Abstract
Background/Aim: Lung squamous cell carcinoma often arises from precancerous lesions where alterations in tumor suppressor genes and subsequent chromosomal instability are often observed due to carcinogen exposure. These tumors are often immunogenic; as such, immune checkpoint inhibitors are a promising therapeutic option. We hypothesized that the DNA damage response in tumor cells induces an immune response, thereby up-regulating programmed death-ligand 1 (PD-L1) expression on tumor cells, which in turn sensitizes them to anti-PD-1 therapy. Patients and Methods: An immunohistochemical analysis was performed in 41 consecutive lung squamous cell carcinoma patients who underwent surgery at our institution between April 2013 and March 2014. Results: The analysis revealed a high PD-L1 expression in 15 patients (37%) (p=0.028). The PD-L1 expression was positively associated with the nuclear γH2AX expression (p=0.02), that was confirmed by immunofluorescent staining. Conclusion: Our findings demonstrate that nuclear γH2AX expression is positively associated with the PD-L1 expression in lung squamous cell carcinoma.
Keywords: Immune checkpoint inhibitor, DNA damage response, lung squamous cell carcinoma
Lung cancer, which is a leading cause of cancer death worldwide, is associated with a poor survival, even when the tumor is surgically removed. In terms of histology, lung squamous cell carcinoma, which accounts for 22% of the cases of resected lung cancer, is associated with poorer overall survival, with a five-year survival rate of approximately 60% in comparison to adenocarcinoma, which is the most common histological type (68%) and which has a five-year survival rate of approximately 75% (1). Molecular targeting therapies have recently been developed and have shown promising results against advanced lung cancer. Among the various types of lung cancer, lung squamous cell carcinoma has fewer treatment options because it is driven by alterations of tumor suppressor genes and subsequent chromosomal instability rather than oncogenic mutations (2). The chromosomal instability results in accumulation of somatic mutations and DNA damage response (3). This may explain why carcinogen-induced cancer, like lung squamous cell carcinoma and melanoma, is often accompanied by inflammation, as the DNA damage response is a major trigger activating the innate immune response. This consequence has been closely examined in the immune elimination process during viral infection and recently in the cancer immunity cycle (4).
The activation of the innate immune response leads to activation of immune checkpoint molecules as a mechanism of immune escape. Programmed death-ligand 1 (PD-L1) is one of the immune checkpoint molecules that are expressed on the surface of tumor cells. Once it is expressed on the tumor cells, PD-L1 binds to its receptor, PD-1, on the membrane of the cytotoxic T cell and inhibits T cell activity, resulting in the escape of the tumor cell from the immune system (5). The recent development of therapies against immune checkpoint molecules, namely, CTLA-4, PD-1 and PD-L1 has shown that PD-1/PD-L1 blockade can improve overall survival in patients with cancer including malignant melanoma, lung squamous cell carcinoma, and lung adenocarcinoma (6-13).
During the DNA damage response process, γH2AX, a unique histone subunit, serves as a sensor of double-stranded DNA damage, thereby gathering other proteins to form DNA damage repair complex foci (14). γH2AX foci are formed through irradiation, UV exposure, and cytotoxic chemotherapy, and the overexpression of γH2AX is common among various types of cancer. We hypothesized that the PD-L1 expression in lung squamous cell carcinoma cells is associated with the γH2AX expression as a marker of double-stranded DNA damage, with the expectation that the level of γH2AX may be a biomarker for PD-1/PD-L1-targeted therapy.
