.P. Recruitment of mast cells to muscle after mild damage. J Neurol Sci. 1996;135(1):10–17. doi: 10.1016/0022-510x(95)00255-z. [DOI] [PubMed] [Google Scholar]
- 21.Fielding R.A., Manfredi T.J., Ding W.J., Fiatarone M.A., Evans W.J., Cannon J.G. Acute-phase response in exercise .3. Neutrophil and Il-1-beta accumulation in skeletal-muscle. Am J Physiol. 1993;265(1):R166–R172. doi: 10.1152/ajpregu.1993.265.1.R166. [DOI] [PubMed] [Google Scholar]
- 22.Hawke T.J., Geary D.J. Myogenic satellite cells: physiology to molecular biology. (vol 91, pg 534, 2001) J Appl Physiol. 2001;91(6) doi: 10.1152/jappl.2001.91.2.534. U36–U36. [DOI] [PubMed] [Google Scholar]
- 23.Arango Duque G., Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dumont N., Bouchard P., Frenette J. Neutrophil-induced skeletal muscle damage: a calculated and controlled response following hindlimb unloading and reloading. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R1831–R1838. doi: 10.1152/ajpregu.90318.2008. [DOI] [PubMed] [Google Scholar]
- 25.Cassatella M.A. Neutrophil-derived proteins: selling cytokines by the pound. Adv Immunol. 1999;73:369–509. doi: 10.1016/s0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- 26.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 27.Fujishima S., Hoffman A.R., Vu T., Kim K.J., Zheng H., Daniel D. Regulation of neutrophil interleukin 8 gene expression and protein secretion by LPS, TNF-alpha, and IL-1 beta. J Cell Physiol. 1993;154(3):478–485. doi: 10.1002/jcp.1041540305. [DOI] [PubMed] [Google Scholar]
- 28.Semple J.W., Kim M., Hou J., McVey M., Lee Y.J., Tabuchi A. Intravenous immunoglobulin prevents murine antibody-mediated acute lung injury at the level of neutrophil reactive oxygen species (ROS) production. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0031357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korthuis R.J., Grisham M.B., Granger D.N. Leukocyte depletion attenuates vascular injury in postischemic skeletal muscle. Am J Physiol. 1988;254(5 Pt 2):H823–H827. doi: 10.1152/ajpheart.1988.254.5.H823. [DOI] [PubMed] [Google Scholar]
- 30.Chazaud B., Brigitte M., Yacoub-Youssef H., Arnold L., Gherardi R., Sonnet C. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc Sport Sci Rev. 2009;37(1):18–22. doi: 10.1097/JES.0b013e318190ebdb. [DOI] [PubMed] [Google Scholar]
- 31.Mortha A., Chudnovskiy A., Hashimoto D., Bogunovic M., Spencer S.P., Belkaid Y. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343(6178):1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epelman S., Lavine K.J., Randolph G.J. Origin and functions of tissue macrophages. Immunity. 2014;41(1):21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okabe Y., Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157(4):832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 35.Tidball J.G. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 36.Biswas S.K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 37.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Geissmann F., Jung S., Littman D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 39.Nahrendorf M., Swirski F.K., Aikawa E., Stangenberg L., Wurdinger T., Figueiredo J.L. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jetten N., Verbruggen S., Gijbels M.J., Post M.J., De Winther M.P., Donners M.M. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17(1):109–118. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 41.Madaro L., Bouche M. From innate to adaptive immune response in muscular dystrophies and skeletal muscle regeneration: the role of lymphocytes. BioMed Res Int. 2014;2014 doi: 10.1155/2014/438675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen S.E., Gerken E., Zhang Y., Zhan M., Mohan R.K., Li A.S. Role of TNF-{alpha} signaling in regeneration of cardiotoxin-injured muscle. Am J Physiol Cell Physiol. 2005;289(5):C1179–C1187. doi: 10.1152/ajpcell.00062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warren G.L., Hulderman T., Jensen N., McKinstry M., Mishra M., Luster M.I. Physiological role of tumor necrosis factor alpha in traumatic muscle injury. FASEB J. 2002;16(12):1630–1632. doi: 10.1096/fj.02-0187fje. [DOI] [PubMed] [Google Scholar]
- 44.Peterson J.M., Bakkar N., Guttridge D.C. NF-kappaB signaling in skeletal muscle health and disease. Curr Top Dev Biol. 2011;96:85–119. doi: 10.1016/B978-0-12-385940-2.00004-8. [DOI] [PubMed] [Google Scholar]
