Abstract
Abnormal deposition of the microtubule-associated protein tau is a common pathological feature of multiple neurodegenerative diseases, including Alzheimer’s disease (AD), and plays critical roles in their pathogenesis. Disruption of calcium homeostasis and the downstream kinase Ca2+/calmodulin-dependent protein kinase II (CaMKII) coincides with pathological phosphorylation of tau in AD brains. However, it remains unclear whether and how dysregulation of CaMKII affects tau toxicity. Using a Drosophila model, we found that CaMKII promotes neurodegeneration caused by tau phosphorylated at the AD-associated sites Ser262/356. Overexpression of CaMKII promoted, while RNA-mediated knockdown of CaMKII and inhibition of CaMKII activity by expression of an inhibitory peptide suppressed, tau-mediated neurodegeneration. Blocking tau phosphorylation at Ser262/356 by alanine substitutions suppressed promotion of tau toxicity by CaMKII, suggesting that tau phosphorylation at these sites is required for this phenomenon. However, neither knockdown nor overexpression of CaMKII affected tau phosphorylation levels at Ser262/356, suggesting that CaMKII is not directly involved in tau phosphorylation at Ser262/356 in this model. These results suggest that a pathological cascade of events, including elevated levels of tau phosphorylated at Ser262/356 and aberrant activation of CaMKII, work in concert to promote tau-mediated neurodegeneration.
Keywords: Ca2+/calmodulin (CaM)-dependent protein kinase II, Drosophila, microtubule-associated protein tau, phosphorylation, tauopathy
The tau protein accumulates in multiple neurodegenerative diseases, including Alzheimer’s disease (AD), progressive supranuclear palsy (PSP), frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17), corticobasal degeneration (CBD), frontotemporal dementia (FTD), Pick’s disease (PiD) and Lewy Body Dementia (LBD) (1). Tau is a microtubule-binding protein that is predominantly localized in axons, where it binds to microtubules to regulate their stability. In the brains of patients with the aforementioned diseases, however, tau is detached from microtubules and phosphorylated at disease-specific sites (2–5). Hyperphosphorylated tau proteins aggregate into bundles of filaments that are deposited as neurofibrillary tangles (NFTs), which are well correlated with the clinical expression of these diseases (6).
Among more than 40 sites in tau that are phosphorylated in disease brains (3–5), tau phosphorylation at Ser262 and Ser356 is one of the pathological changes in the early stages and has a significant impact on the metabolism and toxicity of tau (7–13). Both residues are located in the microtubule-binding domain, and phosphorylation at these sites increases the levels of microtubule-unbound tau (14–17), which is subsequently phosphorylated at other sites (10–13). Thus, tau phosphorylation at Ser262/356 is likely to play an initiating role in tau toxicity (10–13). However, the factors involved in neurodegeneration downstream of tau phosphorylation at Ser262/356 have not been fully elucidated.
Disruption of intracellular Ca2+ homeostasis has been observed in a number of neurodegenerative diseases, and Ca2+/calmodulin (CaM)-dependent protein kinase II (CaMKII) may be a key molecule in the pathological cascade downstream of abnormal Ca2+ signaling (18, 19). CaMKII is activated by the binding of Ca2+/CaM followed by autophosphorylation at Thr286, and this autophosphorylated form retains its catalytic activity beyond the initial stimulation (20). CaMKII phosphorylates tau at several sites including Ser262 and Ser356 in vitro (21), promotes phosphorylation of tau in cultured cells (22–24) and frequently co-localizes with NFTs in AD brains (24–29). Furthermore, CaMKII inhibitors reduce death of primary cortical neurons treated with Alzheimer’s amyloid-β peptides (30). These reports suggest that dysregulation of CaMKII activity may contribute to abnormal metabolism and toxicity of tau under disease conditions.
In this study, we used a Drosophila model to investigate the roles of CaMKII in tau toxicity and its relationship with tau phosphorylation at Ser262 and Ser356. Our results suggest that although CaMKII is not directly involved in tau phosphorylation at Ser262/356, it promotes neurodegeneration caused by tau phosphorylated at Ser262/356.
Materials and Methods
Fly stocks
Flies were maintained in standard cornmeal media at 25 °C. The transgenic fly lines carrying the human 0N4R tau, which has four tubulin-binding domains (R) at the C-terminal region and no N-terminal insert (N), is a gift from Dr. M. B. Feany (Harvard Medical School) (31). GMR-Gal4 was obtained from the Bloomington Stock Center. UAS-CaMKII RNAi is obtained from Vienna Drosophila Resource Center. UAS-CaMKII (32) and UAS-ala (33) are gifts from Dr. Leslie Griffith (Brandeis University). The transgenic fly line carrying UAS-S2Atau was reported previously (10, 11, 34). All experiments were performed using female flies at 3–5 day-old after eclosion unless otherwise indicated. Genotypes are described in Supplementary Table SI.
