Abstract
Background
Patients with chronic hepatitis B virus (HBV) infection who are hepatitis B virus e antigen (HBeAg) positive are increasingly being treated with nucleos(t)ide analogs (NUCs). However, the predictive value of serum hepatitis B virus core antibody (HBcAb) levels for HBeAg seroconversion among patients with high viral load remains unclear.
Methods
This study consisted of 74 patients with high viral load (HBV DNA >1 × 107 copies/mL) enrolled in a multicenter, randomized, controlled trial, treated with lamivudine and adefovir (N = 32) or entecavir (N = 42) for up to 96 weeks. Serum HBV DNA, quantitative hepatitis B virus surface antigen (HBsAg), HBeAg, and HBeAb was tested at each visit. Quantitative HBcAb evaluation was conducted for all the available samples in the trial, by using a newly developed double-sandwich anti-HBc immunoassay.
Results
Serum HBcAb levels were significantly higher in patients with a serum alanine aminotransferase (ALT) level more than five times the upper limit of normal (ULN) compared with patients with ALT levels within 5 × ULN (4.25 ± 0.61 vs. 3.94 ± 0.47 log10 IU/mL, P = 0.0345). Patients with HBeAg seroconversion were associated with a higher level of HBcAb at baseline, compared with those without HBeAg seroconversion (4.38 ± 0.54 vs. 4.02 ± 0.58 log10 IU/mL, P = 0.029). The area under receiver operating characteristic curve of baseline HBcAb for HBeAg seroconversion was 0.71 (95% CI: 0.55–0.86, P = 0.013). When the baseline HBcAb level was >4.375 log10 IU/mL, the sensitivity and specificity to predict HBeAg seroconversion at week 96 were 62.5% and 74.2%, and the positive likelihood ratio (LR) and negative LR were 2.42 and 0.51, respectively. The multivariate analysis result indicated that baseline serum HBcAb level was the only independent predictor for HBeAg seroconversion at week 96, with an odds ratio of 4.78.
Conclusion
Baseline serum HBcAb level >4.375 log10 IU/mL could satisfactorily predict HBeAg seroconversion among patients with high viral load treated with NUC.
Keywords: chronic hepatitis B, HBV: hepatitis B core antibody, hepatitis B e antigen, seroconversion, high viral load
Introduction
It has been estimated that 240 million people worldwide are chronically infected with hepatitis B virus (HBV). People with chronic HBV infection are at increased risk of developing liver cirrhosis, liver failure, and hepatocellular carcinoma (HCC).1 Five nucleos(t)ide analogs (NUCs) have been approved for antiviral treatment of chronic HBV infection in most countries. The aim of antiviral treatment for chronic HBV infection is to suppress HBV replication and to achieve hepatitis B virus e antigen (HBeAg) seroconversion in HBeAg seropositive patients, to improve health-related quality of life, and patient survival.2 The presence of HBeAg seropositivity indicates active replication of HBV and a reflection of high risk of HCC. The relative risk of HCC in chronic HBV infection has been reported as 9.6% for patients who were seropositive for hepatitis B virus surface antigen (HBsAg) alone; the risk increased to 60.2% for patients who were both HBsAg seropositive and HBeAg seropositive.3 Therefore, it is important to monitor HBeAg status in patients with chronic HBV infection during anti-HBV treatment.
Recently, available evidence suggests that patients with chronic HBV infection with high viral load (HVL) are less likely to achieve HBeAg seroconversion and more likely to be associated with high drug-related resistance and treatment failure. In the 2-year GLOBE trial, data suggested that patients with chronic HBV infection with serum HBV DNA levels at baseline <9 log10 copies/mL were confirmed as strong predictors for a favorable virologic response and serological outcomes at week 104, including undetectable HBV DNA viral load and HBeAg seroconversion.4 However, chronic HBV infection is an infectious disease with a pathogenesis and course that depends on the virus and host interaction. Although previous studies have shown that HBV viral load and HBsAg levels can be used to predict treatment outcomes of anti-HBV treatment, these predictors are virus-dependent.5,6 At present, host immune-related predictors for HBeAg seroconversion and immune response in patients with chronic HBV infection with an HVL at baseline are limited. There is currently no specific predictor for HBeAg seroconversion in patients with chronic HBV infection with HVL.
