Abstract
Background/Aim: We investigated the expression of angiogenesis and hypoxia markers in the adenohypophysis and neurohypophysis of patients who died from various acute or chronic diseases. Materials and Methods: Paraffin-embedded material of pituitary glands (97 patients) was investigated immunohistochemically for vascular density (CD31) and the expression of vascular endothelial growth factor (VEGF) and of hypoxia inducible factors HIF1α and HIF2α. Results: Vascular density, and HIF1α/HIF2α reactivity is directly related with VEGF expression in the pituitary gland, suggesting that the HIF pathway may regulate the vascular density and blood flow in the gland under hypoxic conditions. HIF2α appears to be a key regulator in neurohypophysis, whilst in adenohypophysis HIF1α and HIF2α are equally expressed. Chronic conditions, including alcoholism and substance abuse, seem to activate the HIF pathway in both neuro- and adeno-hypophysis. Conclusion: The HIF pathway has an important role in regulating vascular density and blood flow in the pituitary gland.
Keywords: Adenohypophysis, neurohypophysis, HIF1α, HIF2α, VEGF, CD31
The pituitary (hypophysis), a small 0.5-g endocrine gland, is located at the base of the brain, just below the hypothalamus; it is the "master gland" of the neuroendocrine system for it controls the function of other endocrine glands. The pituitary itself is regulated by the hypothalamus that is connected to the pituitary by a thin stalk, the pituitary stalk, which carries a delicate portal venous system. Microscopically, the pituitary is composed of two distinctive parts: the anterior lobe (adenohypophysis) and posterior lobe (neurohypophysis) (1). The anterior lobe is a typical endocrine gland composed of cells that contain hormone-secreting cytoplasmic granules; these, after a conventional H&E staining, characterize the adenohypophyseal cells as acidophils, basophils and chromophobes. By immunohistochemistry, however, the cells are distinguished in somatotrophs (GH cells), prolactin secreting cells (PRL cells), corticotrophs (ACTH cells), thyrotrophs (TSH cells) and gonadotrophs (FSH and LH cells). As to the posterior lobe, this being an extension of the hypothalamus, is composed largely of axons of the hypothalamic neurons, with the cell bodies of the neurons synthesizing oxytocin and antidiuretic hormone (ADH), also known as vasopressin.
Recent studies suggest that hypoxia is a strong regulator of the function of both adeno- and neuro-hypophysis, presumably by regulating transcription of hormone mRNA through specific receptor activation (2,3). Both protracted and intermittent hypoxia have been implicated in the regulation of hypophesial function (4-7). Hypoxia inducible factor 1α and 2α are principal transcription factor sensors of hypoxia (8). Theα-subunits in the cytoplasm of cells dimerize with the β-subunit, enter the nuclei and exert their transcriptional activity on a large number of genes, including the key angiogenesis involved gene VEGF (9) and a variety of genes involved in glycolysis, metabolism, cell cycle and death pathways (10,11).
In the current study, we investigated the vascular density and the expression of HIF1α, HIF2α and VEGF in the adenohypophysis and neurohypophysis of patients deceased from various acute or chronic diseases, in order to assess whether death patterns involving hypoxia have an effect on the hypophyseal sensing of hypoxic conditions.
