Abstract
Background and aim
Direct-acting antiviral (DAA) treatments became available for all people living with hepatitis C virus (HCV) in Australia in March 2016. We assess variations in treatment rates and prescribing patterns across Australia's 338 Statistical Area 3 (SA3) geographical units.
Methods
Geocoded DAA treatment initiation data were analysed for the period 1 March 2016 to 30 June 2017. Regression models tested associations between the population demographics and healthcare service coverage of geographical areas and (a) their treatment rates; and (b) the proportion of prescriptions written by specialists compared to non-specialists.
Results
Across the 320 areas (95%) recording treatments, a median 76 (interquartile range [IQR] 35–207, range 4–3834) per 100,000 were initiated, corresponding to an estimated median 7.9% (IQR 2.9–23.6%, range 0–100%) treatment uptake. Major cities, areas of socioeconomic advantage and areas with lower proportions of the population born overseas had the highest per capita treatment rates. Non-specialists prescribed 46% (20,323/44,382) of treatment initiations. Prescriptions were written by non-specialists only in 163 areas (51%), while in other areas a median 40.0% (IQR 21.8–62.5%) of prescriptions were written by non-specialists. Non-specialist prescribing was higher in regional areas, as well as areas that had greater proportions of Indigenous Australians.
Conclusions
High national-level treatment uptake of 20% in Australia masks underlying health system limitations; more than half of geographical areas may have treated less than 8% of people living with HCV. Areas of socioeconomic disadvantage and areas with a higher proportion of the population born overseas may need targeting with interventions to improve treatment uptake.
Keywords: direct-acting antivirals; elimination; geospatial analysis; hepatitis C virus
Introduction
The recently released World Health Organization (WHO) global health sector strategy on viral hepatitis [1] sets a series of elimination targets for hepatitis C virus (HCV), including process targets for prevention, diagnosis and treatment initiation and outcome targets for reductions in incidence and mortality. Modelling suggests that the outcome target of an 80% reduction in HCV incidence by 2030 can be achieved through treatment scale-up; however, this will require high levels of treatment to be sustained among key risk populations for treatment as prevention to be effective [2]. A decline in treatment initiation will limit population-level prevalence reduction and may lead to ongoing transmission as well as increasing cumulative healthcare and retreatment costs.
Since March 2016, direct-acting antiviral (DAA) treatments for HCV have been listed on the Australian Pharmaceutical Benefits Scheme (PBS), available at low cost (US$5 per month for concession holders) for everyone living with HCV with no restrictions by liver disease or risk factors such as injecting drug use [3]. To facilitate access, prescribing guidelines have allowed general practitioners (GPs) and nurse practitioners to prescribe DAA treatments [4]. Between March 2016 and the end of June 2017, PBS data indicate that 44,382 treatment courses were initiated in Australia, representing approximately 20% of the estimated 227,000 people living with HCV (PLHCV) [5]. Among people who inject drugs (PWID), the key risk group for transmission and infection in Australia, treatment initiation in the first 12 months of DAA availability has also been approximately 20% [6]. This exceeds the estimated 12% of PWID per annum required to achieve the incidence reduction target [2]; however, figures from the first year will be significantly inflated by the backlog of people who were already engaged in care and waiting for treatment to become available.
Although the national figures on treatment uptake in Australia have exceeded both expectations and the uptake level of other countries [7], it is not clear how consistent this has been across the country. A significant challenge is providing a consistent and equitable geographical distribution of services that also targets high-notification areas. Previous work has found that in Australia healthcare services related to HCV are disproportionately low in non-metropolitan areas and areas marked by socioeconomic disadvantage [8]. Moreover, these areas have had historically higher notification rates than their counterparts; in 2015 35% of notifications were outside major cities, despite only 29% of the population residing there [8]. In order to maintain high national-level treatment rates, it will be critical to understand how uptake and prescribing patterns vary by geographical area, and whether or not treatments are reaching areas with the most need.
