Abstract
This study examined the effect of bisphenol A (BPA) exposure on human uterine stromal fibroblast cells (HuF) undergoing decidualization. HuF cells were isolated and cultured for eight days in the presence of a decidualization-inducing cocktail, while concurrently exposed to physiological and supra-physiologic doses of BPA (1 ng/mL, 10 ng/mL, 0.5 (μg/mL, 10 (μg/mL and 20 (μg/mL). Decidualization markers, steroid hormone receptors and cell cycle gene expression were detected by qRT-PCR and cellular proliferation was assessed by KI-67 immunofluorescent staining and MTS assay. BPA impaired decidualization at 10 μg/mL and 20(μg/mL, but not at lower doses. Additionally, BPA at 20 μg/mL decreased progesterone receptor and estrogen receptor-alpha compared to controls. The highest dose of BPA also reduced cellular proliferation and cyclin D2 expression compared to controls. These findings demonstrate that BPA disrupts in vitro decidualization of uterine stromal fibroblasts by altering steroid hormone receptor expression at higher concentrations but not at lower physiological doses.
Keywords: Bisphenol-A, HuF cells, Decidualization, Endocrine disruptor, Endometrium, Fertility, Cell proliferation
1. Introduction
BPA is a known endocrine disruptor which is an extensively used chemical in the production of epoxy resins, polycarbonate plastics and flame retardants [1]. Globally, BPA production is increasing at substantial rates. Nearly 5.5 million tons were produced in 2011, compared to 3.2 million tons in 2003 [2,3]. In 2007, the United States accounted for approximately 30% of the global production of BPA [4]. BPA can leach from these products and enter into the human body by ingestion and absorption through direct contact with BPA or contact with products containing BPA such as metal food cans, dental sealants and plastic drinking containers [1]. Therefore, humans are constantly exposed to BPA and a recent study detected BPA in 95% of human urine samples tested [5]. As an endocrine disruptor, BPA can bind to estrogen receptor-alpha and–beta and can exhibit weak estrogenic activity, but it is potency ranges from 10 to 10,000 fold less than estradiol [6,7]. Due to this action and other significant evidence, BPA was recently classified as a female reproductive toxicant by a panel of experts in the field [8].
Epidemiological studies have linked BPA exposure to pregnancy loss and a higher incidence of polycystic ovarian syndrome, endometriosis and implantation failure in women [9–12]. Furthermore, BPA exposure has been extensively tested in rodent models and in vitro studies which confirm BPA's role as a selective estrogen receptor modulator [8].
The female reproductive system and specifically the uterus is highly sensitive to changes in sex hormone concentrations as the endometrium proliferates and differentiates each month in response to estrogen and progesterone, respectively. During the proliferative stage, estrogen receptor-alpha and progesterone receptor are expressed in the endometrial epithelium and stromal compartments. However, around the mid- to late-secretory stage, estrogen receptor-alpha and progesterone receptor expression are absent in the epithelium, but retained in the stroma [13]. Progesterone receptor A is the predominant progesterone receptor isoform in the uterus and is also highly expressed throughout pregnancy [14]. Aberrant expression of estrogen receptor-alpha and progesterone receptor in the female uterus can lead to infertility, recurrent pregnancy loss and endometrial hyperplasia [15].
Decidualization is the process of stromal cell differentiation in the endometrium that must occur during a successful pregnancy. The decidual cells create an environment that facilitates embryo attachment and placental development [16]. In humans, this process is under maternal control, meaning that decidualization occurs every cycle in the mid- to late-secretory phase due to endocrine and paracrine regulation. Decidual cells are characterized by rounded nuclei, a large cytoplasmic area, significant extracellular matrix production and gap junction formations [17]. Prolactin and IGFBP-1 are produced and secreted by decidual cells and are considered markers to assess the status of stromal cells decidualizing in vitro [16].
Effective decidualization relies upon a harmonized coordination of hormone, hormone receptor and paracrine factors and the perturbation of any of these factors may lead to failed decidualization and ultimately pregnancy loss [16].