Materials and Methods
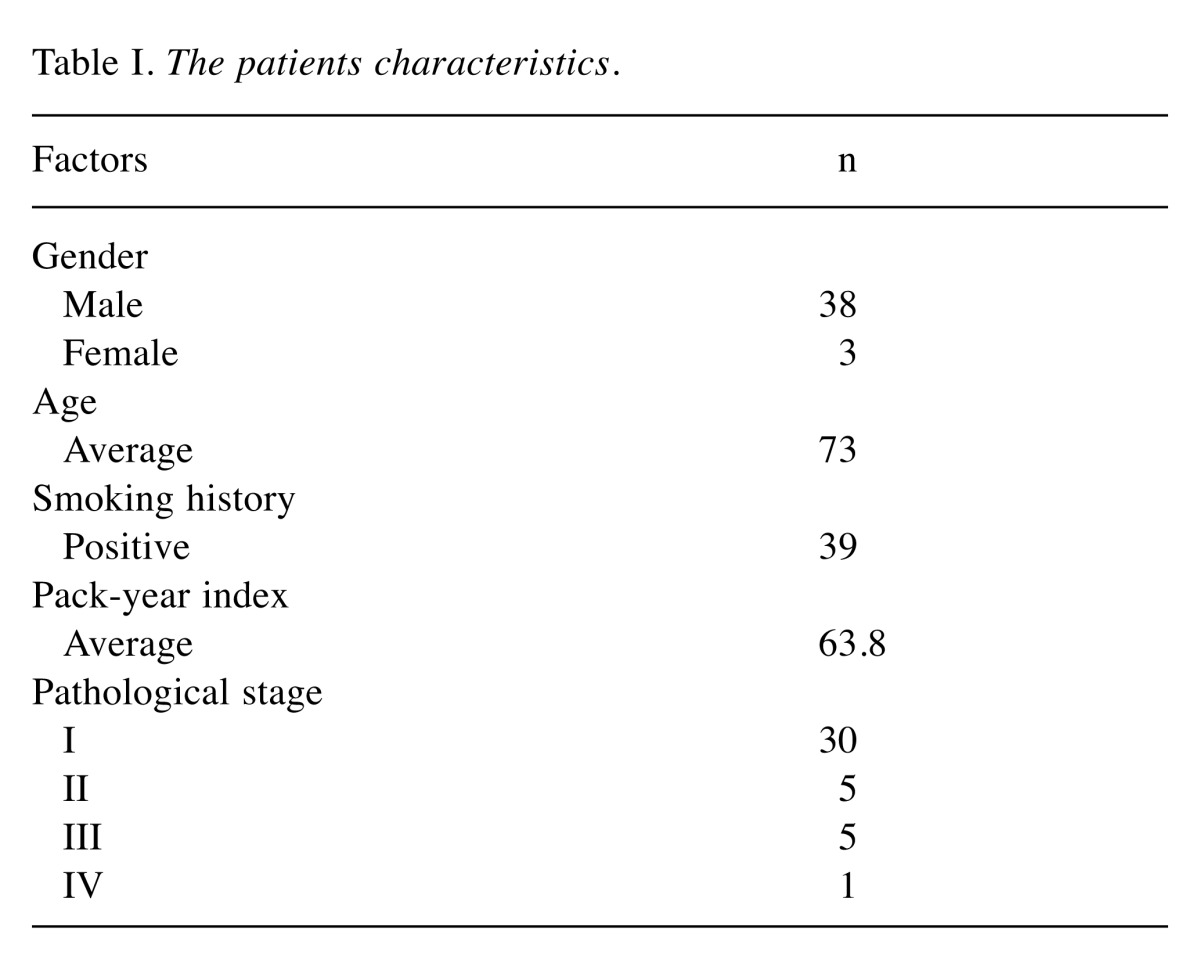
Patients. From April 2013 to March 2014, 41 consecutive patients with lung squamous cell carcinoma underwent lung resection at our institution. The demographic characteristics of the study population are summarized in Table I. Ninety-three percent of the patients were male and 95% had a history of smoking (average pack-years: 63.8). One patient who had stage IV disease, which was confirmed by malignant effusion (M1a) at surgery, underwent the partial resection of the primary tumor for diagnostic purposes and was excluded from the survival analysis. The use of the specimens was approved by the internal review board of Oita University Faculty of Medicine, and informed consent was obtained from each patient.
Table I. The patients characteristics.
