Abstract
Heat shock protein 90 (Hsp90) facilitates the maturation of many newly synthesized and unfolded proteins (clients) via the Hsp90 chaperone cycle, in which Hsp90 forms a heteroprotein complex and relies upon co-chaperones, immunophilins, etc. for assistance in client folding. Hsp90 inhibition has emerged as a strategy for anticancer therapies due to the involvement of clients in many oncogenic pathways. Inhibition of chaperone function results in client ubiquitinylation and degradation via the proteasome, ultimately leading to tumor digression. Small molecule inhibitors perturb ATPase activity at the N-terminus and include derivatives of the natural product geldanamycin. However, N-terminal inhibition also leads to induction of the pro-survival heat shock response (HSR), in which displacement of the Hsp90-bound transcription factor, Heat Shock Factor-1 translocates to the nucleus and induces transcription of heat shock proteins, including Hsp90. An alternative strategy for Hsp90 inhibition is disruption of the Hsp90 heteroprotein complex. Disruption of the Hsp90 heteroprotein complex is an effective strategy to prevent client maturation without induction of the HSR. Cucurbitacin D, isolated from Cucurbita texana, and 3-epi-isocucurbitacin D prevented client maturation without induction of the HSR. Cucurbitacin D also disrupted interactions between Hsp90 and two co-chaperones, Cdc37 and p23.
Keywords: Hsp90, co-chaperone, client, heat shock response (HSR)
INTRODUCTION
Hsp90 is a molecular chaperone responsible for the correct conformation of many newly synthesized polypeptides and the re-maturation of denatured proteins termed “clients” via the Hsp90 chaperone cycle. Hsp90 functions as a homodimer and requires the formation of the heteroprotein complex composed of several immunophilins, co-chaperones and partner proteins for assistance in chaperone activity. Several conformational transitions of the Hsp90 heteroprotein-client complex, coupled to Hsp90 ATPase activity results in correct folding and release of client protein1,2,. Inhibition of the chaperone cycle leads to client protein ubiquiltinylation and subsequent degradation via the proteasome3–5. Hsp90 inhibition has emerged as a strategy for anticancer chemotherapeutics due to the involvement Hsp90-dependent clients in a variety of oncogenic signaling pathways6–11. Many clients (i. e. ErbB2, B-Raf, Akt, steroid hormone receptors, mutant p53, HIF-1, survivin, telomerase, etc.) are contributors to the six hallmarks of cancer. Oncogenic client degradation via Hsp90 inhibition ultimately halts cancer progression and has been observed during clinical trials of Hsp90 inhibitors.
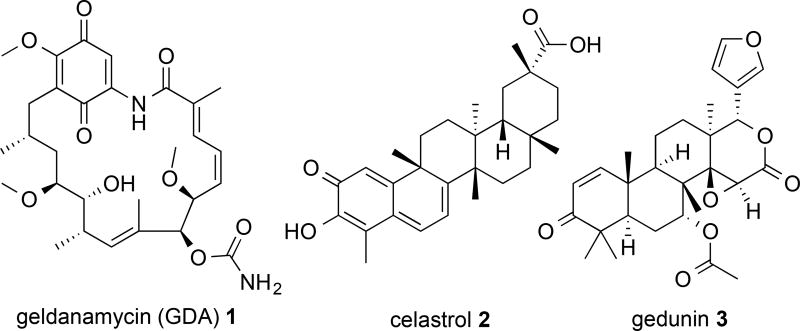
Classic small molecule Hsp90 inhibitors are designed to perturb N-terminal ATPase activity and include derivatives of geldanamycin (1) (Figure 1), radiciol and several purine analogs12. Although Hsp90 N-terminal inhibitors are effective at inhibiting Hsp90 function and lead to tumor digression through client degradation, N-terminal inhibition also leads to displacement of the Hsp90-bound transcription factor, Heat Shock Factor-1 (HSF-1) and induction of the pro-survival heat shock response (HSR)13–16. Upon displacement, HSF-1 trimerizes, translocates to the nucleus and induces transcription of the Heat Shock Element. This leads to increases in the cellular concentration of heat shock proteins, including Hsp90, and resulted in dosing and scheduling problems during clinical trials of N-terminal inhibitors. Therefore, anticancer Hsp90 inhibitors that avoid induction of the HSR are needed.
Figure 1.
Structures of geldanamycin (GDA) (1), celastrol (2) and gedunin (3).
Alternative strategies to inhibit the chaperone cycle include targeting the Hsp90 C-terminus and disruption of the heteroprotein complex. C-terminal inhibitors include derivatives of the coumarin-containing natural product novobiocin17–20. These inhibitors prevent cancer cell proliferation at concentrations similar to N-terminal inhibitors and destabilize Hsp90-client protein interactions without induction of the HSR. Maturation of all clients requires additional proteins that interact with Hsp90 and modulate ATPase activity and/or assist in correct client folding21–22. For example, the co-chaperone Hsp90-Hsp70 organizing protein (HOP) associates with the Hsp90 dimer during the initial stages of the chaperone cycle and facilitates the delivery of certain clients from Hsp70 to Hsp90. Peptidyl-prolyl isomerase co-chaperones (PPIases) assist in the correct folding and 3-diminsional structure of clients and the co-chaperone p23 promotes the optimal Hsp90 conformation for ATP binding and hydrolysis ultimately resulting in client protein release23. In addition, classes of clients require the assistance of specific co-chaperones for maturation (e. g. all Hsp90-dependent kinases require the assistance of the co-chaperone cell division cycle 37 (Cdc37) for maturation). Disruption of interactions between Hsp90 and client-specific co-chaperones may allow for select degradation of clients and avoid systemic client protein degradation, a consequence of Hsp90 inhibitors under current clinical investigation and may be the cause of certain side effects.