Western blotting
Western blotting was carried out as described previously (10, 11, 34). Anti-CaMKIIα phospho-Thr286 (Santa Cruz Biotechnology), anti-dCaMKII (Cosmo Bio), anti-tau antibody (Tau46, Invitrogen), anti-tau phospho-Ser262 (Abcam), anti-tau phospho-Ser356 (Biosource), anti-actin (Sigma) and anti-tubulin (Sigma) were purchased. The signal intensity was quantified using Image J (NIH) or an Odyssey system. Western blots were repeated a minimum of three times with different animals.
Histological analysis
Preparation of paraffin sections, hematoxylin and eosin staining and analysis of neurodegeneration were described previously (10). Serial sections (6 µm thickness) through the entire heads were prepared and examined by bright-field microscopy. Images of the sections that include the lamina were captured with Insight 2 CCD Camera (SPOT), and vacuole area was measured using Image J (NIH). Heads from more than four flies (more than eight hemispheres) were analyzed for each genotype.
In vivo microtubule-binding assay
Microtubule binding assay was performed using a previously reported (10, 34). Protein concentration in each fraction was measured using the BCA Protein Assay Kit (Pierce). The same amount of protein was loaded to each lane of Tris-Glycine gels and analyzed by western blotting using anti-tau antibody (Tau46, Zymed) or anti-tubulin (Sigma).
Statistics
Statistics was done with Microsoft Excel (Microsoft) with Student's t.
Results
Knockdown of CaMKII suppresses, while overexpression of CaMKII promotes, neurodegeneration induced by tau
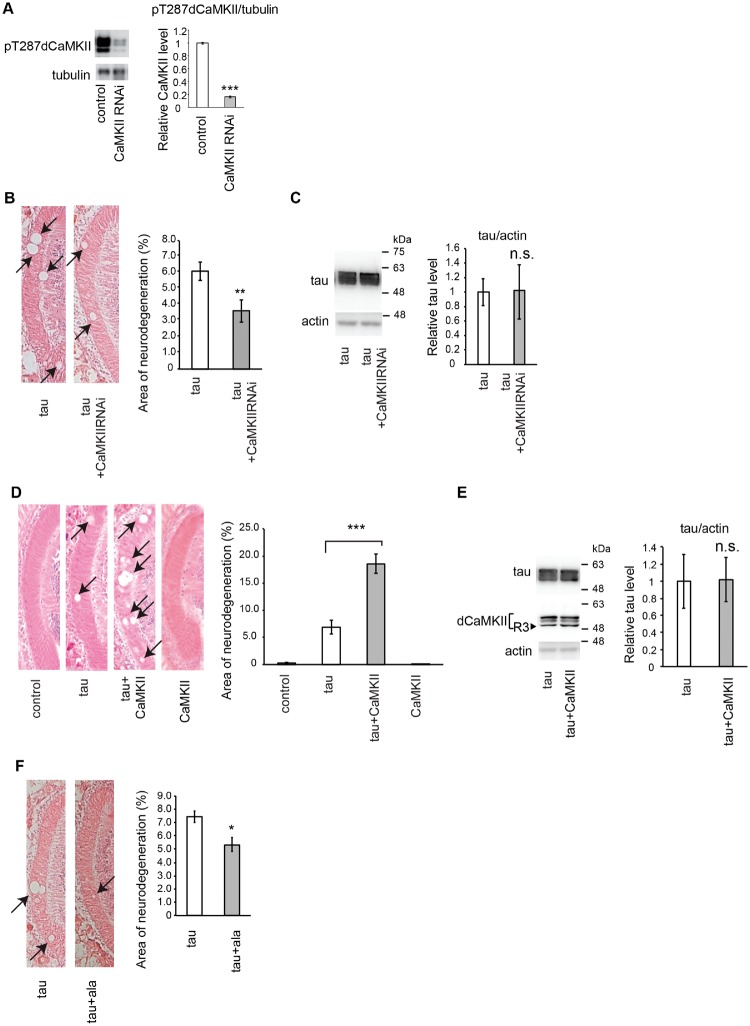
Drosophila has one gene encoding CaMKII that gives rise to at least four protein isoforms, which all share over 85% sequence identity with the α isoform of vertebrate CaMKII and contain the autophosphorylation site T287, which is functionally equivalent to T286 in mammalian CaMKIIα (35). To determine whether CaMKII is involved in neurodegeneration induced by tau, we knocked down CaMKII in a fly model of tauopathy (31). Expression of wild-type human tau in Drosophila eyes using the pan-retinal GMR-GAL4 driver causes age-dependent and progressive neurodegeneration, which is observed as vacuoles in the lamina, the first synaptic neuropil of the optic lobe containing photoreceptor axons (34). RNAi-mediated knockdown of CaMKII efficiently decreased CaMKII activity as indicated by reduced levels of the autophosphorylated form of all isoforms of CaMKII (Fig. 1A), and significantly suppressed neurodegeneration caused by tau (Fig. 1B). This effect was not due to titration of the effectiveness of GAL4-mediated transcription, as CaMKII knockdown did not reduce total levels of tau (Fig. 1C). Moreover, expression of control RNAi (RNAi targeting firefly luciferase) did not affect tau-induced neurodegeneration (34), indicating that suppression of tau toxicity caused by CaMKII RNAi was not due to non-specific effects of RNAi expression.