In 2013, a study by Yuan et al reported that increased serum levels of hepatitis B virus core antibody (HBcAb) may indicate a stronger host-adaptive antiviral immune response in chronic HBV infection.7 This study suggested a possible role for HBcAb as a predictor of host immune response to HBV infection. However, due to the relatively low HBV DNA viral load, the performance of detection of HBcAb as a marker for HBeAg seroconversion in patients with chronic HBV infection and with HVL requires further validation.
The aim of this study was to determine the predictive value of serum HBcAb levels as predictors for HBeAg seroconversion in patients with chronic HBV infection treated with NUCs.
Methods
Patients studied
This study consisted of 74 patients with HVL (an HVL at baseline defined as serum HBV DNA levels >1 × 107 copies/mL) enrolled in a multicenter, randomized, controlled trial, treated with lamivudine (LAM) and adefovir (ADV) (N = 32) or entecavir (ETV) (N = 42) for up to 96 weeks. Patients enrolled in the trial were HBsAg seropositive for at least 6 months, HBeAg-positive with an HVL at baseline, accompanied by alanine aminotransferase (ALT) levels greater than the upper limit of normal (ULN), documented on two separate occasions, at least 2 weeks apart, with the latest ALT ≥2 × ULN.
The exclusion criteria included patients who had received previous treatment with IFN or NUCs; pregnancy; a history of metabolic liver disease; patients who had diagnostic markers of co-infection with hepatitis C virus, hepatitis D virus, or HIV; autoimmune hepatitis; heavy alcohol intake; liver cirrhosis; or HCC.
Serological methods
Serum levels of ALT, HBV serological markers, and HBV DNA viral load were evaluated every 12 weeks from baseline to the end of the study. Levels of HBV serological markers and serum HBV DNA levels were measured at the Central Laboratory of Nanfang Hospital, and serum ALT levels were assessed at local laboratories according to local standard procedures.
Serum ALT levels were measured by automated techniques. HBsAg, HBeAg, and HBcAb were measured using a commercially available radioimmunoassay (ARCHITECT i2000SR; Abbott Laboratories, Abbott Park, IL, USA). Serum HBV DNA viral load was measured using real-time quantitative PCR, with the Cobas Ampliprep and Cobas TaqMan, version 2.0 (CAP/CTM; Hoffman-La Roche Ltd., Basel, Switzerland).8 According to the manufacturer’s report, the HBV DNA linear range was 20 IU/mL to 1.7 × 108 IU/mL (1 IU/mL = 5.82 copies/mL).
Quantitative analysis of serum HBcAb levels was performed by using a newly developed, double-sandwich immunoassay (dynamic range 0.1–2.0 IU/mL; Wantai, Beijing, China), a method previously validated by the World Health Organization HBcAb standards.9 The evaluation was conducted with the investigators blinded to treatment status and other clinical characteristics, for all the available samples in the study. The samples were tested at dilutions of 1:100–1:100000 (10-fold increase) if the HBcAb level was >2.0 IU/mL10 (Figure S1).
In this study, a virologic response was defined as HBV DNA <100 IU/mL; biochemical responses were defined as normalization of ALT levels; HBeAg seroconversion was defined as HBeAg negative and HBeAb positive.
Reproducibility and reliability evaluation of the double-sandwich HBcAb assay
Five serum specimens were used for the evaluation of the reproducibility of the double-sandwich HBcAb assay used in the study. The HBcAb titers of the five specimens were 0.1, 0.3, 0.6, 1.2, and 2.0 IU/mL. The reproducibility was assessed from ten measurements of the five specimens. The coefficient of variation was calculated to estimate the precision of the assay. To evaluate the quantitative accuracy of the assay, 80 randomly selected serum specimens of chronic hepatitis B (CHB) patients were independently measured twice by the assay (Figure S1).
Statistical analysis
The measurements were expressed as mean ± SD for normally distributed data, and median (range), for data showing a non-normal distribution. Categorical data were expressed as a percentage. The HBV DNA levels were expressed in logarithmic units (log10 IU/mL). The χ2-test and Student’s t-test were applied when appropriate. Spearman’s rho tests were used for correlation analyses. We performed the randomization by random numbers generated by Excel. Data analysis and quality control procedures were performed using SPSS for Windows, version 13.0 (SPSS Inc., Chicago, IL, USA) with an alpha level of 0.05.
Ethics statement
The Institutional Review Board of Nanfang Hospital, Southern Medical University approved the study (ID: ZHF2011206). All procedures were in accordance with the ethical standards of the responsible committee on human experimentation and the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients included in the study.
