Abstract
Purpose
Cross-sectional studies have found a positive association between body mass index (BMI) and bone mineral density (BMD), but little is known about the longitudinal relationship in US older adults.
Methods
We examined average annual rate of change in BMD by baseline BMI in the Health, Aging, and Body Composition Study. Repeated measurement of BMD was performed with dual energy x-ray absorptiometry (DXA) at baseline, and years 3, 5, 6, 8, and 10. Multivariate generalized estimating equations were used to predict mean BMD (femoral neck, total hip, and whole body) by baseline BMI (excluding underweight), adjusting for covariates.
Results
The sample (N= 2,570) was 43% were overweight and 24% were obese with a mean baseline femoral neck BMD of 0.743 g/cm2, hip BMD of 0.888 g/cm2, and whole body BMD of 1.09 g/cm2. Change in total hip or whole body BMD over time did not vary by BMI groups. However, obese older adults lost 0.003 g/cm2 of femoral neck BMD per year more compared with normal weight older adults (p<0.001). Femoral neck BMD change over time did not differ between the overweight and normal weight BMI groups (p=0.74). In year 10, adjusted femoral neck BMD ranged from 0.696 g/cm2 among obese, 0.709 g/cm2 among normal weight, and 0.719 g/cm2 among overweight older adults.
Conclusions
Findings underscore the importance of looking at the longitudinal relationship between body composition and bone mineral density among older adults, indicating that high body mass may not be protective for bone loss over time.
Keywords: Obesity, Osteoporosis, Aging, Longitudinal
INTRODUCTION
Numerous studies have found a strong, cross-sectional association between body mass index (BMI) and bone mineral density (BMD) [1–3], but little is known about the longitudinal relationship in the US population. Many studies have found obesity to be protective against bone loss, but the majority have been conducted in non-US populations [4–9] whose cultural differences in exercise and diets, as well as dramatic differences in the prevalence of obesity, make results difficult to generalize to US older adults. Ravn et al.[10] examined this association in a small, US sample of postmenopausal women in the 1990s and found higher BMI categories had a protective effect against bone loss compared to lower BMI categories for total hip and spine BMD. However, these results do not reflect dramatic increases in the prevalence of obesity [11,12] and the development of obesity at younger ages among more recent birth cohorts of older adults [13]. Additionally, most research on the association between BMI and bone loss has focused on underweight older adults, who are at the highest risk for bone loss and fracture [4]. However, underweight individuals represent a small proportion of the total older adult population (around 2%) [14]. Dramatic increases in the prevalence of overweight and obesity in the US necessitate a focus on heavier individuals, who make up the majority of the older adult population [15].
The objective of this study was to examine change in BMD of the femoral neck, total hip, and whole body over time (10 years) by baseline BMI categories among a recent cohort of US older adults. Using data from the Health, Aging, and Body Composition Study (Health ABC), this analysis was able to control for an extensive set of potential confounding variables associated with bone loss and obesity.
METHODS
Study sample
The Health ABC study follows a healthy cohort of 3,075 White and Black well-functioning men and women (age 70–79 at enrollment in 1997–1998) from two US field centers (Pittsburgh, PA and Memphis, TN). Participants in designated zip codes near the two field centers were recruited using a random sample of White Medicare beneficiaries and all age-eligible Black community residents. Only individuals who were disability and cancer free at baseline were eligible to be enrolled in the study.
Participants were screened and determined eligible during a phone interview in 1997. A home interview was conducted before the clinic visit where questionnaire data were obtained on self-reported demographic information, health status, weight history, physical function and activity, work and volunteer activities, appetite and eating behavior, smoking and alcohol use, sleep habits, bodily pain, chronic conditions, cancer history (prior to the past three years as they would be ineligible to participate in the study), osteoporosis and falls, medical conditions, health care, and social support. Clinic visits were then scheduled and included performance measures, fasting blood sample, bone density scan (DXA), measured height and weight, cognition tests, as well as an inventory of medications being taken by the participant. Participants visited the clinic yearly for similar testing and answered questions about items that may have changed since the last visit. A home visit, telephone interview, or a proxy interview may have been conducted if the participant was not able to come into the clinic or was not able to be interviewed.
Ten years of longitudinal follow-up data are available for the baseline sample. The final analytic sample not missing on baseline study variables was N=2,570. Variables with large amounts of missing data included percent weight change from age 50 to baseline (n=105), total fat free and total fat mass (n=106), performance measures (n=95), and prevalence of cardiovascular disease (n=56) and stroke (n=29).