- 45.Chen S.E., Jin B., Li Y.P. TNF-alpha regulates myogenesis and muscle regeneration by activating p38 MAPK. Am J Physiol Cell Physiol. 2007;292(5):C1660–C1671. doi: 10.1152/ajpcell.00486.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhan M., Jin B., Chen S.E., Reecy J.M., Li Y.P. TACE release of TNF-alpha mediates mechanotransduction-induced activation of p38 MAPK and myogenesis. J Cell Sci. 2007;120(Pt 4):692–701. doi: 10.1242/jcs.03372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C.C., Li Y.L., Wu Y.N., Wang L.Y., Wang X.N., Du J. Interleukin-6/Signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J Biol Chem. 2013;288(3):1489–1499. doi: 10.1074/jbc.M112.419788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joe A.W.B., Yi L., Natarajan A., Le Grand F., So L., Wang J. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12(2) doi: 10.1038/ncb2015. 153–U144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kami K., Senba E. Localization of leukemia inhibitory factor and interleukin-6 messenger ribonucleic acids in regenerating rat skeletal muscle. Muscle Nerve. 1998;21(6):819–822. doi: 10.1002/(sici)1097-4598(199806)21:6<819::aid-mus20>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 50.Gallucci S., Provenzano C., Mazzarelli P., Scuderi F., Bartoccioni E. Myoblasts produce IL-6 in response to inflammatory stimuli. Int Immunol. 1998;10(3):267–273. doi: 10.1093/intimm/10.3.267. [DOI] [PubMed] [Google Scholar]
- 51.Kami K., Senba E. In vivo activation of STAT3 signaling in satellite cells and myofibers in regenerating rat skeletal muscles. J Histochem Cytochem. 2002;50(12):1579–1589. doi: 10.1177/002215540205001202. [DOI] [PubMed] [Google Scholar]
- 52.Munoz-Canoves P., Scheele C., Pedersen B.K., Serrano A.L. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 2013;280(17):4131–4148. doi: 10.1111/febs.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin W.J., Walton M., Harper J. Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis Rheum. 2009;60(1):281–289. doi: 10.1002/art.24185. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka S., Aso H., Miyazawa K., Nagai Y., Watanabe K., Ohwada S. Differential cytokine gene expression in CD4+ and CD8+ T cell subsets of calves. Vet Immunol Immunopathol. 2007;118(1–2):84–91. doi: 10.1016/j.vetimm.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 55.Luo G.J., Hershko D.D., Robb B.W., Wray C.J., Hasselgren P.O. IL-1 beta stimulates IL-6 production in cultured skeletal muscle cells through activation of MAP kinase signaling pathway and NF-kappa B. Am J Philos Relig I. 2003;284(5):R1249–R1254. doi: 10.1152/ajpregu.00490.2002. [DOI] [PubMed] [Google Scholar]
- 56.Villalta S.A., Nguyen H.X., Deng B., Gotoh T., Tidball J.G. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet. 2009;18(3):482–496. doi: 10.1093/hmg/ddn376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshioka Y., Yamamuro A., Maeda S. Nitric oxide at a low concentration protects murine macrophage RAW264 cells against nitric oxide-induced death via cGMP signaling pathway. Br J Pharmacol. 2003;139(1):28–34. doi: 10.1038/sj.bjp.0705206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wink D.A., Hanbauer I., Laval F., Cook J.A., Krishna M.C., Mitchell J.B. Nitric-oxide protects against the cytotoxic effects of reactive oxygen species. Ann NY Acad Sci. 1994;738:265–278. doi: 10.1111/j.1749-6632.1994.tb21812.x. [DOI] [PubMed] [Google Scholar]
- 59.Fujii S., Sawa T., Ihara H., Tong K.I., Ida T., Okamoto T. The critical role of nitric oxide signaling, via protein S-guanylation and nitrated cyclic GMP, in the antioxidant adaptive response. J Biol Chem. 2010;285(31):23970–23984. doi: 10.1074/jbc.M110.145441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Filippin L.I., Cuevas M.J., Lima E., Marroni N.P., Gonzalez-Gallego J., Xavier R.M. Nitric oxide regulates the repair of injured skeletal muscle. Nitric Oxide-Biol Chem. 2011;24(1):43–49. doi: 10.1016/j.niox.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 61.Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204(5):1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biswas S.K., Gangi L., Paul S., Schioppa T., Saccani A., Sironi M. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107(5):2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J., Xiao Z., Qu C., Cui W., Wang X., Du J. CD8 T cells are involved in skeletal muscle regeneration through facilitating MCP-1 secretion and Gr1(high) macrophage infiltration. J Immunol. 2014;193(10):5149–5160. doi: 10.4049/jimmunol.1303486. [DOI] [PubMed] [Google Scholar]
- 64.Cheng M., Nguyen M.H., Fantuzzi G., Koh T.J. Endogenous interferon-gamma is required for efficient skeletal muscle regeneration. Am J Physiol Cell Physiol. 2008;294(5):C1183–C1191. doi: 10.1152/ajpcell.00568.2007. [DOI] [PubMed] [Google Scholar]
- 65.Mourkioti F., Rosenthal N. IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol. 2005;26(10):535–542. doi: 10.1016/j.it.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Spencer M.J., Walsh C.M., Dorshkind K.A., Rodriguez E.M., Tidball J.G. Myonuclear apoptosis in dystrophic mdx muscle occurs by perforin-mediated cytotoxicity. J Clin Investig. 1997;99(11):2745–2751. doi: 10.1172/JCI119464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morrison J., Palmer D.B., Cobbold S., Partridge T., Bou-Gharios G. Effects of T-lymphocyte depletion on muscle fibrosis in the mdx mouse. Am J Pathol. 2005;166(6):1701–1710. doi: 10.1016/S0002-9440(10)62480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fu X., Xiao J., Wei Y.N., Li S., Liu Y., Yin J. Combination of inflammation-related cytokines promotes long-term muscle stem cell expansion (vol 25, pg 655, 2015) Cell Res. 2015;25(9):1082–1083. doi: 10.1038/cr.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mildner M., Hacker S., Haider T., Gschwandtner M., Werba G., Barresi C. Secretome of peripheral blood mononuclear cells enhances wound healing. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kyritsis N., Kizil C., Zocher S., Kroehne V., Kaslin J., Freudenreich D. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science. 2012;338(6112):1353–1356. doi: 10.1126/science.1228773. [DOI] [PubMed] [Google Scholar]
