Abstract
Background
CD19-chimericantigen receptor (CAR) modified T cells (CD19-CAR T cells) have been well documented to possess potent anti-tumor properties against CD19-expressingleukemia cells. As a traditional medicine, metformin has been widely used to treat type II diabetes mellitus and more recently has become a candidate for the treatment of cancer. However, no report has revealed the direct effect of metformin on CD19-CAR T cell biological function and its underling mechanisms.
Purpose
The purpose of this research was to explore the effect of metformin on CD19-CAR T cell biological function and the mechanisms involved.
Methods
CD19-CAR T cells proliferation, apoptosis and cytotoxicity were mainly tested by CCK-8 assay, flow cytometry and ELISA. The detection of mechanism primarily used western blot. Bioluminescence imaging is the main application technology of animal studies.
Results
In the current study, it was found that metformin inhibited CD19-CAR T cell proliferation and cytotoxicity and induced apoptosis. Furthermore, our study revealed that metformin activated AMPK and suppressed mTOR and HIF1α expression. By using an AMPK inhibitor, compound C, we demonstrated the crucial roles of AMPK in CD19-CAR T cells when they were treated with metformin. Finally, we verified that metformin suppressed the cytotoxicity of CD19-CAR T cell in vivo.
Conclusion
Taken together, these results indicated that metformin may play an important role in modulating CD19-CAR T cell biological functions in an AMPK-dependent and mTOR/HIF1α-independent manner.
Keywords: Chimeric antigen receptor, metformin, proliferation, apoptosis, cytotoxicity, AMPK
Introduction
Cancer immunotherapy has emerged as a hopeful approach in cancer treatment for the weak side effect and favorable applicability.1 Recently, chimeric antigen receptor (CAR)-modified T cells has been proven to be a promising strategy in cancer therapy.2 Among them, T cells genetically modified with CD19 CARs which specifically bind to normal and malignant B lymphocytes but not to other normal myeloid cells, have been well documented to possess potent antitumor property against CD19-expressing leukemia cells.3,4 Recent studies in clinical trials have reported that the objective tumor response in patients with acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), and multiple myeloma (MM) after infusion with CD19-CAR T cells.5–7 However, various outcomes were found in different clinical trials. These various outcomes might be due to various procedures such as the design of CAR structure, transfect methods, T-cell source and culture conditions, and the dosage of CAR T cells.8 Until now, how to improve the persistence and efficiency is still a challenge for the application of CD19-CAR T cells. However, the crucial factor in regulating the efficiency as well as its potential mechanism still remains unclear.
In recent years, glucose metabolism is gaining increased attention in type II diabetes mellitus and cardiovascular disease, especially in cancers.9–11 Moreover, a study has demonstrated that glucose uptake and metabolism is a key cellular transcription factor in T-cell function, such as activation, proliferation, and differentiation.12 As a traditional medicine, metformin has been widely used in type II diabetes mellitus for over 50 years. It has long been demonstrated in inhibiting glucose production, increasing insulin sensitivity, and reducing insulin secretion by β-pancreatic cells.13,14 Intriguingly, recent reports have focused on the relationship between metformin and cancer. For example, some studies have established the direct suppressive effect of metformin on breast and pancreatic cancer cells.15,16 Further-more, studies have provided evidences that diabetic patients receiving metformin have a reduced risk of cancer progress and mortality.17,18 Meanwhile, metformin has been shown to affect the glycolytic metabolism of immunocytes.19,20 A study further showed the regulatory role of glycolytic metabolism on T-cell proliferation and effective function.21 These findings implied the close relationship between glycolytic metabolism and biological functions of CAR T cells. However, no evidences have evaluated whether the regulatory role of glycolytic metabolism by metformin on T cells could be affected when genetically modified with CD19-CARs, as well as its underlying mechanisms.
It is well established that AMP-activated protein kinase (AMPK) played a key role in maintaining multifunctionality of immunocytes under low nutrient conditions by regulating glycolytic metabolism.20,22 A study also found that mTOR, the downstream kinase of AMPK, functions as a main regulator of glucose metabolism in activated lymphocytes.23 Furthermore, mTOR induction by glycolysis is modulated through the activation of HIF1α.24 Therefore, the AMPK/mTOR/HIF1α pathway is an overt signal pathway to reflect T-cell glycometabolism.
Thus, the aims of the current study were to evaluate the effect of metformin on the biological functions of CD19-CAR T cells and explore the mechanisms involved.
Materials and methods
Cell lines and reagents
The Raji, K562, and 293FT cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The Raji cells were routinely cultured in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific), 100 U/mL penicillin, and 100 μg/mL streptomycin in 5% CO2 at 37°C. Raji-ffluc cells for bioluminescent imaging were generated by transfecting Raji cells with an expression cassette for firefly luciferase. The K562 cells were cultured in Iscove’s modified Dulbecco’s medium (Thermo Fisher Scientific) containing 10% FBS (Thermo Fisher Scientific), 100 U/mL penicillin, and 100 μg/mL streptomycin. 293FT cells were maintained in Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific) supplemented with 10% FBS. All cells were cultured at in 5% CO2 at 37°C. Metformin was purchased from Sigma-Aldrich Co. (St Louis, MO, USA) and then dissolved in PBS. Dorsomorphin (Compound C), a potent AMPK inhibitor, was purchased from Selleck (Houston, TX, USA) and then dissolved in dimethyl sulfoxide.
