SUMMARY
Purpose.
In this study we wanted to observe the improvement in the healing of periodontal tissues in a group of diabetic patients treated with traditional methods compared to another group treated with the addition of oxygen.
The potential of oxygen has long been known in the field of plastic surgery, where it is used to treat burns and skin lesions.
Materials and methods.
This study consists in a split mouth study which involved 30 patients. We carefully treated them with periodontal therapy using manual and mechanical instrumentation. Then, we applied oxygen in half mouth according to randomization list. Finally we checked up patients after some weeks.
Results.
Our results highlight that all areas treated with oxygen application healed more rapidly and better than no treated areas.
Conclusions.
All in all, we have demonstrated that oxygen can improve the outcome of non-surgical periodontal treatment in diabetic subjects.
Keywords: oxygen, periodontitis, diabetes
Introduction
The potential of oxygen has long been known in the field of plastic surgery, where it is used to treat burns and skin lesions. In our bodies, oxygen is mostly bound to hemoglobin, which is normally 97% saturated. One gram of hemoglobin combines with 1.34 ml of oxygen, and as our hemoglobin content is equal to 15 g per 100 ml, it follows that 100 ml of blood transports about 20 ml of oxygen. The remainder of the oxygen is physically dissolved in plasma at a concentration of 0.3 ml per 100 cc of blood. The concentration of oxygen that increases during hyperbaric therapy is the portion dissolved in plasma; every pressure increase of 1 atmosphere leads to an increase in oxygen of 2 volumes percent. Consequently, at a pressure of 3ATA (20 m), the quantity dissolved in plasma is 6 volumes percent, a concentration sufficient to sustain vital cellular processes (1–5).
The use of oxygen by cells increases in certain morbid conditions, such as infections, and it is moreover fundamental in all pathologies whose etiology is associated with impaired vascularization, as in the case, for example, of diabetes. An increase in the availability of oxygen to hypoxic tissues favors various cellular processes, which can be summarized as follows:
increase in processes of repair of ischemic tissues: oxygen delivered in high volumes increases collagen synthesis, enabling a normal hydroxylation of this protein. At lower-than-normal tissue tensions, collagen is not correctly synthesized and ulcers and wounds fail to heal;
increase in the osteogenic stimulus: oxygen delivered in high volumes increases processes of mineralization and synthesis of bone tissue in the case of fractures and osteonecrotic lesions;
antibacterial action: oxygen delivered in high volumes has a double bactericidal action, direct and indirect.
The direct action towards bacteria consists in the production of free radicals (RL), a group of substances deriving from the reduction of oxygen, which degrades the cell wall of pathogenic germs. The indirect action is obtained through the synergy of hyperbaric oxygen with antibiotics in soft tissue or bone infections.
Anti-oedemigenic action: traumas involving areas with non-expandable compartments usually lead to the formation of oedema, which is maintained through a vicious cycle of “trauma-oedema-hypoxia-vasodilatationoedema”. Oxygen delivered in high volumes interrupts this mechanism by causing both arterial vasoconstriction and pressure on the superficial vein wall, thereby facilitating an increase in the venous circle and lymph drainage.
Neo-angiogenic action: after several applications, oxygen delivered in high volumes is capable of performing a neo-angiogenic function – meaning the formation of new vessels – through the release of such factors as the vascular endothelial growth factor (VEGF). This function is essential for restoring microcirculation in compromised vascular situations, such as diabetic foot, and thus re-establishing a vascular flow in hypoxic areas.
The effectiveness of topical oxygen as an additional treatment for wounds has been studied since the 1960s. In a brief pilot study, the Authors tested a modified technique for applying hyperbaric oxygen and observed a 50 and 90% reduction in the dimensions of the wound after 6 and 20 days, respectively. Transdermal oxygen therapy has shown to be optimal in improving cell metabolism and accelerating healing processes, thus reducing irritations, and performing an anti-inflammatory and antibacterial effect. It is well known that diabetes considerably complicates wound healing. This is due both to peripheral vasculopathy, which induces local hypoxia with a decrease in metabolites to cells, and to a decrease in the influx and reactivity of leukocytes and consequent increase in the likelihood of infections (6–14).
In consideration of the foregoing, in this study we wanted to observe the improvement in the healing of periodontal tissues in a group of diabetic patients treated with traditional methods compared to another group treated with the addition of oxygen.
Materials and methods
Study design
This study consists in a split mouth study which involved 30 patients meeting the following inclusion criteria: diagnosis of periodontitis from moderate to severe (PD > 5MM in over 30% of the sites that bleed on probing); age between 35 and 70 years; non-smokers or smokers of fewer than 10 cigarettes a day; diagnosis of diabetes. Diagnosis of diabetes was formulated according to the following criteria:
HbA1c > 6.5% (with certified NGS-aligned assay standardized to the DCCT Assay)
FPG ≥ 126 mg/dl (fasting is defined as no caloric intake for at least 8 hours)
glycaemia after 2 hours during 75 g OGTT ≥ 200 mg/dl (the test was performed as described by the World Health Organization, using a glucose load equivalent to 75 g of anhydrous glucose dissolved in water)
in the presence of typical symptoms of the disease (polyuria, polydipsia, weight loss) the diagnosis was confirmed with the occasional finding of even only one glycaemia value ≥ 200 mg/dl
Exclusion criteria were: patients with additional systemic diseases other than diabetes; patients with uncontrolled diabetes; patients who had received antibiotic therapy during the previous 6 months; patients with a full-arch implant pros-thesis (e.g. Columbus bridge, Toronto bridge). All the patients were selected among subjects with periodontitis who came to our clinic. Informed consent was obtained from all participants.
Periodontal treatment
The following clinical indices were determined using a periodontal probe CP 15: pocket depth (PD), probing attachment level (PAL), bleeding on probing (BoP) and plaque index (PlI).