The Australian government predominately uses centralised data drawn from state-based passive surveillance systems to monitor and respond to infectious diseases and trends in risk behaviour [9]; these data have been used to inform targeted interventions, such as social marketing for testing campaigns, which have differed by state according to need [10]. Similarly, the Australian Needle and Syringe Program Survey [6], the Illicit Drug Reporting System [11] and the Gay Community Periodic Surveys [12,13] are used to monitor trends in drug use and sexual risk behaviour in order to inform policies, many of which are legislated or funded at the state level. However, even within each state, heterogeneity in service access, demographics and epidemic drivers means that more granular analyses would enable better tailoring of responses and more effective intervention. Of note, more recently the Australian Collaboration for Coordinated Enhanced Sentinel Surveillance of Sexually Transmitted Infections and Blood-Borne Viruses (ACCESS) [14] has been funded by the Australian Government. ACCESS will have the capacity to provide the more granular BBV/STI surveillance data in future years.
In this paper we use geo-coded treatment initiation data to analyse the geographical distribution of the first year of treatment scale-up in Australia. We aim to identify associations between the population demographics and selected health service coverage of particular areas and their associated treatment uptake and prescribing patterns. Understanding these relationships will provide valuable insight into where treatment is likely to have had the greatest impact so far, as well as areas where improvements may be needed.
Methods
Geographical units
The geographical areas used were Statistical Area 3 (SA3), as defined by the Australian Bureau of Statistics (ABS) [15]. Australia has 338 SA3s, each of which has a population of approximately 30,000 to 130,000 people; in major cities they represent the area serviced by a major transport and commercial hub, while in regional or remote areas they represent areas recognised as having distinct identities and similar social and economic characteristics [16]. Population sizes and demographic characteristics for each SA3 were obtained from the ABS [17].
Treatment data
Quarterly time-series HCV treatment prescription data were obtained from the PBS for the period January 2013 to June 2017. Prescription data were obtained only for initial prescriptions, rather than repeat prescriptions, meaning that our assessment includes treatment initiation rather than treatment completion. Data were disaggregated by geographical area (SA3), prescriber type and treatment regimen. Prescriber types were classified as specialist or non-specialist according to PBS definitions (specialist prescriber types included ‘gastroenterologist’, ‘hepatologist’ or ‘infectious diseases physician’, while non-specialist prescriber types included ‘GP’, ‘addiction worker’ or ‘other’). The type and location of individual prescribers were assigned according to the prescriber's primary listed specialty and registered postcode within the PBS system. Treatments regimens were classified into three groups: interferon (peginterferon alpha 2a or alpha 2b ± ribavirin), first generation DAA (boceprevir, telaprevir), and interferon-free DAA (sofosbuvir, daclatasvir, sofosbuvir/ledipasvir, sofosbuvir/velpatasvir, paritaprevir/ritonavir/ombitasvir/dasabuvir ± ribavirin, grazoprevir/elbasvir). For this analysis only interferon-free DAAs were considered (henceforth simply ‘DAA’).
The prescription data did not contain any demographic or risk behaviour data, meaning that differences in treatment uptake by age, gender or other factors could not be assessed.
Prevalence data
In 2015 there were an estimated 227,000 PLHCV in Australia [5]. We approximated their geographical distribution based on previous work assessing the geographical distribution of HCV notifications [8]. Briefly, this work used a statistical model to determine correlations between the notification rates of geographical areas and their socioeconomic/demographic factors, health service coverage and geographical remoteness profiles. The characteristics of geographical areas were obtained from a number of sources, including the ABS [17], the Australian Urban Research Infrastructure Network [18] and various government websites, and included: population size [19], socioeconomic status [20], geographical remoteness [21], proportion of the population who were of Aboriginal or Torres Strait Islander (ATSI) descent [22], proportion of the population who were born overseas [19], number of needle and syringe programmes (NSPs) (including primary and secondary sites and vending machines) [23–29], number of GP clinics [18], number of alcohol and other drug services [18], number of hospitals, number of liver specialists [18] and presence or absence of a prison [30–34].