Construction of CD19-CAR
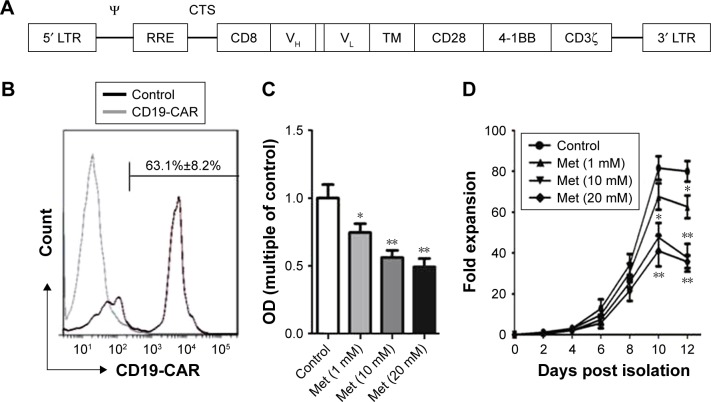
CD19-CAR consists of CD19-binding single-chain fragment variable (scFv), human CD8a molecule transmembrane region and intracellular signaling domains of CD28, CD137, and CD3zeta. The CD19 scFv was obtained from GenBank (No NG_009286.1). The anti-CD19 scFv-CD28-4-1BB-CD3zeta CAR (Figure 1A) was combined with PGK lentivirus vector (Addgene, Cambridge, MA, USA) backbone, in which the CAR sequences took precedence over in-frame by a green fluorescent protein sequence.
Figure 1.
Metformin inhibits CD19-CAR T-cell proliferation. (A) Schematic of construct of CD19-targeted CAR. (B) Transfection efficiency of CD19-CAR T cells. The expression of CD19-targeted CAR was detected by flow cytometry. (C) CD19-CAR T cells were treated with metformin (0, 1, 10, and 20 mM) for 24 hours. The proliferation of CD19-CAR T cells was detected by the CCK-8 assay. (D) Growth curves of CD19-CAR T cells. CD19-CAR T cells treated with metformin (0, 1, 10, and 20 mM) were counted by using a hemocytometer over 12 days of incubation *P<0.05, **P<0.01 versus the control group. The results are represent as mean ± SD from three independent experiments.
Abbreviations: CAR, chimeric antigen receptor; Met, metformin; LTR, long terminal repeat; CTS, Cis-acting termination zone; RRE, rev-response element; VH, variable region of heavy chain; VL, variable region of light chain; TM, transmembrane; OD, optical density.
Lentivirus preparation
293FT cells were used to produce high-titer lentivirus. Briefly, once 293FT cells were 80% confluence, lentiviral vector plasmid, ps-PAX2 packaging plasmid, and pMD2.G envelope plasmid with 4:3:1 ratio were mixed in Opti-MEM culture medium (Thermo Fisher Scientific). Three plasmids blending with lipofectamine 2000 (Thermo Fisher Scientific) were evenly dripped into cultured 293FT cells. Then, the supernatants were collected 60 hours after transfection and preserved in −80°C.
Isolation and cultivation of human primary peripheral blood T cells
Peripheral blood mononuclear cells (PBMCs) were obtained from healthy donors’ whole blood which is checked by routine blood examination. All experiments using healthy donors’ peripheral blood were approved by the Second Hospital of Shandong University Ethics Committee. Donors had signed informed consents. Ficoll density gradient centrifugation was used for PBMC isolation. Then we used CD3+ T-Cell Isolation Kit (Miltenyi Biotec, Teterow, Germany) to obtain purified T cells. The purified T cells were cultured in complete medium including X-VIVO™ 15 serum-free medium (Lonza, Morristown, NJ, USA), 300 U/mL Interleukin-2 (IL-2; PeproTech, Rocky Hill, NJ, USA), CD3/CD28 Dynabeads (Thermo Fisher Scientific) were used for T-cell activation and proliferation. Then CD3/CD28 Dynabeads were removed for the following experiments.
Lentiviral transduction of human primary peripheral blood T cells
After activated for 24 hours, the T cells were transduced in 24-well plates coated with 2 μg/cm2 of retronectin (TaKaRa, Kusatsu, Japan). Briefly, viral supernatants were added into each retronectin-coated wells and centrifuged for 2 hours at 32°C at 3,000 rpm. The viral supernatants were then discarded and the activated T cells were added to relevant wells centrifuged at the same conditions. About 8 μg/mL polybrene (Sigma-Aldrich Co.) was appended into T-cell supernatants. T cells were cultured in 5% CO2 at 37°C.
