Abstract
Cytoplasmic protein O-glycosylation in bacteria is often required for protein maturation, but the dependence of protein export on carbohydrate modifications is less understood. In the current issue of JBC, Chen et al. describe the mechanism for posttranslational modification of a Streptococcus gordonii adhesin and its delivery to the membrane, leading to the first comprehensive model featuring the interplay of glycosyltransferases and the translocation system.
Keywords: bacteria, glycobiology, post-translational modification (PTM), membrane transport, adhesin
Introduction
Protein N- and O-glycosylation occurs in all domains of life (1, 2) and is frequently key to protein function. Studies of bacterial glycosylation pathways can provide simplified enzymatic or regulatory systems to develop mechanistic frameworks and inform our understanding of basic microbial processes such as biofilm formation. Additionally, the finding that numerous pathogenic bacteria glycosylate their proteins has provided a strong biomedical motivation for in-depth research in the last decade. Glycosylation often occurs early in a protein's lifetime and can affect folding, maturation, and trafficking. However, too often these processes are studied in isolation, limiting our understanding of the cross-talk between pathways such as glycosylation and export. The new study by Chen et al. (3) helps to fill this gap, uncovering both the glycosylation pathway of a bacterial adhesion protein and the influence of these glycans on the first steps of protein secretion. This is important because linking these two processes ensures that correct and functional glycoforms are expressed on the cell surface of streptococci.
Bacterial adhesion to host cells and extracellular matrix is mediated by a family of cell wall proteins known as serine-rich repeat (SRR)2 glycoproteins in Gram-positive bacteria (4, 5). These SSR glycoproteins use a dedicated export system, the accessory secretion (Sec) system (6), which contains two homologues of the general Sec pathway (SecA2 and SecY2). The operon containing SecA2 and SecY2 also includes glycosyltransferases and other essential proteins (Asp1 to Asp5) that do not share homology to any protein of known function (7). It is known that these glycosyltransferases, named Nss and Gly in the pathogenic bacterium Streptococcus gordonii, are important for modification of the SRR adhesin GspB and that they act sequentially also in two other Streptococcus species after the general cytosolic O-glycosyltransferase GtfA/B modifies the adhesin's Ser/Thr-rich repeats (7). It is also known that Asp2 is a bifunctional protein required for the transport of the GspB and for its posttranslational modification (8) and that Asp2 has a putative catalytic region possessing a Ser-Asp-His catalytic triad. It has been speculated that the catalytic domain might be responsible for controlling GspB glycosylation, whereas the remainder might be required for glycoprotein transport (6). However, the full role of Asp2, much less than the other Asp proteins, is unclear.
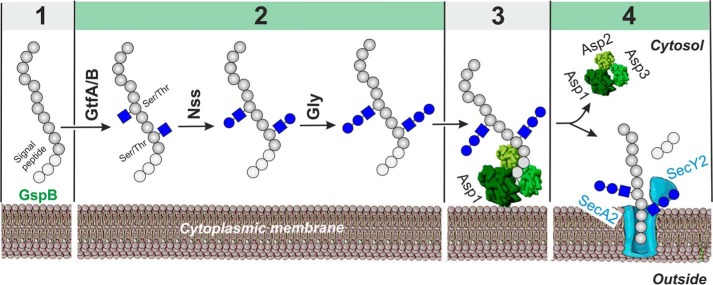
Now, Chen et al. (3) combine in vitro and cellular enzymatic assays with biophysical analysis, structural biology and membrane mimetics to develop a model for the first steps in the export of S. gordonii GspB, in which glycosylation plays a key role (Fig. 1). The authors demonstrate that the adhesin is sequentially modified by three glycosyltransferases, with GftA/B adding an GlcNAc residue to Ser/Thr residues in SRR, Nss transferring a glucose to the GlcNAc residues, and Gly adding further glucose residues. The authors also discover that Gly is an unusual enzyme, showing affinity for its own reaction product. This is explained by the enzyme's multiple domains: The reaction is catalyzed by a GT-D domain, whereas binding to the product is mediated by catalytically inactive GT-A and -B domains. Although the substrate affinity of these domains is high, the fully glycosylated GspB dissociates from Gly in the presence of the Asp1–3 complex. But what is the role of the mysterious Asp proteins, and how does glycosylation mediate this process?
Figure 1.
Scheme of S. gordonii GpsB adhesin O-glycosylation and export. GpsB is glycosylated at multiple Ser/Thr sites through the sequential activities of the glycosyltransferases GtfA/B, Nss, and Gly and then transferred to the Asp1–3 complex in which Asp1 mediates the interaction with the lipid bilayer for targeting the glycoprotein to the SecA2/SecY2 export machinery (3). Sugar residues are shown according to the Symbol Nomenclature for Graphical Representation of Glycans (10). 1, protein production; 2, glycosylation; 3, membrane targeting; 4, export and signal peptide cleavage.
To answer these questions, the authors next solved the structures of Asp1 and the Asp1/Asp3 complex, which indicate the proteins are likely carbohydrate binding proteins, where Asp1 appears to be a catalytically inactive member of the GT-B family of glycosyltransferases and Asp3 is similar to the carbohydrate binding module of glycosidases. This explains how Asp1 and Asp3 could be binding to glycosylated adhesin and mediating release from Gly. The crystal structures of Asp1 and Asp3, combined with trypsin digests and EM data of the Asp1–3 complex, further helped the authors to construct a model for the intact assembly. The results confirm that Asp2 sits at the open end of the Asp1/3 complex and that Asp1 has affinity for negatively charged phospholipids typical of the streptococci lipid bilayer, which facilitates the recruitment of substrate to liposomes in co-flotation experiments.
