Abstract
Aims
Change in the NT-proBNP level is a common surrogate endpoint in early phase heart failure (HF) trials, but whether this endpoint is influenced by atrial fibrillation/flutter (AFF) is unclear.
Methods and results
This analysis included 1358 patients from the ASTRONAUT trial, which randomized patients hospitalized for HF with EF ≤40% to aliskiren or placebo in addition to standard care. Patients were stratified by presence of AFF on baseline ECG. NT-proBNP was measured longitudinally by a core laboratory at baseline, 1 month, 6 months, and 12 months. Compared with non-AFF patients, AFF patients experienced greater reduction from baseline in log-transformed NT-proBNP (interaction P < 0.001), but this difference was not significant after adjustment (interaction P = 0.726). The ability of aliskiren to lower NT-proBNP during follow-up differed by AFF status (interaction P = 0.001), with aliskiren lowering NT-proBNP more than placebo among non-AFF patients only. After adjustment, baseline AFF was not associated with mortality or HF hospitalization at 12 months (all P ≥ 0.152).
Conclusion
In this hospitalized HF cohort, AFF status did not influence post-discharge NT-proBNP levels or clinical outcomes after adjustment for patient characteristics. Aliskiren lowered follow-up NT-proBNP levels in patients without AFF, but had no influence among patients with AFF. This study generates the hypothesis that the ability of a HF trial to meet an NT-proBNP defined endpoint may be influenced by the prevalence of AFF in the population. Because aliskiren did not improve outcomes in patients without AFF, this analysis suggests changes in NT-proBNP induced by investigational therapies may be dissociated from clinical effects.
Keywords: Heart failure, Natriuretic peptide, Atrial fibrillation, Outcomes
Introduction
The natriuretic peptide (NP) level is a well-recognized and powerful prognostic tool in heart failure (HF) care.1 This predictive value is seen across the spectrum of HF care settings, and NP cut-offs are increasingly incorporated into clinical trial selection criteria to identify patients at appropriate risk.2–5 These data have sparked enthusiasm regarding the potential of NPs in guiding titration of HF therapy and have prompted inclusion of change in NP as an endpoint in recent HF clinical trials.3,6,7 However, although the prognostic value is supported by robust and consistent evidence, the ability of a change in NP concentration to serve as a reliable surrogate for morbidity and mortality in HF clinical trials remains uncertain.8
Overall, 30–40% of patients hospitalized for HF (HHF) have comorbid atrial fibrillation or flutter (AFF), a condition that may contribute to an elevated NP level, independent of HF status.9–11 For this reason, in the setting of AFF, a higher NP cut-off for HF diagnosis may be preferred, and some recent trials have specified differing NP inclusion criteria based on presenting rhythm.7,9,12 However, to our knowledge, there are no data systematically evaluating the influence of AFF on longitudinal changes in NP level. Likewise, the hypothesis that the prevalence of AFF in a cohort could influence the ability of an HF trial to meet an NP-defined endpoint remains plausible, but untested. The characterization of such relationships could have significant implications on use of NP target levels in clinical practice, the design of future HF trials, and the usefulness of an NP trial endpoint. The ASTRONAUT (Aliskiren Trial on Acute Heart Failure Outcomes) trial database affords the opportunity to formally study these questions for the first time.3 We hypothesized that AFF status would track with a unique longitudinal NP trajectory and that the influence of aliskiren on the NP endpoint would differ by baseline heart rhythm.
Methods
Study design
The study design and primary results of the ASTRONAUT trial have been published previously.3,13 Briefly, ASTRONAUT was a prospective, multicentre, global, placebo-controlled randomized trial investigating the effect of aliskiren, a direct renin inhibitor, on clinical outcomes among stable HHF patients. All patients were ≥18 years old with LVEF ≤40%, elevated admission NP level (BNP ≥400 pg/mL or NT-proBNP ≥1600 pg/mL), and signs and symptoms of fluid overload that required hospitalization. The trial found that aliskiren, compared with placebo, was associated with a sustained significant reduction in longitudinal NT-proBNP without affecting clinical outcomes. ASTRONAUT was conducted in full accordance with the Declaration of Helsinki and with institutional review board and ethics committee approval at all sites. Informed consent was obtained from all patients.
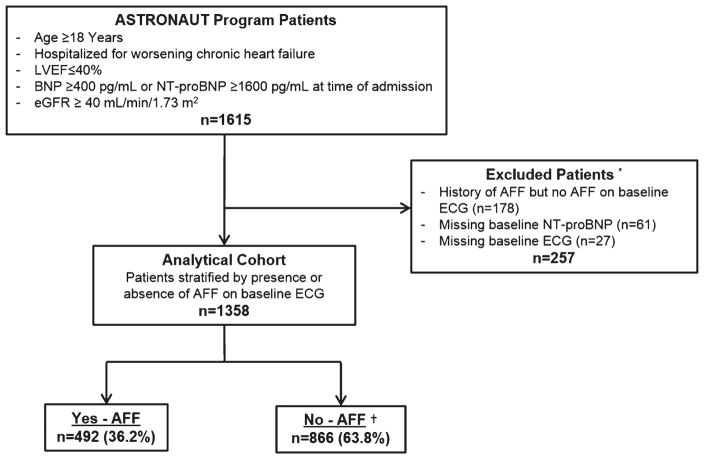
The present analysis included patients in both the aliskiren and placebo study arms. In ASTRONAUT, the presence or absence of AFF at baseline was determined by ECG. To better determine the true influence of rhythm status on longitudinal NT-proBNP and to minimize crossover between study groups during follow-up, patients without AFF on baseline ECG but with history of AFF were excluded from the current analysis. Other exclusion criteria included absence of a baseline ECG and absence of baseline NT-proBNP measurement. Figure 1 details the overall study design and selection of the final analytic cohort.
Figure 1.
Selection of the analytic cohort. Potential ECG findings within the non-atrial fibrillation/flutter (AAF) group, as documented on the trial case report form, included the following: LBBB, RBBB, pathological Q-waves, LV hypertrophy, paced rhythm, and ‘other’. There was no designation for normal sinus rhythm. eGFR, estimated glomerular filtration rate. * Some patients were excluded for multiple reasons; †ECG findings included in the non-AFF group included ‘other’ (n = 338), LBBB (n = 209), LV hypertrophy (n = 193), pathological Q-waves (n = 187), paced rhythm (n = 77), and RBBB (n = 62). Individual patients could have multiple ECG findings.
Natriuretic peptide measurement
The trial protocol specified measurement of NT-proBNP at the time of randomization (i.e. baseline/Visit 2), and 1 month, 6 months, and 12 months post-randomization at a central core laboratory blinded to clinical data. Plasma concentrations of NT-proBNP were measured using the Roche Elecys proBNP assay (Roche Diagnostics GmbH) with a reporting range of 5–35 000 pg/mL. Measurement of NT-proBNP at admission (i.e. screening/Visit 1) was performed locally using the assay of the specific study site and utilized as a study inclusion criterion.