Fig. 1.
Knockdown of CaMKII suppresses, while overexpression of CaMKII promotes, neurodegeneration induced by tau. (A) CaMKII RNAi reduces active CaMKII in Drosophila brain. Western blot analysis of fly heads carrying the pan-neuronal elav-Gal4 driver alone (control) or expressing CaMKII RNAi driven by elav-Gal4 (CaMKII RNAi) with anti-pT286 CaMKII antibody. Tubulin was used as a loading control. Mean ± SD, n = 5, ***, P < 0.005, Student’s t-test. (B) RNAi-mediated knockdown of CaMKII suppresses tau-induced neurodegeneration. (Left) The lamina of flies expressing human tau alone (tau) and co-expressing human tau and CaMKII RNAi (tau+CaMKIIRNAi) driven by GMR-Gal4. Neurodegeneration is indicated by arrows. (Right) Quantification of neurodegeneration, Mean ± SEM, n = 8–12. **, P < 0.01. (C) RNAi-mediated knockdown of CaMKII does not change tau protein levels. Western blot analysis of fly heads expressing human tau alone (tau) and co-expressing human tau and CaMKII RNAi (tau+CaMKIIRNAi) driven by GMR-Gal4 with anti-tau antibody. Actin was used as a loading control. Mean ± SD, n = 5, n.s., P > 0.05. (D) Overexpression of CaMKII enhances tau-induced neurodegeneration. (Left) The lamina of control flies bearing the GMR-Gal4 driver only (control), flies expressing tau alone (tau), co-expressing tau and CaMKII (tau+CaMKII) and CaMKII alone (CaMKII). (Right) Quantification of neurodegeneration. Mean ± SEM, n = 8–12. ***, P < 0.005. (E) Overexpression of CaMKII does not change tau protein levels. Western blot analysis of fly heads expressing human tau alone (tau) and co-expressing human tau and CaMKII (tau+CaMKII) driven by GMR-Gal4 with anti-tau antibody (tau). Expression of exogenous CaMKII (R3) is confirmed by Western blot with anti-dCaMKII antibody (dCaMKII). Please note that this blot reflects all the CaMKII proteins in the head including endogenous CaMKII. Endogenous CaMKII is expressed in multiple isoforms and abundant in all the brain regions, while exogenous CaMKII is R3 isoform (arrowhead) and expressed only in the retina. Mean ± SD, n = 5, n.s., P > 0.05. (F) Inhibition of CaMKII activity suppresses tau-induced neurodegeneration. (Left) The lamina of flies expressing human tau alone (tau) and co-expressing human tau and the inhibitory domain of the rat CaMKII (tau+ala) driven by GMR-Gal4. (Right) Quantification of neurodegeneration. Mean ± SEM, n = 8–12. *, P < 0.05.
We next investigated whether upregulation of CaMKII would promote tau-induced neurodegeneration by using well-established transgenic fly carrying Drosophila R3 isoform of CaMKII (32, 33), whose expression has been shown to increase CaMKII activity in Drosophila neurons (35–42). Co-expression of CaMKII significantly increased neurodegeneration due to tau, whereas CaMKII expression alone did not cause neurodegeneration (Fig. 1D). Co-expression of CaMKII did not alter total tau levels (Fig. 1E), suggesting that this effect was not due to an increase in the tau protein levels.
To determine whether inhibition of CaMKII activity is sufficient to suppress tau toxicity, we expressed the inhibitory domain of the rat CaMKII (ala) (43). Expression of this peptide has been shown to reduce CaMKII activity in Drosophila neurons (35, 37–39, 41–46). Co-expression of CaMKII inhibitory peptide suppressed tau-induced neurodegeneration (Fig. 1F), indicating that the observed promotion of tau toxicity is mediated by the kinase activity of CaMKII.
Taken together, these results indicate that CaMKII activity is involved in neurodegeneration in a Drosophila model of tauopathy.