Previous studies demonstrated that BPA reduced proliferation and altered expression of hallmark genes associated with decidualization in human stromal fibroblasts [18,19]. One study tested doses of BPA above known physiological exposure levels [18] and the other study tested a range of physiological and supra-physiological doses, but only observed significant effects of decidualization at doses above physiological exposure levels [19]. In the current study, we questioned whether exogenous BPA exposure would impair the process of decidualization in uterine stromal cells, as previous studies only assessed BPA's effect on stromal fibroblasts which were pre-decidualized. To address this question, we treated HuF cells (human uterine stromal fibroblasts) during the process of decidualization with vehicle, physiological or supra-physiological doses of BPA and examined the expression of genes associated with decidualization and cell cycle regulation and evaluated several markers of proliferation.
2. Materials and methods
2.1. Reagents
17-β–estradiol (lot # 021M8707 V), medroxyprogesterone acetate (lot# K1293) and dibutyryladenosine cyclic monophosphate (lot # SLBK3830 V) (cAMP) were purchased from Sigma Aldrich (St. Louis, MO). Bisphenol A (99% purity) was obtained as a gift from the National Institute for Environmental Health Sciences to JF. RNA and qPCR reagents including trizol, high capacity cDNA synthesis kit and Power SYBR green PCR master mix were purchased from Life Technologies (Grand Island, NY). The CellTiter 96 Aqueous One Solution Cell Proliferation Assay was purchased from Promega (Madison, WI) and the KI-67 antibody was purchased from BD Pharmingen (Ref. 558616; San Jose, CA).
2.2. HuF cell isolation
Human uterine stromal fibroblasts were isolated from the decidual parietalis of the placental membrane after normal vaginal delivery. The isolation and culture of HuF cells was performed as previously described and each cell line was grown individually and treated with between passages 4–5 [20]. Each cell line (n = 4) is representative of proliferative, undifferentiated stromal fibroblasts. Placental tissues, used to harvest HuF cells, were obtained with informed consent under Institutional Review Board approved protocols at Michigan State University and Spectrum Health System.
2.3. Decidualization and bisphenol A treatment
HuF cells were cultured in RPMI1640 media supplemented with 10% charcoal dextran stripped (CDS) FBS until they reached 70–80% confluence. Subsequently, media supplemented with 2% CDS FBS was used for the eight day treatment period. During the treatment, media was changed every two days. All cells were treated with 17-β–estradiol (36 nM), medroxyprogesterone acetate (1 μM) and dibutyryl cyclic adenosine monophosphate (0.5 mM) (EPC). This hormonal cocktail treatment has been used extensively for in vitro decidualization in our laboratory [20–22]. HuF cells were additionally treated with vehicle or one of several doses of BPA (1 ng/mL, 10 ng/mL, 0.5 μg/mL, 10 μg/mL and 20 μg/mL or 4.38 nM, 43.8 nM, 2.19 μM, 43.8 μM and 87.6 μM, respectively) dissolved in 100% molecular grade ethanol in conjunction with the eight-day EPC treatment. The doses of BPA used in this study were selected based on previous in vitro studies [18,19,23,24]. The doses used were similar to and higher than reported physiological bio-fluid concentrations measured in the population [25]. The higher doses tested in this study may provide insight into the mechanisms involved with very high BPA exposure among industrial workers. In addition, it is difficult to mimic the BPA exposure scenario in humans because exposure is ubiquitous and exposure levels vary significantly within the population. BPA is also metabolized very rapidly in the body making it difficult to accurately quantify exposure [25].
2.4. RNA isolation and quantitative real-time PCR
After completion of the eight day treatment period, HuF cells were washed three times with 1× PBS and 0.5 mL of Trizol reagent was added to each well and the manufacturer's protocol was followed for the isolation of RNA. Next, 1 μg of RNA was converted to cDNA using the protocol outlined in the High Capacity cDNA synthesis kit. For qPCR, 10 ng of cDNA template was combined with primer pairs for the gene of interest (sequences listed in supplemental Table 1) and PowerSYBR master mix. The reaction was completed per the manufacturer's specifications and performed on Viia7 Real-time PCR machine (Life Technologies).