Body mass index
BMI categories were calculated by measured height and weight at baseline (weight in kilograms divided by height in meters squared). BMI categories included normal weight as the reference (18.5 to 24.9), overweight (25.0 to 29.9), and obese (≥ 30.0). Underweight individuals were excluded from all analyses (n=25) as the relationship between this BMI category and bone loss is well understood [4] and they represented a small proportion of the total older adult population (<2%) [14]. Baseline BMI was used as the independent variable, instead of changes in BMI over time, for two reasons. First, this study population was either overweight or obese for most of their older adult lives (evident in % weight change since age 50 in Table 1). Therefore, this study was able to examine the constant, biomechanical loading of these higher body mass categories that were present as the majority of the sample aged. Second, because risk of hip fracture greatly increases after age 80, it is useful to measure body mass prior to age 80 to understand the impact it has had over time on bone mass and therefore risk of fracture [16]. With the baseline age of Health ABC reflecting the 70–79 age period, the sample represents an ideal snapshot of the impact of heavier body mass on bone loss around the time that risk of fracture is most relevant for older adults. Lastly, although weight change (particularly weight loss) is an important factor associated with bone loss [17,18], descriptive and regression analyses found annual changes in weight over the study period were small in this population. Therefore, this study examined the impact of baseline body mass categories on bone loss over time.
Table 1.
Sample Characteristics at Baseline by Body Mass Index Categories, n=2,570
| Total | Normal | Overweight | Obese | p-value | |
|---|---|---|---|---|---|
|
| |||||
| N | 2,570 | 852 | 1,095 | 623 | |
|
| |||||
| Bone mineral density g/cm2 [mean (SD)] | |||||
| Femoral Neck | 0.743 (0.142) | 0.683 (0.135) | 0.750 (0.132) | 0.814 (0.135) | <0.001 |
| Total Hip | 0.888 (0.169) | 0.808 (0.160) | 0.904 (0.158) | 0.968 (0.153) | <0.001 |
| Whole Body | 1.090 (0.141) | 1.058 (0.142) | 1.099 (0.141) | 1.116 (0.134) | <0.001 |
| Age [mean (SD)] | 73.6 (2.9) | 73.9 (2.9) | 73.6 (2.9) | 73.1 (2.8) | <0.001 |
| Female % | 50.8 | 53.5 | 45.0 | 57.3 | <0.001 |
| Black % | 39.7 | 32.2 | 35.9 | 56.7 | <0.001 |
| Education % | 0.001 | ||||
| < High school | 23.9 | 21.4 | 22.7 | 29.5 | |
| High school grad | 32.1 | 29.0 | 32.7 | 35.3 | |
| Post-secondary | 44.0 | 49.7 | 44.6 | 35.2 | |
| Percent weight change from age 50 [mean (SD)] | 6.3 (14.0) | −1.6 (9.9) | 6.0 (11.8) | 17.3 (15.0) | <0.001 |
| Total fat free mass, gm [mean (SD)] | 48,981.3 (10,407.5) | 43,632.5 (8,744.0) | 50,001.3 (9,556.9) | 54,503.2 (10,514.8) | <0.001 |
| Total fat mass, gm [mean (SD)] | 26,562.2 (8,474.5) | 19,114.1 (4,124.0) | 26,377.2 (4,547.7) | 37,073.1 (7,090.1) | <0.001 |
| Diabetes % | 14.5 | 9.0 | 14.5 | 22.0 | <0.001 |
| Cardiovascular disease % | 24.6 | 23.1 | 26.1 | 23.9 | 0.283 |
| Hypertension % | 43.7 | 34.6 | 43.0 | 57.3 | <0.001 |
| Cancer % | 19.2 | 18.8 | 19.9 | 18.6 | 0.746 |
| Stroke % | 1.1 | 0.7 | 1.7 | 0.3 | 0.011 |
| Steroid use % | 2.3 | 2.6 | 2.1 | 1.9 | 0.602 |
| Bisphosphonate use % | 4.0 | 6.3 | 3.1 | 2.3 | <0.001 |
| Vitamin D use % | 8.4 | 11.5 | 7.6 | 5.6 | <0.001 |
| Calcium use % | 18.4 | 24.1 | 17.1 | 13.2 | <0.001 |
| Good health % | 84.8 | 85.7 | 86.8 | 79.9 | <0.001 |
| Physical activity, kcal/week [mean (SD)] | 1081.8 (1904.4) | 1,044.7 (1951.8) | 1,216.6 (2037.0) | 895.4 (1549.3) | 0.233 |
| Short Physical Performance Battery, 0–4 [mean (SD)] | 2.2 (0.5) | 2.3 (0.5) | 2.3 (0.5) | 2.0 (0.6) | <0.001 |
| Cognition (3MS), 0–100 [mean (SD)] | 90.6 (7.8) | 90.7 (8.3) | 90.9 (7.6) | 89.8 (7.6) | 0.007 |
| CESD score, 0–20 [mean (SD)] | 4.6 (5.2) | 4.7 (5.1) | 4.6(5.2) | 4.5 (5.2) | 0.433 |
| Current smoker % | 9.8 | 14.8 | 8.1 | 6.1 | <0.001 |
| Frequent drinking % | 29.5 | 36.2 | 31.1 | 23.0 | <0.001 |
Femoral neck, total hip, and whole body bone mineral density
The outcome variables include repeated measures of femoral neck, total hip, and whole body BMD. Femoral neck and total hip BMD were measured at baseline and at years 3, 5, 8, and 10. Whole body BMD was measured annually from baseline to year 5 and again at years 8 and 10. BMD was obtained from dual energy x-ray absorptiometry (DXA) measurements and measured in units of g/cm2. A number of participants were missing follow-up data for femoral neck, total hip, and whole body DXA (386 people [14.0 %] were missing DXA data in year 3 and 1355 [49.3%] were missing in year 10).