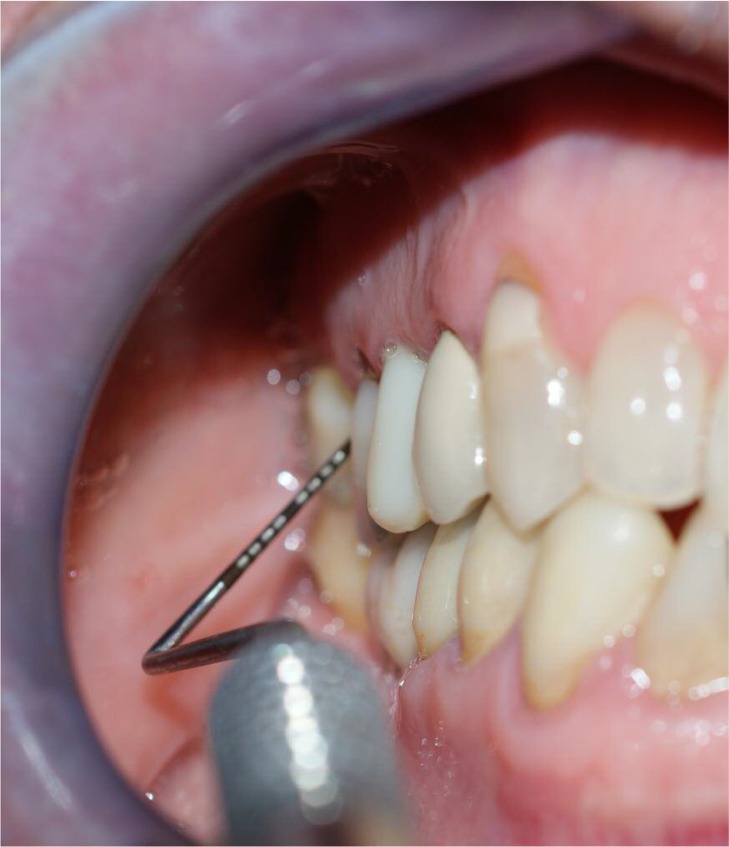
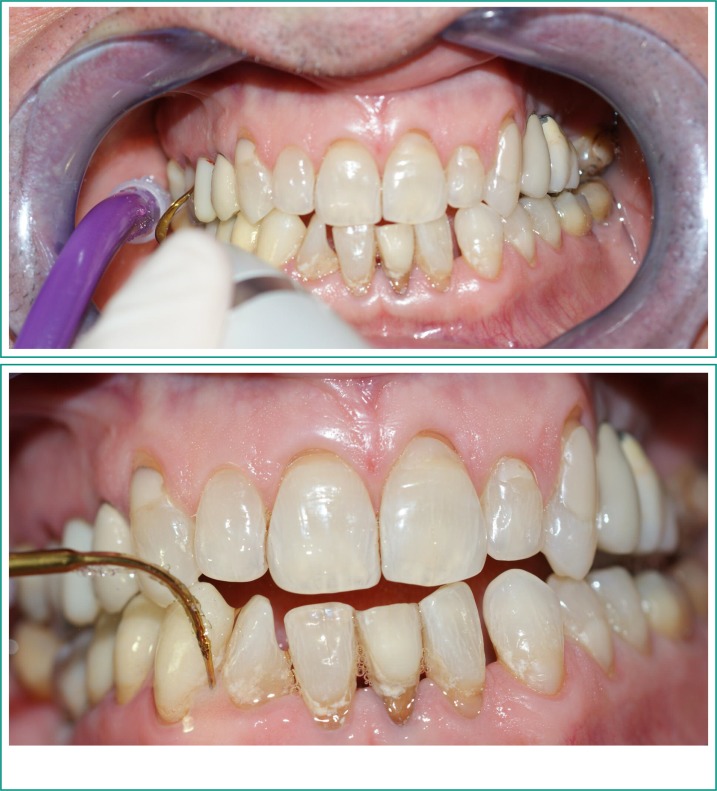
All evaluations were performed on six sites per element: mesial vestibular, vestibular and distal vestibular, mesial lingual/palatal, lingual/palatal and distal lingual/palatal. The probe was kept near the surface of the root and in line with the longitudinal axis of the tooth. The operator was instructed to apply a force of 30 g. Photos were taken in a non-standardized manner (Figure 1). After the indices were determined, a single-sitting full-mouth scaling and root planning protocol was applied to all patients. Glycine powder air polishing was used for plaque removal and universal ultrasonic tips for the removal of supra- and subgingival tartar (S1, P10 Mectron®) (Figures 2, 3).
Figure 1.
Clinical indexes were collected at baseline. Six sites per tooth were examined with a periodontal probe CP 15.
Figures 2–3.
A full mouth scaling and root planning protocol was performed to all patients.
