Abstract
NO3− is present at micromolar concentrations in seawater and must be absorbed by marine plants against a steep electrochemical potential difference across the plasma membrane. We studied NO3− transport in the marine angiosperm Zostera marina L. to address the question of how NO3− uptake is energized. Electrophysiological studies demonstrated that micromolar concentrations of NO3− induced depolarizations of the plasma membrane of leaf cells. Depolarizations showed saturation kinetics (Km = 2.31 ± 0.78 μm NO3−) and were enhanced in alkaline conditions. The addition of NO3− did not affect the membrane potential in the absence of Na+, but depolarizations were restored when Na+ was resupplied. NO3−-induced depolarizations at increasing Na+ concentrations showed saturation kinetics (Km = 0.72 ± 0.18 mm Na+). Monensin, an ionophore that dissipates the Na+ electrochemical potential, inhibited NO3−-evoked depolarizations by 85%, and NO3− uptake (measured by depletion from the external medium) was stimulated by Na+ ions and by light. Our results strongly suggest that NO3− uptake in Z. marina is mediated by a high-affinity Na+-symport system, which is described here (for the first time to our knowledge) in an angiosperm. Coupling the uptake of NO3− to that of Na+ enables the steep inwardly-directed electrochemical potential for Na+ to drive net accumulation of NO3− within leaf cells.
Zostera marina L. is an aquatic angiosperm that grows in a medium with a high salinity—seawater itself—with NaCl concentrations in the region of 0.5 m. Leaf cells of this plant exhibit a plasma membrane potential (Em) of around −160 mV in natural seawater (Fernández et al., 1999). Molecular and physiological evidence indicates that the Em in this halophyte is maintained by the activity of a H+-pump (Fukuhara et al., 1996; Fernández et al., 1999). With this highly negative Em, the uptake of essential anions such as NO3−, which usually occurs in seawater at concentrations of 1 to 500 μm (Riley and Chester, 1971) and is probably below 10 μm in the close environment of the plant (Hernández et al., 1993), must be energized.
Most studies on vascular plants and algae have reported that NO3− transport is powered by the electrochemical potential for protons present across the plasma membrane of plant cells (Ullrich, 1992). Evidence includes simultaneous measurements of NO3− and H+ fluxes (Mistrik and Ullrich, 1996), NO3−-evoked membrane depolarizations (McClure et al., 1990; Ullrich and Novacky, 1990; Glass et al., 1992), and pH dependence of NO3−-elicited inward currents both in intact plants (Meharg and Blatt, 1995) and in oocytes expressing plant NO3− transporters (Tsay et al., 1993; Zhou et al., 1998; Liu et al., 1999). Four different NO3− uptake systems have been identified in higher plants: both low- and high-affinity systems are present and can be constitutive or inducible (Wang and Crawford, 1996; Liu et al., 1999). High-affinity systems show saturation kinetics and operate at concentrations lower than 0.5 mm with Km values in the range of 7 to 100 μm; low-affinity systems show linear kinetics and function at concentrations above 0.5 mm (Wang and Crawford, 1996; Crawford and Glass, 1998).
Energization of solute transport by H+ coupling is prevalent among bacteria, fungi, and plants. Nevertheless, for the plasma membrane of most cells the presence of an inwardly directed electrochemical potential difference for Na+ can potentially be exploited for energization of solute transport through Na+ coupling. Accordingly, a number of Na+-dependent transport systems have been identified. Na+-dependent uptake of Glc and amino acids has been shown in the marine diatom Cyclotella (Hellebust, 1978), and phosphate transport has been reported to be stimulated by Na+ in several green algae (Raven, 1984). In the case of NO3−, Na+-dependent uptake has been described only in the marine diatom Phaeodactylum tricornutum (Rees et al., 1980) and in cyanobacteria (Lara et al., 1993).
During the past decade an emerging number of Na+-coupled transport systems have also been discovered at the plasma membranes of multicellular plants. In freshwater charophyte algae, the influx of K+, urea, and Lys has been shown to be Na+ dependent and/or directly coupled to the transport of Na+ (Smith and Walker, 1989; Walker and Sanders, 1991), and Na+-dependent K+ uptake has also been demonstrated in some aquatic angiosperms such as Egeria, Elodea, and Vallisneria (Walker, 1994; Maathuis et al., 1996). A Na+,K+ symporter, HKT1, has been cloned from wheat (Rubio et al., 1995), but it remains unclear whether Na+ coupling comprises the dominant mode of energizing K+ uptake in terrestrial angiosperms (Maathuis et al., 1996).