Flow cytometry
Flow cytometry was performed as previously described.25 Briefly, cells were washed in phosphate-buffered saline (PBS). Then, they were incubated with fluorescent antibodies reactive to CD3-PE (BD Biosciences, San Jose, CA, USA) at room temperature for 20 minutes. Isotype immunoglobulin Gs were used as negative controls to assess the background fluorescence. Flow cytometry analyses were performed using BD LSRFortessa™ (BD Biosciences), and data were analyzed by FlowJo software (FlowJo, Ashland, OR, USA).
Cytotoxicity assays
CD19-CAR T cells were cocultured with Raji or K562 cells at varying rations for 24 hours. Then the cells were gathered for flow cytometry. CD3-PE (BD Biosciences) antibody was used to stain T cells and 7-AAD (Biolegend, San Diego, CA, USA) was applied for staining apoptotic cells. Keep the cells away from light at room temperature for 20 minutes. Then the cells were analyzed by BD LSRFortessa™ (BD Biosciences), and data were analyzed by FlowJo software (FlowJo).
Cytokine detection assays
CD19-CAR T cells were cocultured with Raji or K562 cells at varying rations for 24 hours. Then the culture supernatants were collected and used for cytokine detection assays. IL-2 and interferon-γ (INF-γ) cytokines were quantified using ELISA kits (Biolegend, San Diego, CA, USA) according to the manufacturer’s instructions. The absorbance at 450 nm was determined using a Microplate Reader (Thermo Fisher Scientific).
Cell apoptosis assay
Cell apoptosis assay was performed as previously described.26 Briefly, CD19-CAR T cells treated with or without metformin were stained with Annexin V-APC and PI (Biolegend) according to the manufacturer’s instructions. Cells were incubated at room temperature for 20 minutes and then analyzed by BD LSRFortessa™ (BD Biosciences), and data were analyzed by FlowJo software (FlowJo).
Western blot analysis
Western blot analysis was performed as previously described.26 The primary antibodies included mTOR, phos-pho-mTOR, AMPKα, phospho-AMPKα, HIF1α antibodies (Cell Signaling Technology, Boston, MA, USA) and β-actin (Sigma-Aldrich Co.).
Animal studies
Six- to eight-week-old male NSG mice purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd were injected via tail vein with 1×106 Raji-ffluc cells. Three days later, 5 mg/mL metformin was dissolved in the drinking water and drank abidingly. The control group drank drinking water. At the same time, each mouse was engrafted with 1×106 CD19-CAR T cells intravenously. For bioluminescence imaging, mice were anesthetized and imaged using a Xenogen IVIS Imaging System (Perkin Elmer Life Sciences, Hopkinton, MA, USA). Bioluminescence imaging was performed on days 3, 10, and 18 posttumor injection. Data were disposed by Living Image® Software, Caliper Life Sciences. The animal study was approved by the Animal Experiment Center of the Second Hospital of Shandong University and followed the Regulations on the management of experimental animals.
Statistical analysis
The data are expressed as mean ± standard deviation (SD). All the experiments were repeated at least three times. Student’s t-test was used for comparing differences of different groups. Comparisons among values of multiple groups were performed by one-way analysis of variance. Survival was compared by a log-rank test. Differences were considered to be significant at P<0.05.
Results
Metformin inhibits CD19-CAR T cell proliferation
The lentiviral expression vectors encoding the CD19-targeted CARs include CD19 scFv, human CD8a molecule transmembrane region, and intracellular signaling domains of CD28, 4-1BB, and CD3zeta and enhanced green fluorescent protein (eGFP) using the “self-cleaving” F2A peptide (Figure 1A). By using flow cytometry, we detected that the anti-CD19-CAR can be expressed efficiently on the T-cell surface. The transduction efficiency was about 63.1%±8.2% (Figure 1B). Based on these results, the CD19-CAR T cells can be used for the following experiments. To evaluate the effect of metformin on CD19-CAR T cells, the CCK-8 assay was used to detect CD19-CAR T-cell proliferation. As shown in Figure 1C, the proliferative ability of CD19-CAR T cells was significantly suppressed by metformin (1, 10, and 20 mM) in a dose-dependent manner. To assess the changes in the actual cell numbers, the effect of metformin on CD19-CAR T-cell proliferation was detected by direct cell counting. As shown in Figure 1D, we found that 1 mM metformin restrained CD19-CAR T-cell proliferation and 10 and 20 mM metformin had greater inhibitive effects. These results indicated that metformin inhibited CD19-CAR T-cell proliferation.
