Abstract
Objective: We conducted a meta-analysis of randomised studies that assessed the effectiveness of directly observed hepatitis C medication therapy delivered in outpatient clinics compared to treatment as usual.
Methods: We completed a systematic literature review up to the end of August 2017, including online databases, study abstracts and references of pertinent articles. We assessed the results of randomised studies using the Cochrane Collaboration risk of bias assessment tool, and observational studies using the ROBINS-I tool. From each study, we extracted the number of patients who did or did not attain sustained virological response (SVR). We utilised a DerSimonian and Laird random effects model for our meta-analysis. This study is registered with PROSPERO (CRD42014012957).
Results: We included six studies with 407 patients in our systematic review; four of those studies (215 patients) used randomisation and were included in our meta-analysis. Overall effect estimates showed that compared to treatment as usual, directly observed therapy demonstrated significantly higher odds of SVR attainment (odds ratio 2.01, 95% confidence interval 1.13–3.59).
Conclusion: Among people who use drugs, directly observed therapy may lead to higher odds of attaining SVR. Further research on the best ways to use directly observed therapy to administer HCV therapy to people who use drugs is warranted.
Keywords: Hepatitis C, treatment, meta-analysis, review
Introduction
Hepatitis C virus (HCV) represents a considerable morbidity and cost burden, contributing to approximately 400,000 deaths annually worldwide [1], and estimated yearly costs of at least US$6.5 billion in the United States [2]. Given the advent of all-oral direct-acting antiviral agents (DAAs) to treat HCV, with lower toxicity and no injections such as those required with interferon-based regimens, it is possible to reduce mortality associated with HCV [3] and to extend therapy to people who may have previously been reticent to undergo treatment [4]. The goal of HCV therapy is attainment of sustained virological response (SVR), defined as undetectable RNA levels in the blood 12 weeks after treatment completion. Patients who attain SVR are considered cured, thus avoiding HCV-related mortality and morbidity [5].
A significant reduction in HCV incidence and prevalence will require treatment for people who use drugs (PWUD), as injection drug use comprises the most common mode of HCV transmission [6]. The mean burden of HCV among PWUD is estimated to be approximately 60%, with HCV prevalence varying between 25% and 90% depending on local prevalence patterns [7]. In addition to curing the therapy recipient of HCV, that person can then no longer transmit HCV, thus preventing HCV transmission to other PWUD [8]. HCV treatment among PWUD can be cost-effective [9], since treatment can negate HCV-related sequelae such as cirrhosis and hepatocellular carcinoma, which HCV-infected PWUD are likely to develop in mid to late adulthood [10].
PWUD may not receive treatment as a result of clinician concerns about compliance with the treatment regimen, and the possibility of HCV re-infection following a course of antiHCV treatment [11,12]. Effectively treating PWUD for HCV requires both a significant increase in treatment availability, for example by incorporating treatment into methadone maintenance therapy, and uptake by patients who may be distrustful of healthcare systems or unable to adhere to regimens due to unmet social needs [4,13]. Additionally, it is important to note that the treatment-as-prevention strategy is successful in lower-HCV prevalence areas compared to those with higher HCV prevalence [14], and thus care models may need to be adapted to local populations. Clearly, creative models of treatment delivery are needed to reach PWUD who are infected with HCV.
Directly observed therapy (DOT) is a care delivery model that was first established for treatment of tuberculosis [15] and was then adapted to deliver treatment to people infected with HIV [16]. DOT has been shown to deliver effective HCV treatment in prisons, primary care clinics, hepatology clinics, drug treatment facilities and multidisciplinary health centres [17–20]. Prior reviews have assessed ways to deliver HCV treatment to PWUD, looking at predictors of treatment completion or HCV treatment delivery mechanisms [21,22]. Given the potential of DOT to deliver treatment among PWUD [9], we conducted a systematic review and summarised existing literature on DOT used in outpatient programmes and SVR attainment in HCV-infected PWUD using meta-analytic techniques. We report our results to augment the available data to inform treatment strategies.