For the present study, the estimated 227,000 PLHCV were distributed across Australia according to the above-described model [8]. The geographical units of analysis of this model were local government areas (LGAs), which are smaller than SA3s. Therefore, the number of PLHCV in each SA3 was calculated as the total number of PLHCV assigned to each of its LGA sub-units. Where LGAs overlapped multiple SA3 areas, the proportion of PLHCV assigned to each SA3 was calculated based on the LGA's population contribution to each SA3 [35].
Mapping
Three measures were used to assess the treatment distributions across the SA3s of Australia: total treatments per 100,000 population; percentage of PLHCV treated; and proportion of treatments prescribed by specialists. The percentage of PLHCV in each SA3 who had initiated treatment was calculated as the total treatments divided by the estimated number of PLHCV.
Statistical analysis
Regression models were used to test for associations between population demographics and healthcare service coverage within each SA3 and their corresponding (a) treatment rates; and (b) proportion of treatments prescribed by specialists. Independent variables in the regressions were: socioeconomic status (as measured by Index of Relative Socio-economic Disadvantage (IRSD) quintiles, with quintile 1 indicating the 20% most socioeconomically disadvantaged areas [20]), Remoteness Area classification (major city, inner regional, outer regional, remote, very remote) [21], the proportion of the population of ATSI descent, proportion of the population born overseas, number of NSPs [23–29], number of GP clinics [18], number of alcohol and other drug services [18], number of hospitals, number of liver specialists [18] and presence or absence of a prison [30–34].
A negative binomial regression model was used to fit the treatment rates since they represent highly skewed count data, while a binomial model was used to fit the proportion of prescriptions written by specialists.
Both socioeconomic status (IRSD score) and Remoteness Area classification were defined only on geographical units smaller than SA3s. Therefore, population-weighted averages from each of the smaller geographical areas were used to create appropriate measures.
A statistical analysis was not undertaken to detect correlations between the percentage of PLHCV treated in each area and our independent variable set due to concerns that our denominators, the number of PLHCV in each area, were based on regression analysis estimates using the same independent variable set (see prevalence data section above), rather than being data-based.
The impact of treatment deferral prior to DAA access
Initial treatment uptake and prescribing patterns are likely to be biased by the large backlog of PLHCV who had already been engaged in care for some time, but with their treatment deferred while waiting for DAAs to become available. Therefore, we re-ran all analyses excluding the first 8 months of treatment availability (the data obtained were quarterly so this analysis was performed on data from 1 October 2016 to 30 June 2017).
Results
Treatments per 100,000 population
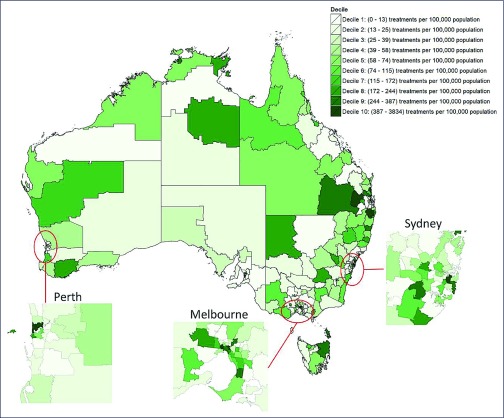
Five percent of SA3s (18/338) had no treatment initiations between March 2016 and July 2017; and these tended to be in rural and remote regions of Australia (Figure 1). Among those recording treatment initiations, there was an uneven distribution of treatment uptake across geographical areas, with a median of 76 (IQR 35–207; range 4–3834) treatment initiations per 100,000 population (Figure 2).
Figure 1.
DAA treatments per capita from March 2016 to June 2017 in each of Australia's Statistical Area 3 geographical regions. Due to the extreme heterogeneity in treatment rates (ranging from 0 to 3834 per 100,000), SA3s were colour-coded by decile to aid visualisation
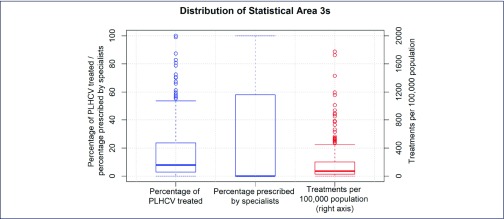
Figure 2.
