Abstract
Capsosiphon fulvescens (green seaweed) and Hizikia fusiforme (brown seaweed) are marine algae consumed as food supplements, especially in Japan, China and Korea, and are considered traditional medicinal tonics for certain ailments. The aim of this study was to investigate the possible inhibitory effects of dietary C. fulvescens and H. fusiforme on azoxymethane (AOM)-induced colorectal cancer (CRC) in rats. F344 male rats (5 weeks, 150 g) were divided into six groups as follows. Group 1: Injected with normal saline solution and fed control diet (untreated control). Group 2: Injected with AOM and fed control diet (treated control). Group 3: Injected with AOM and fed 1% C. fulvescens diet. Group 4: Injected with AOM and fed 2% C. fulvescens diet. Group 5: Injected with AOM and fed 2% H. fusiforme diet. Group 6: Injected with AOM and fed 6% H. fusiforme diet. Test animals received subcutaneous injections of AOM (15 mg/1 ml/kg body weight) once a week for 2 weeks to induce aberrant crypt foci (ACF) in treated control and experimental groups. We evaluated the effects of dietary C. fulvescens and H. fusiforme at two different dose levels: 1 and 2% C. fulvescens, and 2 and 6% H. fusiforme, on colonic carcinogenesis by AOM in rats. Our results suggest that body weights were not significantly different amongst groups. We found that feeding C. fulvescens and H. fusiforme with a control diet significantly (p<0.05) inhibited the development of ACF in experimental groups. C. fulvescens and H. fusiforme in food also significantly (p<0.05) reduced the proliferating cell nuclear antigen labeling index in the colonic tissues of experimental groups. These results demonstrate the chemopreventive potential of C. fulvescens and H. fusiforme against CRC in an AOM-induced rats.
Keywords: Azoxymethane, colorectal cancer, Capsosiphon fulvescens, Hizikia fusiforme, rat
Colorectal cancer (CRC) is the most common cancer, and a significant cause of death, in developed countries, including the United States (1,2). Numerous factors including age, physical activity habits, environment, obesity, diabetes, diet, lifestyle, and genetics play important roles in CRC carcinogenesis (3,4). Many genetic defects/mutations, including the adenomatous polyposis coli (APC) gene, kirsten ras (KRAS) oncogene, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) gene, phosphatase and tensin homolog (PTEN) gene, transforming growth factor (TGFβ) receptor, and tumor protein p53 (TP53) gene, and enzymes such as cyclooxygenase-2 and nitric oxide synthase have been suggested to play vital roles in CRC carcinogenesis (4-6). It is recognized that CRC develops sequentially from polyp to adenocarcinoma. This development may take a long time, as much as 10-17 years (4).
Azoxymethane (AOM) is a tumor-initiating chemical agent commonly used in models of colon cancer (7). Specifically, it can induce the formation of aberrant crypt foci (ACF) by epithelial cells, which then progress to adenomas and malignant adenocarcinomas, that is similar to the pathogenesis of sporadic human colon cancer (8,9). Thus, it has been extensively used in the study of molecular biology, prevention, and treatment of colon cancer.
ACF are clusters of abnormal tube-like glands on the colonic mucosal surface, and are the earliest morphological changes after treatment with chemical carcinogens such as AOM (10). Evidence strongly suggests that ACF are pre-neoplastic lesions in the colon, and they are recognized as intermediate biomarkers of CRC in both rodents and humans (6,11). Chemically- induced ACF in rodents have been used to assess new chemopreventives and diets that might prevent CRC (12).
Capsosiphon fulvescens is a green marine alga consumed as a food supplement, and is considered to possess medicinal activity and high nutritional value (13). Hizikia fusiforme is a brown marine alga, traditionally used as a foodstuff and medicine in Asia (14). Previous research reported that C. fulvescens and H. fusiforme exhibited beneficial medicinal activities, such as antioxidant, anti-diabetic, and antitumor effects (15).
The purpose of the present study was to identify the possible inhibitory effect on colon carcinogenesis of feeding C. fulvescens and H. fusiforme at different dose levels to F344 male rats. We investigated the effects of C. fulvescens at 1 and 2%, and H. fusiforme at 2 and 6% dose levels in the rats’ diet. In addition, we immunohistochemically analyzed the expression of proliferating cell nuclear antigen (PCNA), which plays a vital role in carcinogenesis.
Materials and Methods
Chemicals. All chemicals were of the highest grade and were purchased from commercial suppliers. AOM was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Analytical-grade ethyl ether was purchased from Duksan Pure Chemicals (Ansan, Korea).