Study endpoints and definitions
The pre-specified endpoints for the present study were (i) change from baseline in log-transformed NT-proBNP at 1, 6, and 12 months; (ii) all-cause death within 12 months; and (iii) the composite of cardiovascular death or HHF (CVM/HHF) within 12 months. All clinical endpoints were adjudicated by a blinded clinical event committee (Brigham and Women’s Hospital, Boston, MA, USA). The definition of HHF was presentation requiring overnight hospitalization with signs and symptoms of HF and treatment with intravenous medications (i.e. diuretics, vasodilators, or inotropes), mechanical fluid removal, an intra-aortic balloon pump, or initiation or intensification (i.e. doubling) of the maintenance diuretic dose. Baseline rhythm from a 12-lead ECG was documented by study investigators on the case report form. Aside from heart rate and QRS duration, available ECG documentation fields included atrial fibrillation, atrial flutter, left bundle branch block (LBBB), right bundle branch block (RBBB), pathological Q-waves, left ventricular (LV) hypertrophy, paced rhythm, and other.
Statistical analysis
Eligible patients were grouped by the presence or absence of AFF on baseline ECG. Baseline demographics, vital signs and laboratory values, medical and medication history, and clinical events were compared between groups using χ2, analysis of variance (ANOVA), and Kruskal–Wallis distribution-free tests where appropriate. All continuous variables were reported as mean ± standard deviation or median (interquartile range).
The primary predictor of the present study was AFF status. For assessment of 12-month all-cause death and 12-month CVM/HHF, Kaplan–Meier curves were constructed for each study group and compared using log-rank tests. For clinical endpoints, univariable and multivariable Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the primary predictor. The proportional hazards assumption was confirmed by Kolmogorov-type supremum tests.
For NT-proBNP assessment, a mixed effects model with baseline, 1 month, 6 month, and 12 month study visits as repeated measures variables nested in patients was used to analyse AFF status on baseline ECG, study visit, and aliskiren as predictors of log-transformed NT-proBNP. The full information restricted maximum likelihood algorithm was used to estimate model parameters. No data were imputed. Change from baseline in log-transformed NT-proBNP at each time point was estimated separately for patients with and without AFF. For purposes of visual presentation, estimated NT-proBNP values over time were back-transformed to their raw metric (i.e. pg/mL). Due to a statistically significant interaction between log-transformed change from baseline in NT-proBNP and study treatment, consistent with the overall NT-proBNP results from the main ASTRONAUT trial, a separate multivariable risk-adjusted analysis was performed in placebo patients only. Baseline patient characteristics and their interactions with study visit were used to adjust the estimates for NT-proBNP over time. Quadratic terms of continuous variables were also tested. Interactions with study visit and quadratic terms were not retained if they did not improve model fit by the likelihood ratio test. To evaluate differences in aliskiren treatment effect by AFF status, the influence of aliskiren on change from baseline in NT-proBNP was assessed by three-way interaction (i.e., aliskiren × AFF × study visit).
Multivariable models for clinical outcomes were adjusted for 23 pre-selected baseline covariates: aliskiren treatment, age, gender, ischaemic HF aetiology, NYHA functional class, EF, systolic blood pressure, heart rate, NT-proBNP, serum sodium, serum blood urea nitrogen, QRS duration, medical history (prior HHF, hypertension, CAD, diabetes, COPD), and background therapy [ACE inhibitor/ARB, beta-blocker, mineralocorticoid receptor antagonist (MRA), digoxin, implantable cardioverter-defibrillator (ICD), and CRT]. Given the higher number of outcome events, a more expansive list of covariates and interactions could be included in the NT-proBNP multivariable model (see the legend of Figure 3). The multiple imputation procedure [fully conditional specification methods as implemented in MI and MIANALYZE procedures in SAS (SAS Institute, Cary, NC, USA)] was used for missing covariate data (<5% for all variables). All statistical analyses were performed using SAS version 9.3 (SAS Institute), and two-tailed P < 0.05 was considered to be statistically significant.
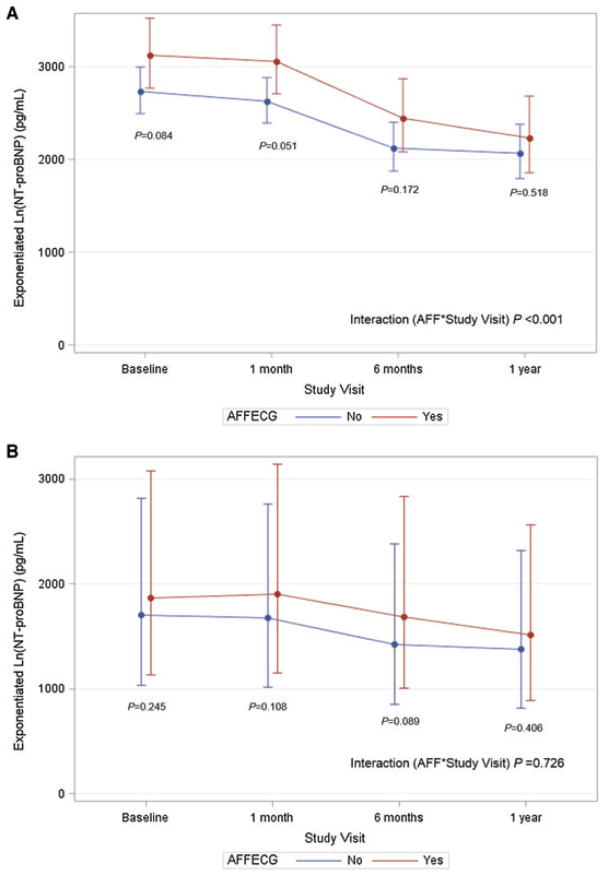
Figure 3.
Longitudinal NT-proBNP level by atrial fibrillation/flutter (AAF) status (placebo patients only), depicted as univariate (A) and multivariate analysis (B). The y-axis represents the estimated NT-proBNP level in pg/mL, derived from exponentiation of the log-transformed NT-proBNP level at each time point. Multivariate analysis adjusted for age, gender, geographic region, ethnicity, ischaemic heart failure (HF) aetiology, NYHA functional class, medical history (prior HF hospitalization, hypertension, CAD, diabetes, chronic renal insufficiency), background therapies (ACE inhibitor/ARB, beta-blockers, mineralocorticoid receptor antagonist, digoxin, implantable cardioverter-defibrillator, CRT), body mass index, EF, systolic blood pressure, QRS duration, serum creatinine, and the following interactions with study visit: gender, ischaemic HF aetiology, NYHA functional class, beta-blocker, implantable cardioverter-defibrillator. AFFECG, atrial fibrillation/flutter on baseline electrocardiogram.