Results
Patient demographics and clinical characteristics
A total of 74 patients who completed a 96-week treatment program with NUCs were included in the study, as shown in Figure 1. At the end of the study, 16 (21.6%) patients achieved HBeAg seroconversion in the cohorts. Of these 74 patients, there were 32 patients receiving LAM and ADV combination therapy with an average age of 32.9 ± 10.6 years; 42 patients receiving ETV therapy, with an average age of 30.1 ± 8.4 years. The baseline HBcAb levels in patients treated with LAM and ADV combination therapy and in ETV therapy were 4.06 ± 0.57 and 4.13 ± 0.61 log10 IU/mL, respectively. The two groups did not differ in the distribution of baseline demographics and clinical characteristics (P > 0.05, Table 1).
Figure 1.
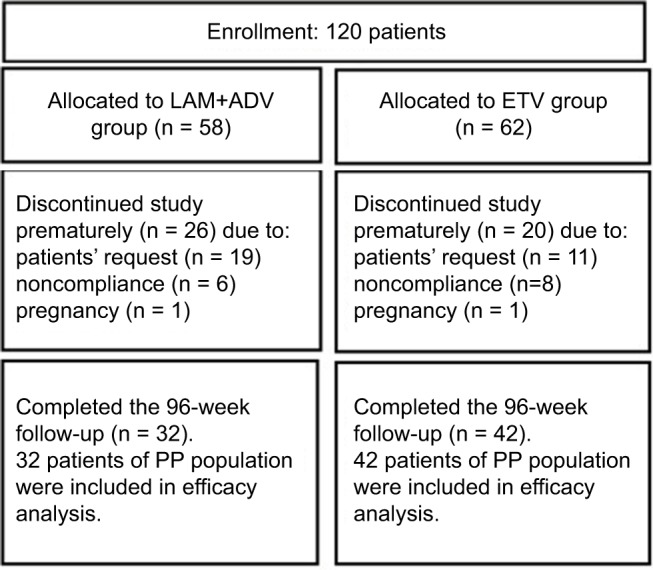
Flow chart of patients included in the study.
Abbreviations: ADV, adefovir; ETV, entecavir; LAM, lamivudine, PP, per-protocol.
Table 1.
Demographic and baseline characteristics by group
| Variables | LAM+ADV group | ETV group | P-value |
|---|---|---|---|
| Sample size | 32 | 42 | – |
| Age (years)a | 32.9 ± 10.6 | 30.1 ± 8.4 | 0.22 |
| Gender (M/F) | 25/7 | 31/11 | 0.67 |
| ALT (U/L)a | 226.1 ± 126.9 | 276.1 ± 144.4 | 0.12 |
| HBcAb level (log10 IU/mL)a | 4.06 ± 0.57 | 4.13 ± 0.61 | 0.59 |
| HBV DNA level (log10 IU/mL)a | 7.99 ± 0.69 | 8.03 ± 0.58 | 0.77 |
Note:
Expressed as mean ± SD.
Abbreviations: ADV, adefovir; ETV, entecavir; LAM, lamivudine; HBV, hepatitis B virus.
To explore the relationship between the levels of serum HBcAb and the activation of host immune response, we first analyzed the correlation between the baseline HBcAb level and ALT, as shown in Figure S2. The baseline levels of serum HBcAb were positively associated with ALT levels (R value 0.174, P = 0.0127). Among the patients with chronic HBV infection, the HBcAb levels in those who had elevated ALT levels of more than 5 × ULN were significantly greater than those who had lower elevated ALT levels (4.25 ± 0.61 vs. 3.94 ± 0.47 log10 IU/mL, P = 0.0345), as shown in Figure S3. We also conducted a correlation between HBsAg levels and HBcAb levels at baseline. However, we did not find a correlation between HBsAg levels and HBcAb levels in our study.
Evaluation of the serum HBcAb levels during antiviral treatment with NUCs
The HBcAb serum levels of patients were stratified according to whether HBeAg seroconversion was confirmed, as shown in Figure 2. At baseline, the mean levels of HBcAb were 4.38 ± 0.54 and 4.02 ± 0.58 log10 IU/mL, respectively (P = 0.029), in patients with and without HBeAg seroconversion. During antiviral treatment, the mean HBcAb level decreased to 3.69 ± 0.39 log10 IU/mL at week 24 and 3.42 ± 0.46 log10 IU/mL at week 48, in patients with HBeAg seroconversion. Similar results were identified for those without HBeAg seroconversion at week 96. The mean HBcAb decreased to 3.53 ± 0.57 and 3.27 ± 0.52 log10 IU/mL at week 24 and week 48, respectively. Although the HBcAb level in both groups decreased during anti-HBV treatment, no difference was found between the two groups with respect to HBcAb levels at week 24 and week 48 in our study.
