Abstract
CONTEXT
Adverse childhood experiences (ACEs) can affect health and well-being across the life course.
OBJECTIVE
This systematic review summarizes the literature on associations between ACEs and risk of cancer in adulthood.
DATA SOURCES
We searched PubMed to identify relevant publications published on or before May 31, 2015.
STUDY SELECTION
We included original research quantifying the association between ACEs and adult cancer incidence. Case reports and reviews were excluded.
DATA ABSTRACTION
Two reviewers independently abstracted and summarized key information (eg, ACE type, cancer type, risk estimates) from included studies and resolved all discrepancies.
RESULTS
Twelve studies were included in the review. In studies in which ACE summary scores were calculated, significant associations were observed between the scores and an increased risk of cancer in adulthood. Of the different types of ACEs examined, physical and psychological abuse victimization were associated with risk of any cancer in 3 and 2 studies, respectively. Two studies also reported significant associations with regard to sexual abuse victimization (1 for cervical cancer and 1 for any cancer). However, 2 other studies reported no significant associations between childhood sexual or physical abuse and incidence of cervical or breast cancer.
LIMITATIONS
Because of heterogeneity across studies, we were unable to compute a summary effect estimate.
CONCLUSIONS
These findings suggest that childhood adversity in various forms may increase a person’s cancer risk. Further research is needed to understand the mechanisms driving this relationship and to identify opportunities to prevent and mitigate the deleterious effects of early adversity on long-term health.
Early life experiences, both positive and negative, can have a lifelong impact on health. The American Academy of Pediatrics promotes using an integrated ecobiodevelopmental (EBD) framework to translate our understanding of the early life origins of many adult diseases into pediatric policies and practices for health and well-being across the life span.1 The EBD framework describes the potential effects of excessive or prolonged early life stress on brain development, which can threaten adaptive capacities and coping skills, thus creating long-term implications for behavioral, educational, economic, and health outcomes decades and even generations later.1
Much of the foundational research examining relationships between childhood adversity and subsequent health outcomes has referred to these early experiences as adverse childhood experiences (ACEs).2 The original ACE study, a collaboration between the Centers for Disease Control and Prevention (CDC) and Kaiser Permanente, examined indicators of child maltreatment and other adverse experiences and found a strong, graded relationship between the breadth of exposure to ACEs and multiple risk factors for several of the leading causes of death in adults (eg, heart disease, cancer).2 Although definitions vary, ACEs also generally include child maltreatment (eg, child abuse and neglect) and other household adversities, such as intimate partner violence among parents, parental separation or divorce, and household mental illness, substance abuse, and incarceration. Recent literature has examined a wider variety of ACEs, including victimization from peer or sibling violence and exposure to community violence.3 A growing body of research suggests that ACEs can impede optimal development and are associated with poorer outcomes across the life course.2,4–6 To date, exposure to ACEs has been linked to >40 negative outcomes, including chronic health conditions (eg, heart disease and cancer2), health behavior risk factors (eg, smoking7), mental health (eg, depressed affect8), life opportunities (eg, education9), and decreased life expectancy.10
Despite the well-documented associations between ACEs and poor adult health outcomes, there are no systematic reviews summarizing the relationships between ACEs and cancer. Given the multifactorial nature of cancer development over the life span,11 a better understanding of how factors during early life influence cancer risk may provide clues to effective prevention strategies to address the growing cancer burden in the United States.12 In this study, we aimed to systematically review the literature reporting associations between ACEs and adult cancer incidence in an effort to synthesize the effects that early adversity may have on the risk of cancer in adulthood and to identify potential research gaps in this area.
METHODS
We conducted this review in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement guidelines.13 Search terms were developed to capture relevant articles related to both ACEs and cancer incidence. The National Cancer Institute’s A-Z cancer list14 was used to ensure inclusion of all potentially relevant cancer search terms. We also included a string of “NOT” terms to exclude irrelevant topics that could inadvertently be captured by the search term “ACE” (eg, angiotensin-converting enzyme inhibitors, auto-capillary electrophoresis). All authors reviewed the final search string for completeness (Supplemental Table 2). We conducted the final PubMed search on May 31, 2015, with no limits on the publication date of the articles.
Study Selection
Studies included in the review were required to be published in peer-reviewed journals and to report the statistical association between experiences considered to meet the definition of ACEs and adult cancer incidence. For the purposes of this review, ACEs were defined as exposure to ≥1 of the following before the age of 18 years: abuse victimization (physical, sexual, or psychological), neglect (failure to provide and failure to supervise), household challenges (parental death or serious illness, parental separation or divorce, parental incarceration, parental substance abuse, domestic violence, mental illness, family financial difficulties, low parental education), and other types of early adversity or trauma. Adult cancer incidence was defined as having been diagnosed with any cancer type at the age of 18 years or older. Case reports and studies that were not original research, did not involve human subjects, or were not written in English were excluded.
An initial PubMed search returned a total of 684 articles, 661 of which did not meet study criteria on the basis of a title and abstract review. The full texts of the remaining 23 articles were then reviewed for relevancy, and 12 articles were determined to be eligible for inclusion. Figure 1 provides a flowchart of the article selection process with reasons for study exclusion.