2.5. MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)assay
HuF cells were cultured for eight days in 96 well plates and after the completion of treatment, 100uL of MTS reagent was added to each well and the plate was incubated for two hours at 37 ° Celsius. After incubation, the plate was read at an absorbance of 490 nm to determine the degree of proliferation.
2.6. Immunocytochemistry
HuF cells were cultured on 4-well chamber slides and were treated for eight days as described above. Briefly, the cells were washed three times with 1× PBS and fixed with 4% Paraformaldehyde for 15 min. After another series of washes with 1× PBS, cells were permeabilized with 0.1% Triton X-100 in 1× PBS for 10 min. Next, cells were rinsed again with 1× PBS and were blocked for 30 min with 5% NGS in 1× PBS. After blocking, 100 μL of antibody conjugated to Alexa Fluor 488 was added for 1 h. Vectashield with DAPI was added to stain the nuclei of the cells and images were captured using confocal microscopy at 488 nm.
2.7. Statistics
Data in the results and figures are expressed as the mean ± SEM. Statistical analyses were performed using GraphPad Prism 5.0 (La Jolla, CA) One-way ANOVA was used to test the null hypothesis of differences between groups, followed by a Student's t-test for comparison between group pairs. P< 0.05 was considered statistically significant.
3. Results
3.1. Bisphenol A disrupts decidualization of HuF cells
HuF cells (n = 4) were cultured with estrogen, progesterone and cAMP (EPC) for eight days in the presence and absence of 1 ng/mL, 10 ng/mL, 0.5 μg/mL, 10 μg/mL and 20 μg/mL BPA. To determine whether BPA influenced the cells ability to decidualize, we examined the gene expression of two well-established markers of decidualization, IGFBP1 and PRL, by quantitative realtime PCR [26]. The physiological doses (1 ng/mL and 10 ng/mL) did not alter the expression of either marker of decidualization (data not shown). As shown in Fig. 1, 0.5 μg/mL BPA did not alter the expression of IGFBP1 or PRL. However, 10 μg/mL of BPA significantly reduced mRNA levels of PRL (p = 0.048), but not IGFBP1 expression compared to controls. Additionally, 20 μg/mL of BPA significantly reduced the expression of PRL (p = 0.005) and caused a trend towards reduced expression of IGFBP1, compared to control (p = 0.07). It is important to note the exponential scale of IGFBP1 expression of Fig. 1 and the significant variability of expression among the vehicle treated cells. It is for this reason that IGFBP1 expression was not significantly different. Morphologically, HuF cells treated with 20 μg/mL of BPA and the decidualization hormone cocktail retained their fibroblastic appearance compared to HuF cells decidualized without BPA, which displayed the hallmark rounded morphology and larger cytoplasmic area characteristic of secretory decidual cells [17](Fig. 1).
Fig. 1.
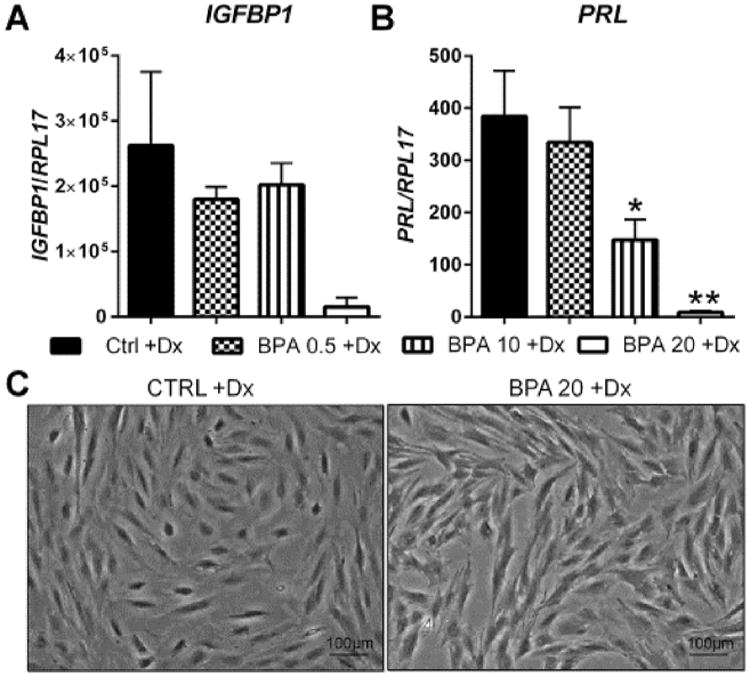
Effect of BPA on in vitro decidualization of uterine stromal fibroblasts. HuF cells were exposed to BPA at several doses (0.5 μg/mL, 10 μg/mL and 20 μg/mL) in the presence of a decidualization-inducing hormonal formulation for eight days. At the conclusion of the treatment, IGFBP1 (A) and prolactin (B) mRNA expression was determined by qRT-PCR which was normalized to the expression of RPL17. Morphological changes (C) were observed in HuF cells treated with 20 μg/mL of BPA as compared to cells with hormone treatment alone. Data are presented as mean ± SEM from four independent experiments from four different cell lines. (*p <0.05, **p <0.01 compared to decidualized controls). Note: Dx in Fig. stands for decidualization.