Figure 2.
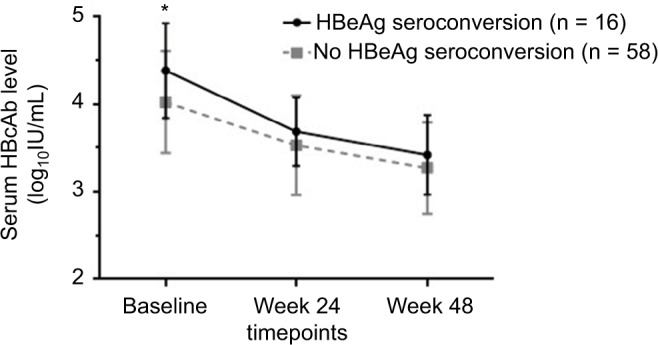
Serum levels of HBcAb at different timepoints according to whether HBeAg seroconversion occurred at week 96.
Notes: Stratification of patients according to whether or not HBeAg seroconversion occurred or not at week 96. The baseline mean quantitative serum HBcAb levels were 4.38 ± 0.54 and 4.02 ± 0.58 log10 IU/mL in groups with and without HBeAg seroconversion, respectively (P = 0.029). The mean serum HBcAb level in the group with HBeAg seroconversion decreased to 3.69 ± 0.39 log10 IU/mL at week 24 and to 3.42 ± 0.46 log10 IU/mL at week 48, and decreased to 3.53 ± 0.57 and 3.27 ± 0.52 log10 IU/mL at weeks 24 and 48 in the group without HBeAg seroconversion.
Analysis of the performance of HBcAb as a predictor of 96-week HBeAg seroconversion
The area under receiver operating characteristic (AUROC) curve was used to determine whether the serum levels of HBcAb could be an indicator for HBeAg seroconversion at week 96 for patients with chronic HBV infection and HVL (Figure 3). The AUROC was 0.71 (P = 0.013) with 95% CI from 0.55 to 0.86. When baseline HBcAb level >4.375 log10 IU/mL, the predictive sensitivity and specificity for HBeAg seroconversion at week 96 during NUC treatment were 62.5% and 74.2%, and the positive likelihood ratio (LR) and negative LR were 2.42 and 0.51, respectively.
Figure 3.
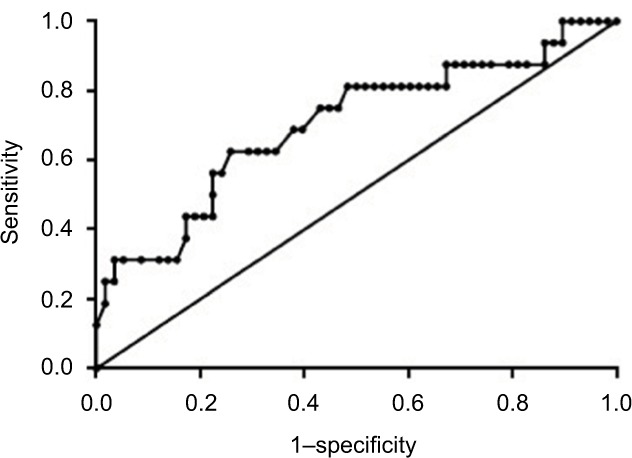
Baseline serum HBcAb levels to predict HBeAg seroconversion at week 96.
Notes: The AUROC curve was 0.71 (95% CI: 0.55–0.86, P = 0.013). When the baseline HBcAb level was >4.375 log10 IU/mL, the sensitivity and specificity to predict HBeAg seroconversion at week 96 during nucleos(t)ide analog treatment were 62.5% and 74.2%, and the positive likelihood ratio (LR) and negative LR were 2.42 and 0.51, respectively.