FIGURE 1.
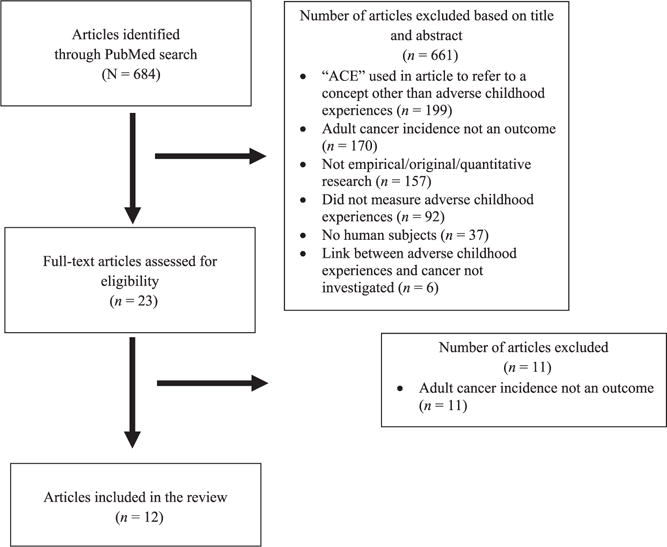
Flowchart of article selection.
Data Abstraction and Synthesis
Two reviewers independently abstracted information from the 12 included studies. For each study, the following data were collected: PubMed ID, title, first author, year of publication, sample size, study population, country, study design, year(s) of data collection, measure(s) of early adversity, measure(s) of cancer incidence, analyses used, variables included in the adjusted analyses, and reported associations between early adversity and cancer outcomes on the basis of adjusted analyses (ie, risk, odds, and hazard ratios). To examine the potential influence of socioeconomic disparities, we also abstracted information on the distribution of both ACEs and cancer by measures of demographic characteristics (eg, race, ethnicity) and socioeconomic factors (eg, education, income), when reported. Both reviewers completed abstraction forms for all 12 studies and then compared their results and resolved any discrepancies. In an effort to identify opportunities for quantitative synthesis of the data, studies were grouped by the ACE measures used (ie, measures of individual ACEs versus ACE summary scores) and cancer measures used (ie, diagnosed with any cancer versus diagnosed with a specific cancer type). Reviewers outlined the key information and findings from each study in a table format and developed a descriptive summary of the observed patterns.
RESULTS
Table 1 provides a summary of the characteristics of each included study and significant associations reported in the adjusted analyses. Summary effect estimates were not computed due to heterogeneity across studies. Of the 12 studies, 7 were conducted in the United States,2,15–20 2 were conducted in Great Britain,21,22 1 in Canada,23 1 in Finland,24 and 1 in Saudi Arabia.25 Six of the studies used a cross-sectional study design,17–20,22,23 collecting data on both ACEs and previous cancer diagnosis at the same point in time. Five studies used a prospective cohort design.15,16,20,21,24 One of the prospective cohort studies21 followed participants from birth; the other 4 prospective cohort studies collected self-reported data on past ACEs at baseline and data on cancer incidence prospectively.15,16,20,24 One additional study25 used a case-control design to compare ACEs among patients with cancer to ACEs among healthy matched controls. Two of the articles reported on data from the ACE study conducted by Kaiser Permanente and the CDC. Felitti et al2 used data from wave 1, and Brown et al15 used data from waves 1 and 2 of the ACE study.
TABLE 1.
Summary of Characteristics and Significant Associations Reported in the Adjusted Analyses of Included Studies
| First Author, Year (Sample Sizea), and Participant Ages | Study Location (Study Design) | Year(s) of Data Collection | ACE Variable(s) Used in Analyses and Types of ACEs Measured | Outcome of Interest | Significant Associations Reportedb |
|---|---|---|---|---|---|
| Bellis, 201522 (3881), 18–69 years | England (cross-sectional) | 2013 | Summary ACE score, based on dichotomous measures of the following variables: • Abuse (physical, psychological, sexual) • Parental separation or divorce • Domestic violence • Mental illness • Alcohol abuse • Drug abuse • Incarceration |
Self-reported cancer diagnosis | ≥4 vs 0 ACEs: HR = 2.38 (1.48–3.83) |
| Brown, 201015 (16 901), ≥18 years | California, United States (prospective cohort) | Past ACEs measured at baseline during 1995–1997; cancer diagnosis measured in 2005 | Summary ACE score, based on dichotomous measures of the following variables: • Abuse (physical, psychological, sexual) • Parental separation or divorce • Domestic violence • Mental illness • Alcohol abuse • Drug abuse • Incarceration |
Lung cancer diagnosis (based on hospital and mortality records) | The observed relationship between ACE score and lung cancer risk was strong and graded. Without controlling for smoking status and parental smoking history: P for trend = 0.001; 3 vs 0 ACEs: RR = 1.92 (1.11–3.33); 4 or 5 vs 0 ACEs: RR = 1.88 (1.04–2.41) When controlling for smoking status and parental smoking history: P for trend = 0.007 |
| Brown, 201317 (4230), ≥18 years | United States (cross-sectional) | 2010 | Components from a factor analysis that included dichotomous measures of the following variables: • Abuse (physical, psychological, sexual) • Parental separation or divorce • Domestic violence • Mental illness • Alcohol abuse • Drug abuse • Incarceration Sexual abuse variables had the highest weights in component 1. ACEs not directed toward the child had highest weights in component 2. Psychological and physical abuse variables had highest weights in component 3. |