Results
Patient characteristics
Of the 1615 patients in the ASTRONAUT efficacy cohort, 1358 (84.1%) were included in the present study, of which 492 (36.2%) had baseline AFF (Figure 1). Table 1 presents baseline demographic, clinical, and laboratory data for patients by AFF status on baseline ECG. Of patients with baseline AFF, 96.3% had a prior history of AFF. Compared with those without AFF, AFF patients generally had higher baseline NT-proBNP level (median 2805 pg/mL vs. 2645 pg/mL, P = 0.052) and more severe NYHA functional status at admission and randomization. Overall, the use of guideline-directed medical therapy among this population was high, with >80% of patients receiving ACE inhibitors/ARBs and beta-blockers at baseline. However, beta-blocker and digoxin use was higher among AFF patients. Rates of therapeutic anticoagulation were 60.2% and 27.3% in AFF and non-AFF patients, respectively. Baseline ECG data for the non-AFF group are provided in the legend of Figure 1.
Table 1.
Baseline characteristics by atrial fibrillation/flutter status
| Atrial fibrillation/flutter on baseline ECG
|
P-value | ||
|---|---|---|---|
| Yes (n = 492) | No (n = 866) | ||
| Demographics | |||
| Age (years) | 68.1 ± 10.9 | 61.6 ± 12.5 | <0.001 |
| Male | 389 (79.1) | 655 (75.6) | 0.150 |
| Race | <0.001 | ||
| White | 428 (87.0) | 490 (56.6) | |
| Black | 12 (2.4) | 49 (5.7) | |
| Asian | 41 (8.3) | 280 (32.3) | |
| Other | 11 (2.2) | 47 (5.4) | |
| Region | <0.001 | ||
| North America | 21 (4.3) | 69 (8.0) | |
| Latin America | 43 (8.7) | 101 (11.7) | |
| Western Europe | 146 (29.7) | 150 (17.3) | |
| Eastern Europe | 209 (42.5) | 214 (24.7) | |
| Asia/Pacific | 73 (14.8) | 332 (38.3) | |
| Time from admission to randomization (days) | 5 (3–8) | 4 (2–7) | <0.001 |
| Hospital length of stay (days) | 10 (7–16) | 7 (4–11) | <0.001 |
| Ejection fraction (%) | 28.6 ± 7.4 | 27.3 ± 7.2 | 0.002 |
| Ischaemic HF aetiology | 302 (61.4) | 566 (65.4) | 0.135 |
| NYHA class at admission | 0.026 | ||
| III | 280 (56.9) | 546 (63.0) | |
| IV | 212 (43.1) | 320 (37.0) | |
| NYHA class at baseline | <0.001 | ||
| I/II | 137 (27.8) | 327 (37.8) | |
| III/IV | 343 (69.7) | 534 (62.7) | |
| Missing | 12 (2.4) | 5 (0.6) | |
| QRS duration on baseline ECG (ms) | 116 ± 40 | 116 ± 37 | 0.905 |
| Vital sign and laboratory data | |||
| Systolic blood pressure (mmHg) | 124.1 ± 13.5 | 122.9 ± 12.9 | 0.127 |
| Heart rate (b.p.m.) | 81.5 ± 17.7 | 77.0 ± 14.7 | <0.001 |
| Weight (kg) | 81.7 ± 19.7 | 75.0 ± 21.1 | <0.001 |
| BMI (kg/m2) | 27.9 ± 5.6 | 26.6 ± 6.4 | <0.001 |
| Haemoglobin (g/dL) | 13.9 ± 1.9 | 13.7 ± 2.0 | 0.017 |
| Albumin (g/dL) | 4.0 ± 0.5 | 3.9 ± 0.5 | 0.015 |
| Serum sodium (mmol/L) | 139.3 ± 3.5 | 138.4 ± 3.7 | <0.001 |
| BUN (mmol/L) | 9.8 ± 3.8 | 8.5 ± 3.7 | <0.001 |
| Creatinine (mmol/L) | 100.9 ± 25.7 | 98.6 ± 27.6 | 0.129 |
| eGFR (mL/min/1.73 m2) | 65.2 ± 18.8 | 68.8 ± 20.5 | 0.002 |
| NT-proBNP at admission (pg/mL)a | 4287 (2734–8084) | 4338 (2705–7886) | 0.571 |
| NT-proBNP at baseline (pg/mL)a | 2805 (1741–5047) | 2645 (1362–5365) | 0.052 |
| Troponin I (ng/mL) | 0.0 (0.0–0.1) | 0.0 (0.0–0.1) | 0.851 |
| PRA (μIU/mL) | 2.7 (0.6–16.1) | 3.3 (0.6–17.4) | 0.382 |
| Past medical history | |||
| Previous HF hospitalization | 350 (71.1) | 553 (63.9) | 0.006 |
| Coronary artery disease | 244 (49.6) | 486 (56.1) | 0.020 |
| Previous PCI | 71 (14.4) | 187 (21.6) | 0.001 |
| Previous CABG | 64 (13.0) | 147 (17.0) | 0.052 |
| Previous myocardial infarction | 170 (34.6) | 398 (46.0) | <0.001 |
| Previous stroke | 55 (11.2) | 66 (7.6) | 0.027 |
| Previous TIA | 18 (3.7) | 23 (2.7) | 0.299 |
| Hypertension | 395 (80.3) | 624 (72.1) | 0.001 |
| Atrial fibrillation | 474 (96.3) | 0 (0.0) | <0.001 |
| Diabetes | 187 (38.0) | 374 (43.2) | 0.062 |
| COPD | 106 (21.5) | 156 (18.0) | 0.113 |
| Baseline therapies | |||
| Diuretic | 470 (95.5) | 837 (96.7) | 0.296 |
| Beta-blocker | 419 (85.2) | 696 (80.4) | 0.027 |
| ACE inhibitor/ARB | 425 (86.4) | 716 (82.7) | 0.073 |
| MRA | 279 (56.7) | 500 (57.7) | 0.712 |
| MRA + ACE inhibitor/ARB | 242 (49.2) | 407 (47.0) | 0.438 |
| Digoxin | 260 (52.8) | 290 (33.5) | <0.001 |
| Anticoagulationb | 296 (60.2) | 236 (27.3) | <0.001 |
| Heparin product | 114 (23.2) | 178 (20.6) | 0.259 |
| Vitamin K antagonist | 229 (46.5) | 69 (8.0) | <0.001 |
| ICD | 71 (14.4) | 120 (13.9) | 0.770 |
| CRT | 29 (5.9) | 39 (4.5) | 0.259 |
| Permanent pacemaker | 57(11.6) | 62 (7.2) | 0.006 |
BMI, body mass index; BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter-defibrillator; MRA, mineralocorticoid receptor antagonist; PRA, plasma renin activity; TIA, transient ischaemic attack
Data available for 649 patients at admission and all 1358 patients at baseline.