Heterogeneity of treatments measures across Australia's Statistical Area 3 geographical regions. The estimated percentage of people living with HCV who were treated (blue, left); percentage of treatments prescribed by specialists (blue, centre); and treatments per 100,000 population (red, right). Boxplot whiskers represent observations greater than the 90th percentile or less than the 10th percentile. The right-hand y-axis scale excludes a single outlier, Adelaide City, which had 3834 treatments per 100,000 (897 treatments). The median of zero on the central bar indicates that greater than 50% of geographical areas had no specialist prescriptions
Higher per capita treatment rates were associated with higher IRSD quintile (increased socioeconomic advantage) and having a greater number of specialists, while lower per capita treatment rates were associated with being in outer regional and remote areas compared to major cities, and having a larger proportion of the population born overseas (Table 1).
Table 1.
Results of the negative binomial regression model for treatment initiations per 100,000 population
| Variable | Adjusted treatment initiation rate ratio | Lower bound | Upper bound | P-value |
|---|---|---|---|---|
| Constant | 100.153 | 68.292 | 146.879 | <0.001*** |
| IRSD (vs quintile 1, the most disadvantaged) | ||||
| Quintile 1 | Ref | — | — | — |
| Quintile 2 | 1.279 | 0.885 | 1.848 | 0.191 |
| Quintile 3 | 1.777 | 1.187 | 2.658 | 0.005** |
| Quintile 4 | 1.385 | 0.955 | 2.009 | 0.086 |
| Quintile 5 | 1.546 | 1.064 | 2.245 | 0.022* |
| Remoteness Area classification (vs major city) | ||||
| Major city | Ref | — | — | — |
| Inner regional | 0.706 | 0.470 | 1.061 | 0.094 |
| Outer regional | 0.585 | 0.350 | 0.978 | 0.041* |
| Remote | 0.402 | 0.172 | 0.938 | 0.035* |
| Very remote | 1.619 | 0.527 | 4.974 | 0.400 |
| Proportion of population of ATSI descent | 1.001 | 0.999 | 1.003 | 0.271 |
| Proportion of population born overseas | 0.997 | 0.995 | 0.999 | <0.001*** |
| Number of NSP sites in SA3 | 0.999 | 0.969 | 1.030 | 0.969 |
| Number of GP clinics in SA3 | 1.001 | 0.995 | 1.007 | 0.805 |
| Number of drug and alcohol services in SA3 | 1.007 | 0.984 | 1.030 | 0.557 |
| Number of hospitals in SA3 | 1.018 | 0.991 | 1.046 | 0.195 |
| Number of specialists in SA3 | 1.007 | 1.004 | 1.010 | <0.001*** |
| Prison (vs no) | 0.805 | 0.563 | 1.151 | 0.234 |
IRSD: Index of Relative Socio-economic Disadvantage
Treatment coverage among people living with HCV
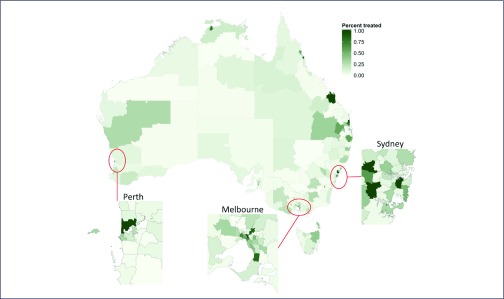
A median of 7.9% (IQR 2.9–23.6%, range 0–100%) of PLHCV were treated within each SA3 (Figure 3). The majority of SA3s (209/338; 62%) had treated fewer than 12% of people living with HCV, which is the estimated scale-up needed among PWID to hit the WHO incidence reduction target.
Figure 3.