The cucurbitacin class of natural products is a group of triterpenoids that are present in the Cucurbitaceae and other closely related families. More than 50 cucurbitacins have been identified and exhibit a wide variety of biological activities that include, but are not limited to, cytotoxicity, anti-proliferation, anti-inflammation, anti-oxidant, anti-hepatotoxicity, anti-bacterial and anti-viral properties, as well as anti-metastatic properties and improved anticancer activity when combined with current chemotherapies24–26. Despite the presence of an α-, β-unsaturated ketone (Michael acceptor; a well-established and non-specific disruptor of general cellular processes via electrophilic chemical reactivity) located within many of the cucurbitacin compounds, several cucurbitacins interact with specific cellular targets involved in gene regulation, transcription and overall cellular fate. Cucurbitacin D (4) reduced the proliferation of T-cell leukemia cells and ultimately led to cancer cell apoptosis27. This cucurbitacin was shown to affect the NF-κB pathway and led to the accumulation of NF-κB and IκB-α within the cytosol. Interestingly, a decrease in the cellular levels of Bcl-xL and Bcl-2 was also observed. Bcl-xL and Bcl-2 levels are regulated by Hsp90 clients that depend on the chaperone cycle for correct folding and function. It has been reported that incubation with N-terminal inhibitors led to cellular decreases in Bcl-xL and Bcl-2 levels28–29. Furthermore, (4), as well as other cucurbitacins, share structural similarities to the natural products celastrol (2) and gedunin (3) which are known inhibitors of client maturation via disruption of the Hsp90 heteroprotein complex (Figure 1). However, these natural products inhibit chaperone function by different mechanisms. 2 covalently binds the Hsp90 N-terminus and disrupts the interaction between Hsp90 and Cdc37 ultimately resulting in kinase client degradation30. 3 binds the co-chapreone p23 and disrupts Hsp90-co-chapreone interactions leading to client degradation across many different cancer cell lines31. Given certain structural similarities between the cucurbitacins, 2 and 3, as well as decreased levels of proteins that depend on functional clients after exposure to cucurbitacins, it was hypothesized that 4, and other cucurbitains, may inhibit the Hsp90 chaperone cycle in a manner similar to 2 and/or 3.
Herein, we report that 4 prevented the maturation of Hsp90-dependent clients by disruption of the Hsp90 heteroprotein complex. With the exception of 23,24-dihydrocucurbitacin D (9) and 23,24-dihydrocucurbitacin B (10), all cucurbitacins exhibited anti-proliferative IC50 values at nanomolar concentrations against the MCF7 breast cancer cell line. Western blot analysis for client levels in the presence of high and low concentrations of each cucurbitacin revealed that incubation with 4 and 3-epi-isocucurbitacin D (6) led to client degradation. 4 also disrupted the interaction between Hsp90 and the co-chaperones Cdc37 and p23; however, 6 did not disrupt these Hsp90-co-chaperone interactions components and caused client degradation through alternative mechanism(s). Furthermore, 4 did not induce the HSR, as no increases in the levels of heat shock proteins Hsp90, Hsp70 or Hsp27 was observed. Client protein degradation, disruption of Hsp90-Cdc37 and Hsp90-p23 interactions as well as a lack of heat shock protein induction, notably Hsp27, has previously been observed after incubation with 3 and suggested 4 inhibited cancer cell growth through Hsp90 client degradation via disruption of Hsp90-co-chaperone interactions by a mechanism comparable to 3.
RESULTS AND DISCUSSION
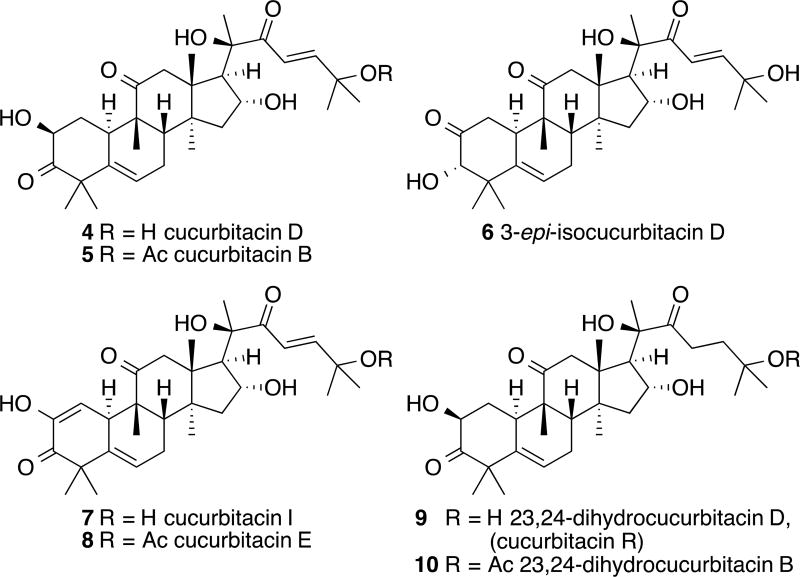
Cucurbitacin D (4) and cucurbitacin B (5), along with isomeric 3-epi-isocucurbitacin D (6) were isolated from the fruits of Cucurbita texana (Cucurbitaceae) following previously described protocols (Figure 2)32–34. Two additional oxidized derivatives, cucurbitacin I (7) and E (8) were obtained as well. These latter compounds along with 6 were obtained in reduced quantities compared to that of 4 and 5. Fortunately, semi-synthetic approaches to these particular compounds have been investigated in the literature and can be seen as a solution to the lack of material for these natural products. In addition, 9, 10 and other reduced C23-24 derivatives have routinely been acquired via hydrogenation conditions. Structures of the isolated and semi-synthesized cucurbitacins of interest are shown in Figure 2.
Figure 2.
Chemical structures of cucurbitacins isolated from Cucurbita texana (Cucurbitaceae) [cucurbitacin D (4) and B (5); 3-epi-isocucurbitacin D (6); cucurbitacin I (7) and E (8)] and the structures of the semi-synthesized natural products [3-epi-cucurbitacin D (6), cucurbitacin I (7), 23,24-dihydrocucurbitacin D (9) (cucurbitacin R) and B (10)].
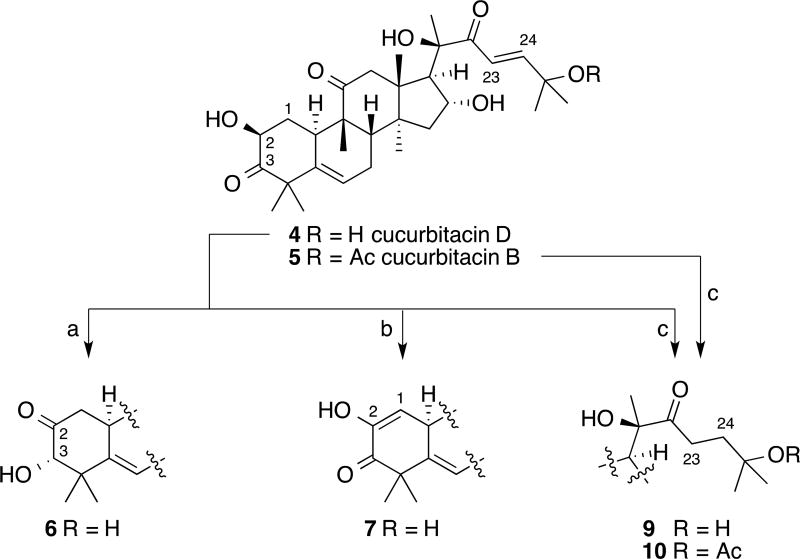
The isomerization of the α-hydroxyketone at C2 and C3 of some cucurbitacins has been determined to take place under both acidic and basic conditions, with the most thorough investigation having been performed by Galindo and co-workers35–36. However, an alternative to Galindo’s procedure was chosen instead, one similar to that reported by Sneden et al. that takes advantage of the slightly acidic nature of silica gel37. Heating a CH2Cl2 solution of 4 and silica gel to 40 °C provided useful quantities of 6 for biological evaluation and the ability to recover unreacted 4 (Scheme 1).