- 71.Han C., Nie Y., Lian H., Liu R., He F., Huang H. Acute inflammation stimulates a regenerative response in the neonatal mouse heart. Cell Res. 2015;25(10):1137–1151. doi: 10.1038/cr.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stout R.D., Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76(3):509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 74.Ruffell D., Mourkioti F., Gambardella A., Kirstetter P., Lopez R.G., Rosenthal N. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci USA. 2009;106(41):17475–17480. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sag D., Carling D., Stout R.D., Suttles J. Adenosine 5'-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181(12):8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tidball J.G., Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol. 2007;578(Pt 1):327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Villalta S.A., Rinaldi C., Deng B., Liu G., Fedor B., Tidball J.G. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Hum Mol Genet. 2011;20(4):790–805. doi: 10.1093/hmg/ddq523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 79.Josefowicz S.Z., Lu L.F., Rudensky A.Y. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burzyn D., Kuswanto W., Kolodin D., Shadrach J.L., Cerletti M., Jang Y. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155(6):1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castiglioni A., Corna G., Rigamonti E., Basso V., Vezzoli M., Monno A. FOXP3+ T cells recruited to sites of sterile skeletal muscle injury regulate the fate of satellite cells and guide effective tissue regeneration. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0128094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuswanto W., Burzyn D., Panduro M., Wang K.K., Jang Y.C., Wagers A.J. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity. 2016;44(2):355–367. doi: 10.1016/j.immuni.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manzur A.Y., Kuntzer T., Pike M., Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008;(1) doi: 10.1002/14651858.CD003725.pub3. [DOI] [PubMed] [Google Scholar]
- 84.De Paepe B., De Bleecker J.L. Cytokines and chemokines as regulators of skeletal muscle inflammation: presenting the case of Duchenne muscular dystrophy. Mediat Inflamm. 2013;2013 doi: 10.1155/2013/540370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tidball J.G., Villalta S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Philos Relig I. 2010;298(5):R1173–R1187. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wehling-Henricks M., Jordan M.C., Gotoh T., Grody W.W., Roos K.P., Tidball J.G. Arginine metabolism by macrophages promotes cardiac and muscle fibrosis in mdx muscular dystrophy. PLoS One. 2010;5(5) doi: 10.1371/journal.pone.0010763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cai B.Y., Spencer M.J., Nakamura G., Tseng-Ong L., Tidball J.G. Eosinophilia of dystrophin-deficient muscle is promoted by perforin-mediated cytotoxicity by T cell effectors. Am J Pathol. 2000;156(5):1789–1796. doi: 10.1016/S0002-9440(10)65050-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wehling-Henricks M., Sokolow S., Lee J.J., Myung K.H., Villalta S.A., Tidball J.G. Major basic protein-1 promotes fibrosis of dystrophic muscle and attenuates the cellular immune response in muscular dystrophy. Hum Mol Genet. 2008;17(15):2280–2292. doi: 10.1093/hmg/ddn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Spencer M.J., Montecino-Rodriguez E., Dorshkind K., Tidball J.G. Helper (CD4(+)) and cytotoxic (CD8(+)) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol. 2001;98(2):235–243. doi: 10.1006/clim.2000.4966. [DOI] [PubMed] [Google Scholar]
- 90.Hauerslev S., Orngreen M.C., Hertz J.M., Vissing J., Krag T.O. Muscle regeneration and inflammation in patients with facioscapulohumeral muscular dystrophy. Acta Neurol Scand. 2013;128(3):194–201. doi: 10.1111/ane.12109. [DOI] [PubMed] [Google Scholar]
- 91.Nagaraju K., Rawat R., Veszelovszky E., Thapliyal R., Kesari A., Sparks S. Dysferlin deficiency enhances monocyte phagocytosis: a model for the inflammatory onset of limb-girdle muscular dystrophy 2B. Am J Pathol. 2008;172(3):774–785. doi: 10.2353/ajpath.2008.070327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.