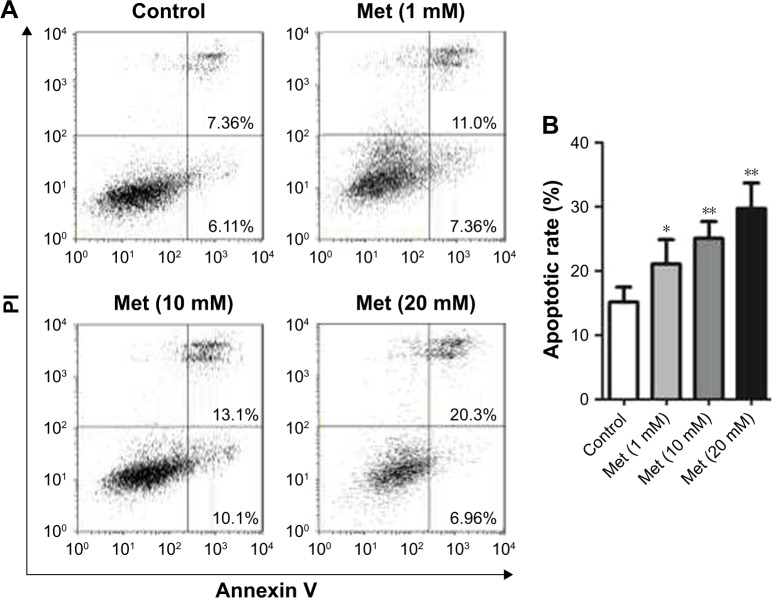
Metformin induces CD19-CAR T-cell apoptosis
To determine whether metformin has a direct effect on CD19-CAR T-cell apoptosis, the cells were incubated with metformin (1, 10, and 20 mM) for 24 hours. As shown in flow cytometry results, metformin (1, 10, and 20 mM) effectively induced CD19-CAR T-cell apoptosis (Figure 2A and B). These data demonstrated the pro-apoptotic role of metformin on CD19-CAR T cells.
Figure 2.
Metformin induces CD19-CAR T-cell apoptosis. (A) CD19-CAR T cells were treated with metformin (0, 1, 10, and 20 mM) for 24 hours. The apoptotic rate of CD19-CAR T cells was detected by flow cytometry. (B) Statistical analysis of the flow cytometry results. The results are represented as mean ± SD from three independent experiments. *P<0.05, **P<0.01 versus the control group.
Abbreviations: CAR, chimeric antigen receptor; Met, metformin; PI, propidium iodide.
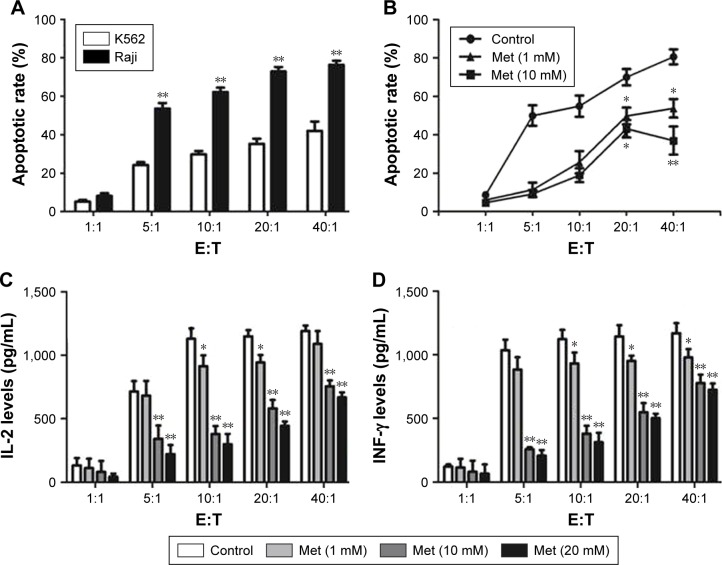
Cytotoxicity of CD19-CAR T cells was inhibited by metformin
To verify the effect of metformin on CD19-CAR T-cell cytotoxicity, CD19-CAR T cells were cocultured with CD19+ Raji cells or CD19− K562 cells for 24 hours. The effector to target cell ratio (E:T) was from 1:1 to 40:1. As shown in Figure 3A, compared to CD19− K562 cells, the cytotoxic effect of CD19-CAR T cells was significantly activated when cocultured with CD19+ Raji cells at various E:T. Then, met-formin was introduced in the cocultured CD19-CAR T cells and CD19+ Raji cells. As shown in Figure 3B, we found that metformin significantly inhibited the cytotoxicity of CD19-CAR T cells at various E:T. Furthermore, the IL-2 and INF-γ release were detected by using ELISA assay. Our data showed that the secretion of IL-2 and INF-γ at various E:T was significantly reduced when treated with metformin (Figure 3C and D). Collectively, these data indicated that metformin inhibited the cytotoxic activity of CD19-CAR T cells.
Figure 3.
Cytotoxicity of CD19-CAR T cells was inhibited by metformin. (A) CD19-CAR T cells were cocultured with Raji and K562 cell lines at various E:T (1:1, 5:1, 10:1, 20:1, and 40:1) for 24 hours. The apoptotic rate was determined by flow cytometry. (B) CD19-CAR T cells were cocultured with Raji cell lines with 0, 1, and 10 mM metformin at various E:T (1:1, 5:1, 10:1, 20:1, and 40:1) for 24 hours. The apoptotic rate was determined by flow cytometry. (C) CD19-CAR T cells were cocultured with Raji cell lines with metformin (0, 1, 10, and 20 mM) at various E:T (1:1, 5:1, 10:1, 20:1, and 40:1) for 24 hours, and the supernatant was obtained from the cocultured system. The IL-2 secretion was detected by ELISA. (D) CD19-CAR T cells were cocultured with Raji cell lines with metformin (0, 1, 10, and 20 mM) at various E:T (1:1, 5:1, 10:1, 20:1, and 40:1) for 24 hours, and the supernatant was obtained from the cocultured system. INF-γ secretion were detected by ELISA. The bar graph represents the results of three independent experiments, *P<0.05, **P<0.01 versus the control group.