Immunohistochemistry. Immunohistochemical studies were performed using formalin-fixed, paraffin-embedded tissue sections. Four-micrometer-thick sections were deparaffinized with xylene and rehydrated in a series of ethanol solutions. Endogenous peroxidase was blocked at room temperature by 3% hydrogen peroxide in methanol for 20 min. Heat-induced epitope retrieval was performed in 0.01 M citrate buffer (pH 6.0) and the samples were autoclaved at 121˚C for 15 min. After blocking with normal goat serum, the slides were incubated with a rabbit monoclonal antibody against PD-L1 (1:200, clone E1L3N; Cell Signaling Technology, MA, USA) or with a mouse monoclonal antibody against γH2AX (1:200, clone JBW301; EMD Millipore, Darmstadt, Germany) overnight at 4˚C. After washing, the sections were treated in goat anti-mouse/rabbit immunoglobulin labeled with horseradish peroxidase (Histofine Simple Stain MAX-PO, Nichirei, Tokyo) for 30 min at room temperature. Staining was completed using diaminobenzidine as a chromogen; the slides were then counterstained with hematoxylin. For the negative controls, the primary antibody was replaced with phosphate buffered saline containing 1% bovine serum albumin. Human thymoma tissue (for PD-L1) and normal human tonsil tissue (for γH2AX) were used as positive controls. At least 400 cancer cells were counted in 4 high-power fields. The H score (the staining intensity multiplied by the percentage of positively stained tumor cells) was used to evaluate PD-L1 expression. The staining intensity was classified as follows: 0, negative; 1, weak; 2, moderate; and 3, strong (Figure 1A). For example, if a tumor was 60% negative and 40% positive with 30% weak staining and 10% moderate staining, the H score would be 50 (60×0 + 30×1 +10×2). We did not evaluate the staining of tumor infiltrating lymphocytes. Nuclear staining was scored to determine γH2AX expression. The IHC evaluations were performed independently by AO and HH, both of whom were blinded to the patient characteristics; their average scores were used for the evaluation. There were few interobserver differences in the staining judgments.
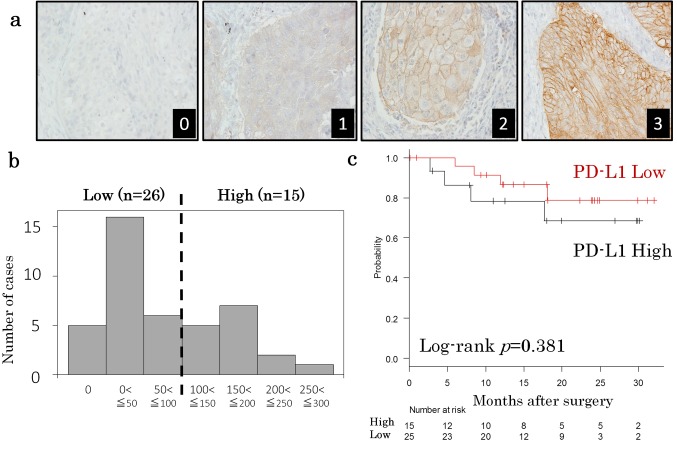
Figure 1. Programmed death- ligand 1 (PD-L1) expression in lung squamous cell carcinoma. (a) Representative PD-L1 staining images. The tumor cell membrane is stained. 0: negative, 1: weak, 2: moderate, and 3: strong. (b) A histogram of the H score distribution. The cut-off value of 100 was chosen because of the bimodal distribution. (c) A Kaplan-Meier plot of recurrence-free survival after surgery. The PD-L1 high and PD-L1 low groups are shown by the black and red lines, respectively. The p values were calculated by a log-rank test.
Immunofluorescent staining. Tumors were embedded in optimum cutting temperature compound, snap frozen and 8-μm-thick sections were cut using a cryostat microtome and fixed in 4% paraformaldehyde in PBS for 15 min. The sections were then rinsed with PBS and permeabilized by treatment with PBS containing 0.3% Triton X-100 (PBS-T) for 10 min. After blocking with 1% BSA in PBS-T for 30 min, the sections were incubated with the primary antibody overnight at 4˚C. The sections were rinsed three times with PBS-T then incubated for 1 h at room temperature with Alexa 568 goat anti-mouse IgG antibody (Molecular Probes, MO, USA) at a concentration of 1:1000 and then rinsed three times in PBS-T. The antibodies were diluted with PBST containing 1% bovine serum albumin. DNA was stained with 100ng/ml DAPI (Molecular Probes, MO, USA). Images were obtained with a confocal laser fluorescence microscope (LSM710, Carl Zeiss, Oberkochen, Germany). Nuclei containing more than 10 foci were classified as positive.
Statistics. Fisher’s exact t-test was used to determine the statistical significance of differences between groups. p-Values of less than 0.05 were considered to indicate statistical significance. All patients were retrospectively analyzed for age and gender, size, smoking history and pack-year index, pathological stage and recurrence-free interval (between lung cancer resection and recurrence, 2nd primary lung cancer or death caused by lung cancer). The probability of survival was analyzed by the Kaplan-Meier method using the date of lung cancer resection as the starting point. The log-rank test was used to determine the significance of differences between the subgroups.