Potential confounders
A number of baseline variables that are associated with both BMI and bone loss were included in regression models to see if they accounted for the association between baseline BMI and change in BMD. Demographic variables such as age, sex, and race are controlled for in regressions models. Education (less than high school, high school graduate, post-secondary [reference]) was included in the analyses as a proxy for socioeconomic status. Health status indicators included physical performance measures based on the Short Physical Performance Battery (SPPB, range 0–4), chronic conditions identified using previously established algorithms based on self-report, clinical data, and medications (diabetes, cardiovascular disease, hypertension, cancer, stroke), self-reported health status (good or fair/poor [reference]), depression measured by the Center for Epidemiologic Studies Depression Scale (CESD, range 0–100), and cognition based on the Modified Mini-Mental State examination (3MS, range 0–20). Factors related to both obesity and bone loss included bone-active medication use (bisphosphonates and steroids), physical activity (kcal/week walking and exercise), vitamin D and calcium supplement use, percent weight change from age 50, smoking status, and frequent alcohol use. A dummy variable indicating Memphis Health ABC site (Pittsburgh reference) was also included in the regression analysis to account for residual differences across study sites.
Statistical Analyses
Descriptive statistics were used to compare study characteristics at baseline by BMI categories. Tests of significance utilized chi-square tests and generalized linear regressions for dichotomous and continuous variables, respectively. Generalized estimating equations (GEE) were used to examine the association between baseline BMI categories and repeated measures of BMD [femoral neck, total hip, and whole body]. Models predicted BMD by obesity and overweight (normal weight reference), time, interaction terms for obesity and time and overweight and time (to determine the direction of the association and slope between BMI categories and BMD) in an unadjusted model as well as a model adjusted for covariates listed in Table 1.
Sensitivity analyses were also conducted to examine whether significant associations between baseline BMI and repeated measures of BMD could be accounted for by demographic (gender, race [white or black (reference)], and age) or body composition differences in the rate of BMD decline. Adjusted per year change in BMD by baseline BMI categories is presented by gender as interaction terms between gender and BMI category were significant in predicting change in femoral neck BMD over time. Sensitivity analyses examined annual changes in weight (kg) over the study period as well as control for baseline body composition (total fat mass [gm] and total fat free mass [gm)]) to see if these adjustments changed the main study findings. Lastly, sensitivity analyses removed individuals who had diabetes, bone-active medication use, and substance use (alcohol and smoking) at baseline to investigate if subgroups already prone to bone loss at baseline could be accounting for the bone loss overtime in certain body mass groups.
RESULTS
The sample included 2,570 subjects of whom 43% were overweight and 24% were obese [Table 1]. The sample had a mean baseline femoral neck BMD of 0.743 g/cm2 (SD=0.142), a mean total hip BMD of 0.888 g/cm2 (SD=0.169), and a mean whole body BMD of 1.09 g/cm2 (SD=0.141). There were no significant differences in missing femoral neck or total hip BMD by BMI category. However, there were significant differences in missing whole body BMD by BMI category in year 3 and 8, with the overweight participants having a higher proportion of missing whole body DXAs than normal or obese participants.
Obese older adults had the highest mean femoral neck (p<0.001), total hip (p<0.001), and whole body BMD (p<0.001) at baseline compared to overweight and normal weight older adults [Table 1]. Obese older adults were more likely to be younger, female, black, less educated, gained more weight since age 50, had more fat free and fat mass, and were more likely to have diabetes or hypertension. Obese older adults were less likely to use bisphosphonates or vitamin D or calcium supplements; less likely to have good health, functional performance, cognition; and less likely to currently smoke or drink frequently.