Materials and Methods
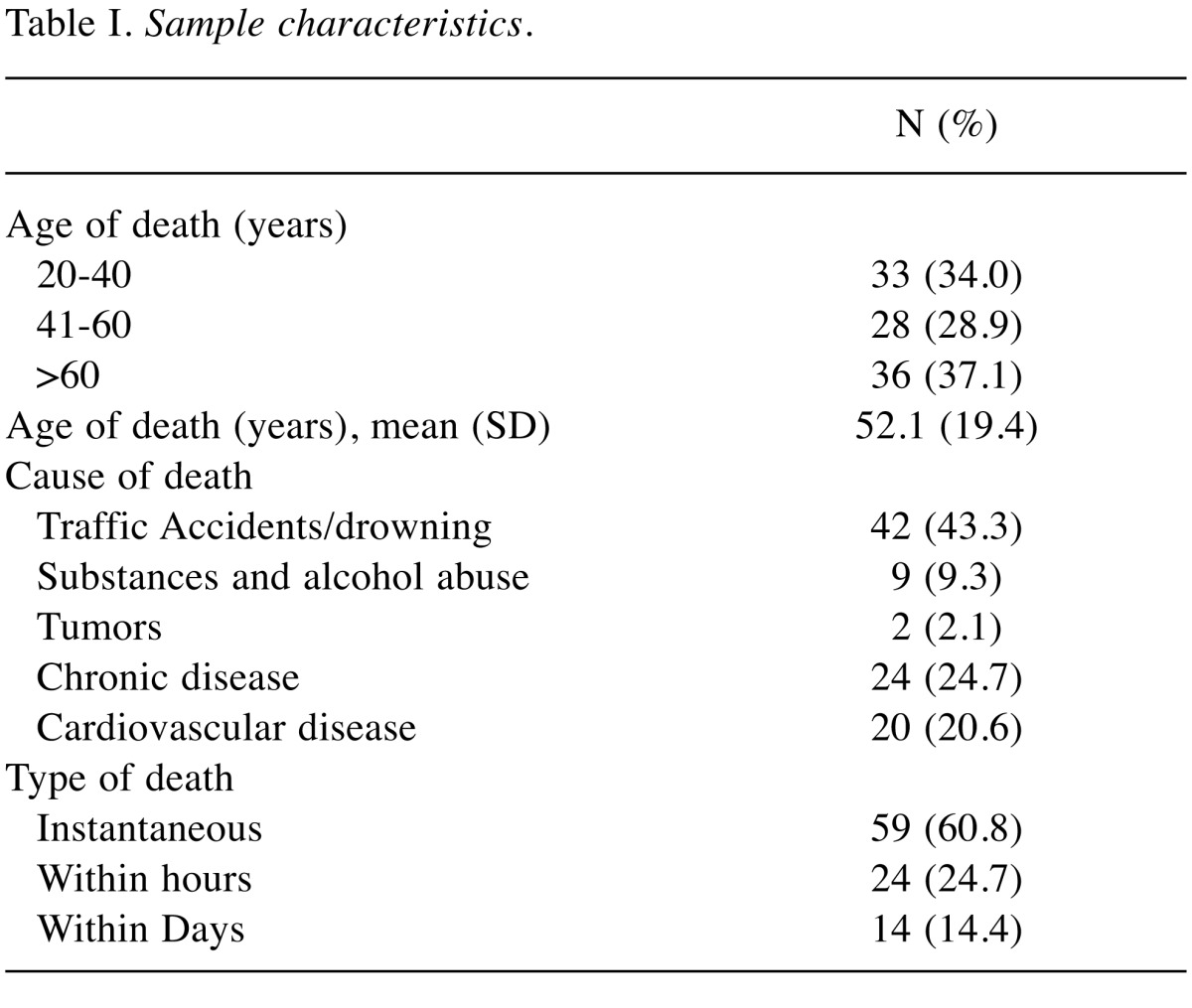
Pituitary glands from 97 cadavars were collected at autopsy. The study has been approved by the local Ethics and Research Committees. The procedure involved opening the sellar diaphragm and fracturing the dorsum of the sella turcica to allow the gland to be removed intact (12). After extraction, the pituitary glands were measured, weighed and fixed in 10% formalin. Subsequently, the tissues were processed routinely to paraffin wax and stained conventionally with hematoxylin-eosin. All the samples were grouped according to the age, cause of death and time-related patterns of death (Table I). The mean age of the individuals at autopsy was 52.1 years (SD=19.4 years), with 37.1% of them being over 60 years of age and 34.0% being between 20 and 40 years. Seventy-five of them were male and 22 were female. Most common cause of death was accident/drowning (43.3%) and the majority of individuals deceased instantly. The Institute Research Ethic Committee approved the collection of hypophyseal tissues and the immunohistochemical analysis (PGNE E.S.1/18-3-2015). Written informed consent from the relatives of the deceased was obtained.
Table I. Sample characteristics.
Immunohistochemistry. The HIF1a protein was detected with mouse monoclonal antibody 1A3 clone, IgG1 isotype (1/1000 dilution) and ab113642 (Abcam Limited, Cambridge, UK). The HIF2a protein identified by using monoclonal antibody ep190b clone, IgG1 isotype (1/100 dilution, overnight) ab8365 (Abcam Limited, Cambridge, UK). VEGF expression was evaluated with mouse monoclonal antibody VG-1 clone, IgG1 isotype (1/100 dilution) ab1316 (Abcam Limited, Cambridge, UK). CD31 expression was assessed with a ready to use FLEX monoclonal mouse antibody (JC/70A clone) from DAKO (Copenhagen, Denmark). For vimentin expression, a ready to use FLEX monoclonal mouse antibody from DAKO was used (V9 clone). S-100 proteins were evaluated with rabbit polyclonal antibody from DAKO (Copenhagen, Denmark) (code Z0311).