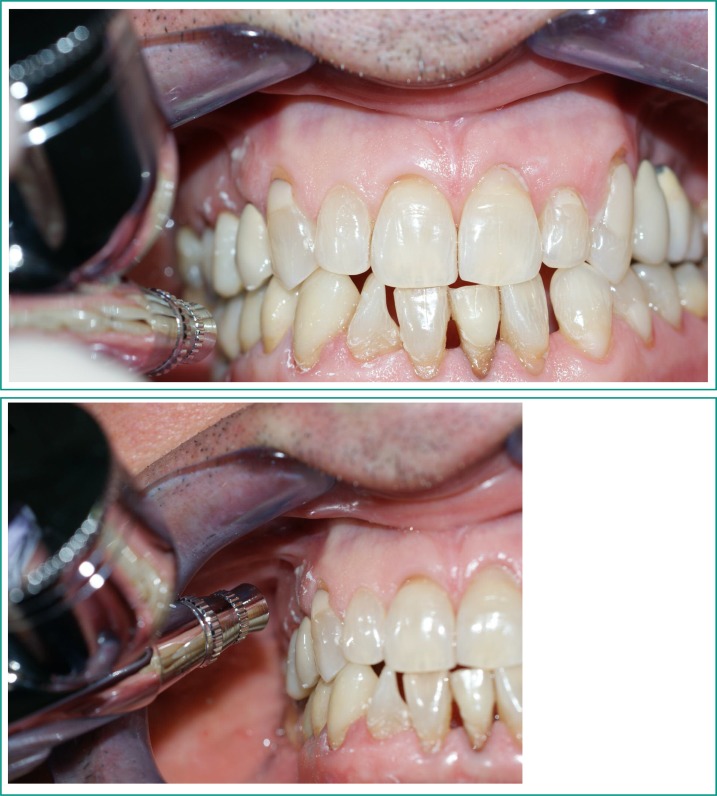
At the end of the treatment, a test side and control side were randomly selected for each patient and recorded in a special form. A specific software application was used to obtain a randomization list (Random Allocation Software version 1.0, downloadable from http://mahmoodsaghaei.tripod.com/Softwares/randalloc.html). An EXEA Genotechnology oxygen therapy device was subsequently used to deliver oxygen to the upper and lower arches on the test side only (Figures 4, 5). The 90% pure oxygen was emitted at a flow rate of 6 volumes/litre for twenty minutes, ten in the upper quadrant and ten in the lower one.
Figures 4–5.
90% pure oxygen was delivered on the test side using EXEA Genotechnology oxygen therapy device.
Each patient was instructed to maintain a good level of oral hygiene using a custom tailored technique (15) duly shown to the patient. A manual toothbrush with medium-soft bristles was selected (Gum® Technique Pro Compact) along with an interdental brush identified with a colour code (Gum® Trav-ler) and the patient was shown how to use them for two-minutes’ brushing twice a day and cleaning of interproximal areas, respectively.
All clinical parameters were recorded after 6 weeks by the same operator, who carefully evaluated the level of oral hygiene.
Results
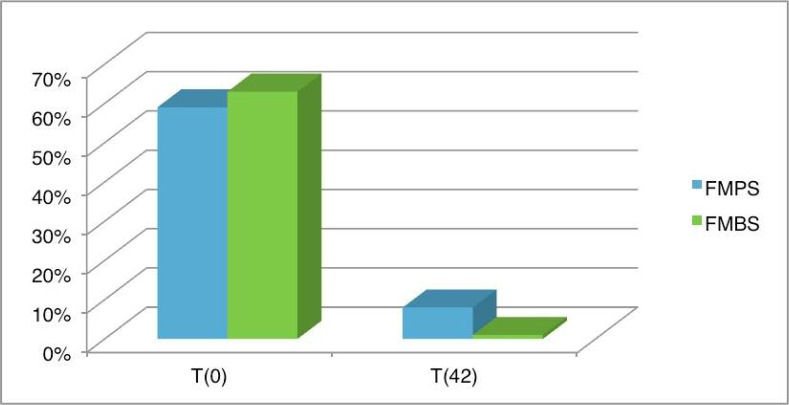
The first stage of therapy included motivation and the complete removal of tartar and supra- and sub-gingival plaque. The full-mouth plaque score (FMPS) was determined after 6 weeks in each patient. The results showed a large reduction compared to pre-treatment values (Figure 6). This is no doubt due to the effectiveness of the home oral hygiene regime and the short period of time between the first stage and the final evaluation. The results are summarized in Tables 1, 2 and 3.
Figure 6.
FMPS and FMBS in both groups at baseline and after 6 weeks.
Table 1.
Changes in FMBS and FMPS in numerical terms.
| values | median | Sd | ||||
|---|---|---|---|---|---|---|
| T(0) | T(42) | T(0) | T(42) | T(0) | T(42) | |
| FMPS | 59% | 8% | 56% | 6% | 3% | 1.00% |
| FMBS | 63% | 1% | 62% | 0.70% | 0% | 0.20% |
Table 2.
PD values found in the two groups in the two evaluations performed.
| values (mm) | median | Sd | ||||
|---|---|---|---|---|---|---|
| PD | T(0) | T(42) | T(0) | T(42) | T(0) | T(42) |
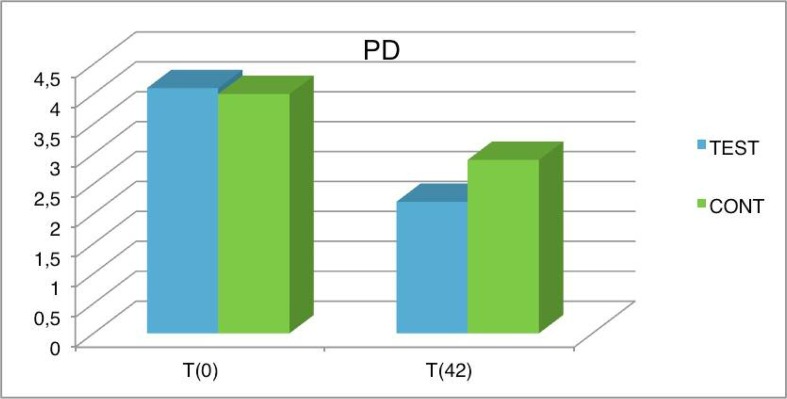
| TEST | 4.1 | 2.2 | 3.8 | 2 | 0.9 | 0.7 |
| CONT | 4 | 2.9 | 3.7 | 2.8 | 0.7 | 0.6 |
Table 3.
