Abstract
Background
Respiratory tract infections (RTIs) are the major causes of mortality and morbidity in children and lead to hospitalization in developing countries. However, little is known about the epidemiology and seasonality of respiratory viruses in the pediatric population in Wuxi, East China.
Material/Methods
We included all patients 14 years of age and below who presented with signs and symptoms of RTIs between January 2010 and December 2016. During this period, a total of 2160 children treated in Wuxi No. 2 People’s Hospital were involved in our study. The clinical and sociodemographic data were recorded to describe the frequency and seasonality. Respiratory specimens were tested by multiplex real-time PCR assays for virus identification.
Results
More than 30% (35.19%, 760 samples) of the specimens showed evidence of infection with viruses, including respiratory syncytial virus (368 samples), influenza virus A (114 samples), influenza virus B (115 samples), parainfluenza virus I (29 samples), parainfluenza virus II (39 samples), parainfluenza virus III (13 samples), and adenovirus (82 samples); 48.99% of the children infected with viruses were under 12 months of age. Viruses were detected throughout all the year, with a peak in winter.
Conclusions
Our study found that RSV is the most important cause of RTIs in our region during winter. Our data provide a comprehensive understanding of the epidemiology and seasonality of virus, which may help to reduce the use of antibiotics and implement an effective approach for prevention, control, and treatment of RTIs, especially during its peak season.
MeSH Keywords: Epidemiology, Pediatrics, Respiratory Tract Infections
Background
Respiratory tract infections (RTI) are a major public health issue in both developed and developing countries, causing nearly 19% of all deaths among children under 5 years and 8.2% of all disability and premature mortality [1–4]. Especially in developing countries, RTIs-induced mortality is high. RTIs are mainly caused by viruses, and the most commonly detected viruses among children with RTIs are respiratory syncytial virus (RSV), influenza virus A (FA), influenza virus B (FB), parainfluenza viruses, and adenoviruses [5,6]. Most infections are limited to the upper respiratory tract and only 5% involve the lower respiratory tract [7]. RTIs are associated with a greater risk of pneumonia and bronchiolitis [8].
Due to lack of vaccines for most of these respiratory viruses, a better understanding of the prevalence of RTIs in children is essential for implementing an effective approach for prevention, control, and treatment. The distribution of respiratory viruses causing RTIs varies based on population, climate, and socioeconomic conditions [9,10]. In China, the prevalence of respiratory viruses has been investigated in Beijing [11], Shanghai [12], Shenzhen [13], and Guangzhou [14], but the epidemiology of respiratory viruses in children with RTIs in other parts of China has not been reported, and there are no reliable population-based data from this region to account for the large numbers of related deaths in children [2].
Wuxi is a major city in Eastern China with a population of 6.5 million people in 2014. It lies on the southern border of Jiangsu province, about 128 kilometers (79.5 miles) northwest of Shanghai, with a typical subtropical monsoon climate. However, limited data are available on the epidemiology of respiratory viruses causing RTIs from children in our region. Our study is the first to assess the epidemiology and seasonality of respiratory viruses in the pediatric population in East China.
Material and Methods
Patients and specimens
The study protocol was approved by the Institutional Review Board of Wuxi No. 2 People’s Hospital and the study was conducted in accordance with the principles for biomedical human research as set by the Declaration of Helsinki. Written consent was obtained from the parents or guardians of the children.
There were 2160 specimens taken from children (≤14-years-old) tested between 1 January 2010 and 31 December 2016 in Wuxi. Selected patients with RTIs admitted to the pediatric wards were enrolled. The inclusion criteria were: cough, hoarseness of voice, and sore throat, combined with a body temperature above 38°C. The nasal and throat swabs (NTS) were analyzed at the Laboratory of Medical Microbiology within 2 h. The patients were divided into 3 age groups: 0–1, 2–3, and 4–14 years. The following underlying conditions in medical records were recorded: bronchitis, pneumonia, asthmatic bronchitis, and other respiratory diseases. The seasons were defined according to the seasonal division method in the northern hemisphere: March to May was considered as spring, June to August was considered as summer, September to November was considered as autumn, and December to February was considered as winter.