3.2. Bisphenol A alters steroid hormone receptors during decidualization
To further investigate the cause of decidualization failure in HuF cells exposed to BPA in the presence of EPC, we evaluated the gene expression of ESR1 and PGR by qPCR. The physiological doses of BPA (1 ng/mL and 10 ng/mL) did not alter the expression of ESR1 or PGR (data not shown). As summarized in Fig. 2 and similar to the decidual marker genes, BPA exposure at 0.5 μg/mL did not alter expression of either receptor compared to vehicle treated decidualized controls. At 10 μg/mL, BPA treated HuF cells exhibited a reduced level of PGR, but not ESR1 compared to vehicle treated decidualized controls. However, 20 μg/mL of BPA significantly reduced both ESR1 and PGR as compared to vehicle treated decidualized HuF cells.
Fig. 2.
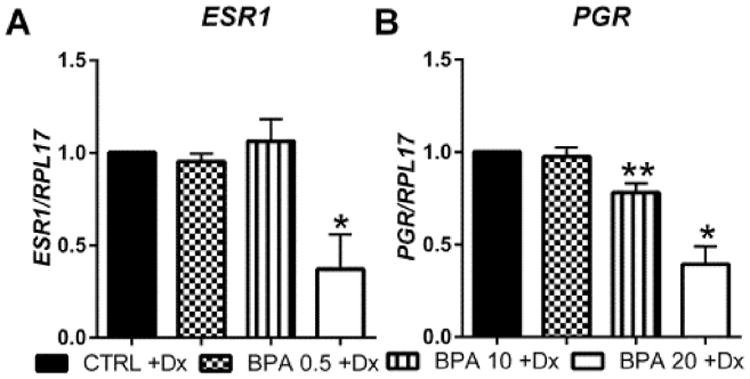
Effect of BPA on steroid hormone receptor expression during decidualization. HuF cells were exposed to BPA at several doses (0.5 μg/mL, 10 μg/mL and 20 μg/mL) in the presence of a decidualization-inducing hormonal formulation for eight days. Estrogen receptor-alpha (A) and progesterone receptor (B) mRNA expression was assessed by qRT-PCR which was normalized to the expression of RPL17. Data are presented as mean ± SEM from four independent experiments from four different cell lines. (*p< 0.05, **p < 0.01 compared to decidualized controls). Note: Dx in Fig. stands for Decidualization.
3.3. Bisphenol A inhibits cell proliferation during in vitro decidualization
Previous in vitro studies have determined that BPA, at doses similar to those used in this study, reduced the proliferative capacity of endometrial stromal fibroblasts and endometrial endothelial cells [18,23]. Thus, we investigated the proliferation of HuF cells undergoing decidualization with and without BPA using MTS assays and KI-67 immunocytochemical staining (Fig. 3). For these experiments, we used 20 μg/mL of BPA in these experiments because it was effective in disrupting decidualization and cell proliferation in experiments described above. After eight days of culture, HuF cells treated with 20 μg/mL BPA and EPC had significantly reduced proliferation as compared to HuF cells treated with EPC alone as measured by absorbance at 490 nm to detect the amount of MTS tetrazolium converted into formazan, which occurs through NADPH metabolism in active proliferating cells. Furthermore, immunofluorescent cell staining for KI-67, a nuclear marker of proliferation which is present during all active stages of the cell cycle, was completely absent in HuF cells treated with 20 μg/mL of BPA and EPC. HuF cells treated with EPC alone stained positively for KI-67.