A modified streptavidin technique was used for immunohistochemistry, as previously reported (13,14). Briefly, sections were deparaffinized and peroxidase was quenched with methanol and 3% H2O2 for 15 min. Microwaving for antigen retrieval was used (3×5 min). The primary antibody was applied overnight. Following washing with TBS, sections were incubated with a secondary antibody (Kwik Kit, Cat. No. 404050, Thermo Shandon, Pittsburgh, PA, USA) for 15 min and washed in TBS. Kwik streptavidin peroxidase reagent was applied for 15 min and sections were again washed in TBS. The color was developed by 15 min incubation with DAB solution and sections were weakly counterstained with hematoxylin. Appropriate positive and negative controls were used.
The percentage of cells expressing the proteins under investigation was assessed in all optical fields at magnification x 200. The percentage of positive cells per optical field was recorded and the mean value of all fields was used to obtain the final score for each case. HIF reactivity is both nuclear and cytoplasmic. The cytoplasmic and the nuclear expression of these proteins was first assessed separately. For CD31+ vascular density assessment, tissue sections were scanned at low x40 magnification and three areas of the highest vascularization were chosen and microvessel counting followed. The final vascular density (VD) was the mean of the vessel counts obtained in these fields.
All staining scoring was performed separately by two independent observers. Any discrepancies were resolved on the conference microscope. The pathologists were blinded to the clinical data.
Statistical analysis. Quantitative variables were expressed as median (interquantile range), while qualitative variables were expressed as absolute and relative frequencies. For the comparison of proportions chi-square and Fisher’s exact tests were used. Mann-Whitney test was used for the comparison of continuous variables between two groups. Kruskall-Wallis test was used for the comparison of continuous variables between two or more groups. Spearman correlations coefficients were used to explore the association of two continuous variables. Correlation coefficient between 0.1 and 0.3 were considered low, between 0.31 and 0.5 moderate and those over 0.5 were considered high. All reported p-values are two-tailed. Statistical significance was set at p<0.05 and analyses were conducted using SPSS statistical software (version 19.0).
Results
All 97 pituitaries were examined microscopically, after stained conventionally with H&E. The following histopathological features were found: Fibrosis 15.5%; necrosis 5.2%; simple cysts 4.1%; benign tumors (one non-functional acidophilic microadenoma and one meningioma) 1%. It was noted that the proportion of cysts was significantly higher in individuals deceased between the age 41 to 60 years (14.3%) compared to those that their age of death ranged between 20 and 40 years (p=0.039) or was above 60 years (p=0.032).
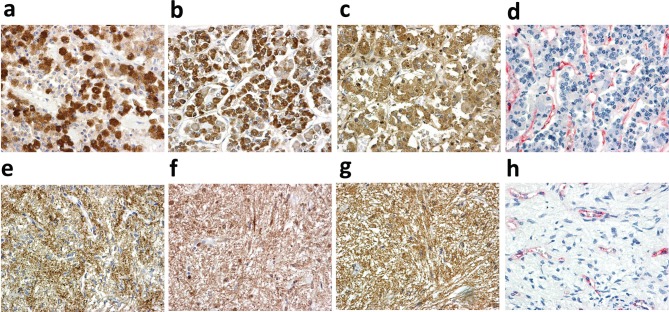
Table II demonstrates the expression patterns of the proteins examined in neurohypophysis and adenohypophysis. A most interesting feature that emerged was the very low ΗΙF1α expression in neurohypophysis, compared to almost equal expression of HIF1α and HIF2α in adenohypophysis. Typical immunohistochemical images are shown in Figure 1.
Table II. Expression of antigens in neurohypophysis and adenohypophysis.