Isolation of RNA
For RNA isolation, a Maxwell 16 Total Viral Nucleic Acid Purification Kit (Promega Corporation, Madison, WI) was used according to the manufacturer’s instructions and the isolate was eluted in 50 μL of RNase-free water.
Conventional Multiplex RT-PCR [15]
To confirm the viruses, the PCR used was a multiplex real-time PCR assay and the RV12 ACE detection kit (Seegene; Seoul, South Korea) was used according to the manufacturer’s instructions. Respiratory syncytial virus (RSV), influenza virus A (FA), influenza virus B (FB), parainfluenza virus I (PIV I), parainfluenza virus II (PIV II), parainfluenza virus III (PIV III), and adenovirus (ADV) were assessed. Random hexamer-primed cDNA synthesis products were generated using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific; Carlsband, CA). Briefly, parallel 20-μL reactions were set up, each containing RV12 mastermix, 8-MOPS contamination control reagent, and 3 μL cDNA. One of each pair was supplemented with 4-mL primer mix A, and the other with 4-mL primer mix B. Thermal cycling conditions were as follows: 15 min at 95°C, followed by 30 cycles of 95°C for 30 s, 60°C for 90 s, and 72°C for 90 s, followed by a single incubation of 10 min at 72°C. Afterward, amplicons were detected by gel electrophoresis.
Statistical analysis
Statistical analysis was performed using SPSS 13. Comparisons of categorical variables among groups were performed using chi-square or Fisher exact test. P<0.05 was considered statistically significant.
Results
Characteristics of the study population
From January 2010 to December 2016, a total of 2160 samples from patients who presented with RTIs were investigated in the study. The median age of our population was 4 years old. The specimens were collected from 1167 males (54.03%) and 993 females (45.97%). Among the studied population, 684 were outpatients and 1476 were inpatients. The demographics of our study population are summarized in Table 1.
Table 1.
Demographic data and respiratory virus-positive patients.
| Case Characteristic | Yr | Total (n=2160) | Respiratory viruses detected (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2010 (n=284) | 2011 (n=296) | 2012 (n=318) | 2013 (n=335) | 2014 (n=318) | 2015 (n=272) | 2016 (n=337) | |||
| Sex | |||||||||
| Male | 150 | 161 | 169 | 172 | 178 | 147 | 190 | 1167 | 420 (35.99%) |
| Female | 134 | 135 | 149 | 163 | 140 | 125 | 147 | 993 | 340 (34.24%) |
| Age, yr | |||||||||
| 0–<1 | 74 | 65 | 82 | 85 | 78 | 55 | 57 | 496 | 243 (48.99%) |
| 2–<3 | 92 | 97 | 105 | 116 | 101 | 89 | 109 | 709 | 206 (29.06%) |
| 4–<14 | 118 | 134 | 131 | 134 | 139 | 128 | 171 | 955 | 311 (32.57%) |
| Settings | |||||||||
| Outpatient | 95 | 96 | 97 | 104 | 101 | 93 | 98 | 684 | 164 (21.97%) |
| Inpatient | 189 | 200 | 221 | 231 | 217 | 179 | 239 | 1476 | 596 (78.42%) |
| Respiratory viruses detected (%) | 91 (32.04%) | 111 (37.5%) | 111 (34.91%) | 133 (39.7%) | 109 (34.28%) | 89 (32.72%) | 116 (34.42%) | 760 (35.19%) | |
Prevalence of respiratory viruses
The presence of the viruses was confirmed in 35.19% of cases, among which RSV predominated. Of the positive samples, RSV was associated with 370 cases that accounted for 17.04% of RTIs and 48.42% of the total viruses. Other respiratory viruses detected were FA (114 samples, 5.28%), FB (115 samples, 5.32%), PIV I (29 samples, 1.34%), PIV II (39 samples, 1.81%), PIV III (13 samples, 0.60%), and ADV (82 samples, 3.8%) (Table 2). Co-infection with respiratory viruses was identified in 36 children. Co-infection of FA and ADV or ADV and FB were detected in 12 samples, while co-infection of RSV and PIV II or PIV II and PIV III were detected in 6 samples (Figure 1). There were no triple or more co-infections. ADV was the most frequently found viral agent in co-infections (66.7%, 24/36) and they were seen in children aged 3–14 years old (66.7%, 24/36). All co-infection samples were from female patients.