Abbreviations: CAR, chimeric antigen receptor; Met, metformin; IL, interleukin; INF, interferon; E:T, effector to target cell ratio.
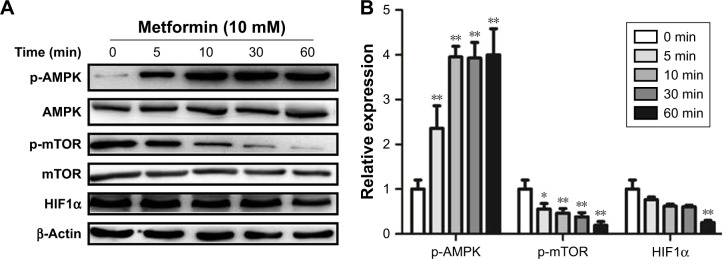
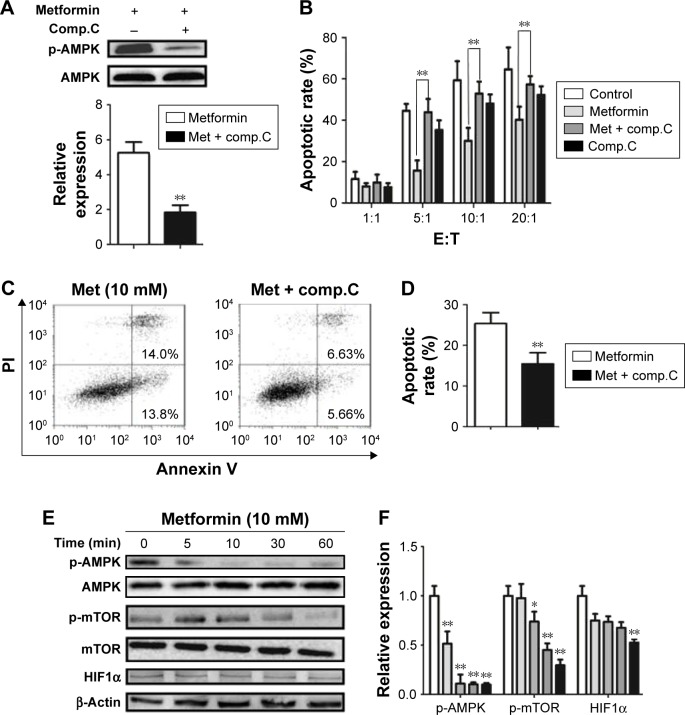
Metformin promotes AMPK phosphorylation and suppresses mTOR and HIF1α expression in CD19-CAR T cells
It is well documented that AMPK/mTOR/HIF1α signal pathway play a critical role in metformin-modulated lymphocytes energetic metabolism.20,24 To further explore the latent mechanism about metformin on CD19-CAR T cells, the AMPK, mTOR, and HIF1α signaling pathway was examined. As shown in Figure 4A, the AMPK phosphorylation was upregulated when treated with 10 mM metformin, whereas mTOR phosphorylation was downregulated. Meanwhile, metformin also downregulated the expression of HIF1α. The regulation was statistically significant, as quantified by densitometry (Figure 4B). These results indicated that AMPK/mTOR/HIF1α signal pathway may take part in the metformin-modulated glucose metabolism of CD19-CAR T cells.
Figure 4.
Metformin increases the expression of p-AMPK and reduces the expression of p-mTOR and HIF1α inCD19-CAR T cells. (A) The CD19-CAR T cells were treated by 10 mM metformin for different times (0, 5, 10, 30, and 60 minutes), and the expression of p-AMPK, p-mTOR, and HIF1α was detected by Western blotting analysis. (B) Statistical analysis of relative p-AMPK, p-mTOR, and HIF1α protein expression results. *P<0.05, **P<0.01 versus the control group. Data are represented as mean ± SD from three independent experiments.
Abbreviations: CAR, chimeric antigen receptor; p-AMPK, phosphor AMP-activated protein kinase.
AMPK pathway is involved in metformin-regulated CD19-CAR T-cell apoptosis and cytotoxicity
To further delineate whether the role of metformin on CD19-CAR T cells was AMPK-dependent, compound C, a potent AMPK inhibitor, was applied to block AMPK phosphorylation (Figure 5A). As shown in Figure 5B–D, inhibition of AMPK phosphorylation with compound C reversed the metformin-modulated cytotoxicity and apoptosis in CD19-CAR T cells, whereas the metformin-suppressed mTOR and HIF1α expression was not affected by compound C (Figure 5E and F). Taken together, these results strengthen the opinion that AMPK pathway is involved in metformin-modulated biological functions of CD19-CAR T cells.
Figure 5.