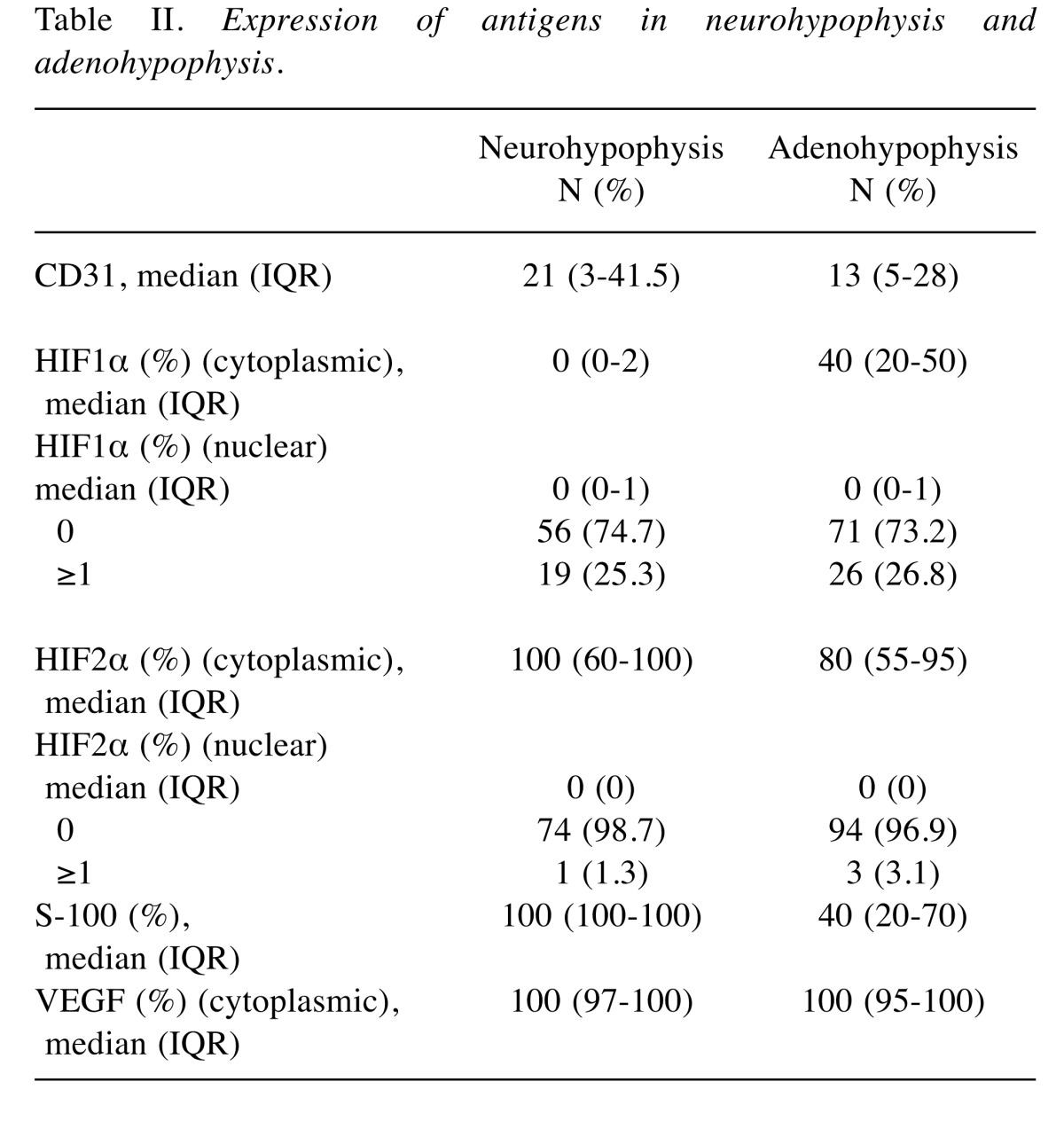
Figure 1. Immunohistochemical staining of tissue sections: (a) HIF1α expression in adenohypophysis; (b) HIF2α expression in adenohypophysis;(c) VEGF expression in adenohypophysis; (d) CD31 expression in adenohypophysis; (e) HIF1α expression in neurohypophysis; (f) HIF2α expressionin neurohypophysis; (g) VEGF expression in neurohypophysis; (h) CD31 expression in neurohypophysis.
Associations between molecular parameters. Neurohypo-physis: High CD31+ vascular density, was significantly associated with high HIF2α, VEGF and S-100 expression (p<0.05). Similarly, a high HIF2α expression was significantly associated with high VEGF and S-100 expression, and a high S-100 expression was significantly associated with a high VEGF positivity (p<0.05).