Scheme 1.
Semi-synthesis of cucurbitacins: 3-epi-isocucurbitacin D (6), cucurbitacin I (7), 23,24-dihydrocucurbitacin D (9) (cucurbitacin R) and 23,24-dihydrocucurbitacin B (10). Reagents and conditions: a) silica gel, CH2Cl2, 40 °C, 25% (30% conversion); b) Pd(OAc)2, PhBr, NaOAc, DMA, 80 °C, 38% (60% conversion); c) H2 (1atm), Pd/C 5%, EtOH, 88%.
While attempting to form cucurbitacin derivatives via a Heck reaction,38 in an effort to improve their biological activity, an undesired product was isolated and later determined by spectroscopic analysis to be 7. Even though this is not the first report of the oxidation of the α-hydroxyketone to a diosphenol moiety for cucurbitacins,39–41 this is the first example of the transformation via a Saegusa oxidation (Scheme 1)42–45. This result can be rationalized via the ability of cucurbitacins to isomerize to form the intermediate enediol, which can be trapped and oxidized to the final diosphenol product. This is the same enediol intermediate that is necessary for the formation of isocucurbitacins. While 7 was not the initially desired product, the resulting extra quantity was now available for biological testing.
To date, Cucurbita texana has not produced any 9 or 10 via natural product isolation. However, hydrogenation of the C23-24 olefin of 4 and 5 has allowed for the ability to obtain useful quantities of the semi-synthesized 9 and 10 from a plant species that does not routinely provide them through natural product isolation (Scheme 1)35,40,46–52.
Select cucurbitacins exhibit potent anti-proliferative activity and decreased Hsp90-dependent client protein levels without induction of the HSR
Consistent with previous data against several cancer cell lines, many of these cucurbitacins exhibited nanomolar IC50 values against the MCF7 breast cancer cell line. The decrease in potency observed for 9 and 10 may be explained by reduction of the α-, β-unsaturated ketone. This would reduce the potential for non-specific, attack by cellular nucleophiles. The IC50 values for each cucurbitacin can be found in Table 1 along with values for 1, an Hsp90 N-terminal inhibitor, 2 and 3.
Table 1.
Calculated IC50 values for the controls geldanamycin (GDA) (1), celastrol (2), gedunin (3) and select cucurbitacins against the MCF7 breast cancer cell line.
| Compounds | MCF7 IC50 Values (µM) |
|---|---|
| GDA (1) | 0.0427 ± 0.018 |
| celastrol (2) | 0.153 ± 0.004 |
| gedunin (3) | 10.9 ± 0.9 |
| cucurbitacin D (4) | 0.598 ± 0.001 |
| cucurbitacin B (5) | 0.0413 ± 0.003 |
| 3-epi-isocucurbitacin D (6) | 0.718 ± 0.009 |
| cucurbitacin I (7) | 0.0607 ± 0.012 |
| cucurbitacin E (8) | 0.055 ± 0.006 |
| 23,24-dihydrocucurbitacin D (9) | 5.13 ± 0.01 |
| 23,24-dihydrocucurbitacin B (10) | 46.3 ± 0.1 |
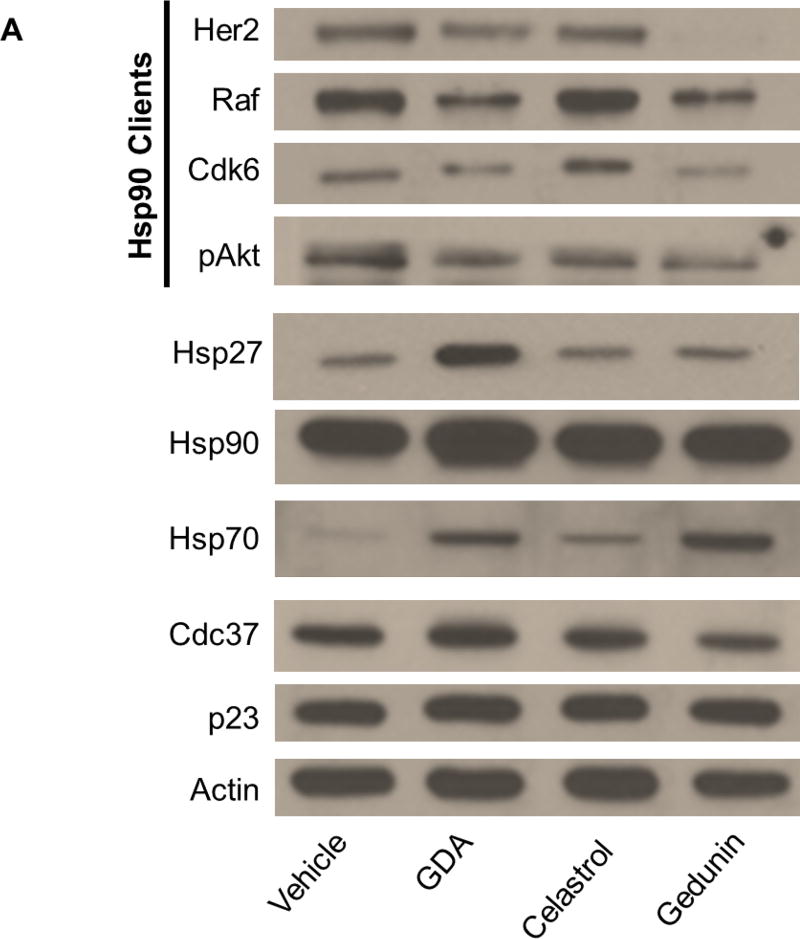
Hsp90 inhibitors and disruptors of the heteroprotein complex prevent cancer cell proliferation at concentrations near the IC50 value by depleting levels of clients necessary for cancer cell growth. To correlate client degradation via inhibition of the chaperone cycle to the IC50 value for each cucurbitacin, client levels from MCF7 cell lysates dosed with high and low concentrations (5-times the IC50 value and half of the IC50 value, respectively) of each cucurbitacin were dectected by Western blot analysis. The levels of heat shock proteins were also detected to account for induction of the HSR as well as the levels of p23 and Cdc37. Incubation with high concentrations of the control compounds 1, 2 and 3 led to client protein degradation and little change in the levels of Cdc37 and p23 (Figure 3A). Consistent with previous reports, incubation with GDA led to increased levels of Hsp90, Hsp70 and Hsp27, indicating induction of the HSR (Figure 3A). Minimal induction of the HSR was observed at the concentrations of 2 and 3 tested; incubation with 3 caused only a slight increase in Hsp70 levels and the levels of Hsp90 and Hsp27 were comparable to vehicle control upon incubation with 2 and 3 (Figure 3A).
