Abstract
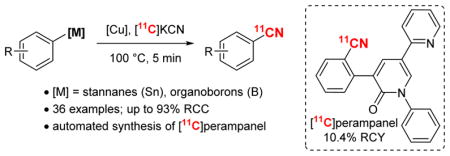
A copper-mediated method for the transformation of diverse arylboron compounds and arylstannanes to aryl–[11C]-nitriles is reported. This method is operationally simple, uses commercially available reagents, and is compatible with a wide variety of substituted aryl- and heteroaryl substrates. This method is applied to the automated synthesis of high specific activity [11C]perampanel in 10% non-decay corrected radiochemical yield.
Graphical Abstract
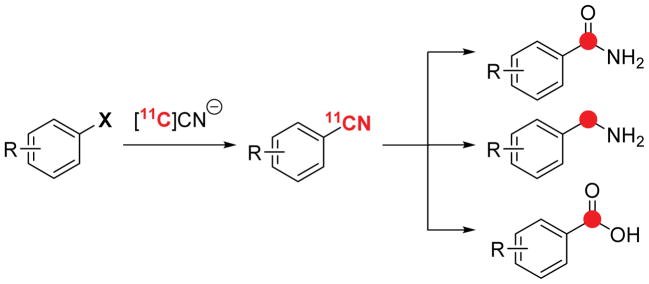
Carbon-11 is a radioisotope that is commonly used for positron emission tomography (PET) imaging.1 The introduction of carbon-11 into PET radiotracers is particularly challenging due to its very short half-life (t1/2 = 20 min).2 A number of methods have been developed for [11C]-radiolabeling, and some of the most attractive involve the late-stage introduction of a [11C]CN substituent (Scheme 1).3 [11C]Cyanide offers an advantage because it can be readily generated from [11C]CO2.1,2 Additionally, the nitrile functionality is common in bioactive molecules4 and can also be rapidly transformed into other important functional groups, including amides, carboxylic acids, and amines (Scheme 1).
Scheme 1.
Diversification of [11C]Nitrile Substrates.
There are currently two major methods for the [11C]cyanation of aromatic and heteroaromatic substrates. The first uses aryl halide precursors in combination with a Pd catalyst to engage [11C]cyanide in aryl–CN cross coupling.5–8 These reactions often provide high yields, exhibit broad scope, and proceed under mild conditions. However, Pd is relatively toxic;9 furthermore, the Pd-aryl intermediates and phosphine ligands required for these reactions can be challenging to handle, particularly in the context of automated radiochemical synthesis.10
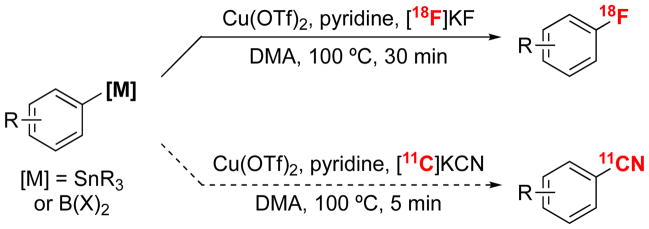
A second [11C]radiocyanation method involves the reaction of aryl halides with [11C]CuCN (i.e., the Rosenmundvon Braun reaction).11–13 This transformation offers the advantages of operational simplicity (i.e., no phosphine ligands, no requirement to preform organometallic intermediates, no need to remove palladium) and the relatively low toxicity of Cu.9 However, it suffers from low yields, modest scope, and forcing reaction conditions (often requiring temperatures of 150–250 °C).2 We sought to develop an alternative Cu-mediated [11C]radiocyanation that would leverage the advantages, while addressing the limitations of the existing Cu method. Our laboratory has recently reported that arylboron compounds and arylstannanes undergo Cu-mediated [18F]radiofluorination with [18F]KF.14–17 We hypothesized that changing the nucleophile from fluoride to cyanide under similar reaction conditions might enable a mechanistically analogous [11C]radiocyanation reaction (Scheme 2).18 We demonstrate here that the combination of Cu(OTf)2, pyridine, and [11C]KCN effectively promotes the [11C]radiocyanation of diverse aryl boronic acids, aryl boronate esters, aryl trifluoroborates, and arylstannanes under relatively mild conditions. We show that this transformation is compatible with a wide variety of functional groups and of aryl/heteroaryl substrates. Furthermore, it is readily translated to the automated synthesis of the radiotracer [11C]perampanel.19 Notably, while this work was underway, the team of Hooker, Vasdev, and Liang published a related method for the Cu-mediated [11C]cyanation of arylboronic acids.20
Scheme 2.