Blocking tau phosphorylation at Ser262/356 abolishes enhancement of tau-mediated neurodegeneration caused by CaMKII
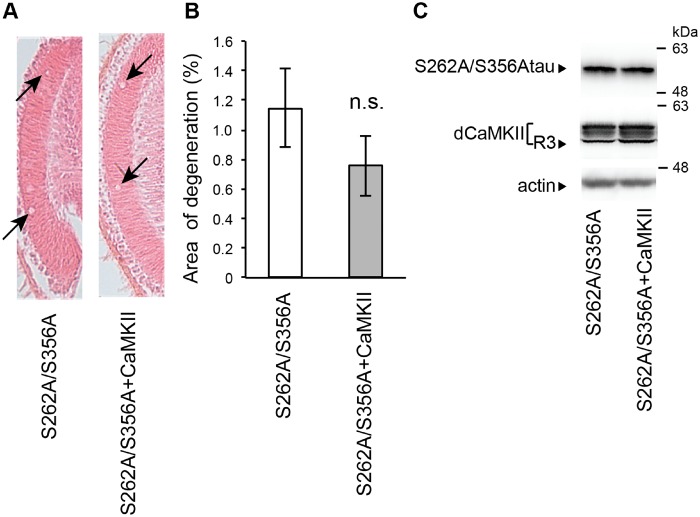
Tau phosphorylation at Ser262 and Ser356 is observed in the early phases of pathological changes in tau (7), and blocking tau phosphorylation at these sites decreases the levels of tau phosphorylated at other sites and attenuates tau toxicity (11–13). Thus, tau phosphorylation at these sites plays a critical role in tau toxicity upstream of other pathological changes in tau. Therefore, we asked whether tau phosphorylation at Ser262 and Ser356 is involved in the enhancement of tau-induced neurodegeneration caused by overexpression of CaMKII. For these experiments, we used transgenic flies carrying human tau with alanine substitutions at both phosphorylation sites (S2A tau). CaMKII overexpression did not augment neurodegeneration caused by S2A tau (Fig. 2), suggesting that tau phosphorylated at Ser262/356 is involved in the promotion of tau-induced neurodegeneration caused by CaMKII.
Fig. 2.
Blocking tau phosphorylation at Ser262/356 abolishes enhancement of tau-mediated neurodegeneration caused by CaMKII. (A) The lamina of flies expressing tau carrying the S262A/S356A double mutation (S262A/S356A) and co-expressing S262A/S356A tau and CaMKII (S262A/S356A+CaMKII) driven by GMR-Gal4. Neurodegeneration is indicated by arrows. (B) Quantification of neurodegeneration. Mean ± SEM, n = 8–10. n.s., P > 0.05, Student's t-test. Flies were 10 days-after-eclosion. (C) Overexpression of CaMKII does not change the levels of S262A/S356A tau. Western blot analysis of fly heads expressing S262A/S356A tau alone (S262A/S356A) and co-expressing S262A/S356A tau and CaMKII (S262A/S356A+CaMKII) driven by GMR-Gal4 with anti-tau antibody (tau) or anti-dCaMKII antibody (dCaMKII).
Neither knockdown nor overexpression of CaMKII alters the levels of tau phosphorylated at Ser262/356
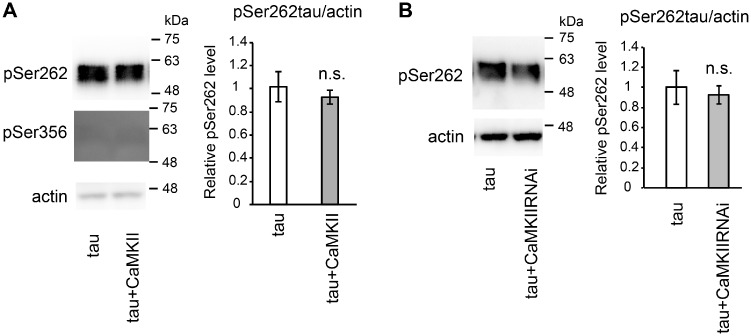
CaMKII phosphorylates tau at Ser262 and Ser356 in vitro (21). Thus, we asked whether overexpression of CaMKII would increase tau phosphorylation at Ser262 and Ser356. Co-expression of CaMKII did not increase the level of tau phosphorylated at Ser262 or at Ser356 (Fig. 3A, also see total tau levels in Fig. 1E).
Fig. 3.
CaMKII does not affect the levels of tau phosphorylated at Ser262/356. (A) Western blot analysis of fly heads expressing human tau alone (tau) and co-expressing human tau and CaMKII (tau+CaMKII) with antibody against pSer262 (pSer262) or an antibody recognizes tau phosphorylated at Ser356 (pSer356). (B) Western blot analysis of fly heads expressing human tau alone (tau) and co-expressing human tau and CaMKII RNAi (tau+CaMKIIRNAi) with an antibody that recognizes tau phosphorylated at Ser262 (pSer262). Actin was used as a loading control. Mean ± SD, n = 5, n.s., P > 0.05, Student’s t-test.
We also investigated whether suppression of tau-induced toxicity by CaMKII was accompanied by a reduction in the levels of tau phosphorylated at Ser262. In our Drosophila model, tau is phosphorylated at Ser262, whereas phosphorylation at Ser356 is not detectable (11). Western blotting using phospho-tau–specific antibody revealed that CaMKII knockdown did not significantly affect the levels of tau phosphorylated at Ser262 (Fig. 3A, also see total tau levels in Fig. 1C). Since neurodegeneration is rescued by CaMKII knockdown (Fig. 1B), these results suggest that the effect of CaMKII on tau toxicity is not associated with the levels of tau phosphorylation at Ser262 in this model.