In order to further evaluate baseline characteristics in predicting HBeAg seroconversion, a multivariate analysis was conducted with inclusion of gender, age, ALT level, HBV DNA levels, HBcAb levels, and type of treatment in the model. The results showed that baseline serum HBcAb level was the only independent strong predictor for HBeAg seroconversion at week 96 with an odds ratio (OR) of 4.78 (P = 0.009), as shown in Table 2.
Table 2.
Baseline variables associated with HBeAg seroconversion
| Variables | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Gender (female/male) | 0.64 | 0.19–2.16 | 0.47 | |||
| Age (year) | 0.99 | 0.94–1.05 | 0.79 | |||
| ALT level (U/N) | 3.28 | 0.84–12.8 | 0.08 | |||
| HBV DNA levels (lg IU/mL) | 1.67 | 0.51–4.43 | 0.39 | |||
| HBcAb levels (lg IU/mL) | 4.78 | 1.48–15.4 | 0.009 | 4.78 | 1.48–15.4 | 0.009 |
| Type of treatment (LAM+ADV/ETV) | 1.96 | 0.64–5.99 | 0.24 | |||
Abbreviation: HBV, hepatitis B virus.
To validate the serum HBcAb cut-off value set as 4.375 log10 IU/mL to predict the HBeAg seroconversion at week 96, the rates of HBeAg seroconversion between patients with different HBcAb levels were compared. The results showed that 40% (10/25) patients with baseline HBcAb >4.375 log10 IU/mL achieved HBeAg seroconversion at week 96 compared with only 12.2% (6/49) of patients with baseline HBcAb level ≤4.375 log10 IU/mL (P = 0.006), as shown in Figure 4.
Figure 4.
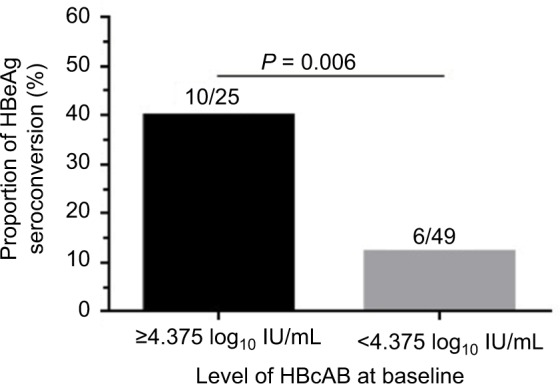
The probability of HBeAg seroconversion based on baseline HBcAb level. Note: There were 25 patients with baseline HBcAb >4.375 log10 IU/mL and 40% of them achieved HBeAg seroconversion at week 96 compared with only 12.2% (6/49) of patients with baseline HBcAb level ≤4.375 log10 IU/mL.
Discussion
Serum HBcAb is one of the most widely used serum markers for HBV infection. However, the clinical significance of serum HBcAb levels is poorly understood. There have been few studies to evaluate the performance of quantitative HBcAb in predicting HBeAg seroconversion among patients with chronic HBV infection with HVL. This study has shown that a baseline serum HBcAb level >4.375 log10 IU/mL could predict which patients with chronic HBV infection and with an HVL experience HBeAg seroconversion at week 96 during treatment with NUCs.
Previous studies have demonstrated that a high HBV DNA level is an independent predictor of patient outcome with NUC treatment, and a predictor of risk of developing HCC.4,6 For patients with chronic HBV infection and with HVL, there remains a clinical challenge to improve the efficacy of antiviral therapy and to achieve desirable therapeutic endpoints.
A previously published study has reported predictive serological markers in chronic HBV infection, including HBV DNA viral load and serum ALT levels.11 However, measurement of serum HBV DNA levels reflects the pathogen load. Chronic viral hepatitis results from pathogen–host interactions, including the immune status of the host. Serum ALT levels may be raised due to a variety of other diseases, such as neuromuscular disease, myocardial injury, and other liver diseases.12–14 However, a high pretreatment ALT level, particularly more than 5 × ULN, is a strong predictor of HBeAg seroconversion in both IFN and NUC therapy.15–17 In CHB patients, the HBcAb level was positively correlated with the ALT level from 0.5 × ULN to 5 × ULN and reached a plateau at ALT levels greater than 5 × ULN, the cut-off value for treatment response prediction.10 In the present study, we demonstrated the practical application of measurement of serum HBcAb levels to predict HBeAg seroconversion in patients with chronic HBV infection and HVL; an elevated ALT level more than 5 × ULN and a baseline HBcAb level of >4.375 log10 IU/mL may be applied practically in real-world clinical practice.