Percentage of people living with HCV who commenced DAA treatment from each of Australia's Statistical Area 3 geographical regions. Denominator numbers of people living with HCV in each region estimated using the statistical regression model from Hainsworth et al. [8]
Five percent of SA3s (18/338) had greater than 100% of PLHCV treated using this measure and were rounded down to 100%. This anomaly is likely to be due to several factors, including the use of a regression model to approximate the distribution of PLHCV across the country [8], as well as some individuals accessing treatment outside their place of residence (e.g. an individual from a regional area commuted to a central city to commence treatment).
Proportion of treatments prescribed by specialists
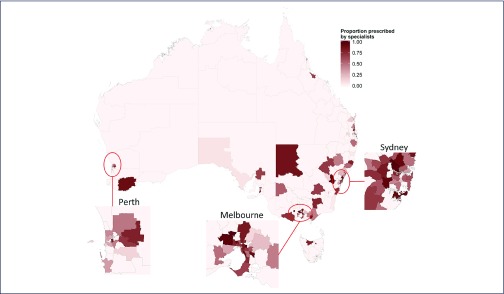
Fifty-four percent (24059/44382) of treatment initiations were prescribed by gastroenterologists, hepatologists or infectious diseases specialists. Of the 320 areas with any treatment initiations, 163 areas (51%) had prescriptions written by non-specialists only. For the 157 areas with a mix of specialist and non-specialists prescribing, a median 60.0% (IQR 37.5–78.2%) of prescriptions were written by specialists (Figure 4).
Figure 4.
Proportion of DAAs prescribed by specialists in each of Australia's Statistical Area 3 geographical regions
The proportion of prescriptions written by specialists was lower in regional areas, in areas with a greater proportion of the population of ATSI descent, and in areas with fewer NSP and GP clinics per capita (Table 2).
Table 2.
Results of binomial regression model for proportion of treatments prescribed by specialists
| Variable | Adjusted odds ratio | Lower bound | Upper bound | p-value |
|---|---|---|---|---|
| Constant (odds ratio) | 0.415 | 0.230 | 0.748 | 0.004** |
| IRSD (vs quintile 1, the most disadvantaged) | ||||
| Quintile 1 | ||||
| Quintile 2 | 1.528 | 0.847 | 2.754 | 0.160 |
| Quintile 3 | 1.517 | 0.790 | 2.912 | 0.211 |
| Quintile 4 | 1.135 | 0.631 | 2.042 | 0.674 |
| Quintile 5 | 0.914 | 0.491 | 1.704 | 0.778 |
| Remoteness Area classification (vs major city)† | ||||
| Major city | ||||
| Inner regional | 0.436 | 0.218 | 0.875 | 0.020* |
| Outer regional | 0.397 | 0.147 | 1.075 | 0.070 |
| Remote | 0.015 | 0.000 | 3.675 | 0.135 |
| Proportion of population of ATSI descent | 0.996 | 0.994 | 0.999 | 0.016* |
| Proportion of population born overseas | 0.999 | 0.997 | 1.002 | 0.693 |
| Number of NSP services in SA3 | 1.055 | 1.003 | 1.110 | 0.038* |
| Number of GP clinics in SA3 | 1.012 | 1.002 | 1.022 | 0.017* |
| Number of drug and alcohol services in SA3 | 0.985 | 0.947 | 1.025 | 0.464 |
| Number of hospitals in SA3 | 0.971 | 0.924 | 1.021 | 0.248 |
| Number of specialists in SA3 | 1.004 | 0.999 | 1.008 | 0.126 |
| Prison (vs no) | 1.709 | 0.936 | 3.123 | 0.082 |
Very Remote category removed as no prescriptions were written by specialists in these areas
IRSD: Index of Relative Socio-economic Disadvantage
The impact of treatment deferral prior to DAA access
When the models were re-run on the amended data set (1 October 2016 to 30 June 2017), no major differences were found; regression coefficients remained within the confidence intervals of Table 1 and Table 2 and the same covariates were found to be statistically significant.