Methods
We performed this meta-analysis following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [23]. Our study protocol is registered with PROSPERO (registration number CRD42014012957).
Data sources
We performed a comprehensive search for eligible studies, searching the Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, and references of relevant articles from the start of each respective database through to the end of August 2017. We also reviewed the conference proceedings and websites of professional organisations such as the American Association for the Study of Liver Diseases (AASLD) and the American Gastroenterological Association (AGA). We used the following search strategy: (‘directly observed therapy’[MeSH Terms] OR (‘directly’[All Fields] AND ‘observed’[All Fields] AND ‘therapy’[All Fields]) OR ‘directly observed therapy’[All Fields]) AND (‘hepatitis c’[MeSH Terms] OR ‘hepatitis c’[All Fields] OR ‘hepacivirus’[MeSH Terms] OR ‘hepacivirus’[All Fields]). We manually searched the reference lists of included studies and related systematic reviews. Two abstractors (CM, CL) evaluated and coded eligible studies. The lead author contacted abstract authors to obtain study results if available.
Study selection
We report our study selection parameters following the PICOTS (Patient population, Intervention, Comparator, Outcome, Timing, Setting) framework. Our study population includes adults (ages 18 and over) with chronic or acute HCV infection of any genotype and who were either receiving opioid maintenance therapy or actively using illicit, injected drugs such as heroin. For our meta-analysis, we included studies that used randomisation and had a comparison arm. The intervention of interest is the randomised DOT assignment for administration of HCV antiviral medications. HCV antiviral medications of interest include pegylated interferon (PEG-IFN)/ribavirin-based regimens as well as the newer direct-acting agents, including NS3/4A protease inhibitors (grazoprevir, paritaprevir, simeprevir), NS5A inhibitors (daclatasvir, elbasvir, ledipasvir, ombitasvir, velpatasvir), or NS5B inhibitors (dasabuvir, sofosbuvir), as single agents or in combination (e.g. ledipasvir/sofosbuvir).
The definition of ‘directly observed therapy’ can be heterogeneous, with different interventions labelled as DOT or similar principles applied without describing them as DOT. For this analysis, we defined DOT as treatment receipt during which time the patient was observed to consume an oral medication and/or receive an injection while in the presence of nursing, community health worker or other trained staff. The comparison group is patients with HCV receiving treatment as usual, receiving at least one of the aforementioned antiviral medications but either not directly observed by study staff or after a time delay for further assessment of subjects' suitability for treatment.
Our outcome of interest is the odds of attaining SVR. We searched for studies that followed patients for at least 12 weeks after treatment completion. We included studies conducted in the setting of outpatient clinics. We excluded studies performed in institutional settings or prisons, as the goal of this analysis is to evaluate the effectiveness of DOT provided in outpatient settings rather than closed systems.
Analysis
Two reviewers (CM, CL) assessed study bias using the Cochrane Collaboration's risk of bias tool for randomised studies and the ROBINS-I tool for observational studies [24]. We assessed the strength of evidence overall using the GRADE scheme [25]. We performed a meta-analysis using the DerSimonian and Laird random-effects model with pooled odds ratios (OR) for the odds of SVR attainment [26]. We performed a sensitivity analysis to see how the inclusion of observational studies comparing DOT and SVT that did not use randomisation affected our findings. We conducted our analysis using Stata version 15 (College Station, TX, USA).