Plant materials and diets. C. fulvescens and H. fusiforme were collected from Jeonnam, Wando, South Korea, and were washed with water and dried. The seaweeds were cut into small pieces and extracted with 95% ethanol at 80˚C for 2 h at 120 rpm, with ethanol at a ratio of 40 ml/g. The extract was centrifuged (8,000 ×g, 10 min), filtered, and concentrated at 60˚C. The experimental diets were prepared by adding 5% brix concentrate to AIN-76A animal diet (ORIENT BIO Inc, Sangdaewon-Dong, Seongnam-si, South Korea) (Table I) with 1 or 2% of C. fulvescens and 2 or 6% of H. fusiforme by dry weight (Table II).
Table I. AIN-76A animal diet for controls and experimental groups.
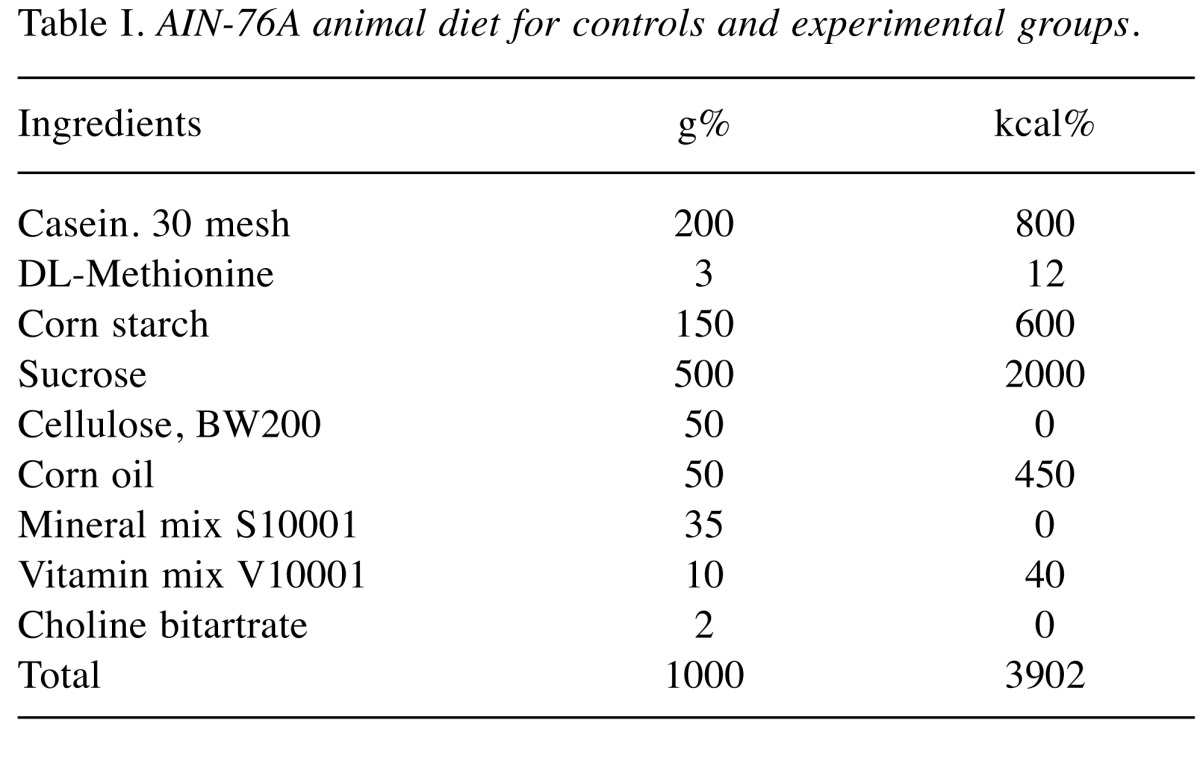
Table II. Experimental animals.