Inspiration from Cu-Mediated [18F]Fluorination of Arylorganometallics
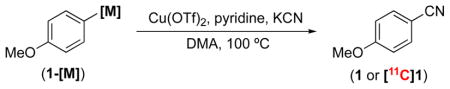
Our initial studies focused on establishing the feasibility of the Cu-mediated cyanation of aryl organometallic reagents under conditions analogous to those for our [18F]radiofluorination reactions.15 Using Cu(OTf)2 and pyridine in DMA at 100 °C, the reaction of 4-methoxyphenyl tributylstannane (1-SnBu3) with KCN afforded 4-methoxybenzonitrile (1) in 28% yield in 1 h, as determined by 1H NMR spectroscopy (Table 1, entry 1). Comparable results were obtained with the corresponding boronic acid (1-B(OH)2) and trifluoroborate (1-BF3K) substrates (32% and 26% yields, respectively), while the boronate ester afforded a lower yield (6%). However, the yield with the boronate ester could be increased to 21% by the addition of 1.2 equiv of KF. This additive likely promotes transmetalation via the formation of a borate intermediate. Notably, no fluorinated product was observed upon the addition of KF.
Table 1.
Initial results with KCN and optimization with [11C]KCN.
| |||
|---|---|---|---|
|
| |||
| entry | [M] | changes from standard | product (yield or RCC) |
| Results with KCNa | |||
| 1 | 1-SnBu3 | none | 1 (28%) |
| 2 | 1-B(OH)2 | none | 1 (32%) |
| 3 | 1-BF3K | none | 1 (26%) |
| 4 | 1-Bpin | none | 1 (6%) |
| 5 | 1-Bpin | KF (1.2 equiv) | 1 (21%) |
| Results with [11C]KCNb | |||
| 6 | 1-SnBu3 | [11C]KCN in DMA | [11C]1 (42%) |
| 7 | 1-SnBu3 | DMA prep, H2Oc | [11C]1 (41%) |
| 8 | 1-SnBu3 | none | [11C]1 (66%) |
| 9 | 1-B(OH)2 | none | [11C]1 (79%) |
| 10 | 1-BF3K | none | [11C]1 (93%) |
| 11 | 1-Bpin | none | [11C]1 (76%) |
Standard conditions: substrate (10 μmol, 1 equiv), Cu(OTf)2 (2 equiv), pyridine (15 equiv), DMA (1 mL, 10 mM).
KCN (2 equiv), 100 °C, 1 h. Yield determined by 1H NMR spectroscopy with 1,3,5-trifluorobenzene as an internal standard.
[11C]KCN in H2O, 100 °C, 5 min. Reported values indicate radiochemical conversion (RCC) of determined by radio-TLC (n ≥ 2).
H2O (0.2 mL, 17% v/v).
We next translated this method to [11C]radiocyanation using anhydrous [11C]KCN. Gratifyingly, the product ([11C]1) was formed in 42% RCC, as determined by radio-TLC with identity confirmed by radio-HPLC (entry 6). Subsequent studies revealed that anhydrous conditions (which were essential for the analogous [18F]radiofluorination with [18F]KF) are not required for [11C]radiocyanation. For example, a comparable 41% RCC was obtained upon the addition of exogenous water (0.2 mL, 17% v/v) to the anhydrously prepared [11C]KCN reaction mixture. Furthermore, an even higher yield (66% RCC, entry 8) was observed when the [11C]KCN was prepared and used directly as an aqueous solution. This modification decreased the overall synthesis time by 4 min (approximately 20% of the 11C half-life). The improved RCC under these conditions is likely due to increased solubility of [11C]KCN.
Using this aqueous [11C]KCN preparation, we next evaluated the radiocyanation of other aryl organometallic substrates. As summarized in Table 1, entries 8–11, various arylboron compounds afforded comparable and/or improved RCCs relative to their stannane counterpart, with the trifluoroborate (1-BF3K) giving the best result (93% RCC). Notably, KF was not required for the radiocyanation of arylboronate ester (1-Bpin), likely due to the decreased amount of KCN available in the reactions.