Table 2.
Detection rates of the 7 respiratory viruses among 3 different age groups.
| Age group (Yr) | Total | Detection rate among all patients (%) | Detection rate among virus-positive patients (%) | |||
|---|---|---|---|---|---|---|
| 0–11 | 2–32** | 4–143* | ||||
| FA | 22 | 22 | 70 | 114 | 15.0% | 5.28% |
| FB | 8 | 5 | 102 | 115 | 15.13% | 5.32% |
| PIV I | 0 | 10 | 19 | 29 | 3.82% | 1.34% |
| PIV II | 15 | 5 | 19 | 39 | 5.13% | 1.81% |
| PIV III | 0 | 0 | 13 | 13 | 1.71% | 0.60% |
| ADV | 15 | 29 | 38 | 82 | 10.79% | 3.80% |
| RSV | 183 | 135 | 50 | 368 | 48.42% | 17.04% |
| Respiratory viruses detected (%) | 243 (48.99%) | 206 (29.06%) | 311 (32.57%) | 760 (35.19%) | ||
| Total cases | 496 | 709 | 955 | 2160 | ||
Group 1 vs. Group 2 (P<0.01);
Group 1 vs. Group 3 (P<0.05).
RSV – respiratory syncytial virus; FA – influenza virus A; FB – influenza virus B; PIV I – parainfluenza virus I; PIV II – parainfluenza virus II; PIV III – parainfluenza virus III; ADV – adenovirus.
Figure 1.
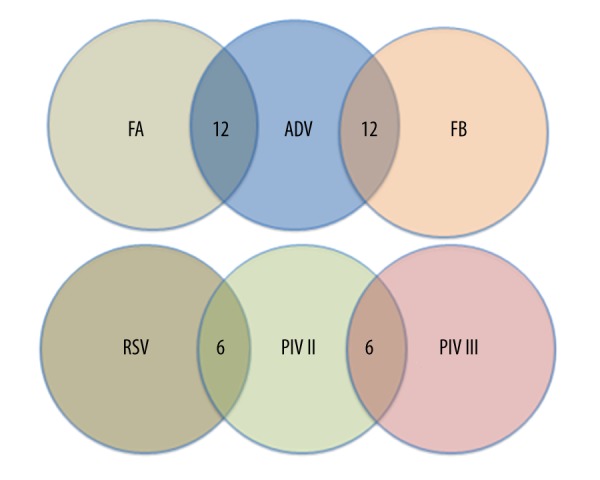
Co-infections with the 7 respiratory viruses. RSV – respiratory syncytial virus; FA – influenza virus A; FB – influenza virus B; PIV II – parainfluenza virus II; PIV III – parainfluenza virus III; ADV – adenovirus
Relationship between respiratory viruses and age
The highest detection rate for viral respiratory pathogens was observed in the 0–1 years group (243/496; 48.99%), followed by the 3–14 years group (311/955; 32.57%) and the 1–3 years group (206/709; 29.06%). RSV was the most frequently found respiratory virus affecting all age groups. In addition, FB diagnoses were made during outbreaks of various intensities that occurred in the 3–14 years group (Table 2).