Fig. 3.
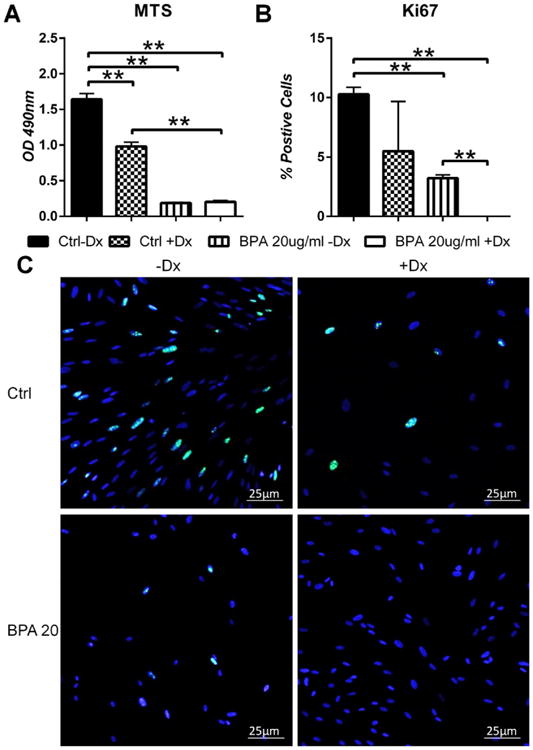
BPA affects proliferation of HuF cells undergoing decidualization. HuF cells were treated with 20 μg/mL of BPA in the presence of the decidualization-inducing hormone formulation for eight days. At the conclusion of the treatment period, an MTS assay was performed to measure proliferation (A). Additionally, cells were fixed and stained for KI-67 (C), a marker of cellular proliferation. Results from KI-67 staining are expressed as percent positive cells (B). Data are presented as mean±SEM from three independent experiments from three different cell lines. (*p<0.05, **p<0.01). Note: Dx in Fig. stands for Decidualization.
3.4. Bisphenol A disrupts cell cycle genes during decidualization
Cellular proliferation is tightly regulated by the cell cycle and its associated regulatory mechanisms which either permit or prohibit its progression [27]. Unperturbed progression through the cell cycle is essential for normal proliferation of cells in vitro. We analyzed the gene expression of several cell cycle genes and regulators of the cell cycle in HuF cells treated with EPC alone or EPC with exposure to BPA to understand the mechanism by which BPA affects proliferation (Fig. 4). Of the cell cycle genes analyzed, BPA at 20 μg/mL significantly reduced expression of CCND2 in HuF cells treated with EPC and decreased, but not significantly at 10 μg/mL of BPA (p = 0.056). BPA did not alter the expression of CCNA2, CCNB1, CCNE1, CDKN1a and CDK4. Cyclin D2, encoded by CCND2, complexes with CDK4 or CDK6 to allow G1/S cell cycle progression. Down-regulation of CCND2 can potentially halt cell cycle progression and may provide a mechanism for the proliferative deficiencies seen in HuF cells treated with BPA and EPC.
Fig. 4.
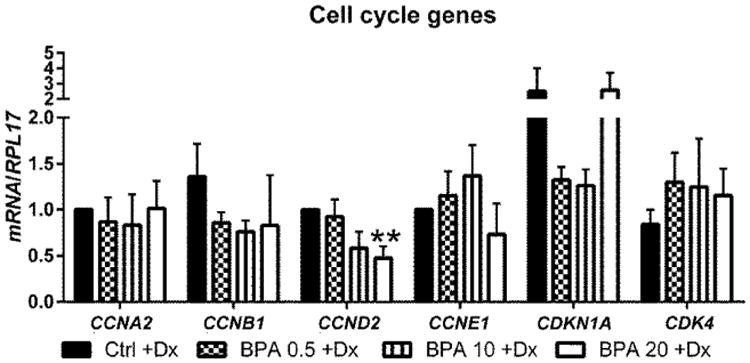
Effect of BPA on cell cycle gene regulation during decidualization. HuF cells were exposed to BPA at several doses (0.5 μg/mL, 10 μg/mL and 20 μg/mL) in the presence of a decidualization-inducing hormonal formulation for eight days. At the conclusion of the treatment, Cyclin A2, Cyclin B1, Cyclin D2, Cyclin E1, CDKN1A, and CDK4 mRNA expression was assessed by qRT-PCR. Data are presented as mean±SEM from four independent experiments from four different cell lines. (*p<0.05, **p<0.01 compared to decidualized controls). Note: Dx in Fig. stands for Decidualization.