CaMKII does not alter tau distribution to the microtubule and cytosol
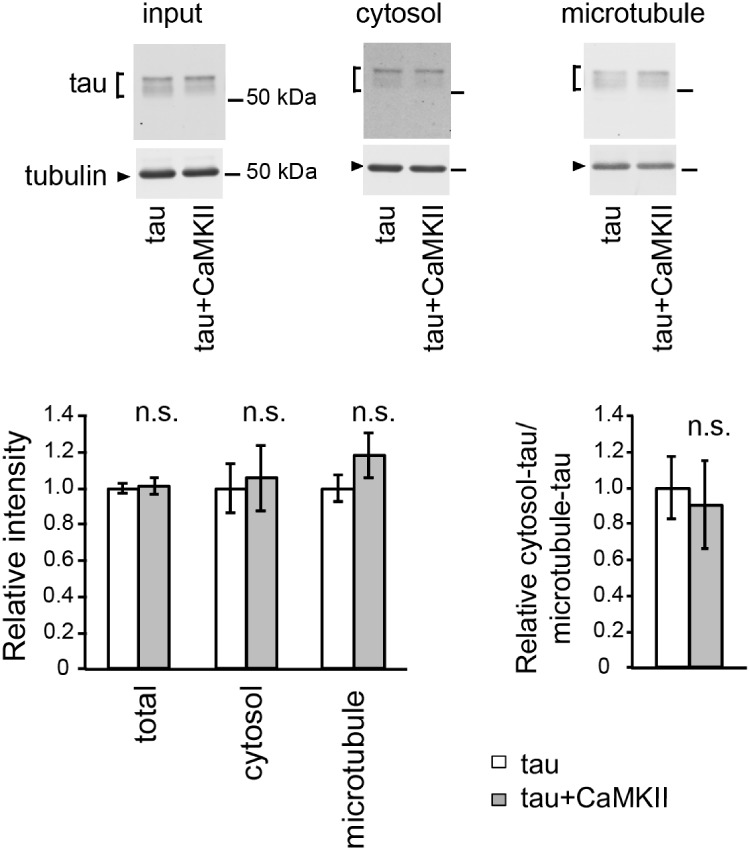
Alterations in the distribution of tau are associated with tau toxicity, and tau phosphorylation at Ser262/356 initiates mismetabolism of tau by increasing the levels of microtubule-unbound free tau (10). Because CaMKII regulates microtubule stability during synaptic activation (47), CaMKII might augment toxicity of tau phosphorylated at Ser262/356 by promoting tau detachment from microtubules. Thus, we tested the effect of CaMKII overexpression on the binding of tau to microtubules. We found that CaMKII overexpression did not significantly affect the distribution of tau on microtubules or in the cytosol (Fig. 4A), indicating that CaMKII does not cause mislocalization of tau in this fly model.
Fig. 4.
CaMKII does not alter tau distribution to the microtubule and cytosol. The levels of tau and tubulin in the lysate of fly heads expressing tau alone (tau) or co-expressing tau and CaMKII (tau+CaMKII) before sedimentation (input), in the supernatant (cytosol) and in the pellet containing microtubules (microtubule) were analyzed by western blotting by using anti-tau and anti-tubulin antibodies. The same amount of proteins from each genotype was loaded. Mean ± SD, n = 5, n.s., P > 0.05, Student’s t-test.
Discussion
Tau accumulation is observed in multiple neurodegenerative diseases, including AD. Abnormal metabolism of tau is induced by post-translational modifications of tau, including phosphorylation (1). Tau phosphorylation at Ser262/356 is thought to be one of the pathological changes that initiate tau mislocalization and mismetabolism (8–13). Phosphorylation at these sites decreases tau binding to microtubules and increases the levels of microtubule-unbound tau, leading to further phosphorylation of tau at other sites, and promotes tau-induced neurodegeneration (9, 11, 14–17). Although this cascade of events plays a central role in the disease pathogenesis, it is not fully understood how this process is modified by other cellular changes under pathological conditions.
Neuronal hyperexcitability and disruption of intracellular calcium homeostasis are observed in the early stages of AD and other neurodegenerative diseases (18, 19). Impairment of cellular pathways involved in Ca2+ buffering under pathological conditions can increase intracellular Ca2+ (48, 49), which may result in prolonged activity of CaMKII and its mislocalization outside of synapses (50). In this study, we demonstrated that excess activity of CaMKII promotes neurodegeneration caused by tau phosphorylated at Ser262/356 using a Drosophila model of tauopathy. Our results suggest that elevated levels of tau phosphorylated at Ser262/356 and aberrant activation of CaMKII work in concert to promote tau-mediated neurodegeneration in disease pathogenesis, and that dysregulation of CaMKII activity, if it coincides with accumulation of tau phosphorylated at Ser262/356, significantly promotes tau-mediated neurodegeneration. Future studies of the mechanisms underlying enhancement of tau toxicity via CaMKII, including possible roles of other phosphorylation sites in tau, will advance our understanding of disease pathogenesis.
Supplementary Data
Supplementary Data are available at JB Online.