Results
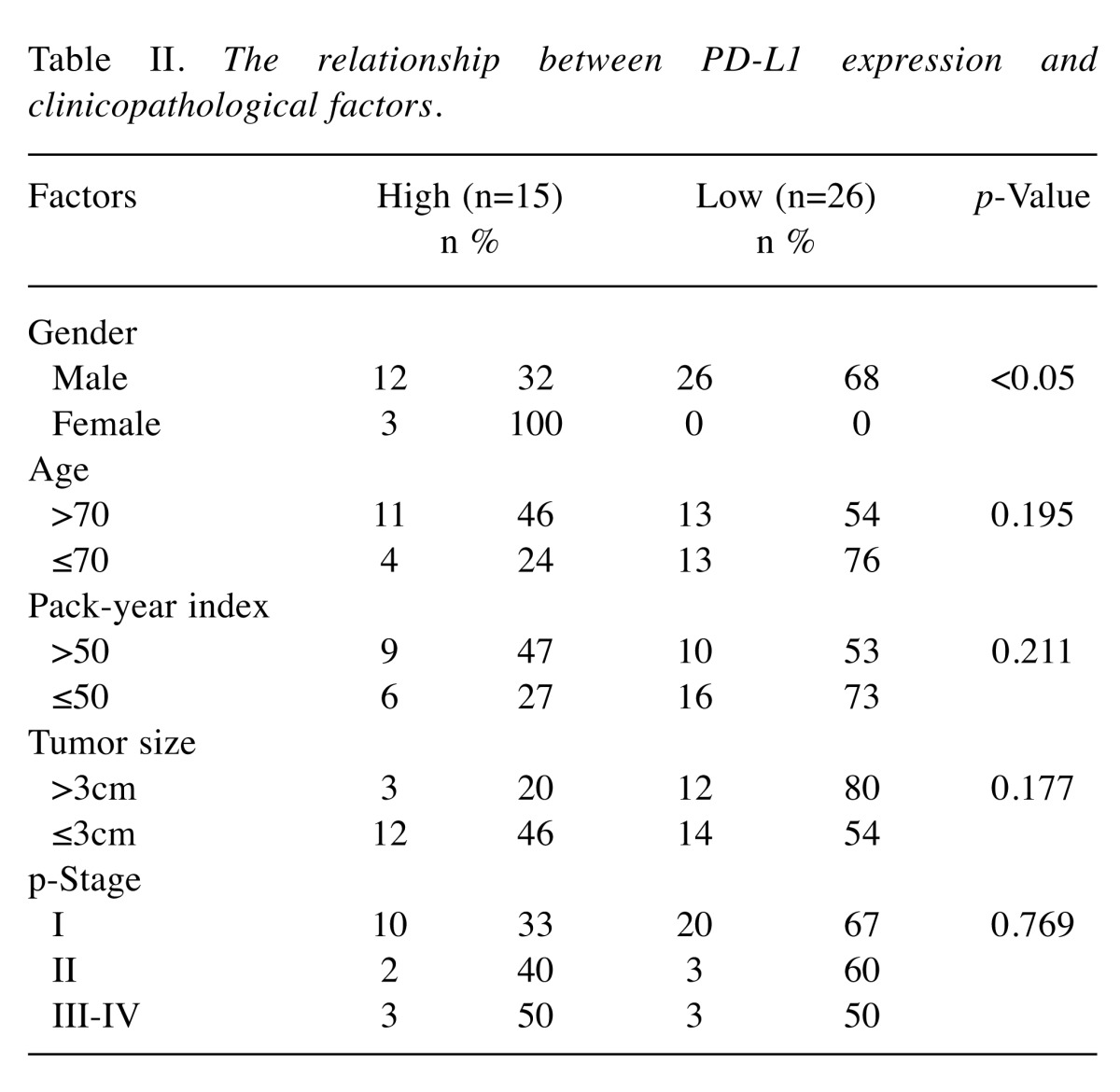
PD-L1 expression in lung squamous cell carcinoma. PD-L1 was mainly stained at the membrane of the tumor cells. The staining intensity ranged from 0 (negative) to 3 (strong) (Figure 1a). A histogram of the PD-L1 staining levels, calculated as H scores, is shown in Figure 1b. The H score was 0 in 5 cases, and 300 in one case. The average score was 84.5. A cut-off value of 100 was chosen because of the bimodal distribution of the histogram. Thus PD-L1 H scores of >100 and ≤100 were therefore used to define PD-L1 high (37%, n=15) and PD-L1 low (67%, n=26), respectively. The relationship between PD-L1 expression and the clinicopathological factors was then analyzed. With the exception of gender, no significant differences were observed among any of the factors (age, smoking history, tumor size or pathological stage) (Table II). A prognostic analysis was performed using 40 completely resected cases. There was no difference in survival curves between patients with PD-L1 high and patients with PD-L1 low (p=0.381, Figure 1c).