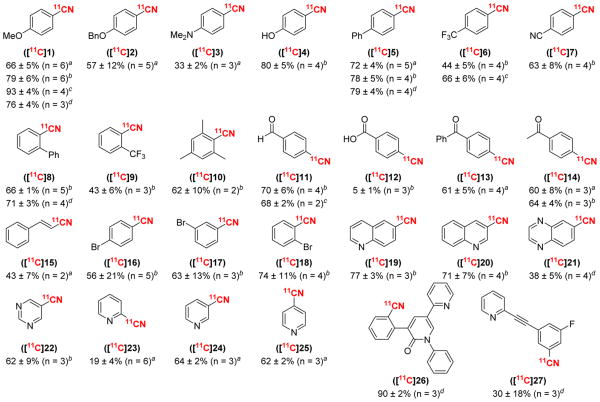
The scope of this transformation was further evaluated using a variety of organoboron and organostannane substrates (denoted by the footnotes in Figure 1). These studies showed that electron-donating ([11C]1–4), -neutral ([11C]5), and -withdrawing ([11C]6–7) substituents on the aromatic ring are all well-tolerated. Ortho-substituted substrates underwent [11C]radiocyanation in comparable yields to their unsubstituted counterparts (compare [11C]8–10).20 In addition, carbonyl groups ([11C]11–14) were compatible with the reaction conditions.19 Significantly, precursors containing unprotected benzoic acid ([11C]12) and phenol ([11C]4) substituents also afforded modest to excellent yields. Aryl bromides were tolerated at various sites around the phenyl ring ([11C]16–18), and could serve as handles for further elaboration of the products. Pyridine derivatives and related nitrogen heterocycles also underwent [11C]radiocyanation in moderate to high yields ([11C]19–25).
Figure 1.
Substrate Scope. Reported values indicate radiochemical conversion (RCC) of determined by radio-TLC (n ≥ 2). General conditions: substrate (0.01 mmol, 1 equiv), Cu(OTf)2 (2 equiv), pyridine (15 equiv), [11C]KCN in H2O (0.1 mL), DMA (10 mM), 100 °C, 5 min. [M] = aSnBu3, bB(OH)2, cBF3K, dBpin.
Overall, the scope of this transformation is broader, and many of the RCCs are higher than those of previously reported methods for the [11C]radiocyanation of aromatic substrates.8,20 As an example, our method affords quinoline product [11C]20 in 71% RCC from 20-B(OH)2. For comparison, recently reported Cu-mediated [11C]radiocyanation conditions provide 18% RCC for the same substrate,20 while a Pd-mediated method affords 46% RCC from the analogous aryl bromide.8 Notably, subjecting 20-B(OH)2 to our related Cu-mediated fluorination affords only trace amounts of product (<10% 19F NMR yield),21 demonstrating that this cyanation reaction also has improved scope relative to fluorination. Finally, it is noteworthy that a number of the products in Figure 1 have not been labeled with [11C]nitrile before, including [11C]3–4, 6, 8–9, 12, 14–15, 17–19, 22, 25, and 27.
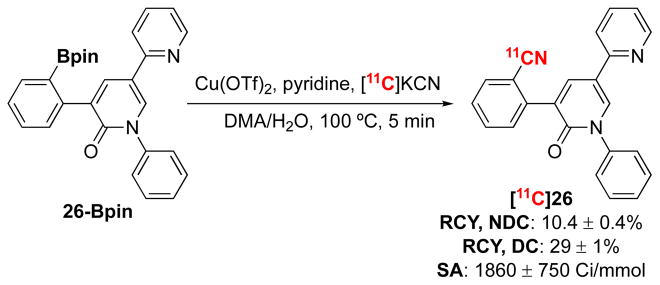
As a final demonstration of this method, we pursued the automated, clinical-scale synthesis of [11C]perampanel. Perampanel, an FDA-approved drug for epilepsy, has been [11C]radiolabeled once before using a Pd-mediated method with an aryl bromide precursor to afford [11C]26 in 40% RCC (manual, radio-TLC) and 9.7% RCY (isolated, non-decay corrected).8 Our manual method provided 90 ± 2% RCC of [11C]26 from the arylboronate ester using approximately 1 mCi of [11C]KCN per reaction. The synthesis was scaled to 450 mCi of [11C]KCN, and the [11C]radiocyanation and subsequent HPLC purification of [11C]26 were conducted using an automated radiosynthesis module. Without further optimization, this procedure afforded [11C]26 in 10.4 ± 0.4% non-decay corrected radiochemical yield (RCY; Scheme 3). The fully automated synthesis lasted approximately 32 min from the end of bombardment.
Scheme 3.
Automation of [11C]Perampanel.
General conditions: 26-Bpin (0.01 mmol, 1 equiv), Cu(OTf)2 (2 equiv), pyridine (15 equiv), [11C]KCN, DMA, 100 °C, 5 min. Radiochemical yield (RCY) determined by isolated material after preparative-HPLC (n = 2). QC was performed to confirm the correct product was formed and purify was >95%. NDC = non-decay corrected. DC = decay corrected. SA = specific activity.