Seasonal variation of different respiratory viruses
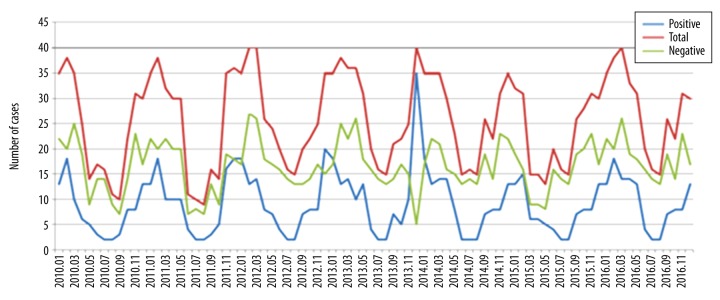
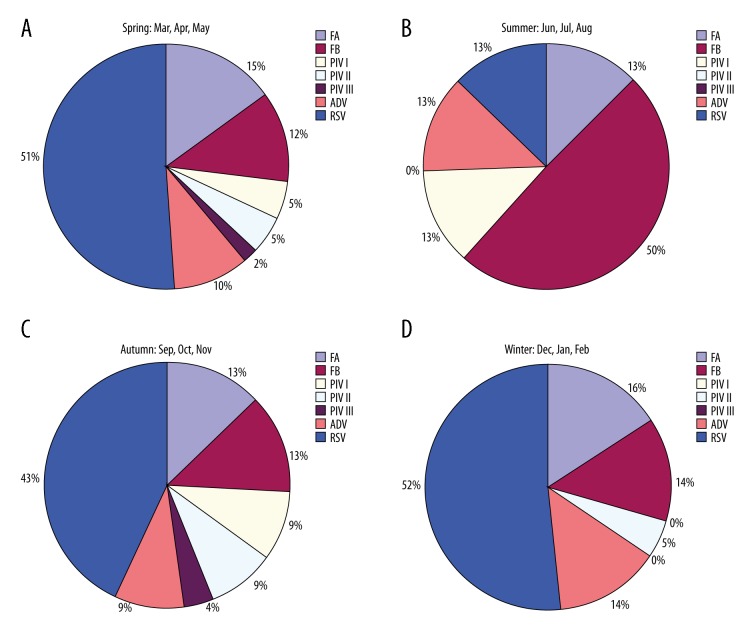
Overall detection rates for respiratory viruses varied by year, ranging from 32.04% in 2010 to 39.7% in 2013 (Table 1). During the study period, viruses were detected throughout the year, with a peak in winter (Figure 2). The most common respiratory viruses detected were RSV (368 samples). We also analyzed the seasonal variation of each respiratory virus (Figure 3). For PIV III, the detection rate was 2% in spring and 4% in autumn, but they were not detected in summer and winter. For FB, the detection rate was higher in summer than in spring, autumn, and winter.
Figure 2.
The monthly distribution and frequency of overall virus detection during 2010–2016.
Figure 3.
Seasonal distribution of 7 respiratory viruses. RSV – respiratory syncytial virus; FA – influenza virus A; FB – influenza virus B; PIV I – parainfluenza virus I; PIV II – parainfluenza virus II; PIV III – parainfluenza virus III; ADV – adenovirus.
Detection rates of respiratory viruses with different respiratory tract diseases
Infection with respiratory viruses was associated with bronchitis, asthmatic bronchitis, bronchiolitis, and pneumonia (Table 3). Of all the respiratory virus-related diseases, pneumonia was associated with 334 cases, accounting for 43.95% of RTIs. Other related diseases were bronchitis (16.32%) and bronchiolitis (18.16%). RSV was the most common pathogen causing pneumonia (170 cases).
Table 3.
Viral detection rates among the different respiratory diseases.
| Bronchitis | Bronchiolitis | Pneumonia | Asthmatic bronchitis | Acute tonsillitis | Others | |
|---|---|---|---|---|---|---|
| FA | 13 | 6 | 46 | 7 | 13 | 26 |
| FB | 32 | 0 | 59 | 13 | 0 | 13 |
| PIV I | 0 | 0 | 20 | 13 | 0 | 0 |
| PIV II | 7 | 7 | 26 | 0 | 0 | 0 |
| PIV III | 0 | 7 | 0 | 6 | 0 | 0 |
| ADV | 39 | 0 | 13 | 7 | 6 | 20 |
| RSV | 33 | 118 | 170 | 33 | 7 | 0 |
| Total (n=760) | 124 | 138 | 334 | 79 | 26 | 59 |
RSV – respiratory syncytial virus; FA – influenza virus A; FB – influenza virus B; PIV I – parainfluenza virus I; PIV II – parainfluenza virus II; PIV III – parainfluenza virus III; ADV – adenovirus.
Discussion
RTIs cause a major health care burden and are associated with high morbidity and mortality, especially in developing countries [16]. Although bacteria and fungi can cause respiratory infections, viruses contribute to a higher proportion of infections in infants and children. Therefore, identifying the prevalence of respiratory viruses is essential to prevention, control, and treatment of RTIs, especially during peak season. Our study aimed to evaluate the epidemiology and seasonality of respiratory viruses in the pediatric population with RTIs from January 2010 to December 2016 in Wuxi, East China.