PAL values found in the two groups in the two evaluations performed.
| values (mm) | median | Sd | ||||
|---|---|---|---|---|---|---|
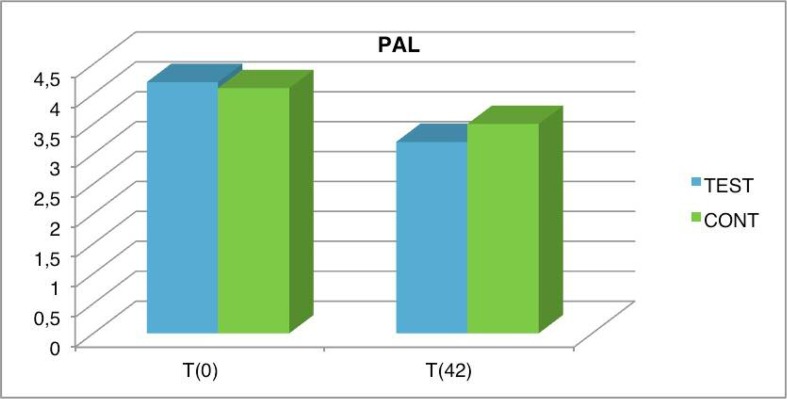
| PAL | T(0) | T(42) | T(0) | T(42) | T(0) | T(42) |
| TEST | 4.2 | 3.2 | 3.9 | 3.2 | 0.9 | 0.5 |
| CONT | 4.1 | 3.5 | 4 | 3.2 | 0.6 | 0.4 |
Similarly, the full-mouth bleeding score (FMBS) was reduced after the periodontal treatment. The results observed after 6 weeks actually confirm the success of the treatment (Figure 6).
After 6 weeks, changes were observed in the PD values compared to the baseline values (Figure 7). Noteworthy improvements were observed on both the test side and control side, indicating that the therapy was successfully implemented. In fact, on the control side, the difference between the PD values at baseline and after the therapy demonstrates that the FMSRP procedures reached the target. Moreover, the test side showed an average PD reduction of 1.9 millimeters, confirming that PD was reduced to a greater degree in this area. Graphic shows the difference between the pre- and post-treatment values.
Figure 7.
PD average values in test and control group at baseline and after 6 weeks.
Analogous considerations apply for the PAL values (Figure 8). The attachment gain after the periodontal treatment improved on both sides. However, better results were obtained on the test side than on the control side. Observing the differences in the PAL values, we may see that the improvements are 0.6 mm on the control side and 1 mm on the test side.
Figure 8.
PAL average values in test and control group at baseline and after 6 weeks.
Differences between the test and control sides were statistically significant according to Fisher’s test (P <0.05).
Discussion
In order to achieve good periodontal regeneration, it is necessary both to prevent the entry of bacteria into the wound and to stabilize coagulation (16–24). This occurs normally in healthy patients. It is well known that this result is much more difficult to obtain in diabetic patients, due both to the decrease in reactivity of neutrophils and leukocytes and the presence of peripheral vasculopathy. A persistence of bacteria within the pocket or on root surfaces seems to undermine healing, since the presence of residual antigenic material on the root surface could result in an increase in the number of neutrophils at the initial healing stage and hence a greater release of proteolitic enzymes, which could in turn delay clot maturation (25–30). Periodontal diseases have certainly an impact on this situation (20, 23, 24, 31–55). This takes on particular significance in diabetic patients, in whom we already see a delay in neutrophilic activity. In addition, tissue healing seems to be enhanced by the use of oxygen at high pressures. Firstly, oxygen performs an anti-edemigenic action. It is well known that edema usually forms after a trauma and persists due to a vicious cycle mechanism of “trauma-edema-hypoxia-vasodilatation-edema”. Oxygen delivered in high volumes interrupts this mechanism by causing both arterial vasoconstriction and pressure on the superficial vein wall, thereby facilitating an increase in the venous circle and lymph drainage. In the periodontal field, this aspect contributes to a lower relaxation of the wound edge and thus greater stability. The edge can therefore protect the underlying clot and – presumably – facilitate healing. The neoangiogenic action obtained through the release of factors such as the vascular endothelial growth factor (VEGF) is essential for restoring microcirculation in compromised vascular situations and re-establishing a vascular flow in hypoxic areas (56–60).
In case of application of high-pressure oxygen on the treatment of periodontal disease in diabetic patients local anesthesia can be performed to sampling patients but it may have relevant side effect and severe complications (61–63).
All of the above-mentioned mechanisms can explain our results, clearly indicating that the application of high-pressure oxygen contributes to improving periodontal parameters after the SRP procedure. With regard to the attachment gain, our data reveal an important difference between the test and control sides. On the test side we achieved a 1 mm average gain versus 0.6 mm on the control side. According to the literature, an evaluation made a few months after SRP therapy may demonstrate an attachment gain up to 0.55 millimeters for 4–6 mm pockets and 1.19 mm in the case of pockets > 7 mm. Considering that in our patients over 30% of the pockets were over 5 mm deep, the results on the control side may be considered more than satisfactory. However, the test side showed a larger gain, supporting the idea that the application of oxygen favors vascularization and reduces the likelihood of infection. Lastly, reducing edema contributes to clot stabilization. It was observed some years ago that the lack of wound stability could lead to the formation of a long junctional epithelium. The formation of a connective attachment on the root surface after reconstructive periodontal surgery is critically dependent on the stability of coagulation. The fundamental role of the stability and adhesion of fibrin clot to the root surface in preventing the apical migration of the gingival epithelium.
In consideration of this, the formation of the junctional epithelium commonly observed after periodontal treatment seems to be a failure of healing (64–70).
Our results also show a noteworthy reduction in the probing depth (PD) values after treatment on both the test and control sides. The reduction in PD values after treatment with mechanical instrumentation is a result of the combination of attachment gain and the occurrence of gingival recession.