Table 2 displays the unadjusted and adjusted baseline BMD (femoral neck, hip, and whole body) by BMI category from multivariable analyses utilizing GEEs. Overweight and obese older adults had 0.07 and 0.14, respectively, higher femoral neck BMD at baseline. Normal weight older adults lost femoral neck BMD at a rate of −0.0032 per year (overweight older adults lost femoral neck BMD at a similar rate). However, there was a significant obesity and time interaction with obese older adults losing 0.003g/cm2 of femoral neck BMD per year more compared with normal weight older adults (p<0.001) for a total excess loss of 0.03 g/cm2 over the study period. Femoral neck BMD change over time did not differ between the overweight and normal weight BMI groups (p=0.75). There were no significant differences in total hip and whole body BMD over time by BMI group.
Table 2.
Baseline Bone Mineral Density (BMD) and Annual Change in BMD by Body Mass Index Category, n=2,570†
| Femoral Neck | Total Hip | Whole Body | ||||
|
| ||||||
| Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | |
|
| ||||||
| Unadjusted Results | ||||||
|
| ||||||
| Baseline BMD (compared to normal weight) | ||||||
|
| ||||||
| Overweight | 0.0692 | <0.0001 | 0.0989 | <0.0001 | 0.0441 | <0.0001 |
| Obesity | 0.1352 | <0.0001 | 0.1679 | <0.0001 | 0.0615 | <0.0001 |
|
| ||||||
| Annual Change in BMD | ||||||
|
| ||||||
| Normal Weight (Time variable) | −0.0032 | <0.0001 | −0.0039 | <0.0001 | −0.0027 | <0.0001 |
| Overweight (Overweight * Time) | 0.0002 | 0.7482 | 0.0003 | 0.7580 | −0.0003 | 0.6789 |
| Obesity (Obesity * Time) | −0.0026 | 0.0032 | −0.0011 | 0.2573 | −0.0005 | 0.5715 |
|
| ||||||
| Adjusted Results± | ||||||
|
| ||||||
| Baseline BMD (compared to normal weight) | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value |
|
| ||||||
| Overweight | 0.0479 | <0.001 | 0.0655 | <0.0001 | 0.0240 | <0.0001 |
| Obesity | 0.1031 | <0.0001 | 0.1322 | <0.0001 | 0.0540 | <0.0001 |
|
| ||||||
| Annual Change in BMD | ||||||
|
| ||||||
| Normal Weight (Time variable) | −0.0026 | <0.001 | −0.0035 | <0.0001 | −0.0023 | <0.0001 |
| Overweight (Overweight * Time) | 0.0000 | 0.9887 | 0.0000 | 0.9916 | −0.0004 | 0.5279 |
| Obesity (Obesity * Time) | −0.0029 | 0.0003 | −0.0015 | 0.0652 | −0.0008 | 0.3250 |
BMD= Bone mineral density
± Adjusted for age, sex, race, education, percent weight change from age 50, diabetes, cardiovascular disease, hypertension, cancer, stroke, vitamin D and calcium supplement intake, bone-active medications, self-reported health status, physical activity, short physical performance battery, cognition, depression, current smoker, frequent alcohol use, and Health ABC site.
In year 10, obese older adults had a mean femoral neck BMD of 0.696 g/cm2 (SE=0.008), overweight older adults had a mean of 0.709 g/cm2 (SE=0.007), and normal weight older adults had a mean femoral neck BMD of 0.719 g/cm2 (SE=0.005) [Figure 1]. This equates to a 3% annual loss in femoral neck BMD for overweight and normal weight older adults and a 7% loss for obese older adults. However, mean BMD values for the obese (p=0.28) and overweight (p=0.27) groups were not statistically different than the normal weight group at year 10.
Figure 1.
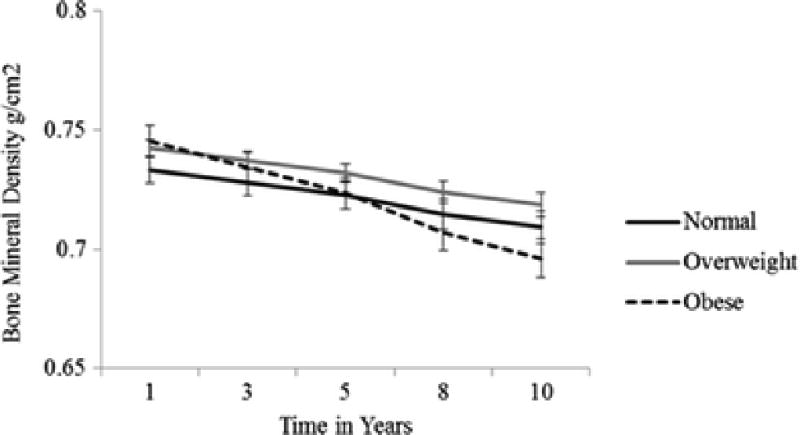
Predicted Mean Femoral Neck Bone Mineral Density by Body Mass Index Category Over Time N=2,570†
† Adjusted for age, sex, race, education, percent weight change from age 50, diabetes, cardiovascular disease, hypertension, cancer, stroke, vitamin D and calcium supplement intake, bone-active medications, self-reported health status, physical activity, short physical performance battery, cognition, depression, current smoker, frequent alcohol use, and Health ABC site.