Results
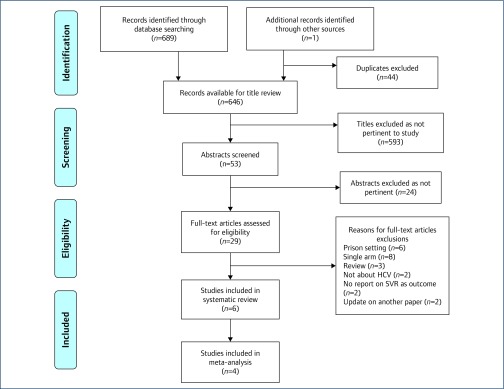
In our database search we identified 690 potentially eligible studies (Figure 1). We eliminated 44 duplicates (e.g. appearing in both Medline and Embase), resulting in 646 titles for review. After conducting a title review to assess appropriateness for this analysis, we eliminated 593 records. We pulled 53 abstracts for abstract review; of these, 24 were not pertinent to our study question. We deemed 28 studies and one abstract to be a potential fit for our inclusion criteria. Following a full-text review, six studies met our inclusion criteria of comparing DOT to treatment as usual and we report the characteristics of those studies in Table 1. For our meta-analysis, we included four studies that randomised patients to treatment (Figure 1). Of the six studies in this systematic review, four studies were performed in the United States, one in Canada, and one in Italy (Table 1). All patients received ribavirin in combination with PEG-IFN. Across all studies, 242 patients received DOT and 165 received treatment as usual. In our meta-analysis, we included a total of 124 subjects receiving DOT and 91 receiving treatment as usual.
Figure 1.
PRISMA flow diagram detailing inclusion and exclusion criteria for studies [23]
Table 1.
Studies identified in systematic review and characteristics of each study population
| Author [ref] | Country | Year | Study type | Outpatient setting | Genotypes | DOT Treatment | TAU | Outcome | Number enrolled | |
|---|---|---|---|---|---|---|---|---|---|---|
| DOT | TAU | |||||||||
| Bonkovsky [40] | USA | 2008 | Randomised open-label | Six study sites; methadone clinics and outpatient clinics within each site | 1–3 | Self-administered RBV, provider-administered weekly PEG-IFN | Self-administered RBV, first PEG-IFN injection provider-administered then self-administered PEG-IFN | SVR | 24 | 24 |
| Bruce [41] | USA | 2012 | RCT | One site with a methadone clinic and a hepatology clinic | 1–4 | Provider administered weekly PEG-IFN with RBV in MEMS container | Self-administered PEG-IFN and RBV in MEMS containers | SVR | 12 | 9 |
| Hilsden [42] | Canada | 2013 | Randomised open-label | Two urban outpatient health clinics | 1–3 | Self-administered RBV, provider-administered weekly PEG-IFN | Self-administered RBV, provider-administered weekly PEG-IFN after a delay in treatment initiation | SVR | 48 | 18 |
| Litwin [43] | USA | 2010 | RCT | Nine outpatient methadone clinics | Not reported | Directly observed RBV, provider-administered weekly PEG-IFN | Self-administered RBV, provider-administered weekly PEG-IFN | SVR | 40 | 40 |
| Cioe [19] | USA | 2013 | Retrospective cohort | Two hospital outpatient clinics: primary care and hepatology | Not reported | Self-administered RBV, provider-administered weekly PEG-IFN | Self-administered PEG-IFN and RBV | SVR | 97 | 58 |
| Nosotti [27] | Italy | 2014 | Prospective cohort | One outpatient drug treatment clinic | Not reported | Directly observed RBV, provider-administered weekly PEG-IFN | Self-administered RBV and PEG-IFN | SVR | 21 | 16 |
DOT: directly observed therapy; MEMS: medication event monitoring system; PEG-IFN: pegylated interferon; RBV: ribavirin; RCT: randomised controlled trial; SVR: sustained virological response; TAU: treatment as usual.