The elevated Na+ concentration present in seawater and the highly negative Em in Z. marina makes feasible the existence of this type of alternative transport system powered by the putative electrochemical potential for Na+. To test this hypothesis, we have investigated NO3− transport at the plasma membrane of leaf cells of Z. marina to determine the possible interactions between Na+ and NO3−. These interactions have been studied with respect to the electrophysiological properties of the plasma membrane and to NO3− depletion in media surrounding whole leaves.
MATERIALS AND METHODS
Plant Material
Zostera marina L. plants were collected off the coast of Málaga (Spain) at a 5-m depth. Plants were maintained in the laboratory in natural seawater at 15°C and a light intensity of 150 μmol m−2 s−1, with a photoperiod of 16 h of light and 8 h of darkness.
Plants were N-starved prior to experiments for at least 3 d in N-free artificial seawater (ASW) adjusted to pH 8.0 with NaOH. The composition of ASW was 0.010 mm KH2PO4, 1.2 mm NaHCO3, 5 mm K2SO4, 12 mm CaCl2, 15 mm MgSO4, 42.5 mm MgCl2, and 500 mm NaCl.
Membrane Potential Measurements
Leaf pieces were excised to remove partially the epidermis, and were mounted in a plexiglass chamber (volume 1.1 mL). Continuous perfusion of the assay medium was maintained at a flux of approximately 10 mL/min. Both epidermal and mesophyll cells were impaled with single-barrel electrodes. Membrane potentials were measured using the standard glass microelectrode technique as described by Felle (1981). Micropipettes were backfilled with 0.5 m KCl. Microelectrodes were fixed to electrode holders containing an Ag/AgCl pellet, and connected to a high-impedance differential amplifier (FD-223, World Precision Instruments, Sarasota, FL). Tip potentials never exceeded −10 mV.
Experiments were carried out in N-free ASW (as above) buffered with 10 mm 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-Tris(hydroxymethyl)-amino-methane (Tris) to pH 8.0. NO3− was added as KNO3. To check the effect of pH, the same buffer was adjusted to pH 6.0 or 7.0. To test the effect of Na+, N-free ASW with a slightly different composition was used (NaCl-ASW: 2.5 mm KHCO3, 2.5 mm K2SO4, 5 mm CaSO4, 5 mm MgSO4, 500 mm NaCl, and 10 mm HEPES-Tris). In Na+-free ASW, NaCl was substituted by 0.8 m sorbitol (sorbitol-ASW). Na+ was added to the medium at increasing concentrations as Na2SO4 or NaCl.
NO3− Depletion Experiments
Whole plants were maintained for 3 d in N-free ASW and were preincubated in the assay medium for 1 h before starting the experiment. The composition of assay medium was the same as for impalements. Whole leaves (0.6–0.9 g fresh weight) were incubated in 100-mL flasks. Three replicates were assayed for each treatment (NaCl-ASW, sorbitol-ASW, sorbitol-ASW + 5 mm Na2SO4, and sorbitol-ASW + 10 mm NaCl). The assay was carried out with slow and constant agitation at 25°C and at a light intensity of 50 μmol m−2 s−1. The first treatment (NaCl-ASW) was also assayed in darkness. KNO3 was added at an initial concentration of 100 μm. Samples were taken at 0, 1, 2, 4, 8, 12, and 24 h. NO3− was analyzed colorimetrically as described in García-Sánchez et al. (1993).
Chemicals
pH buffers and sorbitol were from Sigma-Aldrich (St. Louis). Monensin (Sigma) was dissolved in ethanol at a concentration of 50 mm.
Data Analysis
Data are given as means ± se. Membrane depolarization data were fitted with the Michaelis-Menten equation using a non-linear regression computer program (KaleidaGraph, Synergy Software, Reading, PA).