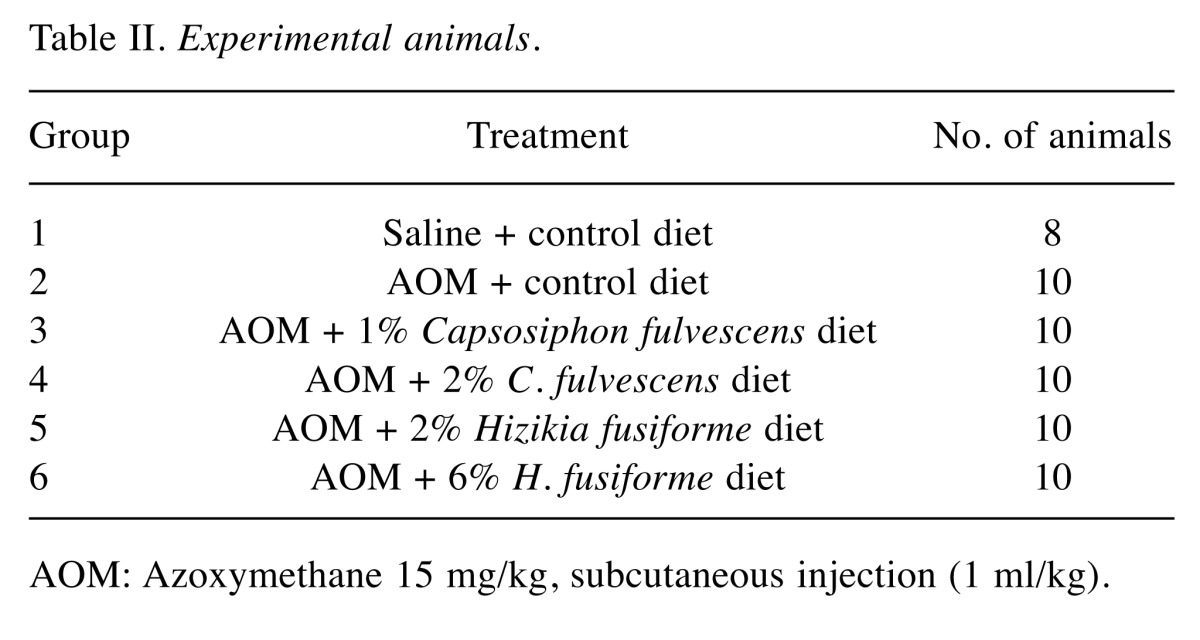
AOM: Azoxymethane 15 mg/kg, subcutaneous injection (1 ml/kg).
Animals. F344 male rats (5 weeks, 150 g) were used in these experiments (Orient Co Ltd, Seoul, Korea) and maintained in an environment with controlled temperature (23±2˚C) and humidity (55±7%) under a 12 h light-dark cycle. All animal experiments were carried out in accordance with the National Institute of Health guidelines.
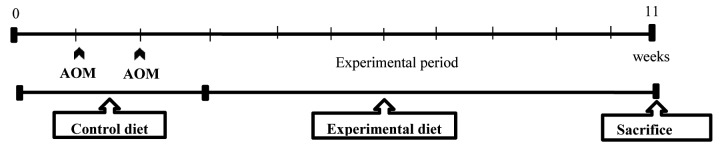
Experimental design. The rats were divided into six groups for experimental procedures as follows. In group 1 (untreated control), saline was injected (1 ml/kg) subcutaneously (sc.) during the first and second weeks of the experimental period. In group 2 (treated control), AOM was injected (15 mg/1 ml/kg) sc. once a week for 2 weeks. The animals in both these groups were fed the control diet throughout for 11 weeks. Groups 3, 4, 5, and 6 (experimental groups) were fed experimental diets containing 1 or 2% C. fulvescens or 2 or 6% H. fusiforme, respectively, for 8 weeks from the third week after the administration of AOM. While group 1 had eight rats, all other groups comprised 10. Body weights and food intake of the animals were measured throughout the experiment. After 11 weeks, all animals were sacrificed for histopathological examination of colonic tissues (Figure 1).
Figure 1. Experimental protocol to evaluate chemopreventive activity in F344 rats. Rats were divided into two control (untreated and treated) groups and four experimental groups. The rats were fed a control diet (AIN-76A) or diet supplemented with different doses of Capsosiphon fulvescens (1 or 2%) and Hizikia fusiforme (2 or 6%), for the 11-week experimental period. Rats in groups 2-6 received subcutaneous injections of azoxymethane (AOM; 15 mg/kg body weight) once a week for 2 weeks. Group 1 served as an untreated control (1 ml saline/kg by subcutaneous injection + control diet) and group 2 served as the treated control (15 mg AOM/kg body weight by subcutaneous injection + control diet).
Histopathological examination of colonic tumor tissues. Animals were sacrificed and colonic tumor tissues were removed immediately. The tissues were washed with normal saline and fixed in 10% neutral formalin solution (pH 7.0). After routine paraffin embedding, 6-8 μm tissue sections were cut, fixed on microscope slides, and deparaffinized, before being hydrated. Finally, sections were stained with hematoxylin and eosin (H&E) for histological examination of colonic cancer specimens.
Determination of ACF. The development of carcinoma was suggested by the detection of ACF, believed to be precancerous lesions, in colonic mucosa. Rectal crypt foci were excised, and the colon was spread, washed with potassium phosphate buffer (0.1 M, pH 7.2), and then fixed in 10% neutral formalin. After fixation and staining with 2.5% methylene blue, ACF were distinguished morphologically by their elliptical shape and increased size of crypts compared to normal crypts and measured microscopically.