The AMPK inhibitor reverses the metformin-regulated apoptosis and cytotoxicity of CD19-CAR T cells but does not affect mTOR and HIF1α expression. (A) CD19-CAR T cells were treated with 10 mM metformin and/or compound C for 24 hours. The expression of p-AMPK was analyzed by Western blotting (up) and the statistical analysis of relative p-AMPK protein expression results is shown (down). (B) CD19-CAR T cells were cocultured with Raji cells with 10 mM metformin and/or compound C at various effector to target cell ratios (1:1, 5:1, 10:1, 20:1, and 40:1) for 24 hours, and the apoptotic rate was determined by flow cytometry. (C) CD19-CAR T cells were treated with 10 mM metformin and/or compound C for 24 hours, and the apoptotic rate of CD19-CAR T cells was detected by flow cytometry. (D) Statistical analysis of the flow cytometry results. (E) CD19-CAR T cells were treated with 10 mM metformin and/or compound C at different times (0, 5, 10, 30, and 60 minutes), and the expression of p-AMPK, p-mTOR, and HIF1α was analyzed by Western blotting analysis. (F) Statistical analysis of the Western blot results. *P<0.05, **P<0.01 versus the control group. Data are represented as mean ± SD from three independent experiments.
Abbreviations: p-AMPK, phosphor AMP-activated protein kinase; CAR, chimeric antigen receptor; Met, metformin; Comp, compound; E:T, effector to target cell ratio; PI, propidium iodide.
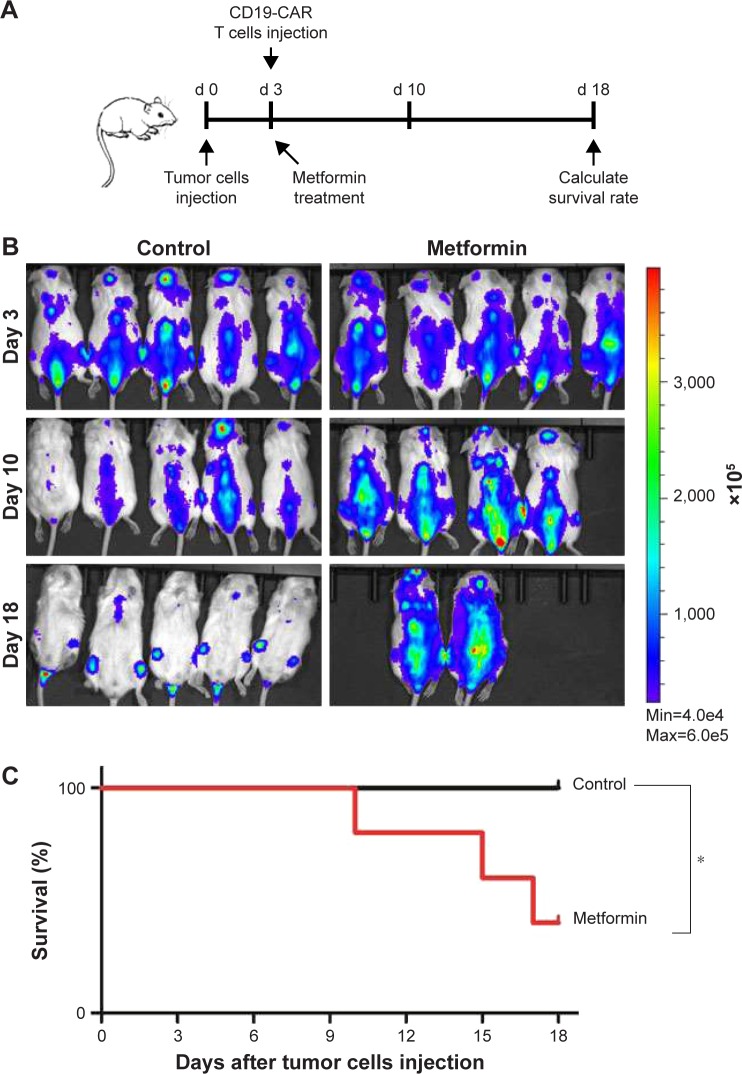
Metformin suppresses the cytotoxicity effect of CD19-CAR T cells in vivo
Having shown the effect of metformin on CD19-CAR T cells in vitro, next we were interested in determining whether metformin had the same role on suppressing the cytotoxicity of CD19-CAR T cells in vivo. NSG mice were engrafted with Raji-ffluc cells intravenously 3 days prior to CD19-CAR T-cell injection (Figure 6A). The data show that metformin treatment exhibits negative effect on the cytotoxicity of CD19-CAR T cells. Three of 5 mice in metformin treatment group showed tumor progression leading to their sacrifice at days 10, 15, and 17, respectively. The other two mice had large clusters of tumor cells but not sacrificed. However, tumor cells in the control group mice were mostly eliminated, and all mice survived at day 18 (Figure 6B). The survival rate of mice was reduced by the treatment of metformin (Figure 6C). Strikingly, the data demonstrated that metformin plays suppressant role on CD19-CAR T-cell cytotoxicity in vivo.
Figure 6.
Metformin suppresses the effect of CD19-CAR T-cell cytotoxicity in vivo. (A) The schematic diagram of the progression of experiment in vivo. (B) Bioluminescence imaging data from Raji-ffluc bearing NSG mice (N=5) that had received 1×106 CD19-CAR T cells and drug treatment. NSG mice (N=5) that received drinking water were used as control. Mice were monitored for 18 days via luminescence imaging to follow tumor progression. (C) Survival curve of mice receiving metformin or no drug treatment. P-values were calculated by log-rank test analysis. *P<0.05, versus the control group. Results represent one independent trial.