Self-reported cancer diagnosis | Component 1: OR = 1.21 (1.03–1.43) |
| Coker, 200918 (4732), 18–88 years | Kentucky, United States (cross-sectional) | 2006–2007 | Dichotomous measure of exposure to sexual abuse | Self-reported cervical cancer diagnosis | Sexual abuse: OR = 2.4 (1.4–4.0) |
| Felitti, 19982 (8011), ≥19 years | California, United States (cross-sectional) | 1995–1996 | Summary ACE score, based on dichotomous measures of the following variables: • Abuse (physical, psychological, sexual) • Domestic violence • Mental illness • Alcohol abuse • Drug abuse • Incarceration |
Self-reported cancer diagnosis | ≥4 vs 0 ACEs: OR = 1.9 (1.3–2.7) |
| Fuller-Thompson, 200923 (12 485), ≥12 years | Manitoba and Saskatchewan, Canada (cross-sectional) | 2005 | Dichotomous measures of exposure to individual ACEs: • Physical abuse • Parental divorce • Parental alcohol or drug addiction • Parental unemployment |
Self-reported cancer diagnosis | Physical abuse: OR = 1.45 (1.05–1.99) Parental unemployment: OR = 1.58 (1.11–2.25) |
| Hyland, 201325 (400), 40–60 years | Dammam, Saudi Arabia (case-control) | 2009–2010 | Dichotomous measures of and frequency of exposure to individual ACEs: • Physical abuse • Psychological abuse |
Any cancer diagnosis (based on hospital records) | Psychological abuse (versus never) Once in my life: RR = 2.20, P = .04 Once every 6 months: RR = 4.62, P = .001 Once a month: RR = 5.52, P = .001 Once a week: RR = 6.19, P = .001 Once every 2–3 days: RR = 4.05, P = .001 Physical abuse (versus never Once a year: RR = 3.60, P = .001 Once every 6 months: RR = 5.17, P = .001 Once a month: RR = 5.32, P = .001 Once a week: RR = 6.42, P = .001 Once every 2–3 days: RR = 3.66, P = .01 |
| Kelly-Irving, 201321 (6138), data collected from birth through age 50 years | Great Britain (prospective birth cohort) | Birth: 1958 (ACEs measured at ages 7, 11, and 16; cancer diagnosis measured at ages 33, 42, 46, and 50) | Summary ACE score, based on dichotomous measures of the following variables: • Physical neglect • Parental death, divorce, or separation • Mental illness • Alcohol abuse • Incarceration or probation of o family member o respondent before age 16 y • Foster care |
Self-reported cancer diagnosis | Men: no significant associations found in the adjusted model Women: ≥2 vs 0 ACEs: OR = 2.14 (1.42–3.21) |
| Korpimäki, 201024 (23 358), 20–54 years at baseline | Finland (prospective cohort) | Past ACEs measured at baseline in 1998; cancer diagnosis measured during 2000–2006 | Dichotomous measures of exposure to individual ACEs: • Parental divorce • Alcohol problems • Prolonged financial difficulties • Serious conflicts in the family • Often afraid of a family member • Family member seriously/chronically ill |
Any cancer diagnosis (based on Finnish Cancer Registry data) | No significant associations found in the adjusted model |
| Morton, 201216 (3032), 25–74 years at baseline (wave 1) | United States (prospective cohort) | Wave 1: 1995–1996; wave 2: 2004–2006; past ACEs measured in wave 1; cancer diagnosis measured in waves 1 and 2 | Dichotomous measures of exposure to individual ACEs and summary ACE score: • Family receipt of welfare for ≥6 months • Physical abuse • Psychological abuse • Parental divorce • Parental death • Lack of male in household • Financially worse off than other families • Less than a high school education for father (or mother if father was absent) • Poor mental or physical health at age 16 years |
Self-reported cancer diagnosis (other than skin) | Men: summary ACE score: OR = 1.307 (1.009–1.693); physical abuse (father): OR = 2.559 (1.173–5.581); frequently psychologically and physically abused by parents: OR = 3.558 (1.467–8.629) Women: physical abuse (mother: OR = 2.211 (1.114–4.388); frequently psychologically and physically abused by parents: OR = 2.184 (1.221–3.905) |
| Ramaswamy, 201119 (204), 34.2 ± 10.1 years (mean ± SD) | Kansas, United States (cross-sectional) | 2010 | Dichotomous measures of exposure to individual ACEs: • Physical abuse • Sexual abuse |
Self-reported cervical cancer diagnosis | No significant associations found in the adjusted model |
| Wise, 201120 (35 728), 21–69 years at baseline | United States (prospective cohort) | Every 2 years during 1995–2009; past ACEs measured in 2005; cancer diagnosis measured in 1997–2009 | Dichotomous measures of exposure to individual ACEs: • Physical abuse • Sexual abuse • Both physical and sexual abuse |
Self-reported breast cancer diagnosis | No significant associations found in the adjusted model |
N = 12 studies. HR, hazard ratio; OR, odds ratio; RR, risk ratio.