Immunohistochemical evaluation. PCNA immunohistochemistry was performed to evaluate the proliferation of tumor cells. Paraffin-embedded colonic tumor tissues cut into 6-8 μm sections. These were then immersed in xylene solution to remove paraffin and rehydrate the tissues while sequentially reducing the ethanol concentration. The slides prepared in this manner were coated with mouse monoclonal antibody against PCNA (Santa Cruz Biotechnology, Santa Cruz, CA, USA), then coated with avidin–biotin peroxidase complex (Zymed Laboratories Inc., San Francisco, CA, USA), and incubated with diaminobenzidine (DAB, Zymed Laboratories Inc.) as a substrate. After washing, slides were examined after being counterstained with Meyer’s hematoxylin and washed with tap water. For the negative control, the primary antibody was replaced with phosphate-buffered saline. Finally, we counted proliferating tumor cells under microscopy.
Statistical analysis. Data are expressed as mean±standard error of the mean (SEM). Data were analyzed using a one-way analysis of variance (ANOVA), followed by Dunnett’s t-test for multiple comparisons using SPSS software (Release 12.0. SPSS Inc., Chicago, IL, USA). Statistical significance was considered at p0.05.
Results
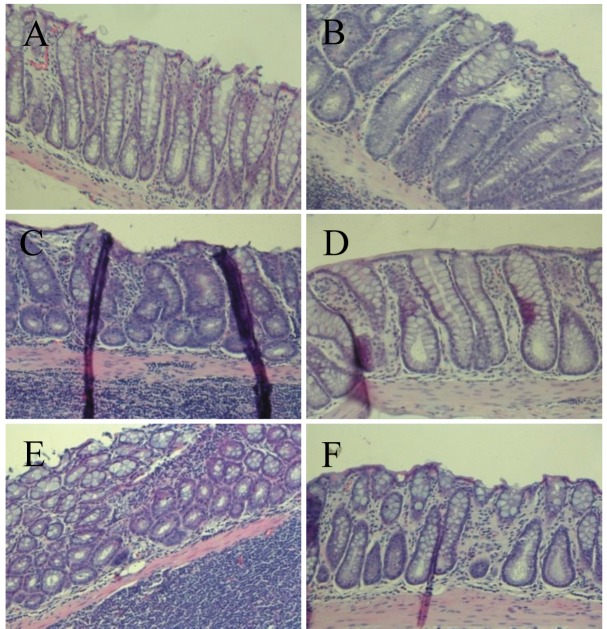
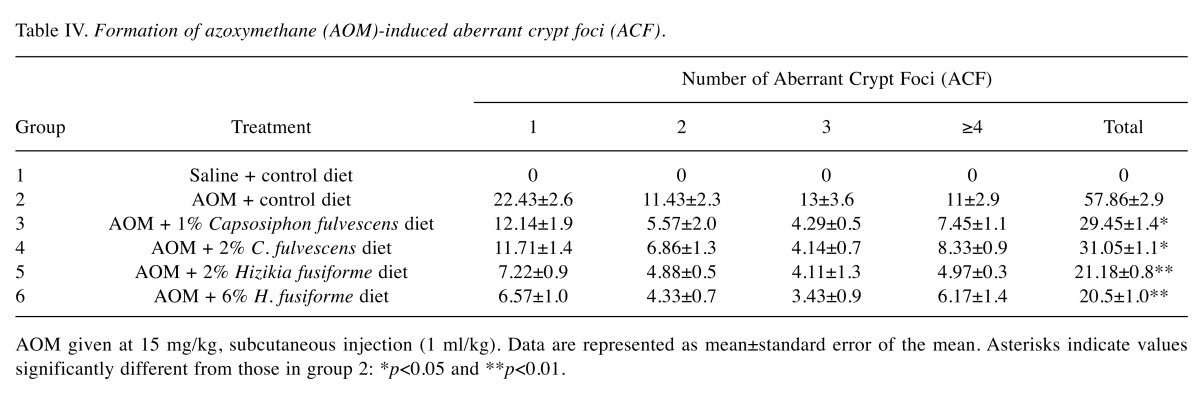
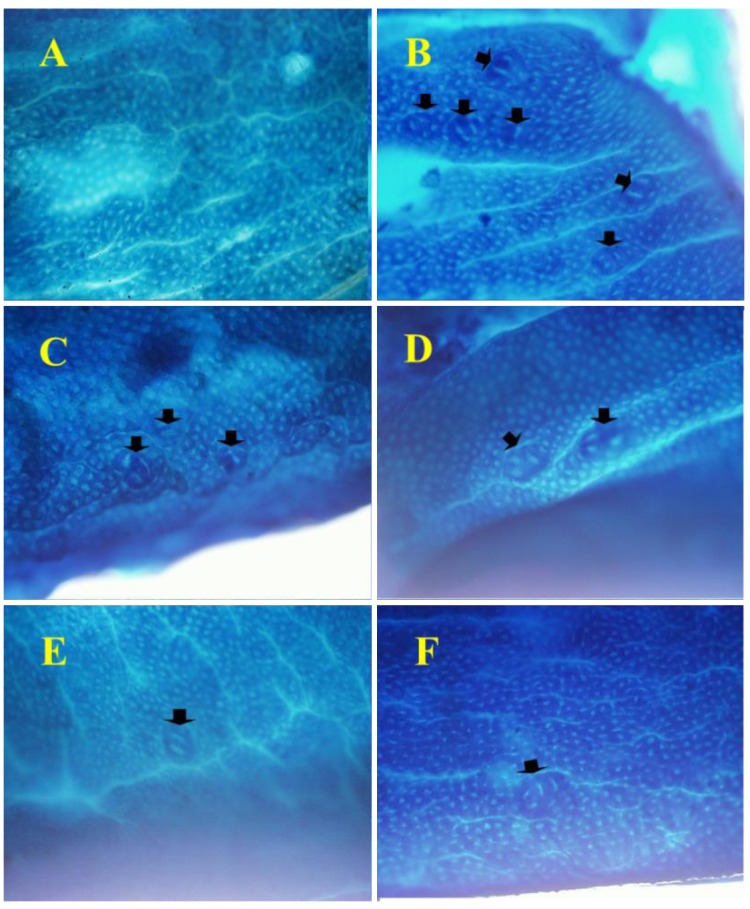
Feeding the experimental diets containing C. fulvescens and H. fusiforme did not produce any toxicity. Histological examinations revealed no morphological evidence of fatty liver (data not shown). The body weights of animals in groups 1 and 2 (control groups) were not significantly greater than those in the experimental groups (Table III) that received s.c. injections of AOM at 15 mg/kg body weight once a week for 2 weeks to investigate the formation of ACF in the colon. H&E staining revealed significant histopathological changes in the colonic tissues of AOM-treated rats. We found histological abnormalities such as ACF including dysplasia and hyperplasia, abnormally shaped lumens and elongated nuclei in the colonic mucosa of the treated control group (group 2), but these findings were significantly (p0.05) less frequent in the experimental groups (groups 3-6) (Figure 2). These results demonstrate that C. fulvescens and H. fusiforme suppress dysplastic