Discussion
In the first year of availability, DAA treatment uptake in Australia has been substantial; however, we have identified large variations in treatment rates and prescribing patterns between geographical areas. Between 1 March 2016 and 30 June 2017, major cities, areas of greater socioeconomic advantage and areas with a lower proportion of the population born overseas had the highest per capita treatment initiation rates. Regional areas had greater proportions of non-specialists prescribing than major cities, and non-specialist prescribing was higher in areas with a greater proportion of Indigenous Australians. This has significant implications for service planning and HCV elimination.
Despite national-level treatment uptake of approximately 20% of PLHCV, when disaggregated by geographical area we estimated that a median of only 7.9% (IQR 2.9–23.6%) of PLHCV were treated within each area. This suggests that national figures have been biased upwards by highly populous major cities, which had statistically significantly higher treatment rates than their outer regional or remote counterparts. This difference in treatment uptake remained in place even after adjustments were made for the initial 6 months of DAAs being available, where it could be expected that major city hospitals were treating large numbers of patients with previously deferred treatment. Major cities are therefore likely to have had treatment uptake in excess of 20% in the first year. As treatment-ready PLHCV are exhausted from these areas, national treatment rates are likely to reduce substantially in coming years if efforts are not made to engage key risk populations in treatment. The global lessonfrom such data is that public health and WHO strategies to eliminate HCV cannot rely on treatment roll-out alone to lead to elimination. Expanded efforts will be required to maintain continued and high levels of HCV treatment uptake [36].
Our findings also highlight the importance of geospatial disaggregation of data; a decline in treatment rates within major cities would appear as a decline in national-level treatment uptake statistics, when in reality treatment rates for many parts of Australia could either have remained steady or increased. It is possible that areas of very high treatment uptake might lead to rapid falls in prevalence, so called micro-elimination, in certain areas. If this occurs, interventions and services to improve treatment delivery will need to become more geographically targeted, with the added challenge of preventing outbreaks of HCV in areas where major reductions in prevalence and incidence have occurred through rapid treatment scale-up.
In order to achieve elimination targets through treatment as prevention, a sustained scale-up of both testing and treatment among risk populations is required, including PWID. For Australia, models have estimated that to reach the WHO elimination targets, treatment coverage at a national level is required to be at least 12% of HCV-infected PWID per year [2] (or 59/1000 PWID given approximately 50% have HCV [6]). Our analyses suggest that this level of treatment uptake is unlikely to have occurred among PLHCV in more than half of the areas considered, and possibly more given that our study period was slightly longer than a year (the data obtained were quarterly, with treatments becoming available on 1st March). Moreover, treatment uptake among PWID may be lower than among the general population of PLHCV as, historically, PWID have been less likely to engage in care [37]. It is not clear what the geographical distribution of injecting drug use is in Australia at this granularity, but in general, illicit drug use is higher per capita outside metropolitan areas and in areas of socioeconomic disadvantage [38]. If this is also indicative of higher per capita rates of injecting drug use, then for Australia to achieve sustained high treatment uptake among PWID, non-metropolitan areas and areas of socioeconomic disadvantage, which are characterised by higher notification rates, lower treatment rates and lower healthcare service coverage [8,39,40], may require additional consideration and/or intervention.
The decision to allow GPs, and more recently nurse practitioners, to prescribe DAAs, whilst not unique to Australia, is uncommon globally [41,42] and appears to be critical to treatment access in a number of areas, particularly those outside major cities. However, national guidelines [43,44] recommend that patients with HCV infection complicated by cirrhosis receive specialist care, and the lack of specialists in regional and remote areas may be a barrier to treatment for this group. This highlights the importance of increasing access to specialist care, for example via telehealth, and supporting ongoing working relationships between GPs and specialist services in rural and regional areas. Appropriate policies should be developed and combined with GP education and support to prevent these patients experiencing poor health outcomes or becoming lost to follow-up.