RESULTS
Effect of NO3− Additions on the Membrane Potential
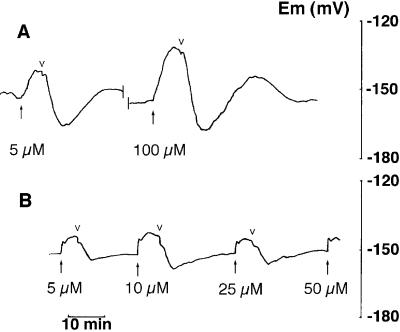
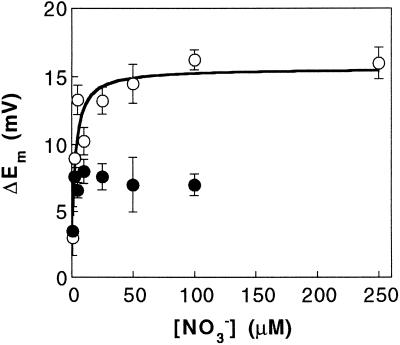
Figure 1A demonstrates that NO3− evoked a rapid membrane depolarization in N-starved plants in the light. Measurable changes in Em were evident even at 1 μm NO3− (not shown). When NO3− was washed from the medium, the membrane first hyperpolarized so that Em was transiently more negative than the original resting potential, and then depolarized with another overshoot to a value close to that before NO3− addition (Fig. 1A). Depolarizations showed saturation at concentrations around 50 μm NO3− (Fig. 2). Because depolarization is an integral function of the net charge carried by the NO3− transport system, the concentration dependence of the depolarization can be taken as a rough guide to the affinity of this system for its substrate. Fitting the data with the Michaelis-Menten equation yielded a Km value of 2.31 ± 0.78 μm NO3− and a maximum depolarization of 15.6 ± 0.9 mV (Fig. 2).
Figure 1.
Effect of the addition of NO3− on Em. A, Tissue in the light, incubated in ASW, showing the response of a single mesophyll leaf cell to the addition of 5 and 100 μm KNO3. B, Tissue in the dark with KNO3 added at the concentrations indicated. Small downward arrowheads indicate the onset of the NO3− wash.
Figure 2.
Membrane depolarizations induced by increasing the NO3− concentration. Mesophyll leaf cells were impaled in ASW in the light (○) or in the dark (●). Data are the means ± se of three independent replicates. Values in the light were fitted with the Michaelis-Menten relationship, as shown by the curve.
NO3−-induced depolarizations were lower in the dark than in the light, with maximum values around 10 mV, and they did not exhibit saturation kinetics (Figs. 1B and 2). The response of Em to NO3− additions was also different (Fig. 1B): the depolarization was very rapid and when NO3− was washed from the medium the Em did not exhibit the overshoot in the depolarizing direction before returning to the resting value.
The depolarizations elicited by NO3− suggest that uptake of this anion involves flow of positive charge into the cell.
Effect of pH on NO3−-Induced Depolarizations
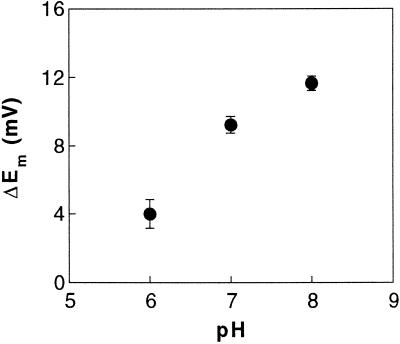
Many H+-coupled transport systems in plants are activated by a rise in external H+ concentration (Bush, 1993), and this enhancement of electrophoretic transport can be reflected in a larger transport-related depolarization as the pH is lowered. We therefore examined the pH dependence of the NO3−-induced depolarization. In contrast to many H+-coupled transport systems, the depolarizations induced by saturating NO3− concentrations (100 μm) were lower at acidic than at alkaline pH (Fig. 3). A shift of external pH from 8.0 to 6.0 decreased the magnitude of the depolarization by a factor of about 3.
Figure 3.
Effect of pH on NO3−-induced depolarizations. Mesophyll leaf cells were impaled in ASW buffered at different pHs and challenged with saturating NO3− concentrations (100 μm KNO3). Values are the means ± se of five independent replicates.
Effect of Na+ on NO3−-Evoked Depolarizations
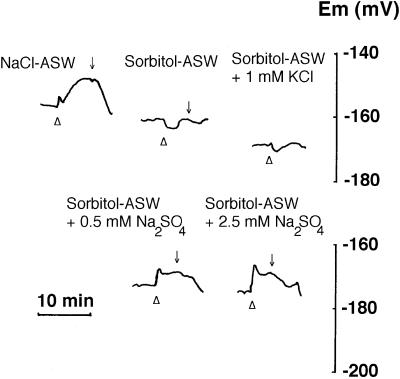
To determine whether Na+ was involved as the coupling ion, we examined the capacity of NO3− to depolarize the membrane in the absence of Na+. Replacement of NaCl in the medium with isosmotic sorbitol produced a slight hyperpolarization of the membrane (data not shown). In Na+-free conditions, NO3−-induced membrane depolarizations were abolished (Fig. 4). The addition of Na+ (as Na2SO4) at concentrations as low as 250 μm to NaCl-free ASW (sorbitol-ASW) restored the NO3−-induced depolarizations observed in NaCl-ASW (Figs. 4 and 5). In contrast, NO3− additions in the presence of KCl did not produce any effect (Fig. 4) and depolarizations were of the same order when Na+ was added as NaCl or Na2SO4 (data not shown).