Table II. The relationship between PD-L1 expression and clinicopathological factors.
γH2AX expression and its relationship with PD-L1 expression. In the γH2AX staining experiments, the tumor cell nuclei were stained and the percentages of positively stained tumor cell were measured. The average percentage was 23.6% (range=0-93.5%). A representative γH2AX staining is shown in Figure 2a. The cut-off level of 20% was chosen, because the phosphorylation of H2AX at Ser-139 is also reported to occur during the S and G2/M phase in the cell cycle (15), and because the normal tonsil nuclei, which served as positive controls, showed <20% γH2AX positivity (data not shown). Thus, the γH2AX levels were low in 24 patients (58.5%) and high in 17 patients (41.5%).
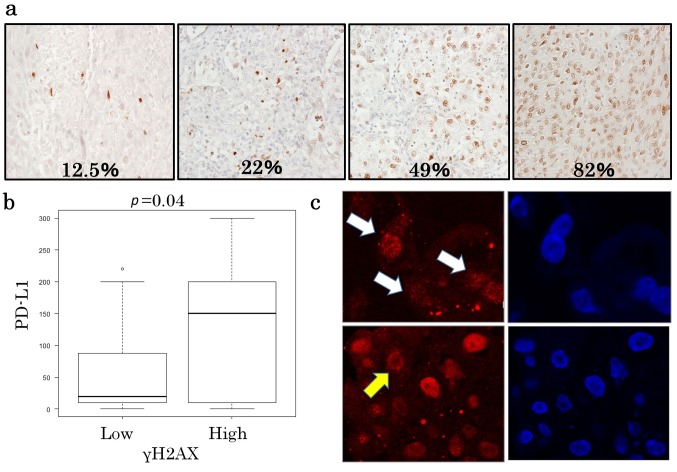
Figure 2. γH2AX expression in lung squamous cell carcinoma. (a) Representative images of γH2AX expression. γH2AX is expressed in the nucleus of the tumor cell. The percentage of positively stained nuclei is noted at the bottom of each image. (b) A box and whisker plot showing the PD-L1 protein levels in the γH2AX low and γH2AX high groups. (c) The γH2AX focus formation was observed by confocal microscopy. The γH2AX foci are identified in the tumor cell nuclei (red, indicated by white arrows). A ring-shaped γH2AX staining is indicated by a yellow arrow. The nuclei in the right panels were stained blue with DAPI.
The relationship between γH2AX and PD-L1 was examined using Fisher’s exact test and the Mann-Whitney U-test. Among the γH2AX-low cases, the PD-L1 expression level was low in 19 cases (79%), while among the γH2AX-high cases, the PD-L1 expression levels were high in 10 cases (58.8%). Thus, a positive relationship was observed between PD-L1 expression and γH2AX expression (Table III, p=0.02). This trend remained significant when the PD-L1 expression levels were compared as continuous variables according to the γH2AX levels (high or low, p=0.04; Figure 2b).
Table III. The relationship between PD-L1 and γH2AX expression.

Immunofluorescent staining for γH2AX was performed to confirm whether or not γH2AX expression reflects the DNA damage response. Five γH2AX high cases for which frozen tumor sections were available were selected for staining with the same antibody that was used for the immunohistochemical analysis. The formation of γH2AX foci in the tumor cell nuclei was observed in all cases, suggesting that γH2AX was activated in response to DNA damage (Figure 2c, upper panel). Interestingly, we noticed sporadic ring-shaped staining patterns, which suggested the existence of small DNA fragments (Figure 2c, lower panel).