The reduction in PD on the test side was 1.9 mm compared to 1.3 mm on the control side. The literature indicates that in pockets initially measuring 4–6 mm, the average reduction in PD is 1.29 mm, whereas in the case of pockets deeper than 7 mm, the reduction is 2.16 mm. The values on the control side seem to be in agreement with the literature, whilst the test side showed a larger reduction. In our view, the application of oxygen might have drastically reduced oedema. This would confirm that the reduction in PD values on the test side is due both to a real attachment gain (as discussed above) and a reduction in gingival edema.
Notwithstanding the limitations of our study, we feel that our results provide new evidence concerning the use of oxygen. In fact, as we have demonstrated, delivering oxygen in large volumes can improve the outcome of non-surgical periodontal treatment in diabetic subjects, probably through vessel neoformation, the removal of anaerobic bacteria, and clot stabilization enabling ideal healing to be achieved. However, further studies are needed to confirm our results, and to clarify exactly which mechanisms are involved in wound healing in diabetic subjects after SRP and whether the application of oxygen can really improve healing from a histological standpoint.
References
- 1.Nagpal R, Yamashiro Y, Izumi Y. The Two-Way Association of Periodontal Infection with Systemic Disorders: An Overview. Mediators Inflamm. 2015;2015:793–898. doi: 10.1155/2015/793898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheiham A. Periodontal Therapy and Systemic Disease: A Reviewer’s Response. J Evid Based Dent Pract. 2015;15(3):142–3. doi: 10.1016/j.jebdp.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Cairo F, Pagliaro U. Regenerative Therapies in the Treatment of Intrabony Defects Show High Clinical Efficacy. J Evid Based Dent Pract. 2015;15(3):108–12. doi: 10.1016/j.jebdp.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Batista MJ, Lawrence HP, de Sousa Mda L. Tooth loss classification: factors associated with a new classification in an adult population group. Cien Saude Colet. 2015;20(9):2825–35. doi: 10.1590/1413-81232015209.17322014. [DOI] [PubMed] [Google Scholar]
- 5.Lucchese A, Guida A, Capone G, Petruzzi M, Lauritano D, Serpico R. Designing a peptide-based vaccine against Porphyromonas gingivalis. Front Biosci (Schol Ed) 2013;5:631–7. doi: 10.2741/s395. [DOI] [PubMed] [Google Scholar]
- 6.Scapoli L, Girardi A, Palmieri A, Testori T, Zuffetti F, Monguzzi R, Lauritano D, Carinci F. Microflora and periodontal disease. Dent Res J (Isfahan) 2012;9(Suppl 2):S202–6. doi: 10.4103/1735-3327.109755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottero A, Lauritano D, Spadari F, Zambellini Artini M, Salvato A. Atrophy of the oro-pharyngeal mucosa caused by vitamin B12 and folic acid deficiency. Etiopathologic aspects and clinico-therapeutic problems. Minerva Stomatol. 1997;46(7–8):359–74. [PubMed] [Google Scholar]
- 8.Petruzzi M, Lucchese A, Campus G, Crincoli V, Lauritano D, Baldoni E. Oral stigmatic lesions of gastroesophageal reflux disease (GERD) Rev Med Chil. 2012;140(7):915–8. doi: 10.4067/S0034-98872012000700014. [DOI] [PubMed] [Google Scholar]
- 9.Petruzzi M, Campus G, Paparusso F, Lucchese A, Lauritano D, De Benedittis M, Serpico R. Analysis of plasma fibronectin levels in patients affected by oral lichen planus. European Journal of Inflammation. 2012;10(1):45–50. [Google Scholar]
- 10.Petruzzi M, Lucchese A, Lajolo C, Campus G, Lauritano D, Serpico R. Topical retinoids in oral lichen planus treatment: an overview. Dermatology. 2013;226(1):61–7. doi: 10.1159/000346750. [DOI] [PubMed] [Google Scholar]
- 11.Di Girolamo M, Arullani CA, Calcaterra R, Manzi J, Arcuri C, Baggi L. Preservation of extraction socket in immediate implant placement: A clinical study. ORAL and Implantology. 2016;9(4):222–32. doi: 10.11138/orl/2016.9.4.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calcaterra R, Di Girolamo M, Mirisola C, Baggi L. Effects of Repeated Screw Tightening on Implant Abutment Interfaces in Terms of Bacterial and Yeast Leakage in Vitro: One-Time Abutment Versus the Multi-screwing Technique. Int J Periodontics Restorative Dent. 2016;36(2):275–80. doi: 10.11607/prd.2082. [DOI] [PubMed] [Google Scholar]
- 13.Calcaterra R, Di Girolamo M, Mirisola C, Baggi L. Expression of Pattern Recognition Receptors in Epithelial Cells Around Clinically Healthy Implants and Healthy Teeth. Implant Dent. 2016;25(3):348–52. doi: 10.1097/ID.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 14.Calcaterra R, Pasquantonio G, Vitali LA, Nicoletti M, Di Girolamo M, Mirisola C, Prenna M, Condo R, Baggi L. Occurrence of Candida species colonization in a population of denture-wearing immigrants. Int J Immunopathol Pharmacol. 2013;26(1):239–46. doi: 10.1177/039463201302600125. [DOI] [PubMed] [Google Scholar]
- 15.Bernardi S, Frascarelli C, Fantozzi G, Caruso S, Gatto R, Nardi GM, Continenza MA. The importance of correct diagnosis and treatment in endoperiodontal lesions: A two cases comparison. Dental Update. 2016;43(8):766–71. [Google Scholar]