Adenohypophysis: A high HIF1α expression was significantly associated with high CD31+ vascular density and a low HIF2α expression (p<0.05).
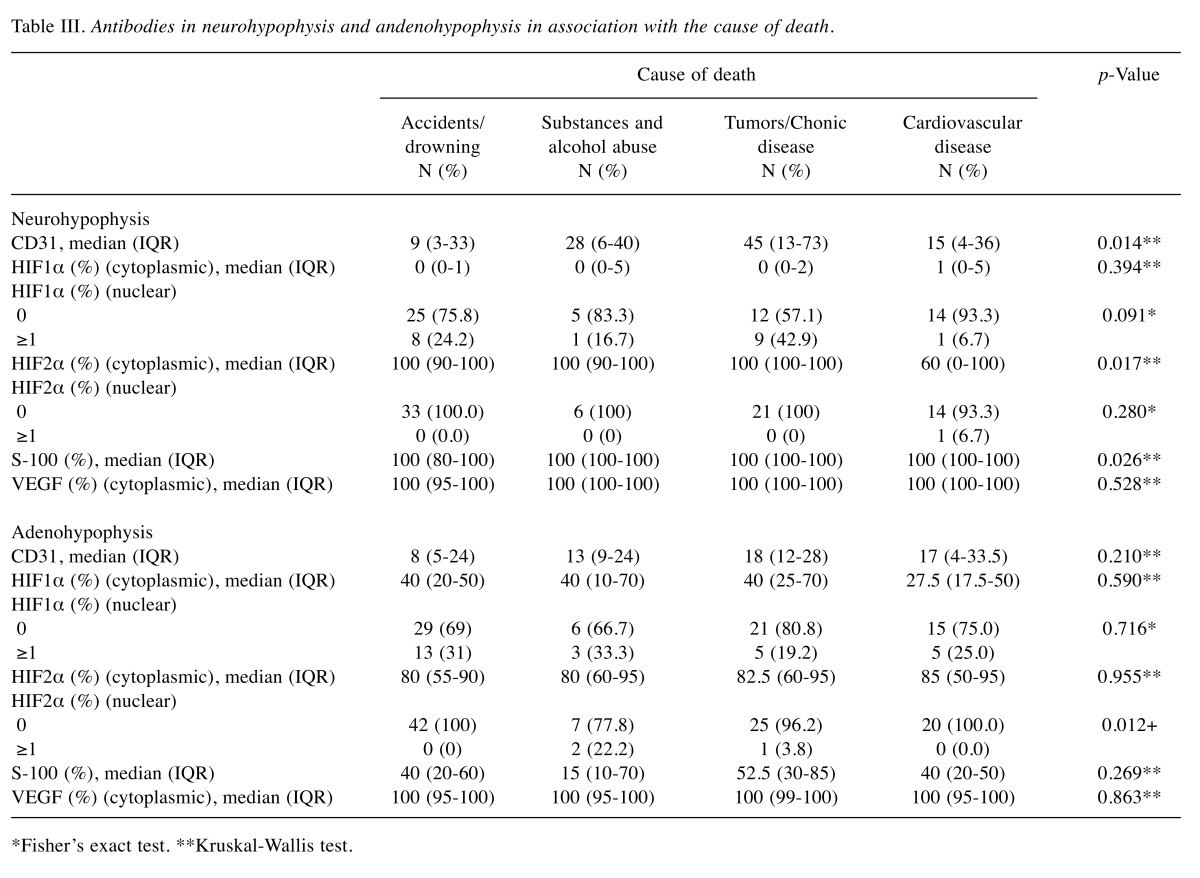
Association with the cause of death. Neurohypophysis: Chronic conditions, including benign tumors, exhibited higher CD31+ vascular density, higher cytoplasmic HIF1α and S-100 protein, compared to cases where the cause of death was accident/drowning (p=0.003, p=0.048 and p=0.025 respectively) (Table III). Furthermore, cytoplasmic HIF1α expression was significantly higher when the cause of death was chronic disease compared to cases where death was due to cardiovascular disease.
Table III. Antibodies in neurohypophysis and andenohypophysis in association with the cause of death.
*Fisher’s exact test. **Kruskal-Wallis test.
Adenohypophysis: Nuclear HIF1α expression (values ≥1) was significantly higher when death was due to chronic substance abuse and alcohol compared to cases where it was caused from accident/drowning (p=0.028) (Table III).