4. Discussion
The aim of this research study was to determine the effect of physiological and supra-physiological doses of BPA exposure in human endometrial stromal cells undergoing decidualization.
In the present study, we established that supra-physiological doses (10 μg/mL and 20 μg/mL), but not physiological doses of BPA can disrupt the ability of endometrial stromal cells to undergo decidualization. Our results are novel in that we identified an effect of BPA on uterine stromal cells undergoing decidualization. Moreover, our data corroborate a previous study where 1 μM of BPA, but not lower doses, was found to disrupt decidualized stromal cells when cells were exposed after decidualization [19]. That study examined the effect of BPA on stromal cells which were previously decidualized [19], whereas our study examined the effect of BPA on stromal cells undergoing decidualization. Regardless, their study demonstrated that 1 μM of BPA reduced the secretion of prolactin in conditioned media after 24 h of exposure. Our cell culture time course was significantly longer at eight days and we determined that prolactin mRNA expression was decreased with 43.8 μM and 87.6 μM (10 μg/mL and 20 μg/mL), but not lower doses of BPA in stromal cells undergoing decidualization.
In our study, exposure to BPA at 20 μg/mL also down-regulated ESR1 and PGR mRNA expression in HuF cells undergoing decidualization as compared to vehicle treated cells. Additionally, BPA at 10 μg/mL reduced expression of PGR, but did not affect ESR1 expression. A previous study documented the ability of BPA to alter estrogen synthesis and down-regulate ESR1 in endometrial stromal fibroblast cells [18]. However, in the same study, PGR expression was unchanged. The dysregulation of ESR1 and PGR as observed in HuF cells treated with BPA in the presence of EPC reveals a potential explanation for decidualization failure. Furthermore, estrogen and progesterone signaling is essential for priming the endometrium for successful implantation and if the receptors of decidual cells are altered during BPA exposure, the blastocyst will face an uninhabitable environment [28]. Therefore, endometrial exposure to BPA at supra-physiological levels used in our study can potentially lead to implantation failure and fertility problems in women.
The decidualization hormone cocktail utilized in our study simulates the levels of estrogen, progesterone and cAMP that coincide with the mid-secretory phase of the cycle. The previous studies investigating the effect of BPA on decidualized stromal cells did not add estrogen in conjunction with progesterone and/or cAMP [18,19] and therefore we believe that our treatment paradigm more accurately reflects the hormonal milieu of the human endometrium as seen during decidualization. A study in non-human primates found that PGR expression was reduced in animals exposed to estradiol and BPA [29]. They concluded that BPA alone exhibited weak estrogenic activity, but in the presence of estradiol, BPA opposed estradiol's ability to up-regulate PGR. These findings are supported by our results in uterine stromal cells exposed to BPA at 20 μg/mL while undergoing decidualization with EPC treatment. Additionally, their findings also highlight the potential difference between treating uterine stromal cells with BPA in the presence (our study) or absence [18,19] of estradiol.
BPA affected the proliferative capacity of HuF cells exposed to the 20 μg/mL dose during in vitro decidualization as measured by MTS assay and KI-67 staining. This result is supported by other studies which also showed decreased proliferation of endometrial stromal cells and endometrial endothelial cells with similar doses of BPA as used in this study [18,23]. In our study, BPA (20 μg/mL) also significantly reduced CCND2 (encodes cyclin D2) mRNA expression in HuF cells and this molecular event coincided with the decrease in proliferation. As the Cyclin D2/CDK complex is required for transition into the S phase of the cell cycle, without sufficient Cyclin D2, the cell will enter a quiescent, non-proliferating state [7]. Proliferation of stromal cells is necessary for decidualization. Thus, it is possible that the lack of proliferation seen in HuF cells exposed to BPA contributes to the reduced decidualization potential in this study.