Although baseline HBcAb levels showed an independent association with clinical outcome after NUC treatment, AUROC values for serum HBcAg were unsatisfactory at only 0.71. Therefore, a combination formula with several indepen dent predictors may be more effective for predicting clinical outcome in patients with chronic HBV infection treated with NUCs. In 2009, Zeuzem et al reported that virological response at week 24 was a strong predictor of clinical outcomes in patients with chronic HBV infection who received telbivudine treatment, although there was no significant early reduction in serum levels of HBcAb detected between patients with and without HBeAg seroconversion.11 The role of HBcAb serum levels in predicting serologic response during NUC treatment should be confirmed in larger, randomized clinical studies.
For patients with chronic HBV infection, the mechanisms underlying the relationship between HBcAb level and host immune status remain poorly understood, but HBV-specific CD4 and CD8 T-cells have an important role in the cellular immune response.18,19 Studies have further shown a role for B-cells; HBcAb is produced by HBcAg-activated B-cells.20 Recently, Zgair et al reported that HBcAb could inhibit or clear HBV through hepatocytotoxic effects associated with HBcAb-secreting B cells.21 Therefore, it is possible that high serum levels of HBcAb may indicate a robust adaptive immune host response against HBV infection, resulting in an improved clinical outcome following NUC treatment.
Another problem is that NUC therapy does not eliminate viral cccDNA and recurrent viremia is the clinical recurring event after treatment cessation in most CHB patients.22,23 Combination therapies of NUC with IFN are associated with increased cure rates with HBsAg loss compared to NUC alone.24 Therefore, seroconversion from HBeAg to HBeAb is an insufficient marker to eliminate HBV. Although HBeAg seroconversion is a good serum marker in the first few years during treatment, suggesting that risk of end-stage hepatic disease in CHB patients has been reduced.25,26 However, for a long-term anti-HBV treatment, HBsAg seroconversion will be a more appropriate marker. A further study about the correlation between HBcAb and HBsAg seroconversion should be done. Recent studies have reported that the innate immune response is critical in the development of adaptive immune response, and multiple innate immune pathways are ultimately induced in chronic HBV infection with relevance to clinical outcome.27–30 The innate immune response and adaptive immune response should also be taken into consideration as research targets in further studies.
This study had several limitations. The major limitation was the relatively small sample size. However, the data collected for this study were collection from only four centers. That could remedy the limitation and reduce bias to some extent. We believe that this preliminary study has resulted in findings that warrant future, larger multicenter controlled studies. The approaches used in this study to measure HBcAb levels should be confirmed in future studies and verified with the use of other methods. Therefore, we recommend that to validate these preliminary results further, and to understand the value of serum HBcAb measurement, these findings should be supplemented with the use of examination of liver biopsies to provide information on the histological response.
In conclusion, the findings of this study have shown that in CHB patients with HVL treated with NUCs, a baseline serum HBcAb level >4.375 log10 IU/mL was predictive for HBeAg seroconversion.
Supplementary materials
Additional performance validation of the double-sandwich anti-HBc assay.
Notes: (A) Reproducibility of the HBcAb assay. (B) Correlation analyses of the results obtained from two independent quantitative HBcAb measurements for 80 chronic hepatitis B serum specimens.
Abbreviations: Con.c, concentration; CV, coefficient of variation.
Correlation between the baseline HBcAb level and ALT.
Note: The baseline HBcAb level was positively associated with ALT (R value = 0.174, P = 0.0127).
Measurement of serum HBcAb levels in patients with chronic hepatitis B virus infection with different ALT levels, separated as four strata (ALT <2 ULN n = 4, 2 ULN ≤ ALT <5 ULN n = 24, 5 ULN ≤ ALT <10 ULN n = 37, 10 ULN ≤ ALT n = 9). The serum HBcAb levels in patients who had elevated ALT levels more than 5 × ULN were significantly greater than in those who had lower elevated levels (4.25 ± 0.61 vs. 3.94 ± 0.47 log10 IU/mL, P = 0.0345).
Abbreviation: ULN, upper limit of normal.
Acknowledgments
This study was supported by grants from Bristol-Myers Squibb and the Chinese Foundation for Hepatitis Prevention and Control (GHF 2011206). We want to thank Dr Yegui Jiang for providing the participants from Southwest Hospital, Third Military Medical University; Dr Yonghong Zhang for providing the participants from Second Xiangya Hospital, Central South University, and Dr Fangfang Lv for providing the participants from Sir Run Run Shaw Hospital, Zhejiang University.