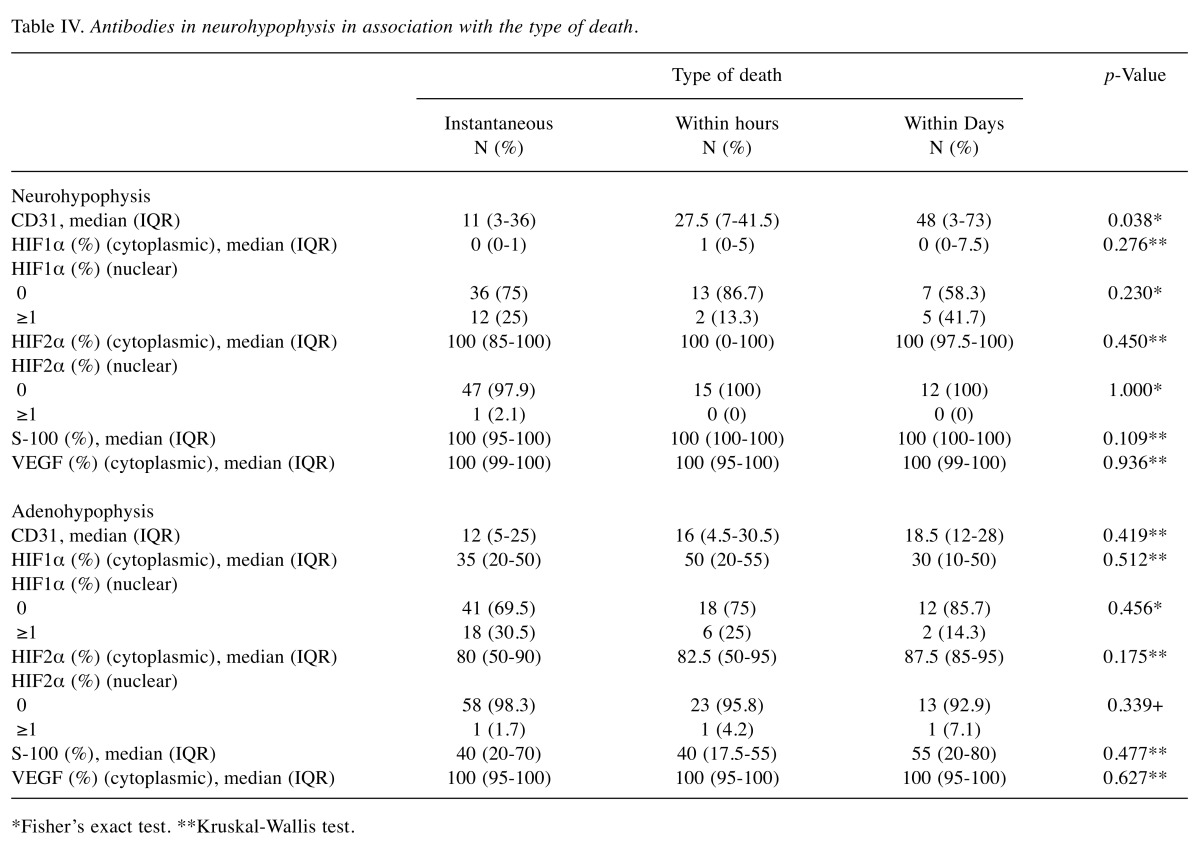
Association with the time-related type of death. Νeurohypophysis: CD31+ vascular density was significantly denser when deaths occurred within days or months rather than instantly (p=0.038) (Table IV).
Table IV. Antibodies in neurohypophysis in association with the type of death.
*Fisher’s exact test. **Kruskal-Wallis test.
Adenohypophysis: There were no significant differences regarding the expression of the molecular variables examined when these were associated with the type of death (Table IV).
Discussion
The role of hypoxia in the adjustment of function of the pituitary gland, both adeno- and neurohypophysis, has been raised in the early ‘70s (13,14). In 1978, Anderson et al. showed that hypoxia induced in dogs by lowering pressure of breathing oxygen induced an antidiuresis as confirmed by increased urine and of plasma arginine vasopressin levels (15). A similar result, with increased arginine vasopressin levels has been also reported in fetal sheep after maternal reduced oxygen delivery (16). An interesting study by Wilson et al., used radiolabelled microspheres to monitor the neurohypophyseal blood flow in dogs (17). A 2-fold increase of the blood flow in the neurohypophysis was documented.