Discussion
We showed, for the first time, that the DNA damage response is associated with the PD-L1 expression in patients with lung squamous cell carcinoma. Several clinical trials have shown the relationship between mutation burden and the efficacy of anti-PD-1/PD-L1 therapy, possibly because a high mutation burden can produce neoantigens, which can be targeted by cytotoxic T cells (16-18). In another study in tumors with mismatch-repair deficiency, the anti-PD-1 antibody pembrolizumab achieved promising outcomes in terms of the response rate and overall survival (19). Our findings add new insight into why tumors with high mutation burdens exhibit fair responses to immune checkpoint inhibitors; tumors with high mutation burdens are always dealing with DNA damage, which is reprehended by antigen presenting cells (APCs), a part of the innate immune system. Furthermore, because mutations found in hypermutators are mainly located in the genes responsible for the DNA damage response (16), tumors have a fair chance of turning apoptotic. The APCs activate cytotoxic T cells, which in turn activate PD-L1 on the tumor cells, so inhibitors of immune checkpoint molecules are effective in tumors with a high mutation burden but not in those with driver mutations, such as EGFR and ALK. Our results may also support the notion of combining anti-PD-1 therapy with other cytotoxic therapies, such as alkylating agents and irradiation (20), which cause DNA double-strand breaks, possibly resulting in the expression of PD-L1 on the tumor cell surface.
Recent studies have shown that the STING pathway is essential to the innate immune response against tumors. Cytosolic double-stranded DNA fragments are recognized by cGAMP, which in turn activates STING, resulting in the activation of APCs (21-23). This activation causes a type I interferon response, and IFNγ activates PD-L1 in tumor cells through the STAT3 or MEK signaling pathways (24-27). Indeed, tumors that developed in STING knock-out mice were not eliminated by immune checkpoint inhibitors, while those that developed in wild type mice were eliminated (23).
H2AX is a subunit of histones and is phosphorylated at Ser-139 (γH2AX) on double-stranded DNA damage through ATM phosphorylation. This phosphorylation also occurs during the S phase of the cell cycle and can be detected as homogenous staining with immunofluorescence. Our results showed the nuclear focus formation of γH2AX, suggesting double-stranded DNA damage repair. Furthermore, we also noticed occasional γH2AX apoptotic rings, which is an indicator of double-stranded DNA fragmentation and impending apoptosis (28,29). This likely activates the STING pathway, which again is essential for PD-1/PD-L1 signaling. Another possible mechanism is as follows: the PI3K-Akt pathway is also reported to be important for PD-L1 activation (30-32), possibly through PTEN inactivation (33,34). It has recently been rediscovered that the PTEN-PI3K-Akt pathway is indispensable in DNA damage repair (35,36). We previously reported that PTEN null lung and breast cell lines exhibit high γH2AX expression levels and are sensitive to olaparib, a polyADP-ribose polymerase inhibitor (37). These extrinsic or intrinsic mechanisms may link the DNA damage response to the PD-L1 expression. Further studies are warranted to clarify the mechanism in detail.
It should also be noted that there is no established method of evaluating PD-L1 expression in the tumor. Several anti-PD-L1 antibodies have been used to evaluate PD-L1 expression in clinical trials, but the cut-off values were not consistent, partly because of the low sensitivity of the antibodies used for immunohistochemistry (38,39). Furthermore, PD-L1 protein expression was not ubiquitous in the tumor tissue samples; some exhibited peripheral staining, another exhibited patchy staining (data not shown), as already reported both in clinical samples and in animal studies (38,40). Because of the heterogeneity of PD-L1 staining intensity/distribution in tumors, we adopted the H-score instead of using the tumor proportion score (TPS), which is used as a biomarker for the anti-PD-1 therapeutic response (6,9). In clinical trials, the TPS was chosen as a simpler method of evaluating the PD-L1 expression than the H-score (41). However, an immunohistochemical analysis of γH2AX expression in the tumor cell nucleus provides clearer results because it is an established marker of DNA double strand breaks, and it is strongly and uniformly expressed in certain cell nuclei (14).
In summary, we showed that PD-L1 expression is relatively common in lung squamous cell carcinoma, and that PD-L1 expression is positively related to γH2AX expression.
Conflicts of Interest
The Authors declare no conflicts of interest in association with the present study.
Acknowledgements
The Authors would like to thank Ms. Yuko Hono for technical assistance and Mr. Brian Quinn for his critical comments on the manuscript. This research is partly funded by Takeda Science Foundation (AO) and OITA Cancer Research Foundation (AO).