Sample size included in the adjusted analyses.
Based on adjusted analyses in the final model; results not listed here were nonsignificant. Values in parentheses are 95% confidence intervals.
Types of ACEs
Figure 2 provides a list of all ACEs included in ≥1 of the studies and the number of studies that included each type as (1) an individual variable in the adjusted analyses, (2) part of an ACE summary score in the adjusted analyses, and (3) both as an individual variable in some adjusted analyses and as part of an ACE summary score in additional analyses. Twenty different types of ACEs were included across the 12 studies. Parental separation or divorce and physical abuse victimization were the most common experiences examined (8 studies), followed by household alcohol abuse (7 studies), sexual abuse (7 studies), and psychological abuse (6 studies). Exposure to domestic violence, household drug abuse, parental incarceration, and household mental illness were each included in 5 of the studies. All other ACEs were only included in 1 or 2 of the studies.
FIGURE 2.
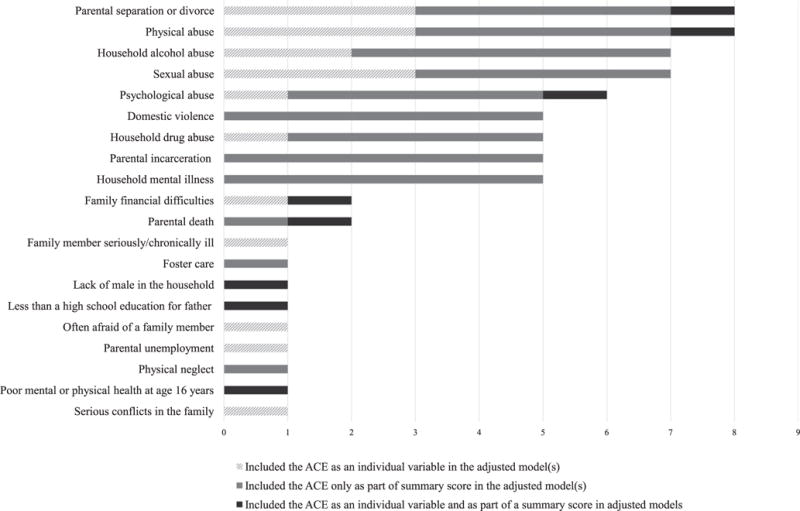
Types of ACEs and the number of studies in which they were included (N = 12 studies).
All of the studies used the data collected on ACEs to create dichotomous variables to indicate whether participants were exposed to the given ACE. In some studies, the researchers included this dichotomous variable in adjusted models to assess the influence of the specific ACE type on cancer risk. In other studies, researchers created a summary score indicating the number of ACEs that participants had experienced and used only the summary score in the adjusted models. In 1 study,16 the researchers used both approaches, estimating the relative cancer risk associated with both individual ACEs and with the overall ACE summary score.
Types of Cancer
In most (8 of 12) studies,2,16,17,21–25 the outcome variable of interest was any type of cancer diagnosis in adulthood. Only 1 study excluded skin cancer.16 Four studies focused on the diagnosis of a specific type of cancer: 2 examined the association between ACEs and cervical cancer,18,19 1 examined breast cancer,20 and 1 examined lung cancer.15 Most studies (9 of 12) relied solely on self-reported cancer incidence,2,16–23 and 3 used cancer registry data, hospital records, and/or mortality records to identify cancer cases.15,24,25
ACEs and Risk of Any Cancer
Of the 8 studies examining the association between ACEs and the risk of any cancer type, 5 included analyses that used an ACE summary score to quantify exposure to early adversity.2,16,17,21,22 All 5 studies found significant associations between ACE summary scores and adult cancer risk. Bellis et al22 and Felitti et al2 both reported a significantly higher cancer risk among those reporting ≥4 ACEs compared with those reporting no ACEs. Kelly-Irving et al21 found a significantly higher cancer risk among those reporting ≥2 ACEs versus those reporting no ACEs, but this association was only observed among women. In contrast, Morton et al16 found that the ACE summary score was significantly associated with cancer risk in men only. The study by Brown et al17 examined the association between ACE summary components from a factor analysis and cancer risk. In that study, the authors found a significant association with adult cancer risk for component 1 only, the component in which sexual abuse variables were given the highest weights. Component 2 (highest weights given to ACEs not directed toward the child) and component 3 (highest weights given to psychological and physical abuse variables) were not significantly associated with adult cancer risk.