Several published studies also support the links between hypoxia and andenohyphyseal pituitary function. In 1981, Semple et al., reported that hypoxia induced during chronic obstructive airway diseases in humans can lead to alterations of the hypothalamic-pituitary function, particularly in the pituitary-testicular axis (18), although these findings were subsequently questioned in a study by Banks et al. (19). Studies in rats and pikas and ewes, exposed to altitude mediate reduced oxygen breathing suggested that hypoxic stress increases release of ACTH via cAMP (4,20). Acute hypoxia seems also to result in increased ACTH release and cortisone blood levels in neonatal rats (21). Intermitent hypoxia has been also shown to suppress GH mRNA expression in adenohypophysis and of GH release in rats (22). ACTH receptor mRNA levels seem also to change under hypoxic conditions in rats, although intermittent hypoxia seems to decrease levels and longer exposure to hypoxia is demanded for an increase to occur (23). Additional studies also suggest that hypoxia has an effect in the control of proopiomelanocortin expression and processing (24,25).
In the current study we investigated the activation status of the Hypoxia Inducible Factor pathway in the pituitary gland, by studying the expression levels of ΗΙF1α, HIF2α and VEGF, in patients deceased by various causes, including acute hypoxia through asphyxia. An impotent finding was that HIF1α seems to be poorly expressed in neurohypophysis, whereas ΗΙF2α seems to be the key hypoxia response element. Indeed, overexpression of HIF2α in neurohypophysis was directly linked with VEGF expression and increased vascular density, which support a role of HIF2α in blood flow and hormone release in this pituitary area. By contrast, HIF1α seems to have a prevalent role in adenohypophysis, where its overexpression was linked with high vascular density.
Studying the cause of death and the patterns of expression of the HIF pathway, we found that chronic diseases, like neoplasms, have a direct effect in increasing the vascular density in neurohypophysis, which is paralleled with an increased expression of HIF1α that normally is very poorly expressed. Chronic substance abuse, like alcoholism, was directly linked with high ΗΙF1α expression in adenohypophysis.
It is concluded that hypoxia inducible factors are expressed in the pituitary gland and are directly linked with VEGF expression and vascular density. This supports that the HIF pathway may have an important role in regulating vascular density and blood flow in the gland under hypoxic stress. Of interest, HIF2α seems to be a key regulator in neurohypophysis compared to HIF1α. Chronic disease like neoplasia or chronic substance abuse like alcoholism seem to activate the HIF pathway in both neuro and adenohypophysis.
Conflicts of Interest
There are no conflicts of interest to declare.
Acknowledgements
The study has been financially supported by the Tumour and Angiogenesis Research Group.
References
- 1. Endocrinology: An Integrated Approach. Chapter 7. The pituitary gland https://www.ncbi.nlm.nih.gov/books/NBK27/ [Google Scholar]
- 2.Xu JF, Chen XQ, Du JZ. CRH receptor type 1 mediates continual hypoxia-induced changes of immunoreactive prolactin and prolactin mRNA expression in rat pituitary. Horm Behav. 2006;49:181–189. doi: 10.1016/j.yhbeh.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Wang TY, Chen XQ, Du JZ, Xu NY, Wei CB, Vale WW. Corticotropin-releasing factor receptor type 1 and 2 mRNA expression in the rat anterior pituitary is modulated by intermittent hypoxia, cold and restraint. Neuroscience. 2004;128:111–119. doi: 10.1016/j.neuroscience.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Ducsay CA, Mlynarczyk M, Kaushal KM, Hyatt K, Hanson K, Myers DA. Long-term hypoxia enhances ACTH response to arginine vasopressin but not corticotropin-releasing hormone in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2009;297:R892–899. doi: 10.1152/ajpregu.00220.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu NY, Chen XQ, Du JZ, Wang TY, Duan C. Intermittent hypoxia causes a suppressed pituitary growth hormone through somatostatin. Neuro Endocrinol Lett. 2004;25:361–367. [PubMed] [Google Scholar]