Figure 3.
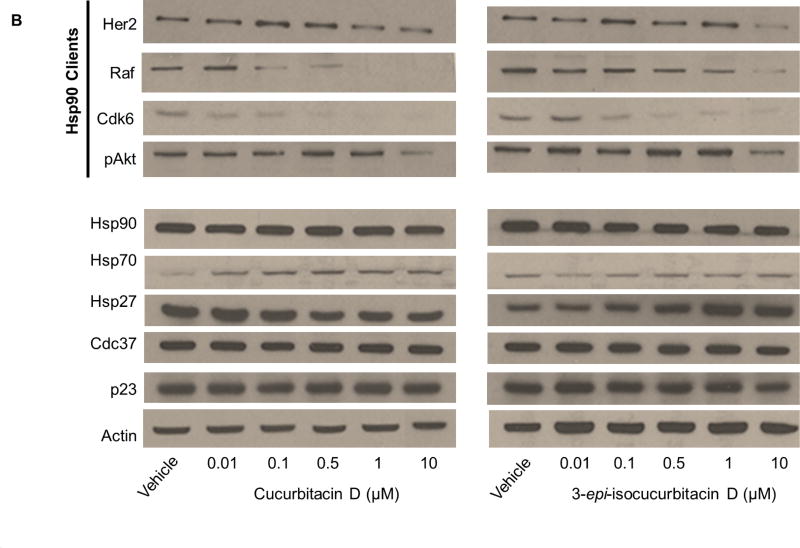
(A) Western blot for the levels of Hsp90 clients (pAkt, Her2, Cdk6 and Raf), heat shock proteins (Hsp90, Hsp70 and Hsp27) co-chaperones (Cdc37 and p23) and actin from MCF7 cell lysates treated for 24 hours with vehicle (0.25% DMSO) or high concentrations (5-times the IC50 value) of the controls geldanamycin (GDA) (1), celastrol (2), gedunin (3) (B) Levels of Hsp90 clients, heat shock proteins and co-chaperones from cell lysates treated with vehicle or increasing concentrations of cucurbitacin D (4) or 3-epi-isocucurbitacin D (6).
Levels of clients and heat shock proteins after incubation with high and low concentrations of each cucurbitacin can be found in Figure 3 B and within the Supporting Information (S1 and S2). Incubation with high concentrations of 4 and 6 led to a decrease in client levels (S3), indicating disruption of client maturation. These cucurbitacins also decreased client levels in a dose-dependent manner at and around their IC50 values (Figure 3B). No changes in Hsp90 or Hsp70 levels were observed; however, Hsp27 levels were increased at high concentrations of 6. The levels of Cdc37 and p23 remained constant at all concentrations (S3 and Figure 3B). Client and heat shock protein levels were unchanged after incubation with high and low concentrations of the remaining cucurbitacins (S1 and S2). No client degradation at high concentrations of compound and constant heat shock protein levels at high and low concentrations indicates the mechanism(s) by which these compounds inhibited MCF7 cancer cell growth is unrelated to Hsp90 inhibition.
Cucurbitacin D, but not 3-epi-isocucurbitacin D, caused client protein degradation through disruption of Hsp90-co-chaperone interactions
Concentration-dependent client degradation void of induction of the HSR indicates an alternative mechanism to traditional N-terminal inhibitors for preventing client maturation. Compounds such as 2 and 3 disrupt the Hsp90 heteroprotein complex thereby preventing co-chaperone assistance during the chaperone cycle. In addition, concentrations of these compounds that destabilize the heteroprotein-client complex do not induce the HSR. There are several reports of 2 and 3 as Hsp90 modulators present in literature. These natural products disrupt the interactions between Hsp90 and co-chaperones involved in both early and late stages of the chaperone cycle. Celastrol (2) was shown to covalently bind the Hsp90 N-terminus thereby disrupting Hsp90 from interacting with Cdc37, a co-chaperone involved during the early stages of the chaperone cycle53. Gedunin (3) binds the co-chaperone p23 and prevents p23 from facilitating progression of the Hsp90 chaperone cycle31. This ultimately results in improper trafficking and localization of clients, such as the steroid receptor, glucocorticoid receptor.
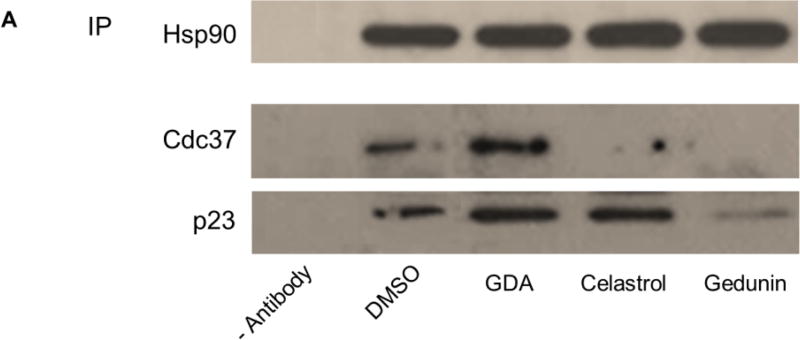
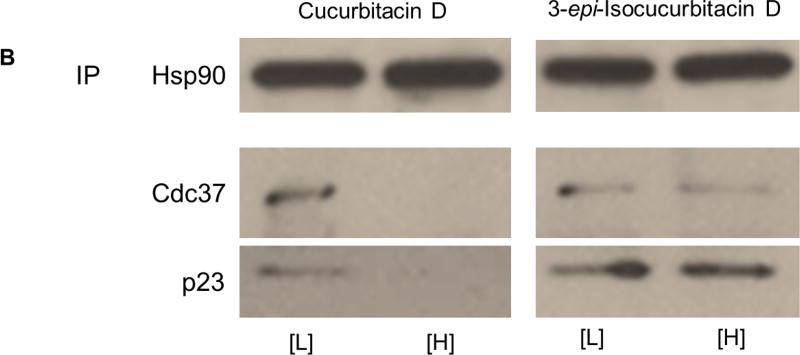
Consistent with previous studies, Figure 4A confirmed that high concentrations of 2 and 3 disrupted components of the heteroprotein complex, while the N-terminal inhibitor 1 did not disrupt these Hsp90-co-chaperone interactions. Co-immunoprecipitation of Cdc37 and p23 was disrupted in the presence of high concentrations of 2 and 3 when using an antibody targeting Hsp90. A high concentration of 4 also disrupted these heteroprotein complex components from Hsp90 (Figure 4B). Incubation with a high concentration of 4 completely disrupted Hsp90-Cdc37 and Hsp90-p23 interactions from MCF7 cell lysates. Interestingly, a high concentration of 6 had no effect on the interactions between Hsp90 and these co-chaperones; Hsp90-Cdc37 and Hsp90-p23 interactions remained intact at high and low concentrations of 6 (Figure 4B).