Non-specialist prescribing also appears to have enhanced treatment initiation in areas with high proportions of ATSI populations. ATSI populations often report feeling marginalised by healthcare systems, and there is a growing body of evidence to indicate that increased engagement in care and better health outcomes can be achieved when community-based services are used rather than specialist or tertiary services [45–48]. This is particularly important, given the high proportion of chronic HCV infection in this group [9]. Community-based services often have the flexibility to be more culturally acceptable, and can utilise peer-driven outreach and communication methods. Flexible models of HCV care should be developed that recognise and utilise the important role of peer educators among marginalised communities.
Limitations
It is difficult to estimate the number of PLHCV in each geographical area. There are limitations to our method that need to be acknowledged as they will have impacted our measure of the percentage of PLHCV treated from each region (the number of PLHCV in each area being the denominator of this measure). We used a regression model to distribute PLHCV as data were unavailable at this granularity. This relied on the use of a series of estimated coefficients that do not extensively capture geographical heterogeneity. We therefore did not perform a statistical analysis on the percentage of PLHCV treated due to concerns of producing misleading results. Nevertheless, values from the regression represent best-fit estimates based on the variables we have used, and as such could be expected to over- and underestimate the number of PLCHV for a roughly equivalent number of areas (i.e. any bias introduced is likely to be approximately equal in direction). Despite these limitations, we believe that there remains utility in these estimates. For example, our analysis demonstrates that heterogeneity in the number PLHCV is likely to exacerbate the heterogeneity in treatment uptake, with non-metropolitan areas and areas of socioeconomic advantage having both higher estimated HCV prevalence and lower treatment rates. Another limitation of our estimates for the percentage of PLHCV treated is that they did not account for reinfections, which would make them optimistic.
Conclusion
Even in a system with minimal drug-cost barriers, challenges in equity of HCV treatment access persist due to geographically heterogeneous population characteristics, health service access and disease burden. High national-level treatment uptake of 20% in Australia masks underlying health system limitations; more than half of geographical areas may have treated less than 8% of people living with HCV. Non-specialist prescribing appears to be significantly facilitating DAA uptake in non-metropolitan areas; however, areas of socioeconomic disadvantage and areas with a higher proportion of the population born overseas may need targeting with interventions to improve treatment uptake.
Acknowledgements
Disclosures
JD, MH, and the Burnet Institute receive investigator-initiated research funding from Gilead Sciences, AbbVie and BMS. JD's institution has received honoraria from Merck, Gilead and BMS. No pharmaceutical grants were received in the development of this study. AP, JD, MH and RSD are the recipients of National Health and Medical Research Council fellowships.
The authors gratefully acknowledge the support provided to the Burnet Institute by the Victorian Government Operational Infrastructure Support Program.
References
- 1. World Health Organization Global health sector strategy on viral hepatitis 2016–2021. Available at: www.who.int/hepatitis/strategy2016-2021/ghss-hep/en ( accessed March 2018).
- 2. Scott N, McBryde ES, Thompson A et al. . Treatment scale-up to achieve global HCV incidence and mortality elimination targets: a cost-effectiveness model. Gut 2017; 66: 1507– 1515. [DOI] [PubMed] [Google Scholar]
- 3. Australian Government Department of Health: The Pharmaceutical Benefits Scheme November 2015 – Positive Recommendations. 2015. Available at: www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/pbac-outcomes/recommendations-pbac-november-2015 ( accessedMarch 2018).
- 4. Australian Government Department of Health: The Pharmaceutical Benefits Scheme General statement for drugs for the treatment of hepatitis C. Available at: www.pbs.gov.au/healthpro/explanatory-notes/general-statement-pdf/general-statement-hepatitis-c.pdf ( accessed March 2018).
- 5. Kirby Institute. Annual Surveillance Report of HIV, viral hepatitis, STIs 2016. Available at: https://kirby.unsw.edu.au/report/annual-surveillance-report-hiv-viral-hepatitis-stis-2016 ( accessed March 2018).