A number of sensitivity analyses were conducted to see if study results remained after accounting for demographic factors. For femoral neck BMD, significant interactions with time were observed for sex (p=0.045) and race (p=0.009), but not age. However, the addition of these interaction terms did not account for the significant obesity and time interaction. Similarly, for total hip BMD, the interaction term for race and time (p=0.014) was significant while the interaction between gender and time was approaching significance (p=0.071). For whole body BMD, there were no significant interactions between time and demographic factors (age, gender, or race).
Adjusted per year change in femoral neck BMD by BMI category is presented in Table 3 to display differences in gender and race and to further explore the significant loss in femoral neck BMD found among obese older adults. It appears that overweight and obese men lose slightly more femoral neck BMD annually compared to overweight and obese women, respectively. Additionally, Black older adults who are normal weight or obese lose more femoral neck BMD than their White BMI category counterparts.
Table 3.
Adjusted Per Year Change in Femoral Neck BMD by Baseline BMI Category, Gender, and Race, n=2,570†
| BMI Category | Gender | Race | ||
|---|---|---|---|---|
|
| ||||
| Women | Men | White | Black | |
| Normal | −0.003 | −0.003 | −0.002 | −0.005 |
| Overweight | −0.002 | −0.003 | −0.003 | −0.003 |
| Obese | −0.005 | −0.006 | −0.005 | −0.006 |
BMD= Bone mineral density (g/cm2), BMI=Body mass index (kg/m2); Adjusted for age, sex, race, education, percent weight change from age 50, diabetes, cardiovascular disease, hypertension, cancer, stroke, vitamin D and calcium supplement intake, bone-active medications, self-reported health status, physical activity, short physical performance battery, cognition, depression, current smoker, frequent alcohol use, and Health ABC site.
Sensitivity analyses also examined weight change over time as well as additional body composition variables as confounders. Annual weight change (kg) over the study period (mean= −1.28) was significantly associated with change in femoral neck BMD over time (p= 0.003), but controlling for weight change did not change the significant obesity and time interaction. There were also no differences in study results when body composition (baseline fat mass and fat free mass [continuous variables, gm units]) variables were added to the adjusted models. Lastly, there were no differences in study results showing that obese older adults, compared to normal weight older adults, lost more femoral neck BMD over time when individuals with diabetes, bone-active medication use, and substance use at baseline were removed from the analytic sample (N=1,239;the obesity and time interaction was still 0.003, p=0,02),
DISCUSSION
In summary, this study found significant differences in femoral neck BMD over time by BMI group but did not find differences in total hip or whole body BMD over time. These results were robust even when annual changes in weight over the study period and body composition variables were included in the regression model. There were significant gender and racial interactions with time in predicting femoral neck BMD, but these interactions did not account for the finding that obese older adults lose more BMD over time.
Study results varied by BMD site with significant BMI category differences over time found in the femoral neck site but not the total hip. Significant results in the femoral neck region could be due to the higher composition of cortical bone compared to the hip region, which has more trabecular bone [17,19]. Obesity is associated with higher levels of trabecular bone and lower levels of volumetric BMD due to adipose tissue [19]. Another likely cause is the expansion of the bone diameter with aging, causing a larger bone with thinner walls and less bone density in the femoral neck [17].
Similar to the current study, Holecki et al. [5] found femoral neck bone loss among obese postmenopausal women. However, these results are inconsistent with other studies that have found higher body mass to be protective against bone loss [4–9,10]. More research in this area is needed, particularly given the shifting demographics within the US aging population that is simultaneously becoming obese at younger ages and is living longer in overweight and obese status [13].
It is important to understand these findings in the context of clinical outcomes. For example, femoral neck BMD levels for all BMI categories in year 10 were below common thresholds for low BMD [0.79 g/cm2 for men and 0.74 g/cm2 for women] [20]. Additionally, obese older adults had a larger total percentage change over the study period in femoral neck BMD than normal and overweight adults (7% loss for obese versus a 3% loss for normal weight and overweight older adults). These results are comparable to other studies that have found about a 0.5–1% annual change in femoral neck BMD among older adults, which equates to a 5–10% loss in femoral neck BMD over a 10 year period [21,22].
It remains unclear why femoral neck bone loss varies by obesity status in older adults. Obesity is associated with chronic inflammation, which is associated with the development and progression of osteoarthritis [15] and with bone loss. Changes in important bone-related health conditions over time that were not accounted for in study analyses (only controlled for baseline health status) may be the driving mechanism underlying the dramatic bone loss. Therefore, dramatic bone loss among obese older adults may in fact be due to post-baseline changes in levels of fat mass [23–25], physical activity [26–28], bone-active medication [29] and vitamin use [30–32], and the severity of diabetes [33]. Future research will need to consider these factors as recent studies have found obesity to be associated with risk of fracture [34].