Four of the studies16,23–25 reported on individual types of ACEs and the risk of any cancer in adulthood. Among these studies, physical and psychological abuse victimization was found to be a significant predictor of cancer risk in adulthood when assessed in multiple studies. Results from 3 studies16,23,25 indicated significant associations between physical abuse and cancer risk. In the study conducted by Morton et al,16 physical abuse by one’s father only was a significant predictor of cancer risk among men, and physical abuse by one’s mother only was a significant predictor among women. The authors also found a significant association between frequent parental psychological and physical abuse and cancer risk among both men and women. Similarly, Hyland et al25 reported a significant association between psychological abuse during childhood and cancer risk in adulthood. Only 1 study conducted in Finland by Korpimäki et al24 failed to find associations between individual types of ACEs and adult cancer risk. The study examined the associations between dichotomous measures of a variety of ACEs (parental divorce, alcohol problems, prolonged financial difficulties, serious conflicts in the family, being often afraid of a family member, and having a seriously/chronically ill family member) but did not examine childhood abuse victimization (physical, psychological, or sexual). Fuller-Thompson and Brennenstuhl23 also found a significant association between parental unemployment during childhood and the risk of cancer in adulthood, but this specific type of adversity was not measured in any of the other studies.
ACEs and Risk of Specific Cancer Types
The other 4 studies included in this review examined the association between ACEs and specific types of cancer: cervical,18,19 breast,20 and lung.15 Findings were mixed in the 2 studies examining cervical cancer risk in relation to ACEs, with 1 study18 finding a significant association between childhood sexual abuse victimization and risk of cervical cancer in adulthood and the other19 reporting no significant associations between either sexual or physical abuse victimization during childhood and risk of cervical cancer in adulthood. The study by Wise et al20 examined breast cancer risk and found no significant associations with childhood sexual or physical abuse victimization. The study by Brown et al15 examined the risk of lung cancer and reported a strong and graded relationship between a summary ACE score and lung cancer risk. Respondents who ever smoked were more likely to report experiencing ACEs, but the association between ACEs and lung cancer risk remained even after controlling for smoking status and parental smoking history.
Distribution of Risk Based on Race, Ethnicity, and Socioeconomic Factors
Three of the 12 studies reported the distribution of ACEs by measures of race, ethnicity, and/or socioeconomic factors.2,17,22 One study reported a significant association between ACE summary score and ethnicity (P < .001), with ACEs being least common among Asians and most common among whites and “other” ethnic groups.22 Another study reported that ACEs were less common among whites than among other racial/ethnic groups (P = .0001) and less common among those with annual incomes of ≥$50 000 than among those with lower incomes (P < .0001).17 A third study found significantly fewer ACEs among white or Asian persons and college graduates (P < .001).2 Three studies examined associations between socioeconomic factors (eg, education and income) and having been diagnosed with cancer.16,21,24 Two of the studies found no associations,16,21 and the other study reported lower cancer risk among those with higher education.24 Two studies examined the association between race/ethnicity and cancer risk.19,23 One found a lower cancer risk among “visible minorities” compared with whites23; the other found lower odds of cervical cancer diagnosis among blacks than among whites.19
DISCUSSION
Across studies, ACE summary scores were associated with an increased risk of cancer in adulthood. Of the different types of ACEs examined, physical and psychological abuse victimization was more frequently associated with adult cancer risk. Two studies also reported significant associations with regard to sexual abuse victimization. Only 1 study (of 8) did not find an association between ACEs and diagnosis of any cancer in adulthood. Most studies included skin cancer as an outcome, which could potentially dilute or inflate the observed relationship between ACEs and cancer risk given the high incidence of nonmelanoma skin cancers in the study populations.26 Only 2 of the 4 studies looking at diagnosis of specific cancer types found an association with ACEs. The lack of consistency in findings regarding specific cancer types may reflect the variation in etiology and causal pathways for different types of cancer. For example, whereas early adversity may have the potential to increase overall cancer risk, the mechanisms by which these effects occur may be more strongly related to certain types of cancer than others.
Potential Mechanisms Linking ACEs to Cancer Risk
The studies do not provide information on the pathways between ACEs and cancer development in adulthood, and research is needed to delineate such pathways, including potential biological, physiologic, and behavioral mechanisms. The current literature indicates that ACEs are associated with an increased risk of certain well-established cancer risk factors (eg, tobacco use,7 alcohol use,27 obesity28). Therefore, some health risk behaviors and chronic conditions may be part of the causal pathway from early adversity to cancer development, pointing to the potential value of health promotion and chronic disease prevention programs for those who have experienced childhood adversity. Emerging research points to additional mechanisms by which childhood maltreatment may influence adult health. For example, evidence from rodent models suggests that maternal care in the first week of postnatal life establishes phenotypes in the offspring through epigenetic changes expressed in the brain, which then shape neuroendocrine and behavioral stress responsivity throughout life.29 Sustained psychosocial stress in early life appears to be associated with shorter telomeres, possibly in a dose-dependent manner.30 Reduced telomere length is considered a proxy for cellular aging and has been associated with many chronic conditions, including cancer.30 Other research findings indicate an association between ACEs and biomarkers of inflammation, another potential mechanism that might contribute to increased cancer risk.31,32 Thus, the promotion of healthy maternal bonding and nurturing by caregivers may reduce cancer risk, as well as prompt attention to any cause of inflammation by care providers.