- 16.Lauritano D, Avantaggiato A, Candotto V, Cura F, Gaudio RM, Martinelli M, Palmieri A. Insulin activity on dental pulp stem cell differentiation: An in vitro study. Journal of Biological Regulators and Homeostatic Agents. 2015;29(3):48–53. [PubMed] [Google Scholar]
- 17.Silvestre FJ, Lauritano D, Carinci F, Silvestre-Rangil J, Martinez-Herrera M, Del Olmo A. Neuroinflammation, Alzheimers disease and periodontal disease: is there an association between the two processes? J Biol Regul Homeost Agents. 2017;31(2 Suppl 1):189–96. [PubMed] [Google Scholar]
- 18.Carramolino-Cuellar E, Lauritano D, Carinci F, Silvestre-Rangil J, Banuls-Morant C, Silvestre FJ, Hernandez-Mijares A. Salivary glucose as a metabolic control marker in patients with type 2 diabetes. J Biol Regul Homeost Agents. 2017;31(2 Suppl 1):181–87. [PubMed] [Google Scholar]
- 19.Lauritano D, Muzio LLO, Gaudio RM, Russo LLO, Mucchi D, Nardi GM, Scapoli L. The ecological catastrophe of oral diseases: A possible link between periodontitis and protozoa. Journal of Biological Regulators and Homeostatic Agents. 2016;30(2):143–47. [PubMed] [Google Scholar]
- 20.Lauritano D, Cura F, Candotto V, Gaudio RM, Mucchi D, Carinci F. Periodontal Pockets as a Reservoir of Helicobacter Pylori Causing Relapse of Gastric Ulcer: A Review of the Literature. J Biol Regul Homeost Agents. 2015;29(3 Suppl 1):123–6. [PubMed] [Google Scholar]
- 21.Lauritano D, Pazzi D, Iapichino A, Gaudio RM, Di Muzio M, Lo Russo L, Pezzetti F. Evaluation of the efficacy of a new oral gel containing carvacrol and thymol for home oral care in the management of chronic periodontitis using PCR analysis: a microbiological pilot study. J Biol Regul Homeost Agents. 2016;30(2 Suppl 1):129–34. [PubMed] [Google Scholar]
- 22.Lauritano D, Bignozzi CA, Pazzi D, Palmieri A, Gaudio RM, Di Muzio M, Carinci F. Evaluation of the efficacy of a new oral gel as an adjunct to home oral hygiene in the management of chronic periodontitis. A microbiological study using PCR analysis. Journal of Biological Regulators and Homeostatic Agents. 2016;30(2):123–28. [PubMed] [Google Scholar]
- 23.Lauritano D, Cura F, Gaudio RM, Pezzetti F, Andreasi Bassi M, Carinci F. Polymerase Chain Reaction to Evaluate the Efficacy of Silica Dioxide Colloidal Solutions in the Treatment of Chronic Periodontitis: A Case Control Study. J Biol Regul Homeost Agents. 2015;29(3 Suppl 1):131–5. [PubMed] [Google Scholar]
- 24.Lauritano D, Cura F, Candotto V, Gaudio RM, Mucchi D, Carinci F. Evaluation of the Efficacy of Titanium Dioxide with Monovalent Silver Ions Covalently Linked (Tiab) as an Adjunct to Scaling and Root Planing in the Management of Chronic Periodontitis Using Pcr Analysis: A Microbiological Study. J Biol Regul Homeost Agents. 2015;29(3 Suppl 1):127–30. [PubMed] [Google Scholar]
- 25.Dahm TS, Bruhn A, LeMaster M. Oral Care in the Long-Term Care of Older Patients: How Can the Dental Hygienist Meet the Need? J Dent Hyg. 2015;89(4):229–37. [PubMed] [Google Scholar]
- 26.Hur Y, Choi SK, Ogata Y, Stark PC, Levi PA. Microbiologic Findings in Relation to Risk Assessment for Periodontal Disease: A Cross-Sectional Study. J Periodontol. 2016;87(1):21–6. doi: 10.1902/jop.2015.140237. [DOI] [PubMed] [Google Scholar]
- 27.Yin X, Li J, Salmon B, Huang L, Lim WH, Liu B, Hunter DJ, Ransom RC, Singh G, Gillette M, Zou S, Helms JA. Wnt Signaling and Its Contribution to Craniofacial Tissue Homeostasis. J Dent Res. 2015;94(11):1487–94. doi: 10.1177/0022034515599772. [DOI] [PubMed] [Google Scholar]
- 28.Jin Y, Chen S, Chao Y, Pu T, Xu H, Liu X, Zhao K, Nie Y, Wei W, Lin D. Dental Abnormalities of Eight Wild Qinling Giant Pandas (Ailuropoda Melanoleuca Qinlingensis), Shaanxi Province, China. J Wildl Dis. 2015;51(4):849–59. doi: 10.7589/2014-12-289. [DOI] [PubMed] [Google Scholar]
- 29.Sato D, Kanazawa M, Kim YK, Yokoyama S, Omura Y, Ozeki M, Minakuchi S, Kasugai S, Baba K. Immediate loading of two freestanding implants placed by computer-guided flapless surgery supporting a mandibular overdenture with magnetic attachments. J Prosthodont Res. 2016;60(1):54–62. doi: 10.1016/j.jpor.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Cruz P, Mehretu AM, Buttner MP, Trice T, Howard KM. Development of a polymerase chain reaction assay for the rapid detection of the oral pathogenic bacterium, Selenomonas noxia. BMC Oral Health. 2015;15:95. doi: 10.1186/s12903-015-0071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauritano D, Martinelli M, Mucchi D, Palmieri A, Muzio LL, Carinci F. Bacterial load of periodontal pathogens among Italian patients with chronic periodontitis: A comparative study of three different areas. Journal of Biological Regulators and Homeostatic Agents. 2016;30(2):149–54. [PubMed] [Google Scholar]
- 32.Lauritano D, Scapoli L, Mucchi D, Cura F, Muzio LLO, Carinci F. Infectogenomics: Lack of association between vdr, il6, il10 polymorphisms and “red Complex” bacterial load in a group of Italian adults with chronic periodontal disease. Journal of Biological Regulators and Homeostatic Agents. 2016;30(2):155–60. [PubMed] [Google Scholar]