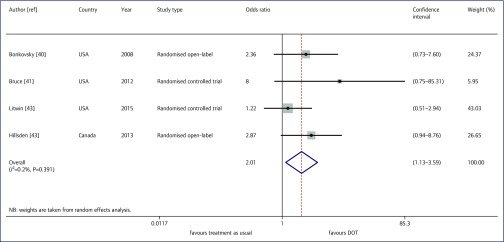
The odds of attaining SVR using DOT were twice that of treatment as usual (odds ratio 2.01, 95% confidence interval [CI] 1.13– 3.59, Figure 2). Our I2 was 0.2% (P=0.39). In our sensitivity analysis, the inclusion of a retrospective cohort study [19] and a prospective study [27] that compared DOT versus treatment as usual without randomisation changed the results. The odds of achieving SVR using DOT were 1.51, but were no longer significant (95% CI 0.97–2.34).
Figure 2.
Odds of sustained virological response attainment for those receiving directly observed therapy versus treatment as usual: Der Simonian and Laird Random Effects Model
We rated the randomised studies as having an unclear risk of bias, and the observational studies as having moderate or critical bias (Table 2). The studies did not report any details about sequence generation or allocation concealment. No studies described blinding of participants or study personnel. Three studies were listed with a ClinicalTrials.gov entry. Two studies were funded by the US National Institute on Drug Abuse, one by the pharmaceutical industry, and one by HealthCanada with medication supplied by a pharmaceutical company. We found the overall strength of evidence to be moderate.
Table 2.
Assessment of bias for each study, using the Cochrane Collaboration's tool for assessing risk of bias for randomised studies and the ROBINS-I bias tool for observational studies
| Randomised studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author [ref] | Sequence generation | Allocation concealment | Blinding | Incomplete outcome reporting | Selective outcome reporting | Other potential threats to validity | Overall rating of bias | |
| Bonkovsky [40] | Unclear | Unclear | No | Yes | Yes | Unclear | Unclear | |
| Bruce [41] | Unclear | Unclear | No | Yes | Yes | Unclear | Unclear | |
| Hilsden [42] | Yes | Unclear | No | No | Unclear | Unclear | Unclear | |
| Litwin [43] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Observational studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author [ref] | Confounding | Selection of participants into the study | Classification of interventions | Deviations from intended intervention | Missing data | Measurement of outcomes | Selection of the reported result | Overall rating of bias |
| Cioe [19] | Moderate | Moderate | Moderate | Moderate | Low | Moderate | Low | Moderate |
| Nosotti [27] | Serious | Critical | Moderate | Serious | Serious | Serious | Serious | Critical |
Discussion
Given the burden of HCV among PWUD and the advent of newer therapies that may decrease this burden [28], research that identifies the most effective strategies to deliver such medication to PWUD is needed. While previously conducted pre–post studies that evaluated DOT as a means to achieve SVR using older HCV medications (e.g. PEG-IFN and ribavirin) showed a positive association between DOT and SVR, with the percentage of patients attaining SVR ranging from 55% [17] to 65% [29], up to 94% [30] and 98% [18], observational studies comparing DOT to treatment as usual have had more mixed results, with patients receiving DOT having similar SVR to those receiving treatment as usual [19,27]. We conducted this meta-analysis to increase sample size available for analysis and summarise the quality of and findings of available studies.
We found a statistically significant increase in attainment of SVR among individuals receiving DOT while receiving opioid maintenance therapy and/or actively injecting drugs. While other systematic reviews and meta-analyses have evaluated treatment completion determinants among PWUD or HCV treatment delivery mechanisms for PWUD [21,22], we filled a knowledge gap by assessing the association between DOT and SVR as an outcome, focusing on randomised studies with a comparator group. In our pooled analysis, 58% of people receiving DOT and 39% of those receiving treatment as usual attained SVR. A recent prospective, observational trial found that while PWUD had a high rate of discontinuation of PEG-IFN/ribavirin therapy, those who completed treatment had SVR attainment rates similar to other cohorts [31].