Figure 4.
(A) Co-immunoprecipitation of Cdc37 and p23 using an anti-Hsp90 antibody (abcam) from MCF7 cell lysates treated for 24 hours with vehicle (0.25% DMSO) or high concentrations (5-times the IC50 value) of the controls geldanamycin (GDA; final concentration = 0.2135 µM) (1), celastrol (final concentration = 0.765 µM) (2), gedunin (final concentration = 54.5 µM) (3). (B) Co-immunoprecipitation of Cdc37 and p23 using an anti-Hsp90 antibody from cell lysates treated with high and low concentrations (5-times the IC50 value and half the IC50 value, respectively) of cucurbitacin D (final [H] concentration = 0.765 µM; final [L] concentration = 0.299 µM) (4) or 3-epi-isocucurbitacin D (final [H] concentration = 3.590 µM; final [L] concentration = 0.359 µM) (6).
These data suggested that, although 4 and 6 both resulted in client protein degradation, the mechanisms by which these compounds disrupted client maturation are different. 4 disrupted Hsp90-Cdc37 and Hsp90-p23 complexes, which ultimately led to a cellular decrease of client levels. 6 also reduced client levels, but did not affect these Hsp90-co-chaperone interactions. This cucurbitacin may directly inhibit Hsp90 and bind an alternative site to the N-terminal ATP-binding pocket (e. g. C-terminal inhibitors, sansalvamide A, etc.), bind and inhibit the function of proteins important for client maturation (e. g. efrapeptins, cruentaren A, etc.) or disrupt interactions of other protein with the Hsp90 heteroprotein complex54–56.
High concentrations of cucurbitacin D did not increase cellular levels of Hsp27
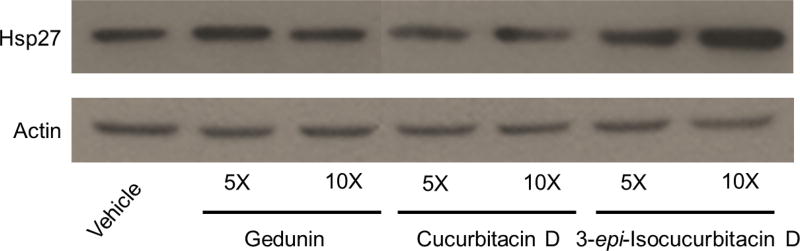
Patwardhan et al. reported that 3 does not induce the HSR in several different cancer cell lines, including the cervical cancer cell line HeLa and the breast cancer cell lines Hs578T and MCF731. More specifically, no increases in the levels of Hsp90, Hsp70 or Hsp27 were observed after incubation with concentrations up to 160 µM of 3. To corroborate Western blot and co-immunoprecipitation data reported here and further verify 4 disrupted client protein maturation in a manner comparable to 3, Hsp27 levels were detected after incubation with high concentrations of 3, 4 and 6. MCF7 cells incubated with 5- and 10-times the IC50 value of each compound and levels of Hsp27 were detected via Western blot analysis (Figure 5). The levels of Hsp27 were unchanged after incubation with high concentrations of 3 and 4 and comparable to vehicle control; however, increased Hsp27 levels were observed at high concentrations of 6 and are consistent with Figure 3B.
Figure 5.
Western blot for Hsp27 and actin levels from MCF7 cell lysates treated for 24 hours with vehicle (0.25% DMSO), 5-times (5×) or 10-times (10×) the IC50 value of the gedunin (final 5× concentration = 54.5 µM; final 10× concentration = 109.0 µM) (3), cucurbitacin D (final 5× concentration = 0.765 µM; final 10× concentration = 5.980 µM) (4) and 3-epi-isocucurbitacin D (final 5× concentration = 3.590 µM; final 10× concentration = 7.180 µM) (6).
Collectively, these data suggest that 4 destabilized client maturation through similar mechanisms to 3 by disruption of Cdc37 and p23 from the Hsp90 heteroprotein complex; however, 4 exhibited a dramatic 18-fold increase in potency when compared to 3. 6 also led to client degradation at nanomolar concentrations, but did not disrupt Hsp90-Cdc37 or Hsp90-p23 interactions and is presumed to cause degradation by alternative mechanisms. Like 3, no increase in Hsp27 levels was observed at high concentrations of 4, yet increased levels of Hsp27 were detected at high concentrations of 6, further supporting alternative mechanisms of client degradation for these cucurbitacins. In conclusion, the curcurbitacins have been shown to affect multiple cellular pathways and exhibit potent inhibitory activity against many cancer cell lines. Here we report the anti-proliferative activity of cucurbitacin D and 3-epi-isocucurbitacin D is partly attributed to disruption of Hsp90 client protein maturation and cucurbitacin D destabilizes components of the Hsp90 heteroprotein complex, ultimately resulting in client degradation.
EXPERIMENTAL SECTION
General Experimental Procedures
Experimental data for each isolated compound was compared to previously reported literature data. NMR spectra were acquired on a Bruker AVANCE-400 MHz NMR spectrometer and compared to previously published data. High-resolution mass spectrometry (HRMS) data was obtained using either electron ionization (EI) on a ThermoFinnigan MAT 95 XL mass spectrometer or electrospray ionization (ESI) on a ThermoFinnigan LCQ Advantage Ion Trap liquid chromatography-mass spectrometer (LC/MS). Melting points were determined on a Vernier Melt Station melting point apparatus. TLC analysis was performed using pre-coated silica gel PE sheets (UV254, 250 µm layer). Compounds were purified via column chromatography using silica gel 40–63 µm (230–400 mesh), preparative normal phase-TLC (silica gel, UV254, 2 µm layer) and reverse phase HPLC using a Dynamax liquid chromatograph (Varian Chromatography Systems) with a PDA-2 photodiode array UV detector (Alltech preparative column, Econosil C18 10 µm, length 250 mm, ID 22 mm). Gradients of MeOH/H2O or ACN/H2O were utilized with a 9 or 13 mL/min flow rate. Analytical separations were optimized on an Alltima C18 (Alltech analytical column, 5 µm, length 250 mm, ID 4.6 mm) HPLC column using a flow rate of 1 mL/min then transferred to preparative scale, All reagents and solvents were obtained from commercial suppliers and used as received.
Isolation and Semi-synthesis of Cucurbitacins
Fruits from Cucurbita texana (Cucurbitaceae) were cut into pieces and homogenized with MeOH, filtered and the solvent was removed under reduced pressure. The residue was subjected to flash column chromatography with gradient elution (hexane/EtOAc and then EtOAc/MeOH of increasing polarity). The fractions containing the same compounds as determined by TLC, were combined and subjected to further purification by one of three methods: normal phase column chromatography, preparative normal phase TLC, or preparative reverse phase HPLC32–35. The spectroscopic data for the pure isolated compounds were compared to published literature data: 1. cucurbitacin D (4)57–59, 2. cucurbitacin B (5)52,60, 3. 3-epi-isocucurbitacin D (6)57,61–62, 4. cucurbitacin I (7)60,63, 5. cucurbitacin E (8)60,63, 6. 23,24-dihydrocucurbitacin D (9)64, 7. 23,24-dihydrocucurbitacin B (10)52,65, 8. data for multiple cucurbitacins66–69.