Defined as receipt of a therapeutic heparin product or vitamin K antagonist; 157 additional patients received an agent during the trial but did not have a start date documented.
Clinical outcomes
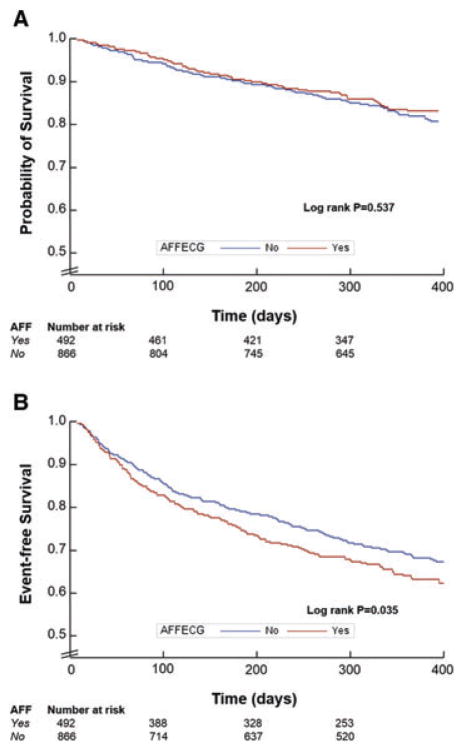
Event rates by AFF status are displayed in Table 2. Times to first event stratified by AFF status were similar by the Kaplan–Meier method for death (P = 0.537), but significantly different for the composite endpoint with decreased event-free survival among AFF patients (P = 0.035) (Figure 2).
Table 2.
Event rates by atrial fibrillation/flutter status
| Atrial fibrillation/flutter on baseline ECG
|
P-value | ||
|---|---|---|---|
| Yes (n = 492) | No (n = 866) | ||
| 12-month event rates | |||
| All-cause mortality | 81 (16.5) | 158 (18.2) | 0.407 |
| CVM or HHF | 190 (38.6) | 293 (33.8) | 0.077 |
| CVM | 71 (14.4) | 150 (17.3) | 0.166 |
| Pump failure | 24 (4.9) | 60 (6.9) | 0.132 |
| Sudden cardiac death | 22 (4.5) | 51 (5.9) | 0.266 |
| Fatal myocardial infarction | 3 (0.6) | 11 (1.3) | 0.402 |
| Presumed sudden death | 3 (0.6) | 5 (0.6) | 1.000 |
| Presumed CV death | 6 (1.2) | 15 (1.7) | 0.462 |
| Other CV death | 1 (0.2) | 1 (0.1) | 1.000 |
| Fatal stroke | 9 (1.8) | 3 (0.3) | 0.011 |
| CV procedural | 0 (0.0) | 2 (0.2) | 0.538 |
| Unknown | 3 (0.6) | 2 (0.2) | 0.359 |
| HHF | 152 (30.9) | 208 (24.0) | 0.006 |
| All-cause rehospitalization | 261 (53.0) | 363 (41.9) | <0.001 |
| CV event | 197 (40.0) | 312 (36.0) | 0.142 |
| Myocardial infarction | 15 (3.0) | 35 (4.0) | 0.350 |
| Stroke | 18 (3.7) | 19 (2.2) | 0.111 |
| 6-month event rates | |||
| All-cause mortality | 50 (10.2) | 95 (11.0) | 0.643 |
| CVM or HHF | 143 (29.1) | 205 (23.7) | 0.029 |
| CVM | 46 (9.3) | 92 (10.6) | 0.455 |
| HHF | 118 (24.0) | 149 (17.2) | 0.003 |
| All-cause rehospitalization | 220 (44.7) | 283 (32.7) | <0.001 |
| 30-day event rates | |||
| All-cause mortality | 7 (1.4) | 17 (2.0) | 0.468 |
| HHF | 31 (6.3) | 43 (5.0) | 0.297 |
| All-cause rehospitalization | 70 (14.2) | 108 (12.5) | 0.357 |
CV, cardiovascular; CVM, cardiovascular mortality; HHF, hospitalization for heart failure
Figure 2.
Kaplan–Meier curves for all-cause mortality (A) and cardiovascular mortality or hospitalization for heart failure (B) at 12 months follow-up by atrial fibrillation/flutter (AAF) status on baseline ECG. Times to events were compared using log-rank tests.
Unadjusted and adjusted outcome analyses are presented in Table 3. Risk of 12-month all-cause death did not significantly differ by AFF status in either unadjusted or adjusted analysis (P ≥ 0.460). Unadjusted estimates of 12-month CVM/HHF demonstrated heightened risk among AFF patients (HR 1.22, 95% CI 1.01–1.46), but this association become non-significant after adjustment (HR 1.16, 95% CI 0.95–1.43).
Table 3.
Relative risk of co-primary end points by presence of atrial fibrillation/flutter on baseline electrocardiogtrama
| Outcome | Unadjusted | Adjustedb |
|---|---|---|
| ACM | 0.92 (0.70–1.20), P = 0.537 | 0.89 (0.66–1.20), P = 0.460 |
| CVM/HHF | 1.22 (1.01–1.46), P = 0.035 | 1.16 (0.95–1.43), P = 0.152 |
Data represent hazard ratios and 95% confidence intervals for risk of primary co-endpoints for patients with atrial fibrillation/flutter on baseline ECG relative to patients without atrial fibrillation/flutter on baseline ECG
Adjusted for aliskiren treatment, age, gender, ischaemic heart failure aetiology, NYHA functional class, EF, systolic blood pressure, heart rate, NT-proBNP, serum sodium, serum blood urea nitrogen, QRS duration, medical history (prior hospitalization for heart failure, hypertension, CAD, diabetes, COPD), and background therapy (ACE inhibitor/ARB, beta-blocker, mineralocorticoid receptor antagonist, digoxin, implantable cardioverter-defibrillator, CRT). ACM, all-cause mortality; CVM/HHF, cardiovascular mortality or hospitalization for heart failure.
Atrial fibrillation, aliskiren, and natriuretic peptide trajectory
Within the placebo group, patients with and without baseline AFF had significant reductions in NT-proBNP from baseline to 12 months (P < 0.001 for both groups). AFF patients experienced greater log-transformed reduction from baseline in NT-proBNP (interaction AFF × study visit P < 0.001) (Table 4, Figure 3A), but this difference was not statistically significant after adjustment for patient characteristics (interaction AFF × study visit P = 0.726) (Figure 3B).
Table 4.