Abbreviations: CAR, chimeric antigen receptor; d, days; min, minimum; max, maximum.
Discussion
The present study documented several new findings about metformin. Metformin can inhibit the proliferation and cytotoxicity of CD19-CAR T cells. Metformin can also induce apoptosis of CD19-CAR T cells. Metformin acts on CD19-CAR T cells through AMPK signaling pathway. In vivo, cytotoxicity of CD19-CAR T cells was restrained by metformin.
In recent years, CD19-CAR T cells have shown valid outcomes in B-cell malignancies.3,4 However, the response rate of CD19-CAR T cells varied in different B-cell malignancies.5–7 Thus, seeking effective drugs to regulate the persistence and efficiency of CD19-CAR T cells and exploring its mechanisms are crucial issues in cancer treatment.
Metformin is frequently used in type II diabetes mellitus treatment for over 50 years. Recent studies have focused on the glucose metabolism in cancers.9–11 Many preclinical and clinical studies have been reported that metformin treatment reduced cancer incidence in type II diabetes patients. For example, Evans et al reported the relationship between metformin usage and cancer incidence in patients with type II diabetes mellitus.27 Also, a meta-analysis revealed that diabetic patients with metformin treatment tend to have a lower incidence of cancer.28 Further studies demonstrated that metformin reduced the risk of various cancers such as colorectal, breast, ovarian, prostate, and endometrial cancer.29–32 Moreover, a study has demonstrated that glucose uptake and metabolism is a key cellular transcription factor in T-cell functions, such as activation, proliferation, and differentiation.12 Although previous studies reported the regulatory effect of metformin, the role of metformin on the biological functions of CD19-CAR T cells as well as its detailed molecular mechanisms has not been clearly elucidated. In the current study, we explored the role of metformin on the proliferation, apoptosis, and cytotoxicity in CD19-CAR T cells. Our research revealed that metformin could significantly suppress the proliferation and cytotoxicity and induce its apoptosis in CD19-CAR T cells. For the first time, our research revealed the negative effect of metformin usage in CD19-CAR T-cell treatment.
However, Eikawa et al pointed out the positive effect of metformin in CD8+ TIL.20 This study verified that the physiological dose of metformin could induce tumor rejection in vivo. Our study indicated that metformin in physiological dose plays a negative role in diminishing CD19+ Raji cells by CD19-CAR T cells in vivo. The differences between these two studies brought interesting thoughts. Maybe metformin can be affected by tumor microenvironment. Perhaps metformin treatment differentially affects CAR and TCR signaling. Further research will be conducted on these details.
It is well documented that metformin was the agonist of AMP-activated protein kinase.33 Studies further revealed that the crucial role of AMPK in regulating the glycolytic metabolism of immunocytes.20,22 Also, studies demonstrated that mTOR promoted CD8+ T-cell differentiation by modulating the transcription factor hypoxia inducible factor 1α (HIF1α).23 Moreover, studies found that HIF1α was caused T-cell activation in mTOR-dependent manner.34–36 In our study, the expression of AMPK, mTOR, and HIF1α in CD19-CAR T cells was examined. We found that the expression of phosphor-AMPK was upregulated, while expressions of phosphor-mTOR and HIF1α expression were downregulated when treated with metformin. To testify that AMPK, mTOR, and HIF1α were involved in metformin-regulated biological function in CD19-CAR T cells, we blocked AMPK activity by compound C, the AMPK inhibitor. Our research revealed that inhibition of AMPK activity by compound C significantly reversed the apoptosis and cytotoxicity of CD19-CAR T cells when treated with metformin. Meanwhile, the expression of mTOR and HIF1α was not affected when AMPK inhibitor was added. These results indicated that AMPK is crucial for metformin-modulated apoptosis and cytotoxicity in CD19-CAR T cells. Also, these results demonstrated that metformin-inhibited mTOR and HIF1α expression might be AMPK-independent.