Implications for Research and Practice
Most articles published on this topic were published in 2009 or later, indicating that this topic is an emerging area of interest among researchers. Although evidence suggests a link between ACEs and cancer risk, there is a need for this relationship to be studied in a more systematic and scientifically rigorous way. For example, in the existing literature, studies typically lack detailed information on the various dimensions of the adverse experiences (eg, age of onset, frequency, duration, or perceived stressfulness of ACEs or the child’s relationship to the perpetrator) and the influence these factors may have on the relationship between ACEs and cancer risk. Most studies also lacked information on the effects of potential protective factors, an understanding of which could help us identify novel prevention strategies. In addition, most studies treated cancer as a single disease outcome when, in reality, cancer refers to a wide range of distinct diseases with different causal factors and etiologic pathways. Examining the association between ACEs and the risk of specific cancer types would likely strengthen our ability to uncover patterns in the exposure-disease relationships. In general, future studies examining cancer risk factors would benefit from including measures of ACEs and further exploring the strength of the association between ACEs and cancer, as well as the interplay between ACEs, other cancer risk factors, protective factors, and cancer outcomes. Studies may also benefit from more careful consideration of the age of the study sample. Among the studies included in this review, participants often spanned a wide range of ages (eg, adults aged ≥18). Among the studies with a narrower participant age range, data on cancer incidence often were not collected beyond age 50 or 60 years. Most cancer cases are not diagnosed until later in life,33 so focusing on cancer outcomes in an older adult population may yield more informative data.
Studying the influence of ACEs on subsequent cancer risk has the potential to uncover new opportunities for prevention. Strategies that prevent or reduce perpetration of child maltreatment (eg, evidence-based family strengthening and home visitation programs)34–37 may have the added benefit of long-term risk reduction for cancer and other poor health outcomes, in addition to the more immediate benefits to the child. Examples of evidence-based programs to stop child maltreatment, as well as guidelines and planning tools, are available on the CDC Web site (http://www.cdc.gov/violenceprevention/childmaltreatment/prevention.html). Pediatricians could be ready to suggest such local resources to their patients’ families. Additional research may help us identify groups that may be at increased risk of cancer or other chronic diseases and who would benefit from supportive interventions to mitigate effects of early life adversities.
Only 3 of the included studies reported on the distribution of ACEs by social or economic descriptors (eg, race/ethnicity, education, income); and 5 examined the relationship between such factors and cancer outcomes, with inconsistent findings. Future research should systematically and consistently include these descriptors to better understand the influence of social and economic conditions on early life experiences as well as on how such conditions affect health and well-being in adulthood. Research indicates that early adversity causes both short-and long-term stress responses that contribute to poor health outcomes.38 Children who have additional stress from environmental conditions, including poverty and racism, may experience greater harmful effects from ACEs and be at greater risk of cancer in adulthood. Furthermore, early adversity can have deleterious effects on brain development within regions tied to regulation of emotion, social behavior, reasoning capacity, and stress reactivity.38 This effect can lead to changes in brain circuitry that can persist into adulthood, possibly contributing to emotional instability, substance abuse, aggression, and stress-related disorders.38 Such long-term effects likely mean that the children of those who experienced early adversity and stressful childhood environments are themselves more likely to experience ACEs. These research findings point to the critical need for public health efforts that address the social and economic contexts. Pediatricians also have a role to play by supporting such public health efforts and by informing their patients’ families of these efforts and the resources they provide. By addressing socioeconomic determinants of health, the public health community and health care providers, including pediatricians, can create healthier environments for today’s children as well as make sustainable improvements in health at the population level for generations to come.39
Pediatricians, pediatric nurses, and other pediatric medical professionals are particularly well positioned to play a role in preventing, identifying, and responding to ACEs; and the American Academy of Pediatrics has used the EBD framework to develop several recommendations specific to addressing early adversity.1,40–42 These recommendations include encouraging pediatricians to adopt a proactive leadership role in educating parents, child care providers, teachers, policy makers, civic leaders, and the general public about the long-term consequences of toxic stress and the potential benefits of preventing or reducing sources of significant adversity in early childhood.1 This process will require embracing a broader vision of pediatric care to address socioemotional health and true primary prevention and health promotion rather than solely treatment of disease.42 Furthermore, it will require “unprecedented levels of collaboration” with community partners, including educators, social service providers, and policy makers.42
This review benefits from the systematic nature of the literature search and synthesis process. However, the review is limited by the heterogeneity of the existing literature (which precludes our ability to compute a summary estimate) and the small number of available publications within the scope of the review. In addition, data on ACEs and on cancer diagnoses often were self-reported and collected retrospectively, introducing the potential for bias. Another important limitation involves the potential for publication bias and the inaccessibility of null results, which may inadvertently exaggerate the strength of association between ACEs and cancer. However, most prevalence estimates of early adversity tend to be vast underestimates,43 so it is possible that the true nature of the association between ACEs and cancer risk is greater than what is reported in the literature.
Conclusions
The findings from our review suggest that child maltreatment and other early adversities may increase a person’s cancer risk. Additional research is needed to better understand the mechanisms driving this relationship. Public health efforts to prevent and mitigate the deleterious effects of childhood adversity may have additional benefits to long-term health. By ensuring safe, stable, nurturing relationships and environments for all children and families, the public health community in partnership with health care providers, including pediatricians, can help prevent early adverse experiences before they occur.44 Transdisciplinary approaches to improve socioeconomic determinants of health will be critical to making this vision a reality. Additional interventions by pediatricians for children who have already experienced adversity could potentially reduce the harmful emotional, behavioral, and health effects, thus resetting their trajectory for lifelong health and reducing the risk of cancer and other chronic diseases and conditions.