Supplementary Material
Acknowledgements
We thank Drs. Mel B. Feany and Leslie Griffith, Bloomington Drosophila Stock Center, Vienna Drosophila Resource Center for fly stocks. We thank Lijuan Zhao, Dr. Michiko Sekiya and Dr. Akira Ishio for technical assistance. The authors declare no conflict of interest associated with this manuscript.
Funding
This work was supported by grants from the National Institutes of Health [R01AG032279-A1] (to K.M.I. and K.A.), and the Alzheimer’s Association [NIRG-10-173189] (to K.A.), and in part by the National Institute of Health [U01AG046170-01] (to K.M.I. and K.A.), The Research Funding for Longevity Sciences (28–26) from National Center for Geriatrics and Gerontology (NCGG), Japan (to K.M.I.), Grants in aid for Scientific Research [JSPS KAKENHI Grant number16K08637] (to K.M.I.), Takeda Science Foundation, Japan (to K.M.I.), research award from Hoansha foundation (to K.A.), Grant-in-Aid for Scientific Research on Innovative Areas (Brain Protein Aging and Dementia Control) [JSPS KAKENHI Grant number 15H01564] (to K.A.) and Grants in aid for Scientific Research [JSPS KAKENHI Grant number 15K06712] (to K.A.), and Takeda Science Foundation, Japan (to K.A.).
Conflict of Interest
None declared.
References
- 1. Holtzman D.M., Carrillo M.C., Hendrix J.A., Bain L.J., Catafau A.M., Gault L.M., Goedert M., Mandelkow E., Mandelkow E.M., Miller D.S., Ostrowitzki S., Polydoro M., Smith S., Wittmann M., Hutton M. (2016) Tau: from research to clinical development. Alzheimer's Dementia: J. Alzheimer's Assoc. 12, 1033–1039 [DOI] [PubMed] [Google Scholar]
- 2. Grundke-Iqbal I., Iqbal K., Tung Y.C., Quinlan M., Wisniewski H.M., Binder L.I. (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 83, 4913–4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hasegawa M., Morishima-Kawashima M., Takio K., Suzuki M., Titani K., Ihara Y. (1992) Protein sequence and mass spectrometric analyses of tau in the Alzheimer's disease brain. J. Biol. Chem. 267, 17047–17054 [PubMed] [Google Scholar]
- 4. Hanger D.P., Betts J.C., Loviny T.L., Blackstock W.P., Anderton B.H. (1998) New phosphorylation sites identified in hyperphosphorylated tau (paired helical filament-tau) from Alzheimer's disease brain using nanoelectrospray mass spectrometry. J. Neurochem. 71, 2465–2476 [DOI] [PubMed] [Google Scholar]
- 5. Morishima-Kawashima M., Hasegawa M., Takio K., Suzuki M., Yoshida H., Titani K., Ihara Y. (1995) Proline-directed and non-proline-directed phosphorylation of PHF-tau. J. Biol. Chem. 270, 823–829 [DOI] [PubMed] [Google Scholar]
- 6. Wang Y., Mandelkow E. (2016) Tau in physiology and pathology. Nat. Rev. Neurosci. 17, 5–21 [DOI] [PubMed] [Google Scholar]
- 7. Augustinack J.C., Schneider A., Mandelkow E.M., Hyman B.T. (2002) Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol. 103, 26–35 [DOI] [PubMed] [Google Scholar]
- 8. Yu W., Polepalli J., Wagh D., Rajadas J., Malenka R., Lu B. (2012) A critical role for the PAR-1/MARK-tau axis in mediating the toxic effects of Abeta on synapses and dendritic spines. Hum. Mol. Genet. 21, 1384–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zempel H., Thies E., Mandelkow E., Mandelkow E.M. (2010) Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J. Neurosci. 30, 11938–11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ando K., Maruko-Otake A., Ohtake Y., Hayashishita M., Sekiya M., Iijima K.M. (2016) Stabilization of microtubule-unbound tau via tau phosphorylation at Ser262/356 by Par-1/MARK contributes to augmentation of AD-related phosphorylation and abeta42-induced tau toxicity. PLoS Genet. 12, e1005917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ando K., Oka M., Ohtake Y., Hayashishita M., Shimizu S., Hisanaga S., Iijima K.M. (2016) Tau phosphorylation at Alzheimer's disease-related Ser356 contributes to tau stabilization when PAR-1/MARK activity is elevated. Biochem. Biophys. Res. Commun. 478, 929–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nishimura I., Yang Y., Lu B. (2004) PAR-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in Drosophila. Cell 116, 671–682 [DOI] [PubMed] [Google Scholar]
- 13. Lasagna-Reeves C.A., de Haro M., Hao S., Park J., Rousseaux M.W., Al-Ramahi I., Jafar-Nejad P., Vilanova-Velez L., See L., De Maio A., Nitschke L., Wu Z., Troncoso J.C., Westbrook T.F., Tang J., Botas J., Zoghbi H.Y. (2016) Reduction of nuak1 decreases tau and reverses phenotypes in a tauopathy mouse model. Neuron 92, 407–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fischer D., Mukrasch M.D., Biernat J., Bibow S., Blackledge M., Griesinger C., Mandelkow E., Zweckstetter M. (2009) Conformational changes specific for pseudophosphorylation at serine 262 selectively impair binding of tau to microtubules. Biochemistry 48, 10047–10055 [DOI] [PubMed] [Google Scholar]