This study had a number of strengths that have not been available in prior research. These include the large sample size, extensive set of covariates to control for confounding, the longitudinal study design that provided 10 years of follow-up data, as well as an examination of this association in a recent US cohort. There are also important limitations to study findings that should be noted. It is challenging to isolate the physiological effect of excess body fat from the effects of associated sequelae in an observational cohort study like Health ABC. This study attempted to control for many of these factors, such as other chronic conditions, bone-related medication use, and behavioral characteristics such as physical activity and substance abuse. However, these factors were only measured at baseline and therefore changes in these factors over the study period could have contributed to the decline in femoral neck BMD observed among obese older adults, particularly as these factors are also highly correlated with obesity. The study sample draws from a healthy cohort of older adults that were disability free at baseline (age 70–79). Study results may not be generalizable to the entire US older adult population. However, Health ABC inclusion criteria are comparable to large population-based studies such as the Longitudinal Study on Aging [LSOA] [35]. The study did not examine change in BMI over time as it relates to bone loss, although sensitivity analyses examined weight change over time. Fluctuations in body weight can greatly impact bone loss [36,37], however the majority of older adults in Health ABC maintained stable weight over time, and only experience small declines after age 70 [38,39]. Lastly, there may have been important differences in those individuals lost to follow-up or missing on study covariates that could have biased study results, especially if missing individuals were sicker than observed participants.
In conclusion, study findings suggest that obese older adults lose more femoral neck BMD over time compared with normal weight older adults. These results are surprising given that many cross-sectional studies, including in this cohort, have found a protective association between obesity and osteoporosis. Study findings underscore the importance of looking at the longitudinal relationship between obesity and bone loss among older adults given increasing prevalence and duration of obesity among older adults.
Acknowledgments
Analysis was supported by the National Institutes of Health (NIH) grants: T32 4G000262, R01AG028556. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging as well as from the Pepper Center (P30AG028747, Shari Waldstein). This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. We would like to thank William G. Hawkes, Ph.D. for his contribution to the methods of this manuscript through his work on Jennifer Lloyd’s dissertation committee.
Footnotes
Disclaimer: The statements contained herein are those of the authors and do not necessarily reflect the views or policies of the Centers for Medicare and Medicaid Services.
Conflict of Interest: Jennifer Tower Lloyd, Dawn E Alley, Marc C Hochberg, Shari R Waldstein, Tamara B Harris, Stephen B Kritchevsky, Ann V Schwartz, Elsa S Strotmeyer, Catherine R Womack, and Denise L Orwig declare that they have no conflict of interest.
References
- 1.Albala C, Yanez M, Devoto E, Sostin C, Zeballos L, Santos JL. Obesity as a protective factor for postmenopausal osteoporosis. Int J Obes Relat Metab Disord. 1996;20:1027–32. [PubMed] [Google Scholar]
- 2.Asomaning K, Bertone-Johnson ER, Nasca PC, Hooven F, Pekow PS. The association between body mass index and osteoporosis in patients referred for a bone mineral density examination. J Womens Health (Larchmt) 2006;15:1028–34. doi: 10.1089/jwh.2006.15.1028. [DOI] [PubMed] [Google Scholar]
- 3.Beck TJ, Petit MA, Wu G, LeBoff MS, Cauley JA, Chen Z. Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the women's health initiative-observational study. J Bone Miner Res. 2009;24:1369–79. doi: 10.1359/JBMR.090307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burger H, de Laet CE, van Daele PL, Weel AE, Witteman JC, Hofman A, Pols HA. Risk factors for increased bone loss in an elderly population: The rotterdam study. Am J Epidemiol. 1998;147:871–9. doi: 10.1093/oxfordjournals.aje.a009541. [DOI] [PubMed] [Google Scholar]