In conclusion, our study for the first time indicated that metformin could affect proliferation, apoptosis, and cytotoxicity of CD19-CAR T cells and validated in vivo. Moreover, the regulatory effect of metformin in CD19-CAR T cells was AMPK-dependent. The results in our study will guide medications on patients with B-cell malignancies and type II diabetes.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81570407) and the Key Research and Development Program of Shandong Province (No. 2016CYJS01A04-2, 2016CYJS01A04-1).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Baxevanis CN, Perez SA, Papamichail M. Combinatorial treatments including vaccines, chemotherapy and monoclonal antibodies for cancer therapy. Cancer Immunol Immunother. 2009;58(3):317–324. doi: 10.1007/s00262-008-0576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116(7):1035–1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garfall AL, Maus MV, Hwang WT, et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med. 2015;373(11):1040–1047. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Jacoby E, Fry TJ. Challenges and opportunities of allogeneic donor-derived CAR T cells. Curr Opin Hematol. 2015;22(6):509–515. doi: 10.1097/MOH.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgos SA, Chandurkar V, Tsoukas MA, et al. Insulin resistance of protein anabolism accompanies that of glucose metabolism in lean, glucose-tolerant offspring of persons with type 2 diabetes. BMJ Open Diabetes Res Care. 2016;4(1):e000312. doi: 10.1136/bmjdrc-2016-000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nascimbeni F, Dalla Salda A, Carubbi F. Energy balance, glucose and lipid metabolism, cardiovascular risk and liver disease burden in adult patients with type 1 Gaucher disease. Blood Cells Mol Dis. 2016;68:74–80. doi: 10.1016/j.bcmd.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Outschoorn UE, Peiris-Pages M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14(1):11–31. doi: 10.1038/nrclinonc.2016.60. [DOI] [PubMed] [Google Scholar]
- 12.Dimeloe S, Burgener AV, Grahlert J, Hess C. T-cell metabolism governing activation, proliferation and differentiation; a modular view. Immunology. 2017;150(1):35–44. doi: 10.1111/imm.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey CJ. Biguanides and NIDDM. Diabetes Care. 1992;15(6):755–772. doi: 10.2337/diacare.15.6.755. [DOI] [PubMed] [Google Scholar]
- 14.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334(9):574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 15.Rico M, Baglioni M, Bondarenko M, et al. Metformin and propranolol combination prevents cancer progression and metastasis in different breast cancer models. Oncotarget. 2017;8(2):2874–2889. doi: 10.18632/oncotarget.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu M, Zhang Q, Wang X, et al. Metformin potentiates anti-tumor effect of resveratrol on pancreatic cancer by down-regulation of VEGF-B signaling pathway. Oncotarget. 2016;7(51):84190–84200. doi: 10.18632/oncotarget.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang P, Li H, Tan X, Chen L, Wang S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 2013;37(3):207–218. doi: 10.1016/j.canep.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35(2):299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng SC, Quintin J, Cramer RA, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345(6204):1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A. 2015;112(6):1809–1814. doi: 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cham CM, Gajewski TF. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J Immunol. 2005;174(8):4670–4677. doi: 10.4049/jimmunol.174.8.4670. [DOI] [PubMed] [Google Scholar]
- 22.Almeida L, Lochner M, Berod L, Sparwasser T. Metabolic pathways in T cell activation and lineage differentiation. Semin Immunol. 2016;28(5):514–524. doi: 10.1016/j.smim.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12(5):325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang K, Chi H. mTOR and metabolic pathways in T cell quiescence and functional activation. Semin Immunol. 2012;24(6):421–428. doi: 10.1016/j.smim.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song H, Wang H, Wu W, et al. Inhibitory role of reactive oxygen species in the differentiation of multipotent vascular stem cells into vascular smooth muscle cells in rats: a novel aspect of traditional culture of rat aortic smooth muscle cells. Cell Tissue Res. 2015;362(1):97–113. doi: 10.1007/s00441-015-2193-9. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Mu Q, Zhou Z, et al. Protective effect of irisin on atherosclerosis via suppressing oxidized low density lipoprotein induced vascular inflammation and endothelial dysfunction. PLoS One. 2016;11(6):e0158038. doi: 10.1371/journal.pone.0158038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011;9:33. doi: 10.1186/1741-7015-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lan B, Zhang J, Zhang P, et al. Metformin suppresses CRC growth by inducing apoptosis via ADORA1. Front Biosci (Landmark Ed) 2017;22:248–257. doi: 10.2741/4484. [DOI] [PubMed] [Google Scholar]
- 30.Alalem M, Ray A, Ray BK. Metformin induces degradation of mTOR protein in breast cancer cells. Cancer Med. 2016;5(11):3194–3204. doi: 10.1002/cam4.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galdieri L, Gatla H, Vancurova I, Vancura A. Activation of AMP-activated protein kinase by metformin induces protein acetylation in prostate and ovarian cancer cells. J Biol Chem. 2016;291(48):25154–25166. doi: 10.1074/jbc.M116.742247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong J, Kelekar G, Shen J, Shen J, Kaur S, Mita M. The expanding role of metformin in cancer: an update on antitumor mechanisms and clinical development. Target Oncol. 2016;11(4):447–467. doi: 10.1007/s11523-016-0423-z. [DOI] [PubMed] [Google Scholar]
- 33.Chen SC, Brooks R, Houskeeper J, et al. Metformin suppresses adipogenesis through both AMP-activated protein kinase (AMPK)-dependent and AMPK-independent mechanisms. Mol Cell Endocrinol. 2016;440:57–68. doi: 10.1016/j.mce.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finlay DK, Rosenzweig E, Sinclair LV, et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med. 2012;209(13):2441–2453. doi: 10.1084/jem.20112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang R, Dillon CP, Shi LZ, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35(6):871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura H, Makino Y, Okamoto K, et al. TCR engagement increases hypoxia-inducible factor-1 alpha protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells. J Immunol. 2005;174(12):7592–7599. doi: 10.4049/jimmunol.174.12.7592. [DOI] [PubMed] [Google Scholar]