Preparation of 3-epi-isocucurbitacin D (6)
Cucurbitacin D (4) (50 mg, mmol) was added to a suspension of silica gel (3 g) in CH2Cl2 (5 mL). The reaction was heated to 40 °C for 72 h. The solution was allowed to cool followed by dilution with EtOAc and filtration through plug of silica gel. The crude mixture was purified via preparative normal phase TLC to provide 3-epi-isocucurbitacin D (6) (13 mg) as a white solid in 25% yield.
Preparation of Cucurbitacin I (8)
Palladium acetate (5 mg, 0.022 mmol, 0.3 equiv) was added to a vial containing cucurbitacin D (4) (44 mg, 0.085 mmol, 1 equiv), NaOAc (4 mg, 0.043 mmol, 0.5 equiv) and bromobenzene (0.01 mL, 0.095 mmol, 1.1 equiv) dissolved in dimethylacetamide (DMA) (0.40 mL). The vial was sealed with a teflon lined cap and the reaction was stirred at 80 °C for 24 h. The reaction was allowed to cool and diluted with EtOAc (10 mL) and H2O (5 mL), followed by extracting the aqueous layer three times with EtOAc. The combined organic layers were washed with brine and dried over anhydrous Na2SO4. The crude product was initially purified via column chromatography using hexane/EtOAc to remove residual DMA. Minor impurities were further removed via preparative reverse phase HPLC using MeOH/H2O, resulting in the isolation of cucurbitacin I (8) (16 mg) as a white solid in 38% yield (unoptimized reaction conditions).
Preparation of 23,24-Dihydrocucurbitacin D (10) (Cucurbitacin R) and 23,24-Dihydrocucurbitacin B (11)
5% palladium on carbon (16 mg) was added to a 25 mL round bottom flask containing the desired cucurbitacin starting material (44.0 mg) dissolved in EtOH (5 mL). A septum was added to the flask, the headspace was flushed with H2 and the reaction was stirred for 4 hours at 1 atm H2. Upon completion, the reaction was filtered through a plug of silica gel using EtOAC. The crude product was purified using column chromatography (hexanes/EtOAc) to provide the desired dihydro compounds as white solids in 76–88% yield.
Antibodies and Reagents
Antibodies targeting Her2, Cdk6, phospho-Akt (pAkt), and Hsp27 were purchased from Cell Signaling Technology. Antibodies targeting Raf and actin were purchased from Santa Cruz Biotechnology. The remaining antibodies are listed and were purchased from the indicated vendors: Hsp90 (Enzo Life Sciences); Hsp70 (Assay Designs); Cdc37 and p23 (abcam). Gedunin was isolated in house. Celastrol was purchased from Cayman Chemical and geldanamycin was purchased from Sigma Aldrich.
Cell Culture
MCF7 cells were maintained in Advanced DMEM/F12 (1:1; Gibco) supplemented with streptomycin (500 µg/mL; corning cellgro), penicillin (100 units/mL; corning cellgro), L-glutamine (2 mM; corning) and 10% FBS (Atlanta Biologicals). Cells were grown in a humidified atmosphere (37 °C, 5% CO2) and passaged when confluent.
Anti-proliferation
Cells were grown to confluence, seeded (2000 cells/well, 100 µL total media) in clear, flat-bottom 96-well plates and allowed to attach overnight. Varying concentrations of compound in DMSO (1% DMSO final concentration; Sigma-Aldrich) was added. Cells were returned to the incubator for an additional 72 h. After 72 h, cell growth was determined using an MTS/PMS cell proliferation kit (Promega) per the manufacturer’s instructions. Cells that incubated in 1% DMSO were used as 100% proliferation (i.e. DMSO = 100% growth) and the relative growth for each compound concentration was compared to 1% DMSO. IC50 values were calculated from two separate experiments performed in triplicate using GraphPad Prism 6.0 (GraphPad Software).
Western Blot
MCF7 cells were grown to confluence and seeded at 0.4 × 106 cells/well/2 mL. Cells were incubated for 24 hours and treated with varying concentrations of compound in DMSO (0.25% DMSO final concentration), or vehicle (DMSO) for 24 hours. Cells were harvested in cold DPBS (corning cellgro) and lysed using MPER (Thermo Scientific) supplemented with protease and phosphatase inhibitors (Roche) according to the manufacturer’s directions. Lysates were clarified at 14,000g for 10 minutes at 4° C. Protein concentrations were determined using the Pierce BCA protein assay kit per the manufacturer’s instructions. Equal amounts of protein (20 µg) were suspended in Laemmli sample buffer (15 µL; BioRad) and boiled at 70°C for 15 minutes. Samples were then electrophoresed under reducing conditions (10% and 12% acrylamide gels; made in house), transferred to PVDF (0.45 µm; Thermo Scientific), and immunoblotted with the corresponding antibody. Membranes were incubated with an appropriate horseradish peroxidase-labeled secondary antibody (Santa Cruz Biotechnology and GE Healthcare), developed with a chemiluminescent substrate, and visualized.
Co-immunoprecipitation
MCF7 cells were grown to confluence and seeded at 2×106 cells/5 mL in 10 cm dishes. Cells were incubated for 24 hours and then treated with compound in DMSO (0.25% DMSO final concentration), or vehicle (DMSO) for 24 hours. Media and cells were collected with DPBS and centrifuged at 200g for 5 minutes at 4°C. Supernatant was aspirated and pellets were washed one time with cold DPBS and centrifuged. Supernatant was aspirated and cell pellets were subsequently suspended in non-denaturing lysis buffer (20 mM Tris-HCl at pH 7.5; Fisher Scientific, 25 mM KCl; Sigma-Aldrich, 2 mM MgCl; Fisher Scientific, 2mM DTT; Sigma-Aldrich, 20 mM sodium molybdate; Sigma-Aldrich, 2mM ATP; Sigma-Aldrich and 0.1% NP-40; US Biological) supplemented with protease and phosphatase inhibitors (Roche) and incubated on ice for 2 hours. Lysates were clarified at 14,000g for 10 min at 4° C. Protein concentrations were determined using the Pierce BCA protein assay kit per the manufacturer’s instructions. Equal protein (400 µg) was incubated with 1 µg of Hsp90 antibody (H90.10; abcam) in 500 µL total volume lysis buffer for approximately 2 hours with rocking at 4 °C. Following incubation, 30 µL of re-suspended MagBeads Protein G (GenScript) was added to each sample and incubated with rocking for 1.5 hours at 4 °C. Protein G beads were washed 3 times with lysis buffer (500 µL), suspended in Laemmli sample buffer (15 µL) and boiled at 70°C for 15 minutes to dissociate proteins from beads. Samples were electrophoresed under reducing conditions (12% acrylamide gels; made in house), transferred to PVDF, and immunoblotted with the indicated antibodies. Membranes were incubated with a species-appropriate horseradish peroxidase-labeled secondary antibody, developed with a chemiluminescent substrate, and visualized.