Figure 4.
NO3−-induced depolarizations in the presence and absence of Na+. Lines show the response of Em of a single mesophyll leaf cell to 100 μm KNO3 addition (▵). The tissue was incubated sequentially in different media: ASW containing NaCl (NaCl-ASW), NaCl-free ASW (sorbitol-ASW), and sorbitol-ASW to which KCl or Na2SO4 were added as indicated. Downward arrows indicate onset of NO3− wash.
Figure 5.
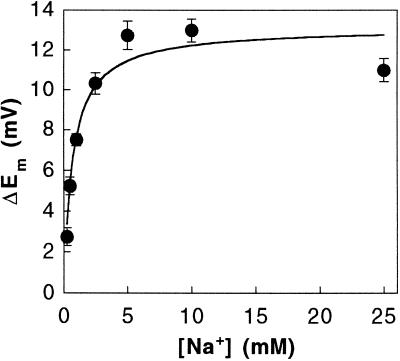
Effect of increasing Na+ concentrations on NO3−-induced depolarization. Leaf cells were incubated in NaCl-free ASW (sorbitol-ASW) and challenged with saturating NO3− concentrations (100 μm KNO3). Na+ was added as Na2SO4. The data are the means ± se of three independent replicates. The solid line represents a non-linear least squares fit of the data to the Michaelis-Menten equation.
The kinetics with respect to Na+ ions was further investigated, with depolarization in response to saturating NO3− concentrations (100 μm) monitored over a range of Na+ concentrations (Fig. 5). The results demonstrate that the response is effectively saturated at around 5 mm Na+. Fitting of the data with the Michaelis-Menten equation yielded a Km of 0.72 ± 0.18 mm Na+ and a maximum depolarization of 13.1 ± 0.7 mV.
Effect of Monensin on NO3−-Induced Depolarizations
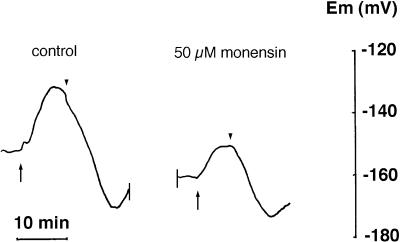
The ionophore monensin, which dissipates the electrochemical potential for Na+ (Pressman, 1976), was added to the assay medium at a concentration of 50 μm to determine its effects on the NO3−-induced depolarization. The presence of monensin in absence of NO3− failed to evoke significant changes in Em (data not shown). However, monensin partially inhibited the depolarization induced by NO3− (Fig. 6), showing a mean inhibition value in tests with 100 μm NO3− of 85.0% ± 20.7% (n = 8).
Figure 6.
Response of Em of a single mesophyll leaf cell to the addition of 100 μm KNO3 (indicated by the long upward arrows) incubated in ASW before (control) and after the addition of 50 μm monensin. ▾, Onset of NO3− wash.
NO3− Uptake Rates in the Absence and Presence of Na+
If NO3− transport in Z. marina is Na+ coupled, then net uptake from the medium should be enhanced in the presence of Na+. Table I shows that NO3− was depleted from the medium by leaves at higher rates when Na+ was present. NO3− uptake rates were measured in NaCl-free ASW (sorbitol-ASW) and in the same medium but with the addition of 10 mm Na+ as Na2SO4 or NaCl. The differences between the mean NO3− uptake rates in the presence and absence of Na+ were significant (t test, P < 0.05), and the addition of Na+ more than doubled the net NO3− uptake rate. Furthermore, there were no significant differences between the means obtained in the presence of Na2SO4 or NaCl.
Table I.
Effect of Na+ on NO3− uptake rates
| Treatment | NO3− Uptake Rate |
|---|---|
| μmol NO3− g−1 fresh wt h−1 | |
| Sorbitol-ASW | 0.12 ± 0.00 |
| Sorbitol-ASW + 5 mm Na2SO4 | 0.35 ± 0.06 |
| Sorbitol-ASW + 10 mm NaCl | 0.28 ± 0.05 |
| NaCl-ASW, light | 0.62 ± 0.09 |
| NaCl-ASW, dark | 0.34 ± 0.05 |
Whole leaves were incubated in different assay media: ASW containing 0.5 m NaCl (NaCl-ASW) or ASW in which NaCl was substituted with 0.8 m sorbitol (sorbitol-ASW). Additions of Na+ were made to sorbitol-ASW as shown. NO3− uptake rates were measured in the light except where indicated. The values shown are the means ± se of three replicates of a representative experiment.