- 6.Kelestimur H, Leach RM, Ward JP, Forsling ML. Vasopressin and oxytocin release during prolonged environmental hypoxia in the rat. Thorax. 1997;52:84–88. doi: 10.1136/thx.52.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stegner H, Leake RD, Palmer SM, Oakes G, Fisher DA. The effect of hypoxia on neurohypophyseal hormone release in fetal and maternal sheep. Pediatr Res. 1984;18:188–191. doi: 10.1203/00006450-198402000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2:336–361. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- 9.Semenza GL. Vascular responses to hypoxia and ischemia. Arterioscler Thromb Vasc Biol. 2010;30:648–652. doi: 10.1161/ATVBAHA.108.181644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semenza GL. Hypoxia-inducible factors: coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. EMBO J. 2017;36:252–159. doi: 10.15252/embj.201695204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semenza GL. Hypoxia. Cross talk between oxygen sensing and the cell cycle machinery. Am J Physiol Cell Physiol. 2011;301:C550–552. doi: 10.1152/ajpcell.00176.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallecillos FJ, Fernández SO. Histopathological features of post-mortem pituitaries: A retrospective analysis. Rev Assoc Med Bras. 2016;62:399–406. doi: 10.1590/1806-9282.62.05.399. [DOI] [PubMed] [Google Scholar]
- 13.Roux C, Capuccio P, Grillo JM. Effect of hypoxia and of anoxia on the posterior pituitary gland of the rat. C R Seances Soc Biol Fil. 1975;169:622–623. [PubMed] [Google Scholar]
- 14.Roychoudhury DK, Banerjee PK. Effect of hypoxia on the anterior lobe of pituitary in guineapigs. Indian J Exp Biol. 1977;15:1208–1210. [PubMed] [Google Scholar]
- 15.Anderson RJ, Pluss RG, Berns AS, Jackson JT, Arnold PE, Schrier RW, McDonald KE. Mechanism of effect of hypoxia on renal water excretion. J Clin Invest. 1978;62:769–777. doi: 10.1172/JCI109188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stegner H, Leake RD, Palmer SM, Oakes G, Fisher DA. The effect of hypoxia on neurohypophyseal hormone release in fetal and maternal Sheep. Pediatr Res. 1984;18:188–191. doi: 10.1203/00006450-198402000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Wilson DA, Hanley DF, Feldman MA, Traystman RJ. Influence of chemoreceptors on neurohypophyseal blood flow during hypoxic hypoxia. Circ Res. 1987;61:94–101. [PubMed] [Google Scholar]
- 18.Semple PD, Beastall GH, Watson WS, Hume R. Hypothalamic-pituitary dysfunction in respiratory hypoxia. Thorax. 1981;36:605–609. doi: 10.1136/thx.36.8.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banks WA, Cooper JA. Hypoxia and hypercarbia of chronic lung disease: minimal effects on anterior pituitary function. South Med J. 1990;83:290–293. doi: 10.1097/00007611-199003000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Du JZ. Hypoxia effects on hypothalamic corticotropin-releasing hormone and anterior pituitary cAMP. Zhongguo Yao Li Xue Bao. 1996;17:489–492. [PubMed] [Google Scholar]
- 21.Bruder ED, Taylor JK, Kamer KJ, Raff H. Development of the ACTH and corticosterone response to acute hypoxia in the neonatal rat. Am J Physiol Regul Integr Comp Physiol. 2008;295:1195–1203. doi: 10.1152/ajpregu.90400.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu NY, Chen XQ, Du JZ, Wang TY, Duan C. Intermittent hypoxia causes a suppressed pituitary growth hormone through somatostatin. Neuro Endocrinol Lett. 2004;25:361–367. [PubMed] [Google Scholar]
- 23.Wang TY, Chen XQ, Du JZ, Xu NY, Wei CB, Vale WW. Corticotropin-releasing factor receptor type 1 and 2 mRNA expression in the rat anterior pituitary is modulated by intermittent hypoxia, cold and restraint. Neuroscience. 2004;128:111–119. doi: 10.1016/j.neuroscience.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 24.Myers DA, Bell PA, Hyatt K, Mlynarczyk M, Ducsay CA. Long-term hypoxia enhances proopiomelanocortin processing in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2005;288:1178–1184. doi: 10.1152/ajpregu.00697.2004. [DOI] [PubMed] [Google Scholar]
- 25.Braems GA, Matthews SG, Challis JR. Differential regulation of proopiomelanocortin messenger ribonucleic acid in the pars distalis and pars intermedia of the pituitary gland after prolonged hypoxemia in fetal sheep. Endocrinology. 1996;137:2731–2738. doi: 10.1210/endo.137.7.8770892. [DOI] [PubMed] [Google Scholar]