- 33.Checchi L, Gatto MR, Checchi V, Carinci F. Bacteria prevalence in a large Italian population sample: A clinical and microbiological study. Journal of Biological Regulators and Homeostatic Agents. 2016;30(2):199–208. [PubMed] [Google Scholar]
- 34.Meynardi F, Pasqualini ME, Rossi F, Dal Carlo L, Biancotti P, Carinci F. Correlation between dysfunctional occlusion and periodontal bacterial profile. Journal of Biological Regulators and Homeostatic Agents. 2016;30(2):115–21. [PubMed] [Google Scholar]
- 35.Lombardo L, Carinci F, Martini M, Gemmati D, Nardone M, Siciliani G. Quantitive evaluation of dentin sialoprotein (DSP) using microbeads - A potential early marker of root resorption. ORAL and Implantology. 2016;9(3):132–42. doi: 10.11138/orl/2016.9.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scapoli L, Girardi A, Palmieri A, Martinelli M, Cura F, Lauritano D, Carinci F. Quantitative Analysis of Periodontal Pathogens in Periodontitis and Gingivitis. J Biol Regul Homeost Agents. 2015;29(3 Suppl 1):101–10. [PubMed] [Google Scholar]
- 37.Scapoli L, Girardi A, Palmieri A, Martinelli M, Cura F, Lauritano D, Pezzetti F, Carinci F. Interleukin-6 Gene Polymorphism Modulates the Risk of Periodontal Diseases. J Biol Regul Homeost Agents. 2015;29(3 Suppl 1):111–6. [PubMed] [Google Scholar]
- 38.Roncati M, Lauritano D, Cura F, Carinci F. Evaluation of light-emitting diode (led-835 nm) application over human gingival fibroblast: An in vitro study. Journal of Biological Regulators and Homeostatic Agents. 2016;30(2):161–67. [PubMed] [Google Scholar]
- 39.Andreasi Bassi M, Andreasi Bassi S, Andrisani C, Lico S, Baggi L, Lauritano D. Light diffusion through composite restorations added with spherical glass mega fillers. ORAL and Implantology. 2016;9:80–89. doi: 10.11138/orl/2016.9.1S.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carinci F, Palmieri A, Girardi A, Cura F, Lauritano D. Aquolab® ozone-therapy is an efficient adjuvant in the treatment of chronic periodontitis: A case-control study. Journal of Orofacial Sciences. 2015;7(1):27–32. [Google Scholar]
- 41.Lauritano D, Petruzzi M, Nardi GM, Carinci F, Minervini G, Di Stasio D, Lucchese A. Single Application of a Dessicating Agent in the Treatment of Recurrent Aphthous Stomatitis. J Biol Regul Homeost Agents. 2015;29(3 Suppl 1):59–66. [PubMed] [Google Scholar]
- 42.Carinci F, Lauritano D, Cura F, Lopez MA, Bassi MA, Confalone L, Pezzetti F. Prevention of bacterial leakage at implant-Abutment connection level: An in vitro study of the efficacy of three different implant systems. Journal of Biological Regulators and Homeostatic Agents. 2016;30(2):69–73. [PubMed] [Google Scholar]
- 43.El Haddad E, Giannì AB, Mancini GE, Cura F, Carinci F. Implant-abutment leaking of replace conical connection nobel biocare® implant system. An in vitro study of the microbiological penetration from external environment to implant-abutment space. ORAL and Implantology. 2016;9(2):76–82. doi: 10.11138/orl/2016.9.2.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mancini GE, Gianni AB, Cura F, Ormanier Z, Carinci F. Efficacy of a new implant-abutment connection to minimize microbial contamination: An in vitro study. ORAL and Implantology. 2016;9(3):99–105. doi: 10.11138/orl/2016.9.3.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliveira DP, Palmieri A, Carinci F, Bolfarini C. Osteoblasts behavior on chemically treated commercially pure titanium surfaces. J Biomed Mater Res A. 2014;102(6):1816–22. doi: 10.1002/jbm.a.34855. [DOI] [PubMed] [Google Scholar]
- 46.Andreasi Bassi M, Lopez MA, Confalone L, Carinci F. Hydraulic sinus lift technique in future site development: clinical and histomorphometric analysis of human biopsies. Implant Dent. 2015;24(1):117–24. doi: 10.1097/ID.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 47.El Haddad E, Lauritano D, Carinci F. Interradicular septum as guide for pilot drill in postextractive implantology: a technical note. J Contemp Dent Pract. 2015;16(1):81–4. doi: 10.5005/jp-journals-10024-1640. [DOI] [PubMed] [Google Scholar]
- 48.Azzi L, Carinci F, Gabaglio S, Cura F, Croveri F, Tettamanti L, Tagliabue A, Segato S. Helicobacter pylori in periodontal pockets and saliva: A possible role in gastric infection relapses. Journal of Biological Regulators and Homeostatic Agents. 2017;31(1):257–62. [PubMed] [Google Scholar]
- 49.Tettamanti L, Gaudio RM, Iapichino A, Mucchi D, Tagliabue A. Genetic susceptibility and periodontal disease: A retrospective study on a large italian sample. ORAL and Implantology. 2017;10(1):20–27. doi: 10.11138/orl/2017.10.1.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tettamanti L, Gaudio RM, Cura F, Mucchi D, Illuzzi N, Tagliabue A. Prevalence of periodontal pathogens among Italian patients with chronic periodontitis: A retrospective study on 2992 patients. ORAL and Implantology. 2017;10(1):28–36. doi: 10.11138/orl/2017.10.1.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gargari M, Comuzzi L, Bazzato MF, Sivolella S, di Fiore A, Ceruso FM. Treatment of peri-implantitis: Description of a technique of surgical 2 detoxification of the implant. A prospective clinical case series with 3-year follow-up. ORAL and Implantology. 2015;8(1):1–11. [Google Scholar]