The uptake of NO3− was also measured in ASW containing 0.5 m NaCl in the light and in the dark. The difference between the mean values was significant (t test, P < 0.1), being 2-fold higher in the light than in the dark (Table I).
DISCUSSION
The depolarizations induced by NO3− in Z. marina indicate that the uptake of this anion is coupled with the inward movement of positive charge. However, since the magnitude of the depolarization is decreased at acid pH, it appeared possible that in this plant NO3− uptake is not coupled with the entrance of H+ as is the rule in other angiosperms (Ullrich, 1992). Nevertheless, the decrease in depolarization observed at low pH might be explained by a direct pH effect on transporter kinetics or by an increase in membrane conductance.
In contrast, the lack of any NO3−-induced depolarization in Na+-free ASW and the restoring of the depolarization when Na+ is added to the medium suggest that NO3− uptake into leaf cells is coupled with the entrance of Na+. The stimulation of NO3− uptake rates by the leaves in the presence of Na+ points to the same conclusion, as does the inhibition of NO3−-evoked depolarizations in the presence of monensin, an ionophore that dissipates the Na+ electrochemical gradient. However, because monensin, like other cation-H+ exchangers, acts as an uncoupler of photosynthesis and respiration (Rottenberg, 1977), the ionophore might also affect NO3− transport indirectly via an effect on nitrogen metabolism.
Na+-coupled NO3− transport has been demonstrated previously only in the marine diatom Phaeodactylum tricornutum (Rees et al., 1980) and in cyanobacteria, where it has been well defined (Lara et al., 1993). Calculated Km values for NO3− (2.31 ± 0.78 μm) and Na+ (0.72 ± 0.18 mm) in Z. marina are quite similar to the values reported in the cyanobacterium Anacystis nidulans, which exhibits a Km for NO3− of 1.6 ± 0.2 μm and for Na+ of 0.36 ± 0.04 mm (Rodríguez et al., 1994). The Km for Na+ in Z. marina is also close to the value reported for the marine diatom (2.58 ± 0.56 mm, Rees et al., 1980). These low-millimolar Km values for Na+ imply that the NO3− transporter of Z. marina, as well as those of the other organisms, will be functioning at saturating Na+ concentrations in a marine environment. It should be noted, though, that the Km for Na+ reported in the present study was derived at a saturating concentration of NO3−, and that a rise in Km as the concentration of the substrate ion is lowered cannot be precluded (Sanders et al., 1984).
Other features of NO3− transport are similar in Z. marina and cyanobacteria. NO3− uptake in cyanobacteria is inhibited by 60% in the presence of 25 μm monensin (Rodríguez et al., 1992); in Z. marina, 50 μm monensin produces an inhibition of the NO3−-induced depolarizations of about 85%. Moreover, a cyanobacterium mutant lacking NO3− reductase activity shows an inhibition of NO3− uptake at concentrations around 25 μm (Rodríguez et al., 1994). In Z. marina NO3−-evoked depolarizations, as well as NO3− uptake, are smaller in the dark and depolarizations reach saturation at lower concentration values than in the light. The lower NO3− uptake rate in the cyanobacterium mutant is explained by apparent substrate inhibition of the transporter (Rodríguez et al., 1994), as NO3− cannot be reduced. This effect might also be invoked here to explain the decrease in NO3− transport in the dark observed in Z. marina, as it is known that NO3− reductase activity declines in the absence of light mainly due to a lack of reducing power (Beevers and Hageman, 1980).
Maximum NO3−-induced depolarizations measured in Z. marina are of the same order as the values reported in Arabidopsis root hairs (Meharg and Blatt, 1995; Wang and Crawford, 1996). The response of Em when NO3− is washed is more complex in Z. marina than in Arabidopsis root hairs, and it is different in the light and in the dark. This suggests a role of N metabolism in determining the effect of NO3− additions on Em (Mistrik and Ullrich, 1996) that could be more important in the light and in photosynthetically active tissue.
A kinetic study of Na+-dependent NO3− transport in A. nidulans indicates that Na+ and NO3− ions are the real substrates of the transporter and a minimum stoichiometry of one Na+ per NO3− and a maximum of two has been proposed (Rodríguez et al., 1994). In Z. marina, the stoichiometry must be >1, since we have recorded membrane depolarization in response to NO3−.