More than 30% (35.19%, 760 samples) of the specimens showed evidence of infection with viruses. RSV was the most common virus detected, with the highest detection rate in children (48.42%). Our data suggest that RSV was the most common pathogen causing pneumonia (170 cases). The prevalence of RSV infections depends on the age of patients, and may be up to 50% in infants [17–19], which indicates that infants are more vulnerable. Thus, children aged 0–1 years should be the major targets to prevent RSV infection. In addition, the ADV detection rate was 3.8% in this study, which was higher than in another study [12]. However, detection rates of FA, FB, and PIV viruses were lower than those in other studies [20,21]. These discrepancies might be caused by differences in population, geographic area, and the socioeconomic status.
A declining infection rate in all viruses was observed with increasing age of children. Specifically, more children in the 1–3 years group appear to have had protective immunity against respiratory viruses than those in the 0–1 years group. However, a higher infection rate was observed in the 3–14 years group. This could be due to the exposure of children in kindergarten and primary schools, where children may have daily contact with other children who have viral infections. Children of this age may have immunity, but antibodies may be insufficient to provide protection against reinfections or infections that can occur in different virus strains. Dual viral infections occurred in a small number of children, which is similar to a previous study in Victoria, Australia [22].
Studies have found associations between respiratory viruses and climate [23]. During the study period, respiratory viruses were detected throughout the year. Detection of respiratory viruses followed a seasonal distribution similar to previous reports in which a temporary peak was observed in winter. Since 2010, a wave of respiratory viruses has occurred every winter. Cold and rainy weather in Wuxi, located in East China, has created the perfect conditions for the replication of respiratory viruses. In 2013, the greatest increase in viral infections, in comparison with previous periods, was detected from children, which may be attributed to extremely cold winter during this period. The research in Changsha, located in the south-central part of China, showed that RSV infection was prevalent in late autumn and winter, but not in spring or summer [24]. However, a study in Guangzhou, located in southern China, found that the highest detection rate of viruses was in spring and the lowest rate was in winter [25]. These conflicting results may be due to geographical differences.
Conclusions
This is the first report to describe the epidemiology of respiratory virus infections in Wuxi, China, including the association of respiratory viruses and age, seasonal variation of respiratory viruses, and respiratory virus-related diseases. In our region, RSV is an important cause of RTIs during winter in children who are 12 months or younger. Our findings provide data for assessing the influence of respiratory virus infections from children in Wuxi, which could help to reduce the use of antibiotics and implement an effective approach for prevention, control, and treatment of RTIs, especially during peak season.
Acknowledgements
We would like to thank Fred Bogott, M.D., Ph.D., at the Austin Medical Center, Mayo Clinic at Austin, Minnesota, USA, for his excellent English editing of the manuscript.
Footnotes
Source of support: This work was supported by the Wuxi Health Department (FYKY201409)
Conflict of interest
None.