In conclusion, this letter describes a Cu-mediated [11C]radiocyanation of diverse aryl organometallic reagents. This method is compatible with a wide range of substrates, including those containing carboxylic acids, phenols, aldehydes, and heterocycles. This method is also amenable to automation on a clinically relevant scale, as demonstrated in the synthesis of [11C]perampanel.
Supplementary Material
Acknowledgments
We acknowledge NIH R01EB021155 (MSS and PJHS) and China Scholarship Council No. 201607060008 (LY and PJHS) for financial support.
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website.
Optimization details, experimental procedures, radio-HPLC/TLC traces, and complete characterization for all new compounds (PDF)
References
- 1.Ametamey SM, Honer M, Schubiger PA. Chem Rev. 2008;108:1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 2.Miller PW, Long NJ, Vilar R, Gee AD. Angew Chem Int Ed. 2008;47:8998–9033. doi: 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- 3.Miller PW, Kato K, Långström B. In: The Chemistry of Molecular Imaging. Long N, Wong W-T, editors. John Wiley & Sons, Inc; 2014. pp. 79–103. [Google Scholar]
- 4.Yan G, Zhang Y, Wang J. Adv Synth Catal. 2017;359:4068–4105. [Google Scholar]
- 5.Andersson Y, Malmborg P, Långström B. J J Label Compd Radiopharm. 1991;30:144–145. [Google Scholar]
- 6.Andersson Y, Långström B. J Chem Soc Perkin Trans. 1;1994:1395–1400. [Google Scholar]
- 7.Andersson Y, Bergström M, Långström B. Appl Radiat Isot. 1994;45:707–714. doi: 10.1016/0969-8043(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 8.Lee HG, Milner PJ, Placzek MS, Buchwald SL, Hooker JM. J Am Chem Soc. 2015;137:648–651. doi: 10.1021/ja512115s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.According to ICH Guideline 3QD: Guideline for Elemental Impurities, Pd has a permitted daily exposure (PDE) of 10 μg/day in parenteral drugs, considerably lower than Cu (PDE = 340 μg/day) and Sn (PDE = 640 μg/day). Due to the very low PDE for Pd, more time and specialized equipment is needed to ensure residual Pd levels in radiotracer doses are below the allowable limit. (http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q3D/Q3D_Step_4.pdf, accessed 18-Feb-2018).
- 10.Such Pd species are often not air stable,6,8 and this needs to be accounted for in radiotracer synthesis. For example, Andersson added Pd to the reaction at the last possible moment to prevent loss in RCY due to oxidation of Pd.6 However, this is not possible when working with multi-Curie production scale levels of 11C in automated synthesis modules that are set up in advance of delivery of 11C to the hot-cell.
- 11.Ponchant M, Hinnen F, Demphel S, Crouzel C. Appl Radiat Isot. 1997;48:755–762. [Google Scholar]
- 12.Siméon F, Sobrio F, Gourand F, Barré L. J Chem Soc Perkin Trans 1. 2001:690–694. [Google Scholar]
- 13.Mathews WB, Monn JA, Ravert HT, Holt DP, Schoepp DD, Dannals RF. J Label Compd Radiopharm. 2006;49:829–834. [Google Scholar]
- 14.Mossine AV, Brooks AF, Makaravage KJ, Miller JM, Ichiishi N, Sanford MS, Scott PJH. Org Lett. 2015;17:5780–5783. doi: 10.1021/acs.orglett.5b02875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makaravage KJ, Brooks AF, Mossine AV, Sanford MS, Scott PJH. Org Lett. 2016;18:5440–5443. doi: 10.1021/acs.orglett.6b02911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z, Zhang C, Lau J, Colpo N, Bénard F, Lin KS. J Label Compd Radiopharm. 2016;59:467–471. doi: 10.1002/jlcr.3436. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Lau J, Kuo HT, Zhang C, Colpo N, Benard F, Lin KS. Bioorg Med Chem Lett. 2017;27:2094–2098. doi: 10.1016/j.bmcl.2017.03.078. [DOI] [PubMed] [Google Scholar]
- 18.Schimler SD, Sanford MS. Synlett. 2016;27:2279–2284. [Google Scholar]
- 19.The co-injection with the authentic product showed that the major peak was the desired product. Uncharacterized side products were noticed in some of the HPLC traces
- 20.Ma L, Placzek MS, Hooker JM, Vasdev N, Liang SH. Chem Commun. 2017;53:6597–6600. doi: 10.1039/c7cc02886e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schimler S. PhD Dissertation. University of Michigan; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.