- 6. Memedovic S, Iversen J, Geddes L, Maher L; for the Kirby Institute Australian NSP Survey National Data Report 2012–2016: prevalence of HIV, HCV and injecting and sexual behaviour among NSP attendees. Available at: https://kirby.unsw.edu.au/report/australian-nsp-survey-national-data-report-2012-2016%20 ( accessed March 2018).
- 7. Hill AM, Nath S, Simmons B.. The road to elimination of hepatitis C: analysis of cures versus new infections in 91 countries. J Virus Erad 2017; 3: 117– 123. [PMC free article] [PubMed] [Google Scholar]
- 8. Hainsworth SW, Dietze PM, Wilson DP et al. . Hepatitis C virus notification rates in Australia are highest in socioeconomically disadvantaged areas and outside of major cities. 2017. Private report submitted to the Australian Blood Borne Viruses and Sexually Transmissible Infections Standing Committee. [DOI] [PMC free article] [PubMed]
- 9. Kirby Institute. Annual Surveillance Report on HIV, viral hepatitis, and STIs in Australia 2017. Available at: https://kirby.unsw.edu.au/report/annual-surveillance-report-hiv-viral-hepatitis-and-stis-australia-2017 ( accessed March 2018).
- 10. Pedrana A, Hellard M, Guy R et al. . Stop the drama downunder: a social marketing campaign increases HIV/sexually transmitted infection knowledge and testing in Australian gay men. Sex Trans Dis 2012; 39: 651– 658. [DOI] [PubMed] [Google Scholar]
- 11. Stafford J, Breen C.. Australian Drug Trends 2016. Findings from the Illicit Drug Reporting System (IDRS). Australian Drug Trend Series No 163. Sydney: University of New South Wales, Australia; 2016. Available at: https://trove.nla.gov.au/work/220581041?selectedversion=NBD59597389 ( accessed March 2018). [Google Scholar]
- 12. Lee E, Mao L, McKenzie T et al. . Gay Community Periodic Survey. Melbourne: University of New South Wales, Australia; 2015. Available at: https://csrh.arts.unsw.edu.au/media/CSRHFile/1_GCPS_2015_Melbourne_FINAL.pdf ( accessed March 2018). [Google Scholar]
- 13. Hull P, Mao L, Kolstee J et al. . Gay Community Periodic Survey. Sydney: University of New South Wales, Australia; 2015. Available at: https://csrh.arts.unsw.edu.au/media/CSRHFile/CSRH_Report__GCPS_Sydney_2015.pdf ( accessed March 2018). [Google Scholar]
- 14. Goller JL, Guy RJ, Gold J et al. . Establishing a linked sentinel surveillance system for blood-borne viruses and sexually transmissible infections: methods, system attributes and early findings. Sex Health 2010; 7: 425– 433. [DOI] [PubMed] [Google Scholar]
- 15. Australian Bureau of Statistics (ABS) Available at: www.abs.gov.au ( accessed 22 Sep 2017).
- 16. Australian Bureau of Statistics Australian Statistical Geography. Available at: www.abs.gov.au/websitedbs/D3310114.nsf/home/geography ( accessed March 2018).
- 17. Australian Bureau of Statistics ABS Statistics. Available at: http://stat.data.abs.gov.au ( accessed March 2018).
- 18. Australian Urban Research Infrastructure Network The National Health Services Directory (‘NHSD’) 2015–2016. Available at: https://aurin.org.au/national-health-services-directory-restricted-data-set-application/ ( accessed March 2018).
- 19. Australian Bureau of Statistics Regional Population Growth, Australia 2014–15. Available at: www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3218.02014-15?OpenDocument ( accessed March 2018).
- 20. Australian Bureau of Statistics Socio-Economic Indexes for Areas 2011. Available at: www.abs.gov.au/websitedbs/censushome.nsf/home/seifa ( accessed March 2018).
- 21. Australian Bureau of Statistics Remoteness Structure 2011. Available at: www.abs.gov.au/websitedbs/D3310114.nsf/home/remoteness+structure ( accessed March 2018).
- 22. Australian Bureau of Statistics Estimates of Aboriginal and Torres Strait Islander Australians 2011. Available at: www