- 5.Holecki M, Chudek J, Titz-Bober M, Wiecek A, Zahorska-Markiewicz B, Dulawa J. Changes of bone mineral density in obese perimenopausal women during 5-year follow-up. Pol Arch Med Wewn. 2012;122:139–47. doi: 10.20452/pamw.1175. [DOI] [PubMed] [Google Scholar]
- 6.Jones G, Nguyen T, Sambrook P, Kelly PJ, Eisman JA. Progressive loss of bone in the femoral neck in elderly people: Longitudinal findings from the dubbo osteoporosis epidemiology study. BMJ. 1994;309:691–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Ribot C, Tremollieres F, Pouilles JM. The effect of obesity on postmenopausal bone loss and the risk of osteoporosis. Adv Nutr Res. 1994;9:257–71. doi: 10.1007/978-1-4757-9092-4_15. [DOI] [PubMed] [Google Scholar]
- 8.Saarelainen J, Kiviniemi V, Kroger H, Tuppurainen M, Niskanen L, Jurvelin J, Honkanen R. Body mass index and bone loss among postmenopausal women: The 10-year follow-up of the OSTPRE cohort. J Bone Miner Metab. 2012;30:208–16. doi: 10.1007/s00774-011-0305-5. [DOI] [PubMed] [Google Scholar]
- 9.Tremollieres F, Pouilles JM, Ribot C. Effect of long-term administration of progestogen on post-menopausal bone loss: Result of a two-year, controlled randomized study. Clin Endocrinol (Oxf) 1993;38:627–31. doi: 10.1111/j.1365-2265.1993.tb02145.x. [DOI] [PubMed] [Google Scholar]
- 10.Ravn P, Cizza G, Bjarnason NH, Thompson D, Daley M, Wasnich RD, McClung M, Hosking D, Yates AJ, Christiansen C. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. early postmenopausal intervention cohort (EPIC) study group. J Bone Miner Res. 1999;14:1622–7. doi: 10.1359/jbmr.1999.14.9.1622. [DOI] [PubMed] [Google Scholar]
- 11.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 12.Ding J, Kritchevsky SB, Newman AB, Taaffe DR, Nicklas BJ, Visser M, Lee JS, Nevitt M, Tylavsky FA, Rubin SM, Pahor M, Harris TB Health ABC Study. Effects of birth cohort and age on body composition in a sample of community-based elderly. Am J Clin Nutr. 2007;85:405–10. doi: 10.1093/ajcn/85.2.405. [DOI] [PubMed] [Google Scholar]
- 13.Leveille SG, Wee CC, Iezzoni LI. Trends in obesity and arthritis among baby boomers and their predecessors, 1971–2002. Am J Public Health. 2005;95:1607–13. doi: 10.2105/AJPH.2004.060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–7. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 15.Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011;6:30–37. doi: 10.1186/1749-799X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Looker AC, Dawson-Hughes B, Tosteson AN, Johansson H, Kanis JA, Melton LJ., 3rd Hip fracture risk in older US adults by treatment eligibility status based on new national osteoporosis foundation guidance. Osteoporosis International. 2010;25:64–71. doi: 10.1007/s00198-010-1288-0. [DOI] [PubMed] [Google Scholar]
- 17.Beck TJ, Oreskovic TL, Stone KL, Ruff CB, Ensrud K, Nevitt MC, Genant HK, Cummings SR. Structural adaptation to changing skeletal load in the progression toward hip fragility: The study of osteoporotic fractures. J Bone Miner Res. 2001;16:1108–19. doi: 10.1359/jbmr.2001.16.6.1108. [DOI] [PubMed] [Google Scholar]
- 18.Cauley JA, Lui LY, Stone KL, Hillier TA, Zmuda JM, Hochberg M, et al. Longitudinal study of changes in hip bone mineral density in caucasian and african-american women. Journal of the American Geriatrics Society. 2005;53:183–189. doi: 10.1111/j.1532-5415.2005.53101.x. [DOI] [PubMed] [Google Scholar]
- 19.Beck TJ, Looker AC, Ruff CB, Sievanen H, Wahner HW. Structural trends in the aging femoral neck and proximal shaft: Analysis of the third national health and nutrition examination survey dual-energy X-ray absorptiometry data. J Bone Miner Res. 2000;15:2297–304. doi: 10.1359/jbmr.2000.15.12.2297. [DOI] [PubMed] [Google Scholar]
- 20.Looker AC, Orwoll ES, Johnston CC, Jr, Lindsay RL, Wahner HW, Dunn WL, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12:1761–1768. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- 21.Greenspan SL, Maitland LA, Myers ER, Krasnow MB, Kido TH. Femoral bone loss progresses with age: A Longitudinal Study in Women Over Age 65. J Bone Miner Res. 1994;9:1959–65. doi: 10.1002/jbmr.5650091216. [DOI] [PubMed] [Google Scholar]
- 22.Cummings SR. Bone Mass and Bone Loss in the Elderly: A Special Case? International Journal of Fertility and Menopausal Studies. 1993;38:92–7. [PubMed] [Google Scholar]