The abstract of this paper was presented at the 26th Annual Conference of APASL (February 15–19, 2017, Shanghai) as an abstract presentation with interim findings. The poster’s abstract was published in Abstracts of the 26th Annual Conference of APASL, February 15–19, 2017, Shanghai, China. Hepatol Int. 2017;11(Suppl 1).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–283. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang HI, Lu SN, Liaw YF, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347(3):168–174. doi: 10.1056/NEJMoa013215. [DOI] [PubMed] [Google Scholar]
- 4.Liaw YF, Gane E, Leung N, et al. 2-Year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136(2):486–495. doi: 10.1053/j.gastro.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Moucari R, Mackiewicz V, Lada O, et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology. 2009;49(4):1151–1157. doi: 10.1002/hep.22744. [DOI] [PubMed] [Google Scholar]
- 6.Cai S, Yu T, Jiang Y, Zhang Y, Lv F, Peng J. Comparison of entecavir monotherapy and de novo lamivudine and adefovir combination therapy in HBeAg-positive chronic hepatitis B with high viral load: 48-week result. Clin Exp Med. 2016;16(3):429–436. doi: 10.1007/s10238-015-0373-2. [DOI] [PubMed] [Google Scholar]
- 7.Yuan Q, Song LW, Liu CJ, et al. Quantitative hepatitis B core antibody level may help predict treatment response in chronic hepatitis B patients. Gut. 2013;62(1):182–184. doi: 10.1136/gutjnl-2012-302656. [DOI] [PubMed] [Google Scholar]
- 8.Cai SH, Lv FF, Zhang YH, Jiang YG, Peng J. Dynamic comparison between Daan real-time PCR and Cobas TaqMan for quantification of HBV DNA levels in patients with CHB. BMC Infect Dis. 2014;14:85. doi: 10.1186/1471-2334-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li A, Yuan Q, Huang Z, et al. Novel double-antigen sandwich immunoassay for human hepatitis B core antibody. Clin Vaccine Immunol. 2010;17(3):464–469. doi: 10.1128/CVI.00457-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song LW, Liu PG, Liu CJ, et al. Quantitative hepatitis B core antibody levels in the natural history of hepatitis B virus infection. Clin Microbiol Infect. 2015;21(2):197–203. doi: 10.1016/j.cmi.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Zeuzem S, Gane E, Liaw YF, et al. Baseline characteristics and early on-treatment response predict the outcomes of 2 years of telbivudine treatment of chronic hepatitis B. J Hepatol. 2009;51(1):11–20. doi: 10.1016/j.jhep.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Peng J, Cao J, Yu T, Cai S, Li Z, Zhang X, Sun J. Predictors of sustained virologic response after discontinuation of nucleos(t)ide analog treatment for chronic hepatitis B. Saudi J Gastroenterol. 2015;21(4):245–253. doi: 10.4103/1319-3767.161645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garvey WT, Mechanick JI, Brett EM, et al. American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guideline for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 14.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Chien RN, Liaw YF, Atkins M. Pretherapy alanine transaminase level as a determinant for hepatitis B e antigen seroconversion during lamivudine therapy in patients with chronic hepatitis B. Asian Hepatitis Lamivudine Trial Group. Hepatology. 1999;30(3):770–774. doi: 10.1002/hep.510300313. [DOI] [PubMed] [Google Scholar]
- 16.Yuen MF, Yuan HJ, Hui CK, et al. A large population study of spontaneous HBeAg seroconversion and acute exacerbation of chronic hepatitis B infection: implications for antiviral therapy. Gut. 2003;52(3):416–419. doi: 10.1136/gut.52.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liaw YF, Jia JD, Chan HL, et al. Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology. 2011;54(5):1591–1599. doi: 10.1002/hep.24555. [DOI] [PubMed] [Google Scholar]