References
- 1.Sawabata N, Miyaoka E, Asamura H, Nakanishi Y, Eguchi K, Mori M, Nomori H, Fujii Y, Okumura M, Yokoi K, Japanese Joint Committee for Lung Cancer R. Japanese lung cancer registry study of 11,663 surgical cases in 2004: Demographic and prognosis changes over decade. J Thorac Oncol. 2011;6(7):1229–1235. doi: 10.1097/JTO.0b013e318219aae2. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research N. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, Kiezun A, Hammerman PS, McKenna A, Drier Y, Zou L, Ramos AH, Pugh TJ, Stransky N, Helman E, Kim J, Sougnez C, Ambrogio L, Nickerson E, Shefler E, Cortes ML, Auclair D, Saksena G, Voet D, Noble M, DiCara D, Lin P, Lichtenstein L, Heiman DI, Fennell T, Imielinski M, Hernandez B, Hodis E, Baca S, Dulak AM, Lohr J, Landau DA, Wu CJ, Melendez-Zajgla J, Hidalgo-Miranda A, Koren A, McCarroll SA, Mora J, Lee RS, Crompton B, Onofrio R, Parkin M, Winckler W, Ardlie K, Gabriel SB, Roberts CW, Biegel JA, Stegmaier K, Bass AJ, Garraway LA, Meyerson M, Golub TR, Gordenin DA, Sunyaev S, Lander ES, Getz G. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pateras IS, Havaki S, Nikitopoulou X, Vougas K, Townsend PA, Panayiotidis MI, Georgakilas AG, Gorgoulis VG. The DNA damage response and immune signaling alliance: Is it good or bad? Nature decides when and where. Pharmacol Ther. 2015;154:36–56. doi: 10.1016/j.pharmthera.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR Jr., Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-pd-l1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, Investigators K- Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 10.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-pd-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, pd-l1-positive, advanced non-small-cell lung cancer (keynote-010): A randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 12.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-pd-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. Gammah2ax and cancer. Nat Rev Cancer. 2008;8(12):957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tu WZ, Li B, Huang B, Wang Y, Liu XD, Guan H, Zhang SM, Tang Y, Rang WQ, Zhou PK. Gammah2ax foci formation in the absence of DNA damage: Mitotic h2ax phosphorylation is mediated by the DNA-pkcs/chk2 pathway. FEBS Lett. 2013;587(21):3437–3443. doi: 10.1016/j.febslet.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 16.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to pd-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 18.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to ctla-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. Pd-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1(9):1325–1332. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 21.Burdette DL, Vance RE. Sting and the innate immune response to nucleic acids in the cytosol. Nat Immunol. 2013;14(1):19–26. doi: 10.1038/ni.2491. [DOI] [PubMed] [Google Scholar]
- 22.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, Huang X, Gajewski TF, Chen ZJ, Fu YX, Weichselbaum RR. Sting-dependent cytosolic DNA sensing promotes radiation-induced type i interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41(5):843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald KA, Alegre ML, Gajewski TF. Sting-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, Saudemont A, Quesnel B. Plasma cells from multiple myeloma patients express b7-h1 (pd-l1) and increase expression after stimulation with ifn-{gamma} and tlr ligands via a myd88-, traf6-, and mek-dependent pathway. Blood. 2007;110(1):296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 25.Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA, Wasik MA. Oncogenic kinase npm/alk induces through stat3 expression of immunosuppressive protein cd274 (pd-l1, b7-h1) Proc Natl Acad Sci USA. 2008;105(52):20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto R, Nishikori M, Tashima M, Sakai T, Ichinohe T, Takaori-Kondo A, Ohmori K, Uchiyama T. B7-h1 expression is regulated by mek/erk signaling pathway in anaplastic large cell lymphoma and hodgkin lymphoma. Cancer Sci. 2009;100(11):2093–2100. doi: 10.1111/j.1349-7006.2009.