cell proliferation in the colonic tissues of AOM-treated rats.The incidence and number of colorectal tumors after 11 weeks are listed in Table IV. In group 1 (untreated control), no colonic tumors were found. However, group 2 (treated control), colonic tissues were found to contain 58 ACF (Figure 3). In the dose–response study, experimental C. fulvescens and H. fusiforme diets reduced the incidence of ACF formation (p0.05) in groups 3 and 4, whereas the occurrence of ACF was significantly (p0.001) lower in groups 5 and 6 compared to the treated control. These findings suggest that C. fulvescens and H. fusiforme suppress ACF formation induced by AOM in the colon of rats.
Table III. Weight gain and feed intake.
AOM: Azoxymethane 15 mg/kg, subcutaneous injection (1 ml/kg).
Figure 2. Histopathological examination of colonic tissues of F344 rats from group 1, saline + control diet (A); group 2, azoxymethane (AOM) + control diet (B); group 3, AOM + 1% Capsosiphon fulvescens-supplemented diet (C); group 4, AOM + 2% C. fulvescens-supplemented diet (D); group 5, AOM + 2% Hizikia fusiforme-supplemented diet (E); and group 6, AOM + 6% H. fusiforme-supplemented diet (F). Representative microscopic examination was performed with hematoxylin and eosin staining. Histological assessment showed the presence of aberrant crypt foci including dysplasia and hyperplasia, abnormally-shaped lumens and elongated nuclei in the colonic mucosa of the treated control group but a marked reduction in all experimental groups. Sections were examined at 200× magnification.
Table IV. Formation of azoxymethane (AOM)-induced aberrant crypt foci (ACF).
AOM given at 15 mg/kg, subcutaneous injection (1 ml/kg). Data are represented as mean±standard error of the mean. Asterisks indicate values significantly different from those in group 2: *p<0.05 and **p<0.01.
Figure 3. Histopathological sections, stained with methylene blue to identify crypts in the colonic tissues of F33 rats, and examined at 200×magnification. In group 1, we did not find formation of any aberrant crypt foci (ACF), while group 2 had the highest number of ACF; with groups 3-6 having significantly fewer crypts after dietary supplementation with different doses of Capsosiphon fulvescens (1 or 2%) or Hizikia fusiforme (2 and 6%), respectively. Arrows indicate ACF.