- 15. Feuillette S., Miguel L., Frebourg T., Campion D., Lecourtois M. (2010) Drosophila models of human tauopathies indicate that Tau protein toxicity in vivo is mediated by soluble cytosolic phosphorylated forms of the protein. J. Neurochem. 113, 895–903 [DOI] [PubMed] [Google Scholar]
- 16. Thies E., Mandelkow E.M. (2007) Missorting of tau in neurons causes degeneration of synapses that can be rescued by the kinase MARK2/Par-1. J. Neurosci. 27, 2896–2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Biernat J., Gustke N., Drewes G., Mandelkow E.M., Mandelkow E. (1993) Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron 11, 153–163 [DOI] [PubMed] [Google Scholar]
- 18. LaFerla F.M. (2002) Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nat. Rev. Neurosci. 3, 862–872 [DOI] [PubMed] [Google Scholar]
- 19. Ghosh A., Giese K.P. (2015) Calcium/calmodulin-dependent kinase II and Alzheimer's disease. Mol. Brain 8, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller S.G., Kennedy M.B. (1986) Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell 44, 861–870 [DOI] [PubMed] [Google Scholar]
- 21. Yoshimura Y., Ichinose T., Yamauchi T. (2003) Phosphorylation of tau protein to sites found in Alzheimer's disease brain is catalyzed by Ca2+/calmodulin-dependent protein kinase II as demonstrated tandem mass spectrometry. Neurosci. Lett. 353, 185–188 [DOI] [PubMed] [Google Scholar]
- 22. Wei Y., Han C., Wang Y., Wu B., Su T., Liu Y., He R. (2015) Ribosylation triggering Alzheimer's disease-like Tau hyperphosphorylation via activation of CaMKII. Aging Cell 14, 754–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang J.Z., Grundke-Iqbal I., Iqbal K. (2007) Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur. J. Neurosci. 25, 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamamoto H., Hiragami Y., Murayama M., Ishizuka K., Kawahara M., Takashima A. (2005) Phosphorylation of tau at serine 416 by Ca2+/calmodulin-dependent protein kinase II in neuronal soma in brain. J. Neurochem. 94, 1438–1447 [DOI] [PubMed] [Google Scholar]
- 25. McKee A.C., Kosik K.S., Kennedy M.B., Kowall N.W. (1990) Hippocampal neurons predisposed to neurofibrillary tangle formation are enriched in type II calcium/calmodulin-dependent protein kinase. J. Neuropathol. Exp. Neurol. 49, 49–63 [DOI] [PubMed] [Google Scholar]
- 26. Mah V.H., Eskin T.A., Kazee A.M., Lapham L., Higgins G.A. (1992) In situ hybridization of calcium/calmodulin dependent protein kinase II and tau mRNAs; species differences and relative preservation in Alzheimer's disease. Brain Res. Mol. Brain Res. 12, 85–94 [DOI] [PubMed] [Google Scholar]
- 27. Simonian N.A., Elvhage T., Czernik A.J., Greengard P., Hyman B.T. (1994) Calcium/calmodulin-dependent protein kinase II immunostaining is preserved in Alzheimer's disease hippocampal neurons. Brain Res. 657, 294–299 [DOI] [PubMed] [Google Scholar]
- 28. Xiao J., Perry G., Troncoso J., Monteiro M.J. (1996) Alpha-calcium-calmodulin-dependent kinase II is associated with paired helical filaments of Alzheimer's disease. J. Neuropathol. Exp. Neurol. 55, 954–963 [DOI] [PubMed] [Google Scholar]
- 29. Wang Y.J., Chen G.H., Hu X.Y., Lu Y.P., Zhou J.N., Liu R.Y. (2005) The expression of calcium/calmodulin-dependent protein kinase II-alpha in the hippocampus of patients with Alzheimer's disease and its links with AD-related pathology. Brain Res. 1031, 101–108 [DOI] [PubMed] [Google Scholar]
- 30. Lin K.F., Chang R.C., Suen K.C., So K.F., Hugon J. (2004) Modulation of calcium/calmodulin kinase-II provides partial neuroprotection against beta-amyloid peptide toxicity. Eur. J. Neurosci. 19, 2047–2055 [DOI] [PubMed] [Google Scholar]
- 31. Wittmann C.W., Wszolek M.F., Shulman J.M., Salvaterra P.M., Lewis J., Hutton M., Feany M.B. (2001) Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293, 711–714 [DOI] [PubMed] [Google Scholar]
- 32. Koh Y.H., Popova E., Thomas U., Griffith L.C., Budnik V. (1999) Regulation of DLG localization at synapses by CaMKII-dependent phosphorylation. Cell 98, 353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Griffith L.C., Greenspan R.J. (1993) The diversity of calcium/calmodulin-dependent protein kinase II isoforms in Drosophila is generated by alternative splicing of a single gene. J. Neurochem. 61, 1534–1537 [DOI] [PubMed] [Google Scholar]