- 23.Marin RV, Pedrosa MA, Moreira-Pfrimer LD, Matsudo SM, Lazaretti-Castro M. Association between lean mass and handgrip strength with bone mineral density in physically active postmenopausal women. J Clin Densitom. 2010;13:96–101. doi: 10.1016/j.jocd.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Reid IR, Ames RW, Evans MC, Sharpe SJ, Gamble GD. Determinants of the rate of bone loss in normal postmenopausal women. J Clin Endocrinol Metab. 1994;79:950–4. doi: 10.1210/jcem.79.4.7962303. [DOI] [PubMed] [Google Scholar]
- 25.Wu F, Ames R, Clearwater J, Evans MC, Gamble G, Reid IR. Prospective 10-year study of the determinants of bone density and bone loss in normal postmenopausal women, including the effect of hormone replacement therapy. Clin Endocrinol (Oxf) 2002;56:703–11. doi: 10.1046/j.1365-2265.2002.01534.x. [DOI] [PubMed] [Google Scholar]
- 26.Etherington J, Harris PA, Nandra D, Hart DJ, Wolman RL, Doyle DV, Spector TD. The effect of weight-bearing exercise on bone mineral density: A study of female ex-elite athletes and the general population. J Bone Miner Res. 1996;11:1333–8. doi: 10.1002/jbmr.5650110918. [DOI] [PubMed] [Google Scholar]
- 27.Rikkonen T, Salovaara K, Sirola J, Karkkainen M, Tuppurainen M, Jurvelin J, Honkanen R, Alhava E, Kroger H. Physical activity slows femoral bone loss but promotes wrist fractures in postmenopausal women - a 15 -year follow-up of ostpre study. J Bone Miner Res. 2010;25:2332–40. doi: 10.1002/jbmr.143. [DOI] [PubMed] [Google Scholar]
- 28.Wilsgaard T, Emaus N, Ahmed LA, Grimnes G, Joakimsen RM, Omsland TK, Berntsen GR. Lifestyle impact on lifetime bone loss in women and men: The tromso study. Am J Epidemiol. 2009;169:877–86. doi: 10.1093/aje/kwn407. [DOI] [PubMed] [Google Scholar]
- 29.Taaffe DR, Cauley JA, Danielson M, Nevitt MC, Lang TF, Bauer DC, Harris TB. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: The health, aging, and body composition study. J Bone Miner Res. 2001;16:1343–52. doi: 10.1359/jbmr.2001.16.7.1343. [DOI] [PubMed] [Google Scholar]
- 30.Jackson RD, LaCroix AZ, Gass M, et al. Women's Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 31.Karkkainen M, Tuppurainen M, Salovaara K, Sandini L, Rikkonen T, Sirola J, Honkanen R, Jurvelin J, Alhava E, Kroger H. Effect of calcium and vitamin D supplementation on bone mineral density in women aged 65–71 years: A 3-year randomized population-based trial (OSTPRE-FPS) Osteoporos Int. 2010;21:2047–55. doi: 10.1007/s00198-009-1167-8. [DOI] [PubMed] [Google Scholar]
- 32.Moschonis G, Katsaroli I, Lyritis GP, Manios Y. The effects of a 30-month dietary intervention on bone mineral density: The postmenopausal health study. Br J Nutr. 2010;104:100–7. doi: 10.1017/S000711451000019X. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz AV, Sellmeyer DE, Strotmeyer ES, Tylavsky FA, Feingold KR, Resnick HE, Shorr RI, Nevitt MC, Black DM, Cauley JA, Cummings SR, Harris TB Health ABC Study. Diabetes and bone loss at the hip in older black and white adults. J Bone Miner Res. 2005;20:596–603. doi: 10.1359/JBMR.041219. [DOI] [PubMed] [Google Scholar]
- 34.Compston JE, Watts NB, Chapurlat R, Cooper C, Boonen S, Greenspan S, Pfeilschifter J, Silverman S, Diez-Perez A, Lindsay R, Saag KG, Netelenbos JC, Gehlbach S, Hooven FH, Flahive J, Adachi JD, Rossini M, Lacroix AZ, Roux C, Sambrook PN, Siris ES Glow Investigators. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med. 2011;124:1043–50. doi: 10.1016/j.amjmed.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corder LS, Manton KG. National surveys and the health and functioning of the elderly: The effects of design and content. Journal of the American Statistical Association. 1991;86:513–25. [Google Scholar]
- 36.Alekel DL, Mortillaro E, Hussain EA, West B, Ahmed N, Peterson CT, Werner RK, Arjmandi BH, Kukreja SC. Lifestyle and biologic contributors to proximal femur bone mineral density and hip axis length in two distinct ethnic groups of premenopausal women. Osteoporos Int. 1999;9:327–38. doi: 10.1007/s001980050155. [DOI] [PubMed] [Google Scholar]
- 37.Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: The framingham study. J Bone Miner Res. 1993;8:567–73. doi: 10.1002/jbmr.5650080507. [DOI] [PubMed] [Google Scholar]
- 38.Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP Cardiovascular Study Research Group. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49:1309–18. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 39.Wallace JI, Schwartz RS. Epidemiology of weight loss in humans with special reference to wasting in the elderly. Int J Cardiol. 2002;85:15–21. doi: 10.1016/s0167-5273(02)00246-2. [DOI] [PubMed] [Google Scholar]