Change from baseline in N-terminal natriuretic peptide among patients with and without atrial fibrillation/flutter
| Time point | Treatment | Atrial fibrillation/flutter = yes | Atrial fibrillation/flutter = no | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| na | Change in Ln(NP) ± SE | Change in NP (pg/mL) | P-value | na | Change in Ln(NP) ± SE | Change in NP (pg/mL) | P-value | ||
| 1 month | Aliskiren | 210 | +0.045 ± 0.049 | +134.34 | 0.356 | 378 | −0.242 ± 0.037 | −576.92 | <0.001 |
| Placebo | 225 | −0.022 ± 0.048 | −67.40 | 0.648 | 371 | −0.040 ± 0.037 | −106.97 | 0.281 | |
| 6 months | Aliskiren | 169 | −0.181 ± 0.075 | −478.86 | 0.016 | 330 | −0.560 ± 0.055 | −1150.61 | <0.001 |
| Placebo | 182 | −0.246 ± 0.072 | −680.59 | <0.001 | 307 | −0.254 ± 0.056 | −613.01 | <0.001 | |
| 12 months | Aliskiren | 126 | −0.148 ± 0.092 | −397.89 | 0.109 | 261 | −0.661 ± 0.066 | −1297.96 | <0.001 |
| Placebo | 139 | −0.337 ± 0.088 | −894.36 | <0.001 | 238 | −0.280 ± 0.068 | −668.29 | <0.001 | |
n represents patients with available data at each time point.
P-values represent change from baseline in Ln (NT-proBNP) within each group. The table represents data from unadjusted analysis.
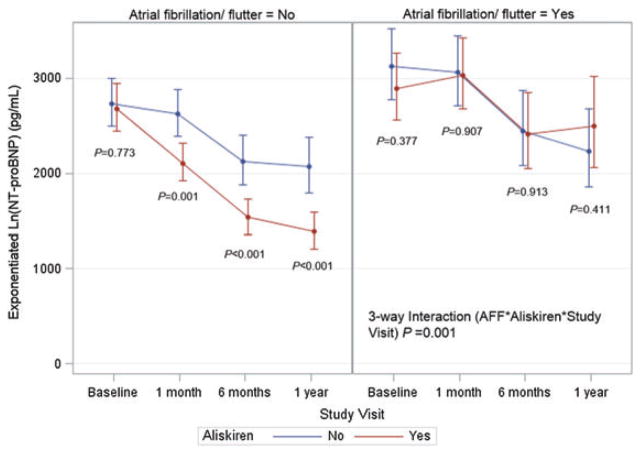
When including patients from both study treatment arms, the ability of aliskiren to reduce the NT-proBNP level during follow-up differed by AFF status (three-way interaction aliskiren × AFF × study visit P = 0.001) (Figure 4). Among AFF patients, compared with placebo, aliskiren patients experienced numerically less reduction in NT-proBNP level at 1, 6, and 12 months (Table 4), but there were no significant differences in absolute NT-proBNP levels at any time point (Figure 4). In contrast, within the non-AFF group, aliskiren patients had numerically larger reductions in NT-proBNP at these same time points, and absolute NT-proBNP concentrations were significantly lower at all post-discharge time points (Figure 4).
Figure 4.
Influence of aliskiren on longitudinal NT-proBNP level by atrial fibrillation/flutter status. The y-axis represents the estimated NT-proBNP level in pg/mL, derived from exponentiation of the log-transformed NT-proBNP level at each time point.
Discussion
In this exploratory analysis of HHF patients with reduced EF, baseline AFF status was associated with distinct clinical profiles but similar risk of post-discharge mortality and HF hospitalization after adjustment for patient characteristics. Within the trial’s placebo group, AFF was a marker of a distinct NT-proBNP trajectory, with greater reductions in NT-proBNP from baseline to 12 months compared with non-AFF patients. However, after accounting for patient factors, there was no independent influence of AFF status on post-discharge NP trajectory. When patients in both trial arms were considered, the ability of aliskiren to reduce NT-proBNP level over time differed by baseline AFF status. Aliskiren decreased the NT-proBNP level more than placebo in non-AFF patients only, and was associated with significantly lower absolute NT-proBNP concentration at 1-, 6-, and 12-month follow-up. With aliskiren as an illustrative example, we believe these results have implications for future drug development programmes of investigational HF therapies.
To our knowledge, we present the first analysis exploring the influence of AFF on the longitudinal NT-proBNP level. Multiple studies have previously documented the association between AFF and higher NP concentration at a single time point.10,14,15 Given that NT-proBNP level reflects myocardial stretch and filling pressures, these data supported the intuitive belief that AFF signalled a drop in cardiac performance.16 Nevertheless, whether AFF is merely a marker or a mediator of higher NT-proBNP level over time remains uncertain. The lack of an independent association between baseline AFF status and NT-proBNP trajectory found here suggests that other patient characteristics (e.g. renal function) tracking with rhythm status may account for differences in longitudinal NT-proBNP concentration and argues against a causal relationship.
A prior post-hoc analysis from ASTRONAUT found no influence of AF on the prognostic value of NT-proBNP concentration at baseline, 1 month, or change from baseline to 1 month.17 Viewing the ASTRONAUT data in aggregate, there appears to be concordance between (i) a lack of independent association of AFF with longitudinal NT-proBNP level; (ii) a lack of independent association between AFF and clinical outcomes; and (iii) similar ability of the NT-proBNP level to predict clinical outcomes irrespective of rhythm status. Existing data on the prognostic significance of co-morbid AFF in HF patients are mixed, with differential results potentially arising from heterogeneity in study design and populations.10,18–20 Notably, although multiple works suggest higher clinical risk with AFF as compared with normal sinus rhythm, the present study adjusted for more prognostic variables than most prior experiences. Moreover, documentation of baseline sinus rhythm was not explicitly required in the ASTRONAUT protocol and the present analysis used ‘non-AFF’ as the comparator group. Thus, the control group of the current study may have included a more heterogeneous collection of rhythms, some of which may have been associated with adverse outcomes and thus attenuated any differences in outcomes between study groups. Additionally, exclusion of patients with history of AFF but no AFF at baseline was a notable methodological difference unique to the present study. However, exclusion of these patients, many of whom probably had paroxysmal AFF, probably selected for inclusion of higher risk AFF patients (i.e. increased proportion of persistent/permanent AFF), and thus would not explain similar clinical outcomes between the AFF and non-AFF groups.21
Despite the influence of AFF on the effect of aliskiren on longitudinal NT-proBNP level, the primary ASTRONAUT results found no interaction between baseline heart rhythm and aliskiren effect on cardiovascular death or HF hospitalizaton.3 The ASTRONAUT data provide a cautionary example of dissociation between NP changes induced by investigational therapies and subsequent clinical outcomes.22 This stands in contrast to NP changes mediated by evidence-based therapies proven to improve outcomes, where treatment-induced lowering of NT-proBNP may reliably correlate with clinical benefits.6 Although the ASTRONAUT study design was novel with statistical power to evaluate NT-proBNP and clinical endpoints simultaneously, traditional HF drug development programmes often reserve NP-based outcomes for phase II trials.7,23 Had this been the case with the ASTRONAUT data, benefits of aliskiren on NT-proBNP reduction (driven by the non-AFF cohort) would have clearly supported investment towards a definitive phase III trial, a study that would have subsequently disappointed with neutral results.