The higher doses which impaired decidualization do not reflect human exposure levels and these concentrations were not selected to mimic human exposure scenarios, but nonetheless they contribute to the growing literature of supra-physiological impacts of BPA in reproduction.
Overall, supra-physiological doses of BPA affected HuF cells ability to proliferate and undergo decidualization. BPA significantly reduced prolactin expression in uterine stromal cells compared to vehicle decidualized cells. A previous study determined that low expression of prolactin was found to be associated with spontaneous recurrent miscarriage in women [30]. Taken together with its ability to reduce the proliferative capacity of uterine stromal cells, high concentrations of BPA disrupted decidualization of stromal cells and exposure to BPA at these levels may lead to significant problems with the establishment of pregnancy in women.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health (NIH P01 ES022848 to JF and NIH R01 HD42280 to AF) and Environmental Protection Agency (RD-83459301).
Footnotes
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.reprotox.2017.07.008.
References
- 1.Chapin RE, Adams J, Boekelheide K, Gray LE, Jr, Hayward SW, Lees PS, McIntyre BS, Portier KM, Schnorr TM, Selevan SG, Vandenbergh JG, Woskie SR. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83(3):157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- 2.Flint S, Markle T, Thompson S, Wallace E. Bisphenol A exposure, effects, and policy: a wildlife perspective. J Environ Manage. 2012;104:19–34. doi: 10.1016/j.jenvman.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Huang YQ, Wong CK, Zheng JS, Bouwman H, Barra R, Wahlstrom B, Neretin L, Wong MH. A. Bisphenol, (BPA) in China: a review of sources, environmental levels, and potential human health impacts. Environ Int. 2012;42:91–99. doi: 10.1016/j.envint.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 4.EPA. Bisphenol-A (BPA) Action Plan (CASRN 80-05-7) [Accessed April 05, 2016];2010 http://www2.epagov/assessing-and-managing-chemicals-under-tsca/bisphenol-bpa-action-plan.
- 5.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population, Environ. Health Perspect. 2005;113(4):391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132(6):2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- 7.Gould JC, Leonard LS, Maness SC, Wagner BL, Conner K, Zacharewski T, Safe S, McDonnell DP, Gaido KW. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol Cell Endocrinol. 1998;142(1-2):203–214. doi: 10.1016/s0303-7207(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 8.Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, Padmanabhan V, Taylor HS, Swan SH, VandeVoort CA, Flaws JA. Bisphenol A and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect. 2014;122(8):775–786. doi: 10.1289/ehp.1307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod. 2005;20(8):2325–2329. doi: 10.1093/humrep/deh888. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y, Taketani Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr J. 2004;51(2):165–169. doi: 10.1507/endocrj.51.165. [DOI] [PubMed] [Google Scholar]
- 11.Cobellis L, Colacurci N, Trabucco E, Carpentiero C, Grumetto L. Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women. Biomed Chromatogr. 2009;23(11):1186–1190. doi: 10.1002/bmc.1241. [DOI] [PubMed] [Google Scholar]
- 12.Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye X, Calafat AM, Petrozza JC, Wright D, Hauser R. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum Reprod. 2012;27(12):3583–3592. doi: 10.1093/humrep/des328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Classen-Linke I, Alfer J, Hey S, Krusche CA, Kusche M, Beier HM. Marker molecules of human endometrial differentiation can be hormonally regulated under in-vitro conditions as in-vivo. Hum Reprod Update. 1998;4(5):539–549. doi: 10.1093/humupd/4.5.539. [DOI] [PubMed] [Google Scholar]