- 34. Iijima-Ando K., Sekiya M., Maruko-Otake A., Ohtake Y., Suzuki E., Lu B., Iijima K.M. (2012) Loss of axonal mitochondria promotes tau-mediated neurodegeneration and Alzheimer's disease-related tau phosphorylation via PAR-1. PLoS Genet. 8, e1002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lu H., Leung H.T., Wang N., Pak W.L., Shieh B.H. (2009) Role of Ca2+/calmodulin-dependent protein kinase II in Drosophila photoreceptors. J. Biol. Chem. 284, 11100–11109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Andersen R., Li Y., Resseguie M., Brenman J.E. (2005) Calcium/calmodulin-dependent protein kinase II alters structural plasticity and cytoskeletal dynamics in Drosophila. J. Neurosci. 25, 8878–8888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chun-Jen Lin C., Summerville J.B., Howlett E., Stern M. (2011) The metabotropic glutamate receptor activates the lipid kinase PI3K in Drosophila motor neurons through the calcium/calmodulin-dependent protein kinase II and the nonreceptor tyrosine protein kinase DFak. Genetics 188, 601–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haghighi A.P., McCabe B.D., Fetter R.D., Palmer J.E., Hom S., Goodman C.S. (2003) Retrograde control of synaptic transmission by postsynaptic CaMKII at the Drosophila neuromuscular junction. Neuron 39, 255–267 [DOI] [PubMed] [Google Scholar]
- 39. Beumer K., Matthies H.J., Bradshaw A., Broadie K. (2002) Integrins regulate DLG/FAS2 via a CaM kinase II-dependent pathway to mediate synapse elaboration and stabilization during postembryonic development. Development 129, 3381–3391 [DOI] [PubMed] [Google Scholar]
- 40. Park D., Coleman M.J., Hodge J.J., Budnik V., Griffith L.C. (2002) Regulation of neuronal excitability in Drosophila by constitutively active CaMKII. J. Neurobiol. 52, 24–42 [DOI] [PubMed] [Google Scholar]
- 41. Nesler K.R., Starke E.L., Boin N.G., Ritz M., Barbee S.A. (2016) Presynaptic CamKII regulates activity-dependent axon terminal growth. Mol. Cell. Neurosci. 76, 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kadas D., Tzortzopoulos A., Skoulakis E.M., Consoulas C. (2012) Constitutive activation of Ca2+/calmodulin-dependent protein kinase II during development impairs central cholinergic transmission in a circuit underlying escape behavior in Drosophila. J. Neurosci. 32, 170–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Griffith L.C., Verselis L.M., Aitken K.M., Kyriacou C.P., Danho W., Greenspan R.J. (1993) Inhibition of calcium/calmodulin-dependent protein kinase in Drosophila disrupts behavioral plasticity. Neuron 10, 501–509 [DOI] [PubMed] [Google Scholar]
- 44. Jin P., Griffith L.C., Murphey R.K. (1998) Presynaptic calcium/calmodulin-dependent protein kinase II regulates habituation of a simple reflex in adult Drosophila. J. Neurosci. 18, 8955–8964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Timmerman C., Suppiah S., Gurudatta B.V., Yang J., Banerjee C., Sandstrom D.J., Corces V.G., Sanyal S. (2013) The Drosophila transcription factor Adf-1 (nalyot) regulates dendrite growth by controlling FasII and Staufen expression downstream of CaMKII and neural activity. J. Neurosci. 33, 11916–11931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sadanandappa M.K., Blanco Redondo B., Michels B., Rodrigues V., Gerber B., VijayRaghavan K., Buchner E., Ramaswami M. (2013) Synapsin function in GABA-ergic interneurons is required for short-term olfactory habituation. J. Neurosci. 33, 16576–16585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lemieux M., Labrecque S., Tardif C., Labrie-Dion E., Lebel E., De Koninck P. (2012) Translocation of CaMKII to dendritic microtubules supports the plasticity of local synapses. J. Cell. Biol. 198, 1055–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stutzmann G.E., Smith I., Caccamo A., Oddo S., Laferla F.M., Parker I. (2006) Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer's disease mice. J. Neurosci. 26, 5180–5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Supnet C., Grant J., Kong H., Westaway D., Mayne M. (2006) Amyloid-beta-(1-42) increases ryanodine receptor-3 expression and function in neurons of TgCRND8 mice. J. Biol. Chem. 281, 38440–38447 [DOI] [PubMed] [Google Scholar]
- 50. Reese L.C., Laezza F., Woltjer R., Taglialatela G. (2011) Dysregulated phosphorylation of Ca(2+)/calmodulin-dependent protein kinase II-alpha in the hippocampus of subjects with mild cognitive impairment and Alzheimer's disease. J. Neurochem. 119, 791–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.