Supplementary Material
Acknowledgments
FUNDING: All authors are federal government employees, and the preparation of the manuscript was entirely funded by the US government.
ABBREVIATIONS
- ACE
adverse childhood experience
- CDC
Centers for Disease Control and Prevention
- EBD
ecobiodevelopmental
Footnotes
Ms Holman and Dr Ports conceptualized and designed the review, conducted the literature search and data abstraction, provided substantial contribution to the interpretation of the results, drafted the initial manuscript, and critically revised the manuscript; Drs Buchanan, Hawkins, and Merrick and Ms Metzler and Dr Trivers conceptualized and designed the review, provided substantial contribution to the interpretation of the results, and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors indicated they have no potential conflicts of interest to disclose.
References
- 1.Garner AS, Shonkoff JP, Committee on Psychosocial Aspects of Child and Family Health; Committee on Early Childhood, Adoption, and Dependent Care; Section on Developmental and Behavioral Pediatrics Early childhood adversity, toxic stress, and the role of the pediatrician: translating developmental science into lifelong health. Pediatrics. 2012;129(1) doi: 10.1542/peds.2011-2662. Available at: www.pediatrics.org/cgi/content/full/129/1/e224. [DOI] [PubMed] [Google Scholar]
- 2.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 3.Finkelhor D, Shattuck A, Turner H, Hamby S. Improving the adverse childhood experiences study scale. JAMA Pediatr. 2013;167(1):70–75. doi: 10.1001/jamapediatrics.2013.420. [DOI] [PubMed] [Google Scholar]
- 4.Anda RFFV, Fleisher VI, Felitti VJ, et al. Childhood abuse, household dysfunction, and indicators of impaired adult worker performance. Perm J. 2004;8(1):30–38. doi: 10.7812/tpp/03-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert LK, Breiding MJ, Merrick MT, et al. Childhood adversity and adult chronic disease: an update from ten states and the District of Columbia, 2010. Am J Prev Med. 2015;48(3):345–349. doi: 10.1016/j.amepre.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Croft JB, Chapman DP, et al. Relationship between adverse childhood experiences and unemployment among adults from five U.S. states. Soc Psychiatry Psychiatr Epidemiol. 2013;48(3):357–369. doi: 10.1007/s00127-012-0554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford ES, Anda RF, Edwards VJ, et al. Adverse childhood experiences and smoking status in five states. Prev Med. 2011;53(3):188–193. doi: 10.1016/j.ypmed.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004;82(2):217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Macmillian R, Hagan J. Violence in the transition to adulthood: adolescent victimization, education, and socioeconomic attainment in later life. J Res Adolesc. 2004;14(2):127–158. [Google Scholar]
- 10.Brown DW, Anda RF, Tiemeier H, et al. Adverse childhood experiences and the risk of premature mortality. Am J Prev Med. 2009;37(5):389–396. doi: 10.1016/j.amepre.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 11.The President’s Cancer Panel. Reducing Environmental Cancer Risk: What Can We Do Now? Bethesda, MD: US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2010. [Google Scholar]
- 12.Weir HK, Thompson TD, Soman A, Møller B, Leadbetter S. The past, present, and future of cancer incidence in the United States: 1975 through 2020. Cancer. 2015;121(11):1827–1837. doi: 10.1002/cncr.29258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Cancer types. Available at: www.cancer.gov/types. Accessed July 8, 2015.