Supplementary Material
Acknowledgments
This work was supported by the NIH grant CA109265 and the NIGMS Biotechnology Pre-doctoral Training Grant Program. We would like to thank South Dakota Board of Regents-2010 Biological Control and Analysis by Applied Photonics [BCAAP] for financial support.
Footnotes
Supporting information includes figures and additional experimental information. This information is available free of charge and can be found at http://pubs.acs.org.
References
- 1.Pratt WB. J. Biol. Chem. 1993;268:21455–21458. [PubMed] [Google Scholar]
- 2.Csermely P, Schnaider T, Soti C, Prohászka Z, Nardai G. Pharmacol. Ther. 1998;79:129–168. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 3.Mimnaugh EG, Chavany C, Neckers L. J. Biol. Chem. 1996;271(37):22796–22801. doi: 10.1074/jbc.271.37.22796. [DOI] [PubMed] [Google Scholar]
- 4.Taipale M, Jarosz DF, Lindquist SL. Nat. Rev. Mol. Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 5.Wandinger SK, Richter K, Buchner J. J. Biol. Chem. 2008;283:18473–18477. doi: 10.1074/jbc.R800007200. [DOI] [PubMed] [Google Scholar]
- 6.Xu W, Neckers L. Clin. Cancer Res. 2007;13:1625–1629. doi: 10.1158/1078-0432.CCR-06-2966. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhury S, Welch TR, Blagg BSJ. ChemMedChem. 2006;1:1331–1340. doi: 10.1002/cmdc.200600112. [DOI] [PubMed] [Google Scholar]
- 8.da Silva VCH, Ramos CHI. J. Proteo. 2012;75:2790–2802. doi: 10.1016/j.jprot.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 9.Holzbeierlein J, Windsperger A, Vielhauer G. Curr. Oncol. Rep. 2010;12:95–101. doi: 10.1007/s11912-010-0086-3. [DOI] [PubMed] [Google Scholar]
- 10.Prodromou C. Curr. Top. Med. Chem. 2009;9:1352–1368. doi: 10.2174/156802609789895656. [DOI] [PubMed] [Google Scholar]
- 11.Whitesell L, Lindquist SL. Nat. Rev. Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 12.Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 13.Kim HR, Kang HS, Kim HD. IUBMB Life. 1999;48(4):429–433. doi: 10.1080/713803536. [DOI] [PubMed] [Google Scholar]
- 14.Winklhofer KF, Reintjes A, Hoener MC, Voellmy R, Tatzelt J. J. Biol. Chem. 2001;276(48):45160–45167. doi: 10.1074/jbc.M104873200. [DOI] [PubMed] [Google Scholar]
- 15.Whitesell L, Bagatell R, Falsey R. Curr. Cancer Drug Targets. 2003;3(5):349–358. doi: 10.2174/1568009033481787. [DOI] [PubMed] [Google Scholar]
- 16.Ali A, Bharadwaj S, O’carroll R, Ovsenek N. Mol. Cell. Biol. 1998;18(9):4949–4960. doi: 10.1128/mcb.18.9.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcu MG, Schulte TW, Neckers L. J. National Cancer Instit. 2000;92(3):242–248. doi: 10.1093/jnci/92.3.242. [DOI] [PubMed] [Google Scholar]
- 18.Zhao H, Blagg BSJ. In: Inhibitors of Molecular Chaperones as Therapeutic Agents. Machajewski T, Gao Z, editors. Royal Society of Chemistry; 2013. pp. 259–301. [Google Scholar]
- 19.Matts RL, Dixit A, Peterson LB, Sun L, Voruganti S, Kalyanaraman P, Hartson SD, Verkhivker GM, Blagg BSJ. ACS Chem. Biol. 2011;6:800–807. doi: 10.1021/cb200052x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu XM, Shen G, Neckers L, Blake H, Holzbeierlein J, Cronk B, Blagg BSJ. J. Amer. Chem. Soc. 2005;127:12778–12779. doi: 10.1021/ja0535864. [DOI] [PubMed] [Google Scholar]
- 21.Röhl A, Rohrber J, Buchner J. Trends Biochem. Sci. 2013;38(5):253–262. doi: 10.1016/j.tibs.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Prodromou C. Biochim. Biophys. Acta. 2012;1823(3):614–623. doi: 10.1016/j.bbamcr.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratzke C, Hellenkamp B, Hugel T. Nat. Comm. 2014;5(4192):1–9. doi: 10.1038/ncomms5192. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Bao J, Guo J, Ding Q, Lu J, Huang M, Wang Y. Anti-Cancer Drug. 2012;23(8):777–787. doi: 10.1097/CAD.0b013e3283541384. [DOI] [PubMed] [Google Scholar]
- 25.Lee HD, Iwanski GB, Thoennissen NH. TheScientificWorldJOURNAL. 2010;10:413–418. doi: 10.1100/tsw.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chao JC, Chiu MH, Nie RL, Cordell GA, Qiu SX. Nat. Prod. Rep. 2005;22:386–399. doi: 10.1039/b418841c. [DOI] [PubMed] [Google Scholar]
- 27.Rios JL, Andujar I, Escandell JM, Giner RM, Recio MC. Curr Pharma.. Design. 2012;14:1663–1676. doi: 10.2174/138161212799958549. [DOI] [PubMed] [Google Scholar]
- 28.Du X, Ruoran M, Quanxin Q, Ye Q, Tianfu Y. Chin. J. Clin. Oncol. 2008;5:113–117. [Google Scholar]
- 29.Caldas-Lopes E, Cerchietti L, Ahn JH, Clement CC, Robles AI, Rodina A, Moulick K, Taldone T, Gozman A, Guo Y, Wu N, de Stanchina E, White J, Gross SS, Ma Y, Varticovski L, Melnick A, Chiosis G. PNAS. 2009;106(20):8368–8373. doi: 10.1073/pnas.0903392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang T, Hamza A, Cao X, et al. Mol. Cancer Therap. 2008;7(1):162–170. doi: 10.1158/1535-7163.MCT-07-0484. [DOI] [PubMed] [Google Scholar]