- 52.Spinelli D, De Vico G, Condò R, Ottria L, Arcuri C. Transcrestal guided sinus lift without grafting materials: A 36 months clinical prospective study. ORAL and Implantology. 2015;8(2–3):74–86. doi: 10.11138/orl/2015.8.2.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartuli FN, Luciani F, Caddeo F, De Chiara L, Di Dio M, Piva P, Ottria L, Arcuri C. Piezosurgery vs High Speed Rotary Handpiece: a comparison between the two techniques in the impacted third molar surgery. Oral Implantol (Rome) 2013;6:5–10. [PMC free article] [PubMed] [Google Scholar]
- 54.Moretto D, Gargari M, Nordsjo E, Gloria F, Ottria L. Immediate loading: a new implant technique with immediate loading and aesthetics: Nobel Active. Oral Implantol (Rome) 2008;1(2):50–5. [PMC free article] [PubMed] [Google Scholar]
- 55.De Vico G, Ottria L, Bollero P, Bonino M, Cialone M, Barlattani A, Jr, Gargari M. Aesthetic and functionality in fixed prosthodontic: sperimental and clinical analysis of the CAD-CAM systematic 3Shape. Oral Implantol (Rome) 2008;1(3–4):104–15. [PMC free article] [PubMed] [Google Scholar]
- 56.Naheeda Asif SM, Padma M, Paul A. Assessment of Periodontal Status of Konda Reddy Tribe in Bhadrachalam, Khammam District, India. J Clin Diagn Res. 2015;9(6):ZC23–5. doi: 10.7860/JCDR/2015/13430.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lauritano D, Petruzzi M, Di Stasio D, Lucchese A. Clinical effectiveness of palifermin in prevention and treatment of oral mucositis in children with acute lymphoblastic leukaemia: a case-control study. Int J Oral Sci. 2014;6(1):27–30. doi: 10.1038/ijos.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lauritano D, Petruzzi M. Decayed, missing and filled teeth index and dental anomalies in long-term survivors leukaemic children: a prospective controlled study. Med Oral Patol Oral Cir Bucal. 2012;17(6):e977–80. doi: 10.4317/medoral.17955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petruzzi M, Lucchese A, Nardi GM, Lauritano D, Favia G, Serpico R, Grassi FR. Evaluation of autofluorescence and toluidine blue in the differentiation of oral dysplastic and neoplastic lesions from non dysplastic and neoplastic lesions: a cross-sectional study. J Biomed Opt. 2014;19(7):76003. doi: 10.1117/1.JBO.19.7.076003. [DOI] [PubMed] [Google Scholar]
- 60.Corsalini M, Di Venere D, Pettini F, Lauritano D, Petruzzi M. Temporomandibular disorders in burning mouth syndrome patients: an observational study. Int J Med Sci. 2013;10(12):1784–9. doi: 10.7150/ijms.6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feltracco P, Gaudio RM, Barbieri S, Tiano L, Iacobone M, Viel G, Tonetti T, Galligioni H, Bortolato A, Ori C, Avato FM. The perils of dental vacation: possible anaesthetic and medicolegal consequences. Med Sci Law. 2013;53(1):19–23. doi: 10.1258/msl.2012.012047. [DOI] [PubMed] [Google Scholar]
- 62.Feltracco P, Gaudio RM, Avato FM, Ori C. Authors’ Response (Letter) Journal of Forensic Sciences. 2012;57(5) doi: 10.1111/j.1556-4029.2012.02187.x. [DOI] [PubMed] [Google Scholar]
- 63.Feltracco P, Barbieri S, Galligioni H, Pasin L, Gaudio RM, Tommasi A, Zucchetto A, Trevisiol P, Ori C, Avato FM. A fatal case of anaphylactic shock during paragliding. J Forensic Sci. 2012;57(6):1656–8. doi: 10.1111/j.1556-4029.2012.02187.x. [DOI] [PubMed] [Google Scholar]
- 64.Levine R. Advancing the scientific basis of oral health education. Community Dent Health. 2015;32(2):66–7. [PubMed] [Google Scholar]
- 65.Parwani SR, Parwani RN. Nitric oxide and inflammatory periodontal disease. Gen Dent. 2015;63(2):34–40. [PubMed] [Google Scholar]
- 66.Biju T, Shabeer MM, Amitha R, Rajendra BP, Suchetha K. Comparative evaluation of serum superoxide dismutase and glutathione levels in periodontally diseased patients: an interventional study. Indian J Dent Res. 2014;25(5):613–6. doi: 10.4103/0970-9290.147105. [DOI] [PubMed] [Google Scholar]
- 67.Gumus P, Emingil G, Ozturk VO, Belibasakis GN, Bostanci N. Oxidative stress markers in saliva and periodontal disease status: modulation during pregnancy and postpartum. BMC Infect Dis. 2015;15:261. doi: 10.1186/s12879-015-1003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giovanella LB, Barletta FB, Felippe WT, Bruno KF, de Alencar AH, Estrela C. Assessment of oxygen saturation in dental pulp of permanent teeth with periodontal disease. J Endod. 2014;40(12):1927–31. doi: 10.1016/j.joen.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 69.Wu W, Yang N, Feng X, Sun T, Shen P, Sun W. Effect of vitamin C administration on hydrogen peroxide-induced cytotoxicity in periodontal ligament cells. Mol Med Rep. 2015;11(1):242–8. doi: 10.3892/mmr.2014.2712. [DOI] [PubMed] [Google Scholar]
- 70.Thomas B, Madani SM, Prasad BR, Kumari S. Comparative evaluation of serum antioxidant levels in periodontally diseased patients: An interventional study. Contemp Clin Dent. 2014;5(3):340–4. doi: 10.4103/0976-237X.137938. [DOI] [PMC free article] [PubMed] [Google Scholar]