In the canonical Sec translocon, SecA helps to recruit substrates to the SecY channel, and the signal sequence of GspB and the adjacent “accessory Sec transport” domain could help to target the glycoprotein to the accessory Sec system and initiate translocation in a similar fashion. However, the new data from Chen et al. (3) suggest the Asps could facilitate the transfer of glycosylated GspB to the membrane, with the handoff mediated by the known interaction between the Asp complex and SecA2 (6). Indeed, previous efforts to purify soluble SecA2 were unsuccessful, even in the presence of detergent, suggesting that SecA2 requires a lipid environment to maintain its native conformation and that it is permanently bound to the membrane (5). This is supported by a homology model showing SecA2 has a pronounced positively charged surface patch, which could mediate its interaction with negatively charged phospholipids in the membrane (5). Thus, the Asp complex could serve as a soluble SecA substitute in the accessory Sec system. Once substrate has been recruited to the SecA2/SecY2 complex it is likely translocated across the membrane by a mechanism similar to that of the SecA1 canonical system (6).
Altogether, this study describes the sequence of cytosolic reactions in the export of GspB adhesin of S. gordonii (3), representing an example of an uncommon protein glycosylation system in bacteria without involvement of a lipid carrier (9). The requirement for the coupling of modification and export may explain the co-evolution of the SRR glycoproteins with their specialized glycan modifying and export systems. Still, the described system holds some secrets, such as how the handoff of the GspB glycoprotein to the Sec complex functions.
Considering that SRR adhesins have been linked to virulence for a variety of infections, including streptococcal endocarditis, the suggested model may pinpoint future targets for innovative antimicrobials.
- SRR
- serine-rich repeat
- Sec
- secretion.
References
- 1. Schäffer C., and Messner P. (2017) Emerging facets of prokaryotic glycosylation. FEMS Microbiol. Rev. 41, 49–91 10.1093/femsre/fuw036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Varki A., Cummings R. D., Esko J. D., Stanley P., and Hart G. W. (2017) Essentials of Glycobiology, 3rd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York: [PubMed] [Google Scholar]
- 3. Chen Y., Bensing B. A., Seepersaud R., Mi W., Liao M., Jeffrey P. D., Shajahan A., Sonon R. N., Azadi P., Sullam P. M., and Rapoport T. A. (2018) Unraveling the sequence of cytosolic reactions in the export of GspB adhesin from Streptococcus gordonii. J. Biol. Chem. 293, 5360–5373 10.1074/jbc.RA117.000963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou M., and Wu H. (2009) Glycosylation and biogenesis of a family of serine-rich bacterial adhesins. Microbiology 155, 317–327 10.1099/mic.0.025221-0 [DOI] [PubMed] [Google Scholar]
- 5. Seepersaud R., Bensing B. A., Yen Y. T., and Sullam P. M. (2012) The accessory Sec protein Asp2 modulates GlcNAc deposition onto the serine-rich repeat glycoprotein GspB. J. Bacteriol. 194, 5564–5575 10.1128/JB.01000-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park E., and Rapoport T. A. (2012) Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu. Rev. Biophys. 41, 21–40 10.1146/annurev-biophys-050511-102312 [DOI] [PubMed] [Google Scholar]
- 7. Chen Y., Seepersaud R., Bensing B. A., Sullam P. M., and Rapoport T. A. (2016) Mechanism of a cytosolic O-glycosyltransferase essential for the synthesis of a bacterial adhesion protein. Proc. Natl. Acad. Sci. U.S.A. 113, E1190–E1199 10.1073/pnas.1600494113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seepersaud R., Sychantha D., Bensing B. A., Clarke A. J., and Sullam P. M. (2017) O-acetylation of the serine-rich repeat glycoprotein GspB is coordinated with accessory Sec transport. PLoS Pathog. 13, e1006558 10.1371/journal.ppat.1006558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwarz F., Fan Y. Y., Schubert M., and Aebi M. (2011) Cytoplasmic N-glycosyltransferase of Actinobacillus pleuropneumoniae is an inverting enzyme and recognizes the NX(S/T) consensus sequence. J. Biol. Chem. 286, 35267–32274 10.1074/jbc.M111.277160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varki A., Cummings R. D., Aebi M., Packer N. H., Seeberger P. H., Esko J. D., Stanley P., Hart G., Darvill A., Kinoshita T., Prestegard J. J., Schnaar R. L., Freeze H. H., Marth J. D., Bertozzi C. R., Etzler M. E., Frank M., Vliegenthart J. F., Lütteke T., Perez S., Bolton E., Rudd P., Paulson J., Kanehisa M., Toukach P., Aoki-Kinoshita K. F., Dell A., Narimatsu H., York W., Taniguchi N., and Kornfeld S. (2015) Symbol nomenclature for graphical representations of glycans. Glycobiology 25, 1323–1324 10.1093/glycob/cwv091 [DOI] [PMC free article] [PubMed] [Google Scholar]