Mechanistically, it remains unclear why aliskiren was able to cause significant sustained reductions in the NT-proBNP level among non-AFF patients only. From the primary ASTRONAUT trial results, one could speculate that analogous to other experiences with incremental renin–angiotensin–aldosterone system inhibition in HF, aliskiren tends to exert favourable long-term effects on the heart, compatible with long-term reduction in congestion and NT-proBNP.24,25 Although the present study showed that AFF patients within the placebo group tended to have greater unadjusted reductions from baseline in NT-proBNP level during follow-up, AFF appeared to negate any potential additive NT-proBNP lowering from aliskiren. Viewing these data in isolation, one could speculate that AFF patients are potentially easier to decongest, and that this made significant incremental decongestion with aliskiren (i.e. incremental NT-proBNP lowering) difficult to detect. Alternatively, and in conjunction with the current multivariate results discussed above, it is possible that the clinical profile of AFF patients exerted an added upward force on the longitudinal NT-proBNP level, negating any added lowering with aliskiren. However, the present data alone cannot prove these hypotheses.
Future clinical trial implications
With few notable exceptions, the last decade of HF trials has witnessed disappointing phase III results despite a myriad of promising phase II studies, highlighting a potential disconnect in the translational process and poor alignment between phase II and phase III trial endpoints.4,26,27 In this regard, change from baseline in log-transformed NT-proBNP represents an increasingly utilized surrogate endpoint across the spectrum of early phase HF trials.7,23,28,29 The present analysis, with aliskiren as an example of an investigational HF therapy, demonstrates how AFF can further complicate interpretation of an NP-defined endpoint and generates the hypothesis that the ability of a study therapy to meet such an endpoint may depend on the prevalence of co-morbid AFF in the population. This finding supports the increasing attention towards the heterogeneity of the HHF syndrome as a key reason for the lack of successful drug discovery in this population.27,30,31
Identifying surrogate endpoints for early phase HF trials is an important but challenging task. By definition, early stage trials are not powered for clinical endpoints, but rather are meant to inform pivotal phase III programmes where effects on ‘hard’ clinical outcomes are definitely assessed. To date, no surrogate endpoint for HF populations has been shown to be a perfect substitute for clinical events.8 Presently, despite limitations and documented potential for discordance between NP-defined endpoints and clinical outcomes, change from baseline NT-proBNP may still be among the most practical endpoints for early phase trials.3 However, the present results suggest a need to interpret such results with caution, paying particular attention to the AFF status of the population. Investigational therapies may exert varying efficacy by NT-proBNP level, which may track with AFF status.32,33 Recent trial designs have already begun to differentiate NP inclusion criteria by AFF status, and a similar rationale may be appropriate in defining NP-based endpoints.5,7,32 Alternatively, it may be appropriate to pre-specify study stratification by AFF status, or to refer patients with AFF to separate studies altogether. While exclusion of AFF patients would eliminate a substantial proportion of HF patients from trial enrolment, inclusion may run the risk of diluting positive signals in non-AFF patients. Our data are not the first suggesting a differential treatment effect of a HF therapy by AFF status. Mounting evidence suggests that beta-blockers do not improve clinical outcomes in HF patients with concurrent AFF, adding further plausibility to the potential of AFF to influence surrogate endpoints such as NP level.34,35
Limitations
Limitations of the present study should be acknowledged. First, absolute NT-proBNP levels decreased in both AFF and non-AFF groups over time and these trajectories must be interpreted in the context of ongoing patient death and loss to follow-up. Secondly, multivariate analysis for change from baseline NT-proBNP did not account for changes in time-dependent patient characteristics. However, this decision was pre-specified given the lack of data on follow-up rhythm status in order to ensure patient characteristics and AFF status were accurately aligned. Likewise, due to the lack of longitudinal ECG data, it was impossible to determine persistence of AFF during follow-up or to exclude potential crossover between AFF and non-AFF groups. For this reason, and to enrich the population with patients with perhaps a better likelihood of maintaining baseline rhythm during follow-up, patients with history of AFF but no AFF at baseline were excluded. Thirdly, despite rigorous multivariable modelling, this retrospective analysis is unable to test definitively cause–effect relationships, and inclusion of patients from a trial of HHF with reduced EF may limit the applicability of these findings to chronic ambulatory HF and HF with preserved EF populations. Fourthly, although the size of this cohort is comparable with multiple existing studies of AFF in HF populations, given the directionality of the HR and 95% CI, it is conceivable that AFF may have shown an independent association with CVM/HHF in a larger sample.19,20 Fifthly, by virtue of its design, this analysis was based on comparison of subgroups and the size of the AFF group was modest. Thus, we cannot exclude the possibility of chance findings, and this study should be considered hypothesis generating only.
Conclusions
This exploratory analysis within a HHF with reduced EF cohort suggests AFF may be a marker of a distinct longitudinal NT-proBNP trajectory, but found no significant difference in post-discharge NT-proBNP levels or clinical outcomes by AFF status after adjustment for patient characteristics. Aliskiren lowered follow-up NT-proBNP levels among non-AFF patients only. With aliskiren as a potential example, this study generates the hypothesis that the ability of a HF trial to meet an NT-proBNP-defined endpoint may be influenced by the prevalence of AFF in the population. These results should be validated in other HF populations and with other medications, but may be considered in the design of future HF drug development programmes.
Acknowledgments
Funding
Financial and material support for the ASTRONAUT trial was provided by Novartis Pharma AG (Basel, Switzerland). Haris Subacius conducted all final analyses for this report with funding from the Center for Cardiovascular Innovation, Northwestern University Feinberg School of Medicine, Chicago, IL, USA, and takes responsibility for the integrity of the data.
Footnotes
Conflict of interest: G.C.F. reports significant consulting for Novartis, and modest consulting for Amgen, Bayer, Gambro, Medtronic, and Janssen; holds the Eliot Corday Chair of Cardiovascular Medicine at UCLA; and is also supported by the Ahman-son Foundation (Los Angeles, CA). S.D.S. has received grant funding, consultant fees, and travel support from Novartis. A.P.A. was funded by National Institutes of Health T-32 training grant #5 T32 HL 069749-12. A.P.M. has served on committees of clinical studies sponsored by Amgen, Bayer, Abbott Vascular, Cardiorentis, John-son & Johnson, and Novartis Pharma AG. M.B. has served as a consultant for AstraZeneca, Bayer, Boehringer-Ingelheim, Daiichi-Sankyo, AWD Dresden, Berlin-Chemie, MSD, Novartis, Pfizer, Sanofi-Aventis, and Servier. F.Z. has received grant funding from Novartis, BG Medicine, and Roche Diagnostics; served on a board for Boston Scientific; and served as a consultant for Novartis, Takeda, AstraZeneca, Boehringer-Ingelheim, GE Healthcare, Relypsa, Servier, Boston Scientific, Bayer, Johnson & Johnson, and ResMed. M.G. has been a consultant for Abbott Laboratories, Astellas, AstraZeneca, Bayer HealthCare AG, CorThera, Cytokinetics, DebioPharm S.A., Errekappa Terapeutici, GlaxoSmithKline, Ikaria, Johnson & Johnson, Medtronic, Merck, Novartis Pharma AG, Otsuka Pharmaceuticals, Palatin Technologies, Pericor Therapeutics, Protein Design Laboratories, Sanofi-Aventis, Sigma Tau, Solvay Pharmaceuticals, Takeda Pharmaceutical, and Trevena Therapeutics. All other authors have no conflicts to declare.