- 31.Patwardhan CA, Fauq A, Peterson LB, Miller C, Blagg BSJ, Chadli A. J. Biol. Chem. 2013;288(10):7313–7325. doi: 10.1074/jbc.M112.427328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartalis J, Halaweish FT. J. Chromatogr. B. 2005;818:159–166. doi: 10.1016/j.jchromb.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 33.Halaweish FT. J. Chem. Ecol. 1993;19:29–37. doi: 10.1007/BF00987468. [DOI] [PubMed] [Google Scholar]
- 34.Bartalis J. Ph.D. Thesis. South Dakota State University; Brookings, SD: 2005. Hepatoprotective Activity of Cucurbitacin. [Google Scholar]
- 35.Galindo A, Villegas N, Mansilla H. Nat. Prod. Lett. 1999;13:285–292. [Google Scholar]
- 36.Snatzke G, Enslin PR, Hozapfel CW, Norton KB. J. Chem Soc. C. 1967:972–976. [Google Scholar]
- 37.Kupchan SM, Meshulam H, Sneden AT. Phytochemistry. 1978;17:767–769. [Google Scholar]
- 38.Heck RF, Nolley JP., Jr J. Org. Chem. 1972;37:2320–2322. [Google Scholar]
- 39.Lavie D, Shvo Y. J. Am. Chem. Soc. 1959;81:3058–3061. [Google Scholar]
- 40.Kupchan SM, Gray AH, Grove MD. J. Med. Chem. 1967;10:337–340. doi: 10.1021/jm00315a011. [DOI] [PubMed] [Google Scholar]
- 41.Lang KL, Silva IT, Zimmermann LZ, Machado VR, Teixeira MR, Lapuh MI, Galetti MA, Palermo JA, Cabrera GM, Bernardes LSC, Simões CMO, Schenkel EP, Caro MSB, Durán FJ. Bioorg. Med. Chem. 2012;20:3016–3030. doi: 10.1016/j.bmc.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Ito Y, Hirao T, Saegusa T. J. Org. Chem. 1978;43:1011–1013. [Google Scholar]
- 43.Williams DR, Turske RA. Org. Lett. 2000;2:3217–3220. doi: 10.1021/ol006410+. [DOI] [PubMed] [Google Scholar]
- 44.Diao T, Wadzinski TJ, Stahl SS. Chem. Sci. 2012;3:887–891. doi: 10.1039/C1SC00724F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Zhu J, Jiang H, Wang W, Li J. Chem. Comm. 2010;46:415–417. doi: 10.1039/b922351g. [DOI] [PubMed] [Google Scholar]
- 46.Fang X, Phoebe CH, Jr, Pezzuto JM, Fong HHS, Farnsworth NR. J. Nat. Prod. 1984;47:988–993. doi: 10.1021/np50036a013. [DOI] [PubMed] [Google Scholar]
- 47.Schlegel W, Melera A, Noller CR. J. Org. Chem. 1961;26:1206–1210. [Google Scholar]
- 48.Eisenhut WO, Noller CR. J. Org. Chem. 1958;23:1984–1990. [Google Scholar]
- 49.Mata R, Castañeda P, Camacho MDR. J. Nat. Prod. 1998;51:836–839. doi: 10.1021/np50059a003. [DOI] [PubMed] [Google Scholar]
- 50.Achenbach H, Waibel R, Hefter-Bübl U, Constenla MA. J. Nat. Prod. 1993;56:1506–1519. [Google Scholar]
- 51.Kupchan SM, Smith RM, Aynehchi Y, Maruyama M. J. Org. Chem. 1970;35:2891–2894. doi: 10.1021/jo00834a007. [DOI] [PubMed] [Google Scholar]
- 52.Jacobs H, Singh T, Reynolds WF, McLean S. J. Nat. Prod. 1990;53:1600–1605. [Google Scholar]
- 53.Sreeramulu S, Lakshmi G, Göbel M, Schwalbe H. Angew. Chem. Int. Ed. 2009;48(32):5853–5855. doi: 10.1002/anie.200900929. [DOI] [PubMed] [Google Scholar]
- 54.Ardi VC, Alexander LD, Johnson VA, Mcalpine SR. ACS Chem. Biol. 2011;6(12):1357–1366. doi: 10.1021/cb200203m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hall JA, Kusuma BR, Brandt GEL, Blagg BSJ. ACS Chem. Biol. 2014;9(4):976–85. doi: 10.1021/cb400906e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Papathanassiu A, Macdonald N, Emlet D, Vu H. Cell Stress Chap. 2011;16(2):181–193. doi: 10.1007/s12192-010-0231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mei WL, Lin F, Zuo WJ, Wang H, Dai HF. Chin. J. Nat. Med. 2012;10:234–237. [Google Scholar]
- 58.Kim DK, Choi SH, Lee JO, Ryu SY, Park DK, Shin DH, Jung JH, Pyo SK, Lee KR, Zee OP. Arch. Pharm. Res. 1997;20:85–87. doi: 10.1007/BF02974048. [DOI] [PubMed] [Google Scholar]
- 59.Seger C, Sturm S, Haslinger E, Stuppner H. Monatsh. Chem. 2005;136:1645–1649. [Google Scholar]
- 60.Wu PL, Lin FW, Wu TS, Kuoh CS, Lee KH, Lee SJ. Chem. Pharm. Bull. 2004;52:345–349. doi: 10.1248/cpb.52.345. [DOI] [PubMed] [Google Scholar]
- 61.Sarker SD, Whiting P, Šik V, Dinan L. Phytochemistry. 1999;50:1123–1128. [Google Scholar]
- 62.Monte FJQ, Papa SMA, Kintzinger JP, Braz-Filho R. Magn. Reson. Chem. 2000;38:809–812. [Google Scholar]
- 63.Seger C, Sturm S, Mair ME, Ellmerer EP, Stuppner H. Magn. Reson. Chem. 2005;43:489–491. doi: 10.1002/mrc.1570. [DOI] [PubMed] [Google Scholar]
- 64.Halim OBA, Marawan ESM, El-Gamal AA, Zaghloul MG. Z. Naturforsch. 2008;63b:1415–1420. [Google Scholar]
- 65.Wu S, Yang L, Gao Y, Liu X, Liu F. J. Chromatogr. A. 2008;1180:99–107. doi: 10.1016/j.chroma.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 66.Afifi MS, Ross SA, ElSohly MA, Naeem ZE, Halaweish FT. J. Chem. Ecol. 1999;25:847–859. [Google Scholar]
- 67.Che CT, Fang X, Phoebe CH, Jr, Kinghorn AD, Farnsworth NR. J. Nat. Prod. 1985;48:429–434. doi: 10.1021/np50039a010. [DOI] [PubMed] [Google Scholar]
- 68.Velde VV, Lavie D. Tetrahedron. 1983;39:317–321. [Google Scholar]
- 69.Halaweish FT, Tallamy DW. J. Chem. Ecol. 1993;19:1135–1141. doi: 10.1007/BF00987375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.