- 18.Bayard F, Malmassari S, Deng Q, Lone YC, Michel ML. Hepatitis B virus (HBV)-derived DRB1*0101-restricted CD4 T-cell epitopes help in the development of HBV-specific CD8+ T cells in vivo. Vaccine. 2010;28(22):3818–3826. doi: 10.1016/j.vaccine.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Furuichi Y, Tokuyama H, Ueha S, Kurachi M, Moriyasu F, Kakimi K. Depletion of CD25+CD4+T cells (Tregs) enhances the HBV-specific CD8+ T cell response primed by DNA immunization. World J Gastroenterol. 2005;11(24):3772–3777. doi: 10.3748/wjg.v11.i24.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliviero B, Cerino A, Varchetta S, et al. Enhanced B-cell differentiation and reduced proliferative capacity in chronic hepatitis C and chronic hepatitis B virus infections. J Hepatol. 2011;55(1):53–60. doi: 10.1016/j.jhep.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Zgair AK, Ghafil JA, Al-Sayidi RH. Direct role of antibody-secreting B cells in the severity of chronic hepatitis B. J Med Virol. 2015;87(3):407–416. doi: 10.1002/jmv.24067. [DOI] [PubMed] [Google Scholar]
- 22.Wu X, Cai S, Li Z, et al. Potential effects of telbivudine and entecavir on renal function: a systematic review and meta-analysis. Virol J. 2016;13:64. doi: 10.1186/s12985-016-0522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng J, Yin J, Cai S, Yu T, Zhong C. Factors associated with adherence to nucleos(t)ide analogs in chronic hepatitis B patients: results from a 1-year follow-up study. Patient Prefer Adherence. 2015;9:41–45. doi: 10.2147/PPA.S71510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcellin P, Ahn SH, Ma X, et al. Combination of tenofovir disoproxil fumarate and peginterferon alpha-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology. 2016;150(1):134–144. doi: 10.1053/j.gastro.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 25.Cai S, Cao J, Yu T, Xia M, Peng J. Effectiveness of entecavir or telbivudine therapy in patients with chronic hepatitis B virus infection pretreated with interferon compared with de novo therapy with entecavir and telbivudine. Medicine (Baltimore) 2017;96(22):e7021. doi: 10.1097/MD.0000000000007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng J, Cai S, Liu J, Xue X, Wu X, Zheng C. Dynamic changes in liver stiffness measured by transient elastography predict clinical outcomes among patients with chronic hepatitis B. J Ultrasound Med. 2017;36(2):261–268. doi: 10.7863/ultra.15.12054. [DOI] [PubMed] [Google Scholar]
- 27.Maini MK, Gehring AJ. The role of innate immunity in the immunopathology and treatment of HBV infection. J Hepatol. 2016;64(1 Suppl):S60–70. doi: 10.1016/j.jhep.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Lan P, Zhang C, Han Q, Zhang J, Tian Z. Therapeutic recovery of hepatitis B virus (HBV)-induced hepatocyte-intrinsic immune defect reverses systemic adaptive immune tolerance. Hepatology. 2013;58(1):73–85. doi: 10.1002/hep.26339. [DOI] [PubMed] [Google Scholar]
- 29.Han Q, Zhang C, Zhang J, Tian Z. The role of innate immunity in HBV infection. Semin Immunopathol. 2013;35(1):23–38. doi: 10.1007/s00281-012-0331-y. [DOI] [PubMed] [Google Scholar]
- 30.Martinet J, Dufeu-Duchesne T, Bruder Costa J, et al. Altered functions of plasmacytoid dendritic cells and reduced cytolytic activity of natural killer cells in patients with chronic HBV infection. Gastroenterology. 2012;143(6):1586–1596. doi: 10.1053/j.gastro.2012.08.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional performance validation of the double-sandwich anti-HBc assay.
Notes: (A) Reproducibility of the HBcAb assay. (B) Correlation analyses of the results obtained from two independent quantitative HBcAb measurements for 80 chronic hepatitis B serum specimens.
Abbreviations: Con.c, concentration; CV, coefficient of variation.
Correlation between the baseline HBcAb level and ALT.
Note: The baseline HBcAb level was positively associated with ALT (R value = 0.174, P = 0.0127).
Measurement of serum HBcAb levels in patients with chronic hepatitis B virus infection with different ALT levels, separated as four strata (ALT <2 ULN n = 4, 2 ULN ≤ ALT <5 ULN n = 24, 5 ULN ≤ ALT <10 ULN n = 37, 10 ULN ≤ ALT n = 9). The serum HBcAb levels in patients who had elevated ALT levels more than 5 × ULN were significantly greater than in those who had lower elevated levels (4.25 ± 0.61 vs. 3.94 ± 0.47 log10 IU/mL, P = 0.0345).
Abbreviation: ULN, upper limit of normal.