Z. marina, as an angiosperm, evolved from terrestrial species that presumably, like extant species, transported NO3− from the soil using H+-coupled transport systems. Therefore the question arises as to why Na+-coupled NO3− transport evolved in Z. marina. This question might best be addressed by considering the thermodynamic aspects of cation-coupled NO3− transport in a marine environment, and that requires knowledge of the respective NO3−, Na+, and H+ electrochemical potentials across the plasma membrane. Cytosolic concentrations of NO3− ([NO3−]c) and Na+ ([Na+]c) have been estimated in some marine plants, showing a range for Na+ of 1 to 50 mm and for NO3− of 0 to 1 mm (Raven, 1984). More accurate cytosolic measurements in non-marine plants give values of 3 to 5 mm for NO3− in barley root epidermal cells (Walker et al., 1995; van der Leij et al., 1998) and 50 mm for Na+ in a salt-tolerant Chara species (Kiegle and Bisson, 1996). (Although total internal Na+ concentrations have been estimated in two species of seagrass, being around 100 mm in epidermal leaf cells and showing higher values in other tissues [Beer et al., 1980], such estimates will reflect primarily the composition of the large central vacuole rather than that of the cytosol.) We might therefore take as reasonable estimates of [NO3−]c and [Na+]c in Z. marina respective values of 3 and 50 mm. The cytosolic pH has been measured as 7.3 in leaf cells of this species (Fernández et al., 1999) and the membrane potential is typically −160 mV. Normal seawater concentrations of NO3− and Na+ can be taken as 10 μm and 550 mm, respectively, and the pH is typically 8.0. The generalized free energy relationship for a plasma membrane cation-NO3− symporter operating with a stoichiometry of n cations per NO3− is given (in millivolts) as
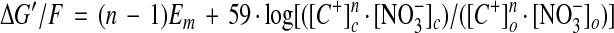 |
1 |
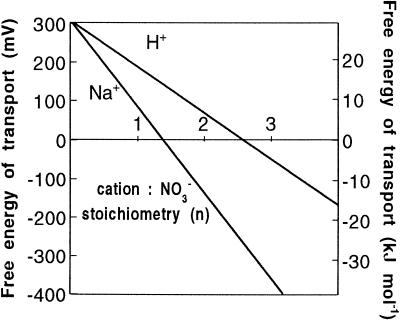
where C+ is the coupling cation (either Na+ or H+) and subscripts o and c denote the external solution and cytoplasm, respectively. Figure 7 shows the resulting free energy relationships for hypothetical cation-NO3− symporters operating in conditions appropriate to Z. marina. Clearly, in the case of H+ coupling, a stoichiometry of two is not sufficient to drive net uptake (free energy > 0), and even a stoichiometry of three yields only a modest inward driving force of −5 kJ mol−1. It is likely that a still higher value of n would be required to guarantee NO3− influx in varying external conditions. By contrast, Na+-coupled transport would be strongly inwardly directed (−13 kJ mol−1), with a stoichiometry of just 2Na+:NO3− (Fig. 7).
Figure 7.
Thermodynamic relationship of hypothetical NO3− transporters employing either H+ or Na+ as the coupling ion. Calculations were performed with Equation 1, with parameter values assigned as discussed in the text.
NO3− transport kinetics in Z. marina indicates the existence of a high-affinity transport system, with a much lower Km (2.31 ± 0.78 μm) than has to date been reported for other angiosperms (where values reside in the range 6–20 μm, Crawford and Glass, 1998), although the value in Z. marina is in the same range (2–13 μm) as that described for marine algae (DeBoer, 1985), the fungus Aspergillus nidulans (1 μm, A.J. Miller, personal communication), and cyanobacteria (Rodríguez et al., 1994). The low Km observed in Z. marina is very likely to be an adaptation to an environment in which NO3− concentrations are typically <10 μm.
In higher plants, two high-affinity systems for NO3− have been defined: one inducible and another one constitutive (Wang and Crawford, 1996; Crawford and Glass, 1998). We have found that the Em response to NO3− addition in N-starved plants does not show any lag, which might indicate the existence of a constitutive high-affinity system. However, we have also detected an increase in the magnitude of depolarizations after repeated additions of NO3− (data not shown), which could be the manifestation of an inducible transport system.
In conclusion, several lines of evidence indicate that a Na+-dependent high-affinity NO3− transport system operates in Z. marina. This is the first report of Na+-dependent NO3− transport in an angiosperm and points to the potential relevance of Na+-coupled transport in halophytic species. The Na+ dependence of NO3− transport in Z. marina also suggests that other anions could enter the cell using a similar mechanism, and studies of other anion transporters are now in progress.