- 15.Brown DW, Anda RF, Felitti VJ, et al. Adverse childhood experiences are associated with the risk of lung cancer: a prospective cohort study. BMC Public Health. 2010;10(20):1–12. doi: 10.1186/1471-2458-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morton PM, Schafer MH, Ferraro KF. Does childhood misfortune increase cancer risk in adulthood? J Aging Health. 2012;24(6):948–984. doi: 10.1177/0898264312449184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown MJ, Thacker LR, Cohen SA. Association between adverse childhood experiences and diagnosis of cancer. PLoS One. 2013;8(6):e65524. doi: 10.1371/journal.pone.0065524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coker AL, Hopenhayn C, DeSimone CP, Bush HM, Crofford L. Violence against women raises risk of cervical cancer. J Womens Health (Larchmt) 2009;18(8):1179–1185. doi: 10.1089/jwh.2008.1048. [DOI] [PubMed] [Google Scholar]
- 19.Ramaswamy M, Kelly PJ, Koblitz A, Kimminau KS, Engelman KK. Understanding the role of violence in incarcerated women’s cervical cancer screening and history. Women Health. 2011;51(5):423–441. doi: 10.1080/03630242.2011.590875. [DOI] [PubMed] [Google Scholar]
- 20.Wise LA, Palmer JR, Boggs DA, Adams-Campbell LL, Rosenberg L. Abuse victimization and risk of breast cancer in the Black Women’s Health Study. Cancer Causes Control. 2011;22(4):659–669. doi: 10.1007/s10552-011-9738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly-Irving M, Lepage B, Dedieu D, et al. Childhood adversity as a risk for cancer: findings from the 1958 British birth cohort study. BMC Public Health. 2013;13(767):1–13. doi: 10.1186/1471-2458-13-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellis MA, Hughes K, Leckenby N, Hardcastle KA, Perkins C, Lowey H. Measuring mortality and the burden of adult disease associated with adverse childhood experiences in England: a national survey. J Public Health (Oxf) 2015;37(3):445–454. doi: 10.1093/pubmed/fdu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuller-Thomson E, Brennenstuhl S. Making a link between childhood physical abuse and cancer: results from a regional representative survey. Cancer. 2009;115(14):3341–3350. doi: 10.1002/cncr.24372. [DOI] [PubMed] [Google Scholar]
- 24.Korpimäki SK, Sumanen MP, Sillanmäki LH, Mattila KJ. Cancer in working-age is not associated with childhood adversities. Acta Oncol. 2010;49(4):436–440. doi: 10.3109/02841860903521103. [DOI] [PubMed] [Google Scholar]
- 25.Hyland ME, Alkhalaf AM, Whalley B. Beating and insulting children as a risk for adult cancer, cardiac disease and asthma. J Behav Med. 2013;36(6):632–640. doi: 10.1007/s10865-012-9457-6. [DOI] [PubMed] [Google Scholar]
- 26.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 27.Strine TW, Dube SR, Edwards VJ, et al. Associations between adverse childhood experiences, psychological distress, and adult alcohol problems. Am J Health Behav. 2012;36(3):408–423. doi: 10.5993/AJHB.36.3.11. [DOI] [PubMed] [Google Scholar]
- 28.Danese A, Tan M. Childhood maltreatment and obesity: systematic review and meta-analysis. Mol Psychiatry. 2014;19(5):544–554. doi: 10.1038/mp.2013.54. [DOI] [PubMed] [Google Scholar]
- 29.Weaver IC. Shaping adult phenotypes through early life environments. Birth Defects Res C Embryo Today. 2009;87(4):314–326. doi: 10.1002/bdrc.20164. [DOI] [PubMed] [Google Scholar]
- 30.Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biol Psychiatry. 2013;73(1):15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Archer JA, Hutchison IL, Dorudi S, Stansfeld SA, Korszun A. Interrelationship of depression, stress and inflammation in cancer patients: a preliminary study. J Affect Disord. 2012;143(1–3):39–46. doi: 10.1016/j.jad.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 32.Crosswell AD, Bower JE, Ganz PA. Childhood adversity and inflammation in breast cancer survivors. Psychosom Med. 2014;76(3):208–214. doi: 10.1097/PSY.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. Published 2013. Available at: http://seer.cancer.gov/archive/csr/1975_2010/. Accessed August 27, 2015.
- 34.Klevens J, Barnett SB, Florence C, Moore D. Exploring policies for the reduction of child physical abuse and neglect. Child Abuse Negl. 2015;40:1–11. doi: 10.1016/j.chiabu.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daro D, Dodge KA. Creating community responsibility for child protection: possibilities and challenges. Future Child. 2009;19(2):67–93. doi: 10.1353/foc.0.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaffin M, Funderburk B, Bard D, Valle LA, Gurwitch R. A combined motivation and parent-child interaction therapy package reduces child welfare recidivism in a randomized dismantling field trial. J Consult Clin Psychol. 2011;79(1):84–95. doi: 10.1037/a0021227. [DOI] [PubMed] [Google Scholar]
- 37.Olds DL, Eckenrode J, Henderson CR, Jr, et al. Long-term effects of home visitation on maternal life course and child abuse and neglect: fifteen-year follow-up of a randomized trial. JAMA. 1997;278(8):637–643. [PubMed] [Google Scholar]
- 38.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 39.Frieden TR. A framework for public health action: the health impact pyramid. Am J Public Health. 2010;100(4):590–595. doi: 10.2105/AJPH.2009.185652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson SB, Riley AW, Granger DA, Riis J. The science of early life toxic stress for pediatric practice and advocacy. Pediatrics. 2013;131(2):319–327. doi: 10.1542/peds.2012-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Malley DM. The Affordable Care Act, science, and childhood adversity: a call for pediatric nurses and physicians to lead. Nurs Adm Q. 2013;37(3):216–221. doi: 10.1097/NAQ.0b013e318295f5d8. [DOI] [PubMed] [Google Scholar]
- 42.Garner AS, Forkey H, Szilagyi M. Translating developmental science to address childhood adversity. Acad Pediatr. 2015;15(5):493–502. doi: 10.1016/j.acap.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Wildeman C, Emanuel N, Leventhal JM, Putnam-Hornstein E, Waldfogel J, Lee H. The prevalence of confirmed maltreatment among US children, 2004 to 2011. JAMA Pediatr. 2014;168(8):706–713. doi: 10.1001/jamapediatrics.2014.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. Child maltreatment: prevention strategies. Published 2015. Available at: www.cdc.gov/violenceprevention/childmaltreatment/prevention.html. Accessed August 17, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