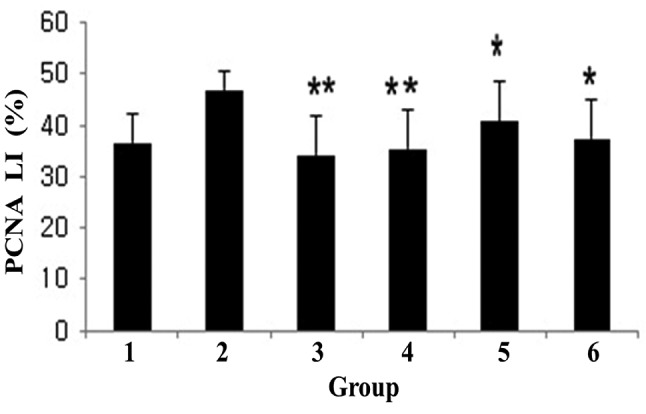
To investigate the effects of dietary C. fulvescens and H. fusiforme, we used immunohistochemistry to analyze the expression of PCNA, a marker of tumor cell proliferation and apoptosis, at week 11. The colonic adenocarcinoma PCNA labeling index was lower (p0.05) in groups 4 and 5, but in groups 3 and 4 more significantly (p0.001) lower than in the treated control group (Figure 5).
Figure 5. Proliferating cell nuclear antigen (PCNA) labeling index (%) of colonic mucosa in F344 rats. Group 1 was the untreated control, group 2 had the highest index, while groups 3-6 demonstrated the significant inhibitory effect of dietary Capsosiphon fulvescens (**p<0.01) and Hizikia fusiforme (*p<0.05) on PCNA labeling index compared to group2. The data represent the mean±standard error of the mean.
Discussion
Seaweeds are rich in bioactive substances that may be useful in treating a wide spectrum of diseases. Seaweeds contain health-promoting compounds such as phytochemicals, soluble dietary fibers, lipids, peptides, and minerals that hold potential as dietary supplements and that may reduce the risk of cancer (16,17). Moreover, macroalgae have been used as food supplements, particularly in Japan, China, and Korea (18). The long life expectancy of Japanese people has been partly attributed to their dietary habits, including the regular consumption of seaweed (17).
Our experimental data suggest that C. fulvescens and H. fusiforme are effective chemopreventive agents for CRC. These chemopreventive effects may be due to the high levels of minerals such as Na, Mg, K, Ca, and Fe in both algae (15). AOM has the potential to induce colonic carcinomas in rats by promoting ACF formation. Carcinogenesis is conventionally defined by three stages: initiation, promotion, and progression (19,20). During the initiation stage, normal cells experience DNA damage (21,22). During the second stage, promotion, the initiated cells affect normal cells, forming preneoplastic lesions (23). Finally, in the progression stage, malignant tumors develop, leading to metastasis (24).
In the present study, we demonstrated that dietary administration of C. fulvescens and H. fusiforme caused no significant difference in body weight in groups 3-6 over the experimental period compared with that in groups 1 (untreated control group) and 2 (treated control group). Additionally, there was no significant difference in feed intake between any of the experimental groups. This suggests that C. fulvescens and H. fusiforme may both have beneficial effects (14) (Table III).
H&E staining of colonic tissues was carried out for histological analyses. Throughout the colonic mucosa of the AOM-treated rats, we observed abnormalities, consistent with molecular aberrations in the treated control group (group 2), but such histological abnormalities were not found in the experimental groups (Figure 2). This demonstrates that dietary C. fulvescens and H. fusiforme have chemopreventive effects.
The ACF assay evaluates crypts with abnormal morphology, such as those found in group 2. In groups 3-6, dietary administration of C. fulvescens and H. fusiforme resulted in significantly less ACF formation than in the treated control group (Figure 3), suggesting that these agents counteract the carcinogenic activities of AOM.
Dietary administration of C. fulvescens and H. fusiforme prevented AOM-induced colorectal proliferative lesions in rats (Figure 4). Experimental groups exhibited pronounced suppression of cell proliferation compared with the treated control group. Groups 3-6 had significantly lower CRC PCNA labeling indices in comparison with that of group 2, suggesting that C. fulvescens and H. fusiforme might both modulate cell proliferation (Figure 5).