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berthon C, Driss V, Liu J, Kuranda K, Leleu X, Jouy N, Hetuin D, Quesnel B. In acute myeloid leukemia, b7-h1 (pd-l1) protection of blasts from cytotoxic t cells is induced by tlr ligands and interferon-gamma and can be reversed using mek inhibitors. Cancer Immunol Immunother. 2010;59(12):1839–1849. doi: 10.1007/s00262-010-0909-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quanz M, Chassoux D, Berthault N, Agrario C, Sun JS, Dutreix M. Hyperactivation of DNA-pk by double-strand break mimicking molecules disorganizes DNA damage response. PLoS One. 2009;4(7):e6298. doi: 10.1371/journal.pone.0006298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solier S, Pommier Y. The nuclear gamma-h2ax apoptotic ring: Implications for cancers and autoimmune diseases. Cell Mol Life Sci. 2014;71(12):2289–2297. doi: 10.1007/s00018-013-1555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crane CA, Panner A, Murray JC, Wilson SP, Xu H, Chen L, Simko JP, Waldman FM, Pieper RO, Parsa AT. Pi(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene. 2009;28(2):306–312. doi: 10.1038/onc.2008.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang X, Zhou J, Giobbie-Hurder A, Wargo J, Hodi FS. The activation of mapk in melanoma cells resistant to braf inhibition promotes pd-l1 expression that is reversible by mek and pi3k inhibition. Clin Cancer Res. 2013;19(3):598–609. doi: 10.1158/1078-0432.CCR-12-2731. [DOI] [PubMed] [Google Scholar]
- 32.Lastwika KJ, Wilson W 3rd, Li QK, Norris J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, Liu LN, Gills JJ, Dennis PA. Control of pd-l1 expression by oncogenic activation of the akt/mtor pathway in non-small cell lung cancer. Cancer Res. 2016;76(2):227–238. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 33.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO. Loss of tumor suppressor pten function increases b7-h1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 34.Xu C, Fillmore CM, Koyama S, Wu H, Zhao Y, Chen Z, Herter-Sprie GS, Akbay EA, Tchaicha JH, Altabef A, Reibel JB, Walton Z, Ji H, Watanabe H, Janne PA, Castrillon DH, Rustgi AK, Bass AJ, Freeman GJ, Padera RF, Dranoff G, Hammerman PS, Kim CF, Wong KK. Loss of lkb1 and pten leads to lung squamous cell carcinoma with elevated pd-l1 expression. Cancer Cell. 2014;25(5):590–604. doi: 10.1016/j.ccr.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, Yin Y. Essential role for nuclear pten in maintaining chromosomal integrity. Cell. 2007;128(1):157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 36.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the pten tumour suppressor. Nat Rev Mol Cell Biol. 2012;13(5):283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 37.Osoegawa A, Gills JJ, Kawabata S, Dennis PA. Oncotarget. 2007;doi:10.18632/oncotarget.19667. doi: 10.18632/oncotarget.19667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerr KM, Tsao MS, Nicholson AG, Yatabe Y, Wistuba II, Hirsch FR, Committee IP. Programmed death-ligand 1 immunohistochemistry in lung cancer: In what state is this art. J Thorac Oncol. 2015;10(7):985–989. doi: 10.1097/JTO.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 39.Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, Richardson W, Towne P, Hanks D, Vennapusa B, Mistry A, Kalamegham R, Averbuch S, Novotny J, Rubin E, Emancipator K, McCaffery I, Williams JA, Walker J, Longshore J, Tsao MS, Kerr KM. Pd-l1 immunohistochemistry assays for lung cancer: Results from phase 1 of the blueprint pd-l1 ihc assay comparison project. J Thorac Oncol. 2017;12(2):208–222. doi: 10.1016/j.jtho.2016.11.2228. [DOI] [PubMed] [Google Scholar]
- 40.Maute RL, Gordon SR, Mayer AT, McCracken MN, Natarajan A, Ring NG, Kimura R, Tsai JM, Manglik A, Kruse AC, Gambhir SS, Weissman IL, Ring AM. Engineering high-affinity pd-1 variants for optimized immunotherapy and immuno-pet imaging. Proc Natl Acad Sci USA. 2015;112(47):E6506–6514. doi: 10.1073/pnas.1519623112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolled-Filhart M, Roach C, Toland G, Stanforth D, Jansson M, Lubiniecki GM, Ponto G, Emancipator K. Development of a companion diagnostic for pembrolizumab in non-small cell lung cancer using immunohistochemistry for programmed death ligand-1. Arch Pathol Lab Med. 2016 doi: 10.5858/arpa.2015-0542-OA. [DOI] [PubMed] [Google Scholar]