- 14.Jabbour HN, Critchley HO. Potential roles of decidual prolactin in early pregnancy. Reproduction. 2001;121(2):197–205. doi: 10.1530/rep.0.1210197. [DOI] [PubMed] [Google Scholar]
- 15.Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34(1):130–162. doi: 10.1210/er.2012-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35(6):851–905. doi: 10.1210/er.2014-1045. [DOI] [PubMed] [Google Scholar]
- 17.Kajihara T, Tanaka K, Oguro T, Tochigi H, Prechapanich J, Uchino S, Itakura A, Sucurovic S, Murakami K, Brosens JJ, Ishihara O. Androgens modulate the morphological characteristics of human endometrial stromal cells decidualized in vitro. Reprod Sci. 2014;21(3):372–380. doi: 10.1177/1933719113497280. [DOI] [PubMed] [Google Scholar]
- 18.Aghajanova L, Giudice LC. Effect of bisphenol A on human endometrial stromal fibroblasts in vitro. Reprod Biomed Online. 2011;22(3):249–256. doi: 10.1016/j.rbmo.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mannelli C, Szostek AZ, Lukasik K, Carotenuto C, Ietta F, Romagnoli R, Ferretti C, Paulesu L, Wolczynski S, Skarzynski DJ. Bisphenol A modulates receptivity and secretory function of human decidual cells: an in vitro study. Reproduction. 2015;150(2):115–125. doi: 10.1530/REP-14-0601. [DOI] [PubMed] [Google Scholar]
- 20.Kim JJ, Jaffe RC, Fazleabas AT. Comparative studies on the in vitro decidualization process in the baboon (Papio anubis) and human. Biol Reprod. 1998;59(1):160–168. doi: 10.1095/biolreprod59.1.160. [DOI] [PubMed] [Google Scholar]
- 21.Strakova Z, Srisuparp S, Fazleabas AT. Interleukin-1beta induces the expression of insulin-like growth factor binding protein-1 during decidualization in the primate. Endocrinology. 2000;141(12):4664–4670. doi: 10.1210/endo.141.12.7810. [DOI] [PubMed] [Google Scholar]
- 22.Strakova Z, Mavrogianis P, Meng X, Hastings JM, Jackson KS, Cameo P, Brudney A, Knight O, Fazleabas AT. In vivo infusion of interleukin-1beta and chorionic gonadotropin induces endometrial changes that mimic early pregnancy events in the baboon. Endocrinology. 2005;146(9):4097–4104. doi: 10.1210/en.2005-0380. [DOI] [PubMed] [Google Scholar]
- 23.Bredhult C, Sahlin L, Olovsson M. Gene expression analysis of human endometrial endothelial cells exposed to Bisphenol A. Reprod Toxicol. 2009;28(1):18–25. doi: 10.1016/j.reprotox.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 24.An BS, Ahn HJ, Kang HS, Jung EM, Yang H, Hong EJ, Jeung EB. Effects of estrogen and estrogenic compounds, 4-tert-octylphenol, and bisphenol A on the uterine contraction and contraction-associated proteins in rats. Mol Cell Endocrinol. 2013;375(1-2):27–34. doi: 10.1016/j.mce.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118(8):1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178(3):357–372. doi: 10.1677/joe.0.1780357. [DOI] [PubMed] [Google Scholar]
- 27.Gali-Muhtasib H, Bakkar N. Modulating cell cycle: current applications and prospects for future drug development. Curr Cancer Drug Targets. 2002;2(4):309–336. doi: 10.2174/1568009023333809. [DOI] [PubMed] [Google Scholar]
- 28.Aplin JD, Kimber SJ. Trophoblast-uterine interactions at implantation. Reprod Biol Endocrinol. 2004;2:48. doi: 10.1186/1477-7827-2-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldad TS, Rahmani N, Leranth C, Taylor HS. Bisphenol-A exposure alters endometrial progesterone receptor expression in the nonhuman primate. Fertil Steril. 2011;96(1):175–179. doi: 10.1016/j.fertnstert.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garzia E, Clauser R, Persani L, Borgato S, Bulfamante G, Avagliano L, Quadrelli F, Marconi AM. Prolactin and proinflammatory cytokine expression at the fetomaternal interface in first trimester miscarriage. Fertil Steril. 2013;100(1):108–115 (e1-2). doi: 10.1016/j.fertnstert.2013.02.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