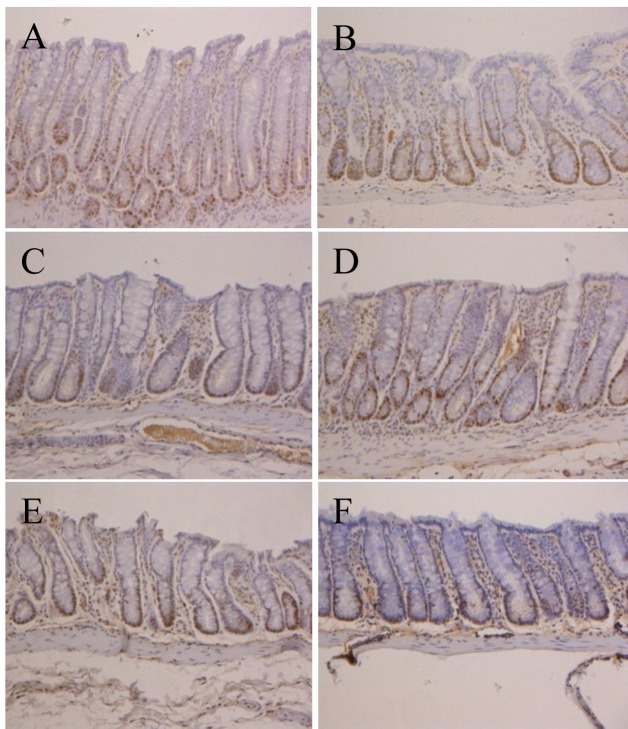
Figure 4. Immunohistochemical analysis of proliferating cell nuclear antigen (PCNA) in colonic tissue of F344 rats from groups 1-6. In group 1,we observed PCNA-negative cells (blue color); in group 2, we found PCNA-positive cells (brown color); but experimental groups 3-6 had fewer PCNA-positive cells than the treated controls (group 2). Colonic tissues were examined at 200× magnification.
Although the mechanism of inhibition of AOM-induced ACF formation in rats by C. fulvescens and H. fusiforme has not been established, one possibility is through interference with the cytochrome P450 2E1 (CYP2E1) pathway, due to their potent antioxidant activity (14,15,25-27). It has been suggested that suppression of CYP2E1 inhibits chemical carcinogenesis (4,28). In vivo, AOM is largely metabolized by isoform CYP2E1 (4,29,30). A previous study established that AOM-induced ACF formation was significantly lower in Cyp2e1, knockout mice (29). In the present study, we demonstrated that dietary C. fulvescens and H. fusiforme both suppressed formation of AOM-induced ACF in rats.
In conclusion, many chemopreventive agents have been shown to exhibit inhibitory effects on cancer in preliminary studies. Our results clearly revealed the potential benefits of C. fulvescens and H. fusiforme at inhibiting ACF formation. However, the optimal dose for an anticancer effect needs to be identified. Further studies on determination of the optimum dose of C. fulvescens and H. fusiforme in inhibiting ACF formation are required.
Conflicts of interest
The Authors declare no conflict of interest in regard to this study.
Acknowledgements
This work was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), which is funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C0001).
References
- 1.Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fung KY, Cosgrove L, Lockett T, Head R, Topping DL. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br J Nutr. 2012;108:820–831. doi: 10.1017/S0007114512001948. [DOI] [PubMed] [Google Scholar]
- 3.Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int J Mol Sci. 2017;18:197. doi: 10.3390/ijms18010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Huang XF. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biol Ther. 2009;8:1313–1317. doi: 10.4161/cbt.8.14.8983. [DOI] [PubMed] [Google Scholar]
- 5.Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5:19. [PMC free article] [PubMed] [Google Scholar]
- 6.Murillo G, Kosmeder JW, Pezzuto JM, Mehta RG. Deguelin suppresses the formation of carcinogen-induced aberrant crypt foci in the colon of CF-1 mice. Int J Cancer. 2003;104:7–11. doi: 10.1002/ijc.10901. [DOI] [PubMed] [Google Scholar]
- 7.Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2007;2:1998–2004. doi: 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- 8.Poulin EJ, Shen J, Gierut JJ, Haigis KM. Pathology and molecular pathology of colorectal cancer. Pathology and Epidemiology of Cancer. Springer. 2017;Cham:409–446. [Google Scholar]
- 9.Hao X, Xiao H, Ju J, Lee MJ, Lambert JD, Yang CS. Green tea polyphenols inhibit colorectal tumorigenesis in azoxymethane-treated F344 Rats. Nutr Cancer. 2017;69:623–631. doi: 10.1080/01635581.2017.1295088. [DOI] [PubMed] [Google Scholar]
- 10.De Robertis M, Arigoni M, Loiacono L, Riccardo F, Calogero RA, Feodorova Y, Tashkova D, Belovejdov V, Sarafian V, Cavallo F, Signori E. Novel insights into Notum and glypicans regulation in colorectal cancer. Oncotarget. 2015;6:41237. doi: 10.18632/oncotarget.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLellan EA, Bird RP. Aberrant crypts: potential preneoplastic lesions in the murine colon. Cancer Res. 1988;48:6187–6192. [PubMed] [Google Scholar]