Footnotes
This work was supported by project PB95–0476 from Dirección General de Enseñanza Superior e Investigación Científica (Spain) and by a European Union Framework Programme 4 grant (no. PL960775 to D.S.).
LITERATURE CITED
- Beer S, Eshel A, Waisel Y. Carbon metabolism in seagrasses. III. Activities of carbon-fixing enzymes in relation to internal salt concentrations. J Exp Bot. 1980;31:1027–1033. [Google Scholar]
- Beevers L, Hageman RH. Nitrate and nitrite reduction. In: Miflin BJ, editor. The Biochemistry of Plants. Vol. 5. New York: Academic Press; 1980. pp. 115–168. [Google Scholar]
- Bush DR. Proton-coupled sugar and amino acid transporters in plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:513–542. [Google Scholar]
- Crawford NM, Glass DM. Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci. 1998;3:389–395. [Google Scholar]
- DeBoer JA. Nutrients. In: Lobban CS, Harrison PJ, Duncan JJ, editors. The Physiological Ecology of Seaweeds. New York: Cambridge University Press; 1985. pp. 356–392. [Google Scholar]
- Felle HH. A study of the current-voltage relationships of electrogenic and passive membrane elements in Riccia fluitans. Biochim Biophys Acta. 1981;646:151–160. doi: 10.1016/0005-2736(81)90282-0. [DOI] [PubMed] [Google Scholar]
- Fernández JA, García-Sánchez MJ, Felle HH. Physiological evidence for a proton pump and sodium exclusion mechanisms at the plasma membrane of the marine angiosperm Zostera marinaL. J Exp Bot. 1999;50:1763–1768. [Google Scholar]
- Fukuhara T, Pak J-Y, Ohwaki Y, Tsujimura H, Nitta T. Tissue-specific expression of the gene for a putative plasma membrane H+-ATPase in a seagrass. Plant Physiol. 1996;110:35–42. doi: 10.1104/pp.110.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sánchez MJ, Fernández JA, Niell FX. Biochemical and physiological responses of Gracilaria tenuistipitataunder two different nitrogen treatments. Physiol Plant. 1993;88:631–637. doi: 10.1111/j.1399-3054.1993.tb01382.x. [DOI] [PubMed] [Google Scholar]
- Glass ADM, Shaff JE, Kochian LV. Studies of the uptake of nitrate in barley. Plant Physiol. 1992;99:456–463. doi: 10.1104/pp.99.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellebust JA. Uptake of organic substrates by Cyclotella cryptica(Bacillariophyceae): effects of ions, ionophores and metabolic and transport inhibitors. J Phycol. 1978;14:79–83. [Google Scholar]
- Hernández I, Corzo A, Gordillo FJ, Robles MD, Saez E, Fernández JA, Niell FX. Seasonal cycle of the gametophytic form of Porphyra umbilicalis: nitrogen and carbon. Mar Ecol Prog Ser. 1993;99:301–311. [Google Scholar]
- Kiegle EA, Bisson MA. Plasma membrane Na+transport in a salt tolerant charophyte: isotopic fluxes, electrophysiology, and thermodynamics in plants adapted to saltwater and freshwater. Plant Physiol. 1996;111:1191–1197. doi: 10.1104/pp.111.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara C, Rodríguez R, Guerrero MG. Sodium-dependent nitrate transport and energetics in cyanobacteria. J Phycol. 1993;29:389–395. [Google Scholar]
- Liu K-H, Huang C-Y, Tsay Y-F. CHL1 is a dual-affinity nitrate transporter of Arabidopsisinvolved in multiple phases of nitrate uptake. Plant Cell. 1999;11:865–874. doi: 10.1105/tpc.11.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJ, Verlin D, Smith A, Sanders D, Fernández JA, Walker NA. The physiological relevance of Na+-coupled K+-transport. Plant Physiol. 1996;112:1609–1616. doi: 10.1104/pp.112.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure PR, Kochian LV, Spanswick RM, Shaff JE. Evidence for cotransport of nitrate and protons in maize roots. I. Effects of nitrate on the membrane potential. Plant Physiol. 1990;93:281–289. doi: 10.1104/pp.93.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meharg AA, Blatt MR. NO3− transport across the plasma membrane of Arabidopsis thalianaroot hairs: kinetic control by pH and membrane voltage. J Membr Biol. 1995;145:49–66. doi: 10.1007/BF00233306. [DOI] [PubMed] [Google Scholar]