References
- 1.Leowski J. Mortality from acute respiratory infections in children under 5 years of age: Global estimates. World Health Stat Q. 1986;39:138–44. [PubMed] [Google Scholar]
- 2.Shann F, Woolcock A, Black R, et al. Introduction: Acute respiratory tract infections – the forgotten pandemic. Clin Infect Dis. 1999;28:189–91. doi: 10.1086/515107. [DOI] [PubMed] [Google Scholar]
- 3.Williams BG, Gouws E, Boschipinto C, et al. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2:25–32. doi: 10.1016/s1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 4.Morikawa S, Hiroi S, Kase T. Detection of respiratory viruses in gargle specimens of healthy children. J Clin Virol. 2015;64:59–63. doi: 10.1016/j.jcv.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulino RDS, Benega MA, Santos KCDO, et al. Differential diagnosis of respiratory viruses by using real time RT-PCR methodology. Rev Inst Med Trop Sao Paulo. 2013;55:432. doi: 10.1590/S0036-46652013000600012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwofie TB, Anane YA, Nkrumah B, et al. Respiratory viruses in children hospitalized for acute lower respiratory tract infection in Ghana. Virol J. 2012;9:78. doi: 10.1186/1743-422X-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yousif TK, Khaleq BA. Epidemiology of acute respiratory tract infections (ARI) among children under five years old attending tikirit general teaching hospital. Middle East J Fam Med. 2006;4:4–23. [Google Scholar]
- 8.Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835–45. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perezruiz M, Pedrosacorral I, Sanbonmatsugamez S, Navarromari J. Laboratory detection of respiratory viruses by automated techniques. Open Virol J. 2012;6:151–59. doi: 10.2174/1874357901206010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noh JY, Song JY, Cheong HJ, et al. Laboratory surveillance of influenza-like illness in seven teaching hospitals, South Korea: 2011–2012 season. PLoS One. 2013;8:e64295. doi: 10.1371/journal.pone.0064295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren L, Gonzalez R, Wang Z, et al. Prevalence of human respiratory viruses in adults with acute respiratory tract infections in Beijing, 2005–2007. Clin Microbiol Infect. 2009;15:1146–53. doi: 10.1111/j.1469-0691.2009.02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge WZ, Feng XU, Zhao ZH, et al. Association between diurnal temperature range and respiratory tract infections. Biomed Environ Sci. 2013;26:222–25. doi: 10.3967/0895-3988.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Zheng Y, Deng J, et al. Prevalence of respiratory viruses among children hospitalized from respiratory infections in Shenzhen, China. Virol J. 2016;13:39. doi: 10.1186/s12985-016-0493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Chen D, Liu Q, et al. Detection of human bocavirus from children and adults with acute respiratory tract illness in Guangzhou, southern China. BMC Infect Dis. 2011;11:345. doi: 10.1186/1471-2334-11-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alayed MSZ, Asaad AM, Qureshi MA, Ameen MS. Viral etiology of respiratory infections in children in southwestern Saudi Arabia using multiplex reverse-transcriptase polymerase chain reaction. Saudi Med J. 2014;35:1348–53. [PMC free article] [PubMed] [Google Scholar]
- 16.Gooskens J, Der Ploeg VV, Sukhai RN, et al. Clinical evaluation of viral acute respiratory tract infections in children presenting to the emergency department of a tertiary referral hospital in the Netherlands. BMC Pediatr. 2014;14:297. doi: 10.1186/s12887-014-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baraldi E, Lanari M, Manzoni P, et al. Inter-society consensus document on treatment and prevention of bronchiolitis in newborns and infants. Ital J Pediatr. 2014;40:65. doi: 10.1186/1824-7288-40-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mejias A, Dimo B, Suarez NM, et al. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med. 2013;10:e1001549. doi: 10.1371/journal.pmed.1001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicolai A, Nenna R, Stefanelli P, et al. Bordetella pertussis in infants hospitalized for acute respiratory symptoms remains a concern. BMC Infect Dis. 2013;13:526. doi: 10.1186/1471-2334-13-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuypers J, Wright N, Ferrenberg J, et al. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006;44:2382–88. doi: 10.1128/JCM.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright PF, Deatly AM, Karron RA, et al. Comparison of results of detection of rhinovirus by PCR and viral culture in human nasal wash specimens from subjects with and without clinical symptoms of respiratory illness. J Clin Microbiol. 2007;45:2126–29. doi: 10.1128/JCM.02553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Druce J, Tran T, Kelly H, et al. Laboratory diagnosis and surveillance of human respiratory viruses by PCR in Victoria, Australia, 2002–2003. J Med Virol. 2005;75:122–29. doi: 10.1002/jmv.20246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shatizadeh S, Yavarian J, Rezaie F, et al. Epidemiological and clinical evaluation of children with respiratory virus infections. Med J Islam Repub Iran. 2014;28:102. [PMC free article] [PubMed] [Google Scholar]
- 24.Hatipoğlu N, Somer A, Badur S, et al. Viral etiology in hospitalized children with acute lower respiratory tract infection. Turkish J Pediatr. 2011;53:508–16. [PubMed] [Google Scholar]
- 25.Zhang D, He Z, Xu L, et al. Epidemiology characteristics of respiratory viruses found in children and adults with respiratory tract infections in southern China. Int J Infect Dis. 2014;25:159–64. doi: 10.1016/j.ijid.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]