- 12.Kensara OA, El-Shemi AG, Mohamed AM, Refaat B, Idris S, Ahmad J. Thymoquinone subdues tumor growth and potentiates the chemopreventive effect of 5-fluorouracil on the early stages of colorectal carcinogenesis in rats. Drug Des Dev Ther. 2016;10:2239. doi: 10.2147/DDDT.S109721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Islam MN, Choi SH, Moon HE, Park JJ, Jung HA, Woo MH, Woo HC, Choi JS. The inhibitory activities of the edible green alga Capsosiphon fulvescens on rat lens aldose reductase and advanced glycation end products formation. Eur J Nutr. 2014;53:233–242. doi: 10.1007/s00394-013-0521-y. [DOI] [PubMed] [Google Scholar]
- 14.Tong T, Ko DO, Kim BS, Ham KS, Kang SG. Beneficial effect of seaweed on high-fat diet-induced oxidative stress and insulin resistance in rats. Food Sci Biotechnol. 2015;24:2185–2191. [Google Scholar]
- 15.Tong T, Zhang C, Ko DO, Kim SB, Jung KJ, Kang SG. Effects of the addition of Hizikia fusiforme, Capsosiphon fulvescens, and Undaria pinnatifida sporophyll on antioxidant and inhibitory potential against enzymes related to type 2 diabetes of vegetable extract. Korean J Food Preserv. 2014;21:460–467. [Google Scholar]
- 16.Matos J, Cardoso C, Bandarra NM, Afonso C. Microalgae as a healthy ingredient for functional food: A review. Food Funct. 2017;8:2672–2685. doi: 10.1039/c7fo00409e. [DOI] [PubMed] [Google Scholar]
- 17.Cardoso SM, Pereira OR, Seca AM, Pinto DC, Silva A. Seaweeds as preventive agents for cardiovascular diseases: From nutrients to functional foods. Mar Drugs. 2015;13:6838–6865. doi: 10.3390/md13116838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jibri SM, Jakada BH, Umar HY, Ahmad TA. Importance of some algal species as a source of food and supplement. Int J Curr Microbiol Appl Sci. 2016;5:186–193. [Google Scholar]
- 19.Martín MA, Goya L, Ramos S. Preventive effects of cocoa and cocoa antioxidants in colon cancer. Diseases. 2016;4:6. doi: 10.3390/diseases4010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pritchard CC, Grady WM. Colorectal cancer molecular biology moves into clinical practice. Gut. 2011;4:116–129. doi: 10.1136/gut.2009.206250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Ng J, Arozulllah A, Ewing R, Llor X, Carroll RE, Benya RV. Aberrant crypt focus size predicts distal polyp histopathology. Cancer Epidemiol Biomarkers Prev. 2008;17:1155–1162. doi: 10.1158/1055-9965.EPI-07-2731. [DOI] [PubMed] [Google Scholar]
- 22.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 23.Willis S, Sunkara R, Willis Z, Smith L, Hester F, Reid HM, Patel P, McCollum M, Shackelford L, Onwasigwe E, Walker LT. Chemopreventive potential of select herbal teas and spices on azoxymethane-induced aberrant crypt foci in Fisher 344 male rats. Food Nutr Sci. 2017;8:348. [Google Scholar]
- 24.Cooper GM. The Cell: A Molecular Approach. Sunderland, MA, USA. 2000 [Google Scholar]
- 25.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aoi W, Naito Y, Takagi T, Kokura S, Mizushima K, Takanami Y, Kawai Y, Tanimura Y, Hung LP, Koyama R, Ichikawa H. Regular exercise reduces colon tumorigenesis associated with suppression of iNOS. Biochem biophys Res Commun. 2010;399:14–19. doi: 10.1016/j.bbrc.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Chiou YS, Tsai ML, Nagabhushanam K, Wang YJ, Wu CH, Ho CT, Pan MH. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J Agric Food Chem. 2011;59:2725–2733. doi: 10.1021/jf2000103. [DOI] [PubMed] [Google Scholar]
- 28.Brady JF, Xiao F, Wang MH, Li Y, Ning SM, Gapac JM, Yang CS. Effects of disulfiram on hepatic P450IIE1, other microsomal enzymes, and hepatotoxicity in rats. Toxicol Appl pharmacol. 1991;108:366–373. doi: 10.1016/0041-008x(91)90125-x. [DOI] [PubMed] [Google Scholar]
- 29.Sohn OS, Fiala ES, Requeijo SP, Weisburger JH, Gonzalez FJ. Differential effects of CYP2E1 status on the metabolic activation of the colon carcinogens azoxymethane and methylazoxymethanol. Cancer Res. 2001;61:8435–8440. [PubMed] [Google Scholar]
- 30.Shimpo K, Chihara T, Beppu H, Ida C, Kaneko T, Hoshino M, Kuzuya H. Inhibition of azoxymethane-induced DNA adduct formation by Aloe arborescens var. natalensis. Asian Pac J Cancer Prev. 2003;4:247–252. [PubMed] [Google Scholar]