DOT in conjunction with other services can help connect PWUD with treatment while meeting social and medical needs. One study randomised pharmacies in Scotland to screen for HCV among people receiving outpatient methadone therapy, then allowed pharmacists to prescribe ledipasvir/sofosbuvir to facilitate HCV treatment [32]. Another study used community health workers to observe HCV therapy among patients, varying the medications received while all subjects received DOT. Patients receiving sofosbuvir plus PEG-IFN and ribavirin achieved 100% SVR while 68% of patients receiving sofosbuvir plus ribavirin attained SVR [33]. As all study subjects received DOT simultaneously without a comparison to those not receiving DOT, this study was not included in our analysis.
While the dominant paradigm has been to deliver HCV treatment to PWUD in the setting of methadone clinics, furthering the reach of HCV treatment, such as using pharmacists and innovative health models, reaches more PWUD. Treatment among PWUD with newer agents may be cost-effective, but this depends on the number of PWUD who are reached with treatment [34]. A recent systematic review showed that community-based HCV treatment, when compared to treatment received in a tertiary care centre such as a hospital, is effective with respect to treatment uptake and SVR achievement [35]. As DAA receipt is most effective in PWUDs when liver fibrosis has not progressed [36], delivering care in this population in a timely fashion following diagnosis may help decrease HCV-related complications.
Our study has multiple strengths, including a focus on randomised studies with a comparator group, interventions delivered in outpatient settings rather than closed settings such as prisons, and assessing the relationship between DOT and SVR. We also note several limitations to our study. As few studies met our inclusion criteria, we were unable to control for confounding using meta-regression techniques. Accordingly, the studies that included the smallest number of participants were assigned less weight in the meta-analysis. All studies included herein involved PEG-IFN/ribavirin, which has a more significant side-effect profile and disutility associated with injection when compared to the newer, oral agents. Future evaluations that include only oral agents delivered by DOT may find even greater increased odds of achieving SVR, and should be the focus of future research in PWUD.
Treating PWUD is an essential component of any plan to eradicate HCV in the future [37], especially since prevalence of HCV is rising in some areas of the world and has only seen a small decline in other parts [1]. Care models that provide HCV treatment to PWUD must be flexible and incorporate different services to meet the needs of this population [38], as noted in a recent call for further research to include evaluation of models of care to reach PWUD and enhance treatment [39]. This study augments the available literature indicating that DOT may further facilitate treatment uptake among PWUD, and provides researchers and policymakers with additional information to inform future interventions.
Acknowledgements
Previous presentation
This work was previously presented as a poster at the 2014 Society of Medical Decision Making Annual Meeting.
Research support
CMcD has received support from postdoctoral fellowship NHLBI T32 HL125195-02 and a pre-doctoral dissertation award from the PhRMA Foundation.
References
- 1. Hill AM, Nath S, Simmons B. The road to elimination of hepatitis C: analysis of cures versus new infections in 91 countries. J Virus Erad 2017; 3: 117– 123. [PMC free article] [PubMed] [Google Scholar]
- 2. Razavi H, Elkhoury AC, Elbasha E et al. . Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology 2013; 57: 2164– 2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chhatwal J, Wang X, Ayer T et al. . Hepatitis C disease burden in the United States in the era of oral direct-acting antivirals. Hepatology 2016; 64: 1442– 1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grebely J, Dore GJ. Can hepatitis C virus infection be eradicated in people who inject drugs? Antiviral Res 2014; 104: 62– 72. [DOI] [PubMed] [Google Scholar]