References
- 1.Januzzi JL, Jr, Sakhuja R, O’Donoghue M, Baggish AL, Anwaruddin S, Chae CU, Cameron R, Krauser DG, Tung R, Camargo CA, Jr, Lloyd-Jones DM. Utility of amino-terminal pro-brain natriuretic peptide testing for prediction of 1-year mortality in patients with dyspnea treated in the emergency department. Arch Intern Med. 2006;166:315–320. doi: 10.1001/archinte.166.3.315. [DOI] [PubMed] [Google Scholar]
- 2.Maisel A, Xue Y, Greene SJ, Pang PS, Januzzi JL, Pina IL, DeFilippi C, Butler J. The potential role of natriuretic peptide-guided management for patients hospitalized for heart failure. J Card Fail. 2015;21:233–239. doi: 10.1016/j.cardfail.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Bohm M, Greene SJ, Fonarow GC, Lewis EF, Zannad F, Solomon SD, Baschiera F, Botha J, Hua TA, Gimpelewicz CR, Jaumont X, Lesogor A, Maggioni AP. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309:1125–1135. doi: 10.1001/jama.2013.1954. [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 5.Smith SA, Mentz RJ, Roessig L, Mebazza A, Longrois D, Gheorghiade M, Pitt B, Zannad F, Butler J, Abraham WT. Using natriuretic peptides for selection of patients in acute heart failure clinical trials. Am J Cardiol. 2015;116:1304–1310. doi: 10.1016/j.amjcard.2015.07.049. [DOI] [PubMed] [Google Scholar]
- 6.Felker GM, Ahmad T, Anstrom KJ, Adams KF, Cooper LS, Ezekowitz JA, Fiuzat M, Houston-Miller N, Januzzi JL, Leifer ES, Mark DB, Desvigne-Nickens P, Paynter G, Pina IL, Whellan DJ, O’Connor CM. Rationale and design of the GUIDE-IT study: Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure. JACC Heart Fail. 2014;2:457–465. doi: 10.1016/j.jchf.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Greene SJ, Butler J, Filippatos G, Lam CS, Maggioni AP, Ponikowski P, Shah SJ, Solomon SD, Kraigher-Krainer E, Samano ET, Muller K, Roessig L, Pieske B. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the SOCRATES-REDUCED randomized trial. JAMA. 2015;309:2251–2262. doi: 10.1001/jama.2015.15734. [DOI] [PubMed] [Google Scholar]
- 8.Zannad F, Garcia AA, Anker SD, Armstrong PW, Calvo G, Cleland JG, Cohn JN, Dickstein K, Domanski MJ, Ekman I, Filippatos GS, Gheorghiade M, Hernandez AF, Jaarsma T, Koglin J, Konstam M, Kupfer S, Maggioni AP, Mebazaa A, Metra M, Nowack C, Pieske B, Pina IL, Pocock SJ, Ponikowski P, Rosano G, Ruilope LM, Ruschitzka F, Severin T, Solomon S, Stein K, Stockbridge NL, Stough WG, Swedberg K, Tavazzi L, Voors AA, Wasserman SM, Woehrle H, Zalewski A, McMurray JJ. Clinical outcome endpoints in heart failure trials: a European Society of Cardiology Heart Failure Association consensus document. Eur J Heart Fail. 2013;15:1082–1094. doi: 10.1093/eurjhf/hft095. [DOI] [PubMed] [Google Scholar]
- 9.Richards M, Di Somma S, Mueller C, Nowak R, Peacock WF, Ponikowski P, Mockel M, Hogan C, Wu AH, Clopton P, Filippatos GS, Anand I, Ng L, Daniels LB, Neath SX, Shah K, Christenson R, Hartmann O, Anker SD, Maisel A. Atrial fibrillation impairs the diagnostic performance of cardiac natriuretic peptides in dyspneic patients: results from the BACH study (Biomarkers in ACute Heart Failure) JACC Heart Fail. 2013;1:192–199. doi: 10.1016/j.jchf.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Mentz RJ, Chung MJ, Gheorghiade M, Pang PS, Kwasny MJ, Ambrosy AP, Vaduganathan M, O’Connor CM, Swedberg K, Zannad F, Konstam MA, Maggioni AP. Atrial fibrillation or flutter on initial electrocardiogram is associated with worse outcomes in patients admitted for worsening heart failure with reduced ejection fraction: findings from the EVEREST Trial. Am Heart J. 2012;164:884–892e2. doi: 10.1016/j.ahj.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Lip GY, Laroche C, Popescu MI, Rasmussen LH, Vitali-Serdoz L, Dan GA, Kalarus Z, Crijns HJ, Oliveira MM, Tavazzi L, Maggioni AP, Boriani G. Heart failure in patients with atrial fibrillation in Europe: a report from the EURObservational Research Programme Pilot survey on Atrial Fibrillation. Eur J Heart Fail. 2015;17:570–582. doi: 10.1002/ejhf.254. [DOI] [PubMed] [Google Scholar]
- 12.Knudsen CW, Omland T, Clopton P, Westheim A, Wu AH, Duc P, McCord J, Nowak RM, Hollander JE, Storrow AB, Abraham WT, McCullough PA, Maisel A. Impact of atrial fibrillation on the diagnostic performance of B-type natriuretic peptide concentration in dyspneic patients: an analysis from the breathing not properly multinational study. J Am Coll Cardiol. 2005;46:838–844. doi: 10.1016/j.jacc.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 13.Gheorghiade M, Albaghdadi M, Zannad F, Fonarow GC, Bohm M, Gimpelewicz C, Botha J, Moores S, Lewis EF, Rattunde H, Maggioni A. Rationale and design of the multicentre, randomized, double-blind, placebo-controlled Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT) Eur J Heart Fail. 2011;13:100–106. doi: 10.1093/eurjhf/hfq209. [DOI] [PubMed] [Google Scholar]
- 14.Morello A, Lloyd-Jones DM, Chae CU, van Kimmenade RR, Chen AC, Baggish AL, O’Donoghue M, Lee-Lewandrowski E, Januzzi JL., Jr Association of atrial fibrillation and amino-terminal pro-brain natriuretic peptide concentrations in dyspneic subjects with and without acute heart failure: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. Am Heart J. 2007;153:90–97. doi: 10.1016/j.ahj.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Silvet H, Young-Xu Y, Walleigh D, Ravid S. Brain natriuretic peptide is elevated in outpatients with atrial fibrillation. Am J Cardiol. 2003;92:1124–1127. doi: 10.1016/j.amjcard.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Iwanaga Y, Nishi I, Furuichi S, Noguchi T, Sase K, Kihara Y, Goto Y, Nonogi H. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol. 2006;47:742–748. doi: 10.1016/j.jacc.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 17.Greene SJ, Maggioni AP, Fonarow GC, Solomon SD, Bohm M, Kandra A, Prescott MF, Reimund B, Hua TA, Lesogor A, Zannad F, Gheorghiade M. Clinical profile and prognostic significance of natriuretic peptide trajectory following hospitalization for worsening chronic heart failure: findings from the ASTRONAUT trial. Eur J Heart Fail. 2015;17:98–108. doi: 10.1002/ejhf.201. [DOI] [PubMed] [Google Scholar]