- Mistrik I, Ullrich CI. Mechanism of anion uptake in plant roots: quantitative evaluation of H+/NO3− and H+/H2PO4stoichiometries. Plant Physiol Biochem. 1996;34:629–636. [Google Scholar]
- Pressman BC. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Raven JA. Energetics and Transport in Aquatic Plants. MBL Lectures in Biology. Vol. 4. New York: Alan R. Liss; 1984. [Google Scholar]
- Rees TA, Cresswell RC, Syrett PJ. Sodium-dependent uptake of nitrate and urea by a marine diatom. Biochim Biophys Acta. 1980;596:141–144. doi: 10.1016/0005-2736(80)90178-9. [DOI] [PubMed] [Google Scholar]
- Riley JP, Chester R. Introduction to Marine Chemistry. London: Academic Press; 1971. [Google Scholar]
- Rodríguez R, Guerrero MG, Lara C. Mechanism of sodium/nitrate symport in Anacystis nidulansR2. Biochim Biophys Acta. 1994;1187:250–254. [Google Scholar]
- Rodríguez R, Lara C, Guerrero MG. Nitrate transport in the cyanobacterium Anacystis nidulansR2: kinetic and energetic aspects. Biochem J. 1992;282:639–643. doi: 10.1042/bj2820639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. Proton and ion transport across the thylakoid membranes. In: Trebst A, Avron M, editors. Photosynthesis I, Encyclopaedia of Plant Physiology. Vol. 5. Berlin: Springer-Verlag; 1977. pp. 338–349. [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- Sanders D, Hansen U-P, Gradmann D, Slayman CL. Generalized kinetic analysis of ion-dependent cotransport systems: a unified interpretation of selective ionic effects on Michaelis parameters. J Membr Biol. 1984;77:123–152. doi: 10.1007/BF01925862. [DOI] [PubMed] [Google Scholar]
- Smith FA, Walker NA. Transport of potassium in Chara australis. I. A symport with sodium. J Membr Biol. 1989;108:125–137. doi: 10.1007/BF01869452. [DOI] [PubMed] [Google Scholar]
- Tsay Y-F, Schroeder JI, Feldman KA, Crawford NM. The herbicide sensitivity gene CHL1 of Arabidopsisencodes a nitrate-inducible nitrate transporter. Cell. 1993;72:705–713. doi: 10.1016/0092-8674(93)90399-b. [DOI] [PubMed] [Google Scholar]
- Ullrich CI, Novacky AJ. Extra- and intracellular pH and membrane potential changes induced by K+, Cl−, H2PO4, and NO3− uptake and fusicoccin in root hairs of Limnobium stoloniferum. Plant Physiol. 1990;94:1561–1567. doi: 10.1104/pp.94.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich WR. Transport of nitrate and ammonium through plant membranes. In: Mengel K, Pilbeam DJ, editors. Nitrogen Metabolism of Plants. Oxford: Clarendon Press; 1992. pp. 121–137. [Google Scholar]
- van der Leij M, Smith SJ, Miller AJ. Remobilisation of vacuolar stored nitrate in barley root cells. Planta. 1998;205:64–72. [Google Scholar]
- Walker DJ, Smith SJ, Miller AJ. Simultaneous measurement of intracellular pH and K+ or NO3−in barley root cells using triple-barreled, ion-selective microelectrodes. Plant Physiol. 1995;108:743–751. doi: 10.1104/pp.108.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker NA. Sodium-coupled symports in the plasma membrane of plant cells. In: Blatt MR, Leigh RA, Sanders D, editors. Membrane Transport in Plants and Fungi: Molecular Mechanisms and Control. Society for Experimental Biology Symposium XLVIII. Cambridge, UK: The Company of Biologists; 1994. pp. 179–192. [PubMed] [Google Scholar]
- Walker NA, Sanders D. Sodium-coupled solute transport in charophyte algae: a general mechanism for transport energisation in plant cells? Planta. 1991;185:443–445. doi: 10.1007/BF00201070. [DOI] [PubMed] [Google Scholar]
- Wang R, Crawford NM. Genetic identification of a gene involved in constitutive, high-affinity nitrate transport in higher plants. Proc Natl Acad Sci USA. 1996;93:9297–9301. doi: 10.1073/pnas.93.17.9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J-J, Theodoulou FL, Muldin I, Ingemarsson B, Miller AJ. Cloning and functional characterization of a Brassica napustransporter that is able to transport nitrate and histidine. J Biol Chem. 1998;273:12017–12023. doi: 10.1074/jbc.273.20.12017. [DOI] [PubMed] [Google Scholar]