- 5. Ng V, Saab S. Effects of a sustained virologic response on outcomes of patients with chronic hepatitis C. Clin Gastroenterol Hepatol 2011; 9: 923– 930. [DOI] [PubMed] [Google Scholar]
- 6. Metts J, Carmichael L, Kokor W, Scharffenberg R. Hepatitis C: prevalence, transmission, screening, and prevention. FP Essent 2014; 427: 11– 17. [PubMed] [Google Scholar]
- 7. Rafiq SM, Banik GR, Khan S, Rashid H, Khandaker G. Current burden of hepatitis C virus infection among injecting drug users: A mini systematic review of prevalence studies. Infect Disord Drug Targets 2014; 14: 93– 100. [DOI] [PubMed] [Google Scholar]
- 8. Hickman M, De Angelis D, Vickerman P, Hutchinson S, Martin NK. Hepatitis C virus treatment as prevention in people who inject drugs: testing the evidence. Curr Opin Infect Dis 2015; 28: 576– 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martin NK, Vickerman P, Grebely J et al. . Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals. Hepatology 2013; 58: 1598– 1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith DJ, Combellick J, Jordan AE, Hagan H. Hepatitis C virus (HCV) disease progression in people who inject drugs (PWID): a systematic review and meta-analysis. Int J Drug Policy 2015; 26: 911– 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asher AK, Portillo CJ, Cooper BA, Dawson-Rose C, Vlahov D, Page KA. Clinicians' views of hepatitis C virus treatment candidacy with direct-acting antiviral regimens for people who inject drugs. Subst Use Misuse 2016; 51: 1218– 1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Midgard H, Bjoro B, Maeland A et al. . Hepatitis C reinfection after sustained virological response. J Hepatol 2016; 64: 1020– 1026. [DOI] [PubMed] [Google Scholar]
- 13. Lima VD, Rozada I, Grebely J et al. . Are interferon-free direct-acting antivirals for the treatment of HCV enough to control the epidemic among people who inject drugs? PLoS One 2015; 10: e0143836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis 2013; 57( Suppl 2): S39– S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bayer R, Wilkinson D. Directly observed therapy for tuberculosis: history of an idea. Lancet 1995; 345: 1545– 1548. [DOI] [PubMed] [Google Scholar]
- 16. Woodward WC. Should directly observed therapy be considered for treatment of HIV? JAMA 1996; 276: 1956. [PubMed] [Google Scholar]
- 17. Grebely J, Genoway K, Khara M et al. . Treatment uptake and outcomes among current and former injection drug users receiving directly observed therapy within a multidisciplinary group model for the treatment of hepatitis C virus infection. Int J Drug Policy 2007; 18: 437– 443. [DOI] [PubMed] [Google Scholar]
- 18. Waizmann M, Ackermann G. High rates of sustained virological response in hepatitis C virus-infected injection drug users receiving directly observed therapy with peginterferon alpha-2a (40KD) (PEGASYS) and once-daily ribavirin. J Subst Abuse Treat 2010; 38: 338– 345. [DOI] [PubMed] [Google Scholar]
- 19. Cioe PA, Stein MD, Promrat K, Friedmann PD. A comparison of modified directly observed therapy to standard care for chronic hepatitis C. J Community Health 2013; 38: 679– 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saiz de la Hoya P, Portilla J, Marco A et al. . Directly observed therapy for chronic hepatitis C: a randomized clinical trial in the prison setting. Gastroenterol Hepatol 2014; 37: 443– 451. [DOI] [PubMed] [Google Scholar]
- 21. Dimova RB, Zeremski M, Jacobson IM et al. . Determinants of hepatitis C virus treatment completion and efficacy in drug users assessed by meta-analysis. Clin Infect Dis 2013; 56: 806– 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aspinall EJ, Corson S, Doyle JS et al. . Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis 2013; 57( Suppl 2): S80– S89. [DOI] [PubMed] [Google Scholar]
- 23. Liberati A, Altman DG, Tetzlaff J et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151: W65– S94. [DOI] [PubMed] [Google Scholar]
- 24. Sterne JA, Hernan MA, Reeves BC et al. . ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berkman ND, Lohr KN, Ansari MT et al. . Grading the strength of a body of evidence when assessing health care interventions: an EPC update. J Clin Epidemiol 2015; 68: 1312– 1324. [DOI] [PubMed] [Google Scholar]