- 18.Swedberg K, Olsson LG, Charlesworth A, Cleland J, Hanrath P, Komajda M, Metra M, Torp-Pedersen C, Poole-Wilson P. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long-term treatment with beta-blockers: results from COMET. Eur Heart J. 2005;26:1303–1308. doi: 10.1093/eurheartj/ehi166. [DOI] [PubMed] [Google Scholar]
- 19.Chamberlain AM, Redfield MM, Alonso A, Weston SA, Roger VL. Atrial fibrillation and mortality in heart failure: a community study. Circ Heart Fail. 2011;4:740–746. doi: 10.1161/CIRCHEARTFAILURE.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cullington D, Goode KM, Zhang J, Cleland JG, Clark AL. Is heart rate important for patients with heart failure in atrial fibrillation? JACC Heart Fail. 2014;2:213–220. doi: 10.1016/j.jchf.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Steinberg BA, Hellkamp AS, Lokhnygina Y, Patel MR, Breithardt G, Hankey GJ, Becker RC, Singer DE, Halperin JL, Hacke W, Nessel CC, Berkowitz SD, Mahaffey KW, Fox KA, Califf RM, Piccini JP. Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: results from the ROCKET-AF Trial. Eur Heart J. 2015;36:2882–2896. doi: 10.1093/eurheartj/ehu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gheorghiade M, Pang PS. Are BNP changes during hospitalization for heart failure a reliable surrogate for predicting the effects of therapies on post-discharge mortality? J Am Coll Cardiol. 2009;53:2349–2352. doi: 10.1016/j.jacc.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 23.Pitt B, Anker SD, Bohm M, Gheorghiade M, Kober L, Krum H, Maggioni AP, Ponikowski P, Voors AA, Zannad F, Nowack C, Kim SY, Pieper A, Kimmeskamp-Kirschbaum N, Filippatos G. Rationale and design of MinerAlocorticoid Receptor antagonist Tolerability Study-Heart Failure (ARTS-HF): a randomized study of finerenone vs. eplerenone in patients who have worsening chronic heart failure with diabetes and/or chronic kidney disease. Eur J Heart Fail. 2015;17:224–232. doi: 10.1002/ejhf.218. [DOI] [PubMed] [Google Scholar]
- 24.McKelvie RS, Yusuf S, Pericak D, Avezum A, Burns RJ, Probstfield J, Tsuyuki RT, White M, Rouleau J, Latini R, Maggioni A, Young J, Pogue J. Comparison of candesartan, enalapril, and their combination in congestive heart failure: randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. The RESOLVD Pilot Study Investigators. Circulation. 1999;100:1056–1064. doi: 10.1161/01.cir.100.10.1056. [DOI] [PubMed] [Google Scholar]
- 25.Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, Matsui T, Kinoshita M. Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol. 2001;37:1228–1233. doi: 10.1016/s0735-1097(01)01116-0. [DOI] [PubMed] [Google Scholar]
- 26.Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–385. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 27.Vaduganathan M, Greene SJ, Ambrosy AP, Gheorghiade M, Butler J. The disconnect between phase II and phase III trials of drugs for heart failure. Nat Rev Cardiol. 2013;10:85–97. doi: 10.1038/nrcardio.2012.181. [DOI] [PubMed] [Google Scholar]
- 28.Felker GM, Butler J, Collins SP, Cotter G, Davison BA, Ezekowitz JA, Filippatos G, Levy PD, Metra M, Ponikowski P, Soergel DG, Teerlink JR, Violin JD, Voors AA, Pang PS. Heart failure therapeutics on the basis of a biased ligand of the angiotensin-2 type 1 receptor. Rationale and design of the BLAST-AHF study (Biased Ligand of the Angiotensin Receptor Study in Acute Heart Failure) JACC Heart Fail. 2015;3:193–201. doi: 10.1016/j.jchf.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 30.Greene SJ, Gheorghiade M. Matching mechanism of death with mechanism of action: considerations for drug development for hospitalized heart failure. J Am Coll Cardiol. 2014;64:1599–1601. doi: 10.1016/j.jacc.2014.06.1199. [DOI] [PubMed] [Google Scholar]
- 31.Gheorghiade M, Pang PS, O’Connor CM, Prasad K, McMurray J, Teerlink JR, Fiuzat M, Sabbah H, Komajda M. Clinical development of pharmacologic agents for acute heart failure syndromes: a proposal for a mechanistic translational phase. Am Heart J. 2011;161:224–232. doi: 10.1016/j.ahj.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 32.Kao DP, Lewsey JD, Anand IS, Massie BM, Zile MR, Carson PE, McKelvie RS, Komajda M, McMurray JJ, Lindenfeld J. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail. 2015;17:925–935. doi: 10.1002/ejhf.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cleland JG, McMurray JJ, Kjekshus J, Cornel JH, Dunselman P, Fonseca C, Hjalmarson A, Korewicki J, Lindberg M, Ranjith N, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J. Plasma concentration of amino-terminal pro-brain natriuretic peptide in chronic heart failure: prediction of cardiovascular events and interaction with the effects of rosuvastatin: a report from CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure) J Am Coll Cardiol. 2009;54:1850–1859. doi: 10.1016/j.jacc.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 34.Rienstra M, Damman K, Mulder BA, Van Gelder IC, McMurray JJ, Van Veldhuisen DJ. Beta-blockers and outcome in heart failure and atrial fibrillation: a meta-analysis. JACC Heart Fail. 2013;1:21–28. doi: 10.1016/j.jchf.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JG, Lip GY, Coats AJ, Andersson B, Kirchhof P, von Lueder TG, Wedel H, Rosano G, Shibata MC, Rigby A, Flather MD. Efficacy of beta blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet. 2014;384:2235–2243. doi: 10.1016/S0140-6736(14)61373-8. [DOI] [PubMed] [Google Scholar]