- 26. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177– 188. [DOI] [PubMed] [Google Scholar]
- 27. Nosotti L, Fagetti R, Rocchi L et al. . Prevalence of HCV infection and adherence to DOT therapy in Italian and non-Italian IV drug users in Rome, Italy. Heroin Addiction and Related Clinical Problems 2014; 16: 41– 44. [Google Scholar]
- 28. Grebely J, Robaeys G, Bruggmann P et al. . Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy 2015; 26: 1028– 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lindenburg CE, Lambers FA, Urbanus AT et al. . Hepatitis C testing and treatment among active drug users in Amsterdam: results from the DUTCH-C project. Eur J Gastroenterol Hepatol 2011; 23: 23– 31. [DOI] [PubMed] [Google Scholar]
- 30. Krook AL, Stokka D, Heger B, Nygaard E. Hepatitis C treatment of opioid dependants receiving maintenance treatment: results of a Norwegian pilot study. Eur Addict Res 2007; 13: 216– 221. [DOI] [PubMed] [Google Scholar]
- 31. Robaeys G, Christensen S, Lucidarme D et al. . Chronic hepatitis C treatment in patients with drug injection history: findings of the INTEGRATE prospective, observational study. Infect Dis Ther 2017; 6: 265– 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Radley A, Tait J, Dillon JF. DOT-C: A cluster randomised feasibility trial evaluating directly observed anti-HCV therapy in a population receiving opioid substitute therapy from community pharmacy. Int J Drug Policy 2017; 47: 126– 136. [DOI] [PubMed] [Google Scholar]
- 33. Solomon SS, Sulkowski MS, Amrose P et al. . Directly observed therapy of sofosbuvir/ribavirin +/- peginterferon with minimal monitoring for the treatment of chronic hepatitis C in people with a history of drug use in Chennai, India (C-DOT). J Viral Hepat 2018; 25: 37– 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bennett H, Gordon J, Jones B et al. . Hepatitis C disease transmission and treatment uptake: impact on the cost-effectiveness of new direct-acting antiviral therapies. Eur J Health Econ 2017; 18: 1001. [DOI] [PubMed] [Google Scholar]
- 35. Wade AJ, Veronese V, Hellard ME, Doyle JS. A systematic review of community based hepatitis C treatment. BMC Infect Dis 2016; 16: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cousien A, Tran VC, Deuffic-Burban S et al. . Hepatitis C treatment as prevention of viral transmission and liver-related morbidity in persons who inject drugs. Hepatology 2016; 63: 1090– 1101. [DOI] [PubMed] [Google Scholar]
- 37. Hellard M, Doyle JS, Sacks-Davis R et al. . Eradication of hepatitis C infection: the importance of targeting people who inject drugs. Hepatology 2014; 59: 366– 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis 2013; 57( Suppl 2): S56– S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grebely J, Bruneau J, Lazarus JV et al. . Research priorities to achieve universal access to hepatitis C prevention, management and direct-acting antiviral treatment among people who inject drugs. Int J Drug Policy 2017; 47: 51– 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bonkovsky HL, Tice AD, Yapp RG et al. . Efficacy and safety of peginterferon alfa-2a/ribavirin in methadone maintenance patients: randomized comparison of direct observed therapy and self-administration. Am J Gastroenterol 2008; 103: 2757– 2765. [DOI] [PubMed] [Google Scholar]
- 41. Bruce RD, Eiserman J, Acosta A et al. . Developing a modified directly observed therapy intervention for hepatitis C treatment in a methadone maintenance program: implications for program replication. Am J Drug Alcohol Abuse 2012; 38: 206– 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hilsden RJ, Macphail G, Grebely J et al. . Directly observed pegylated interferon plus self-administered ribavirin for the treatment of hepatitis C virus infection in people actively using drugs: a randomized controlled trial. Clin Infect Dis 2013; 57( Suppl 2): S90– S96. [DOI] [PubMed] [Google Scholar]
- 43. Litwin AH, Li X, Moonseong H et al. . Strategies to enhance HCV assessment and adherence to therapy among people who use drugs. Suchtmedizin in Forschung und Praxis 2013; 15: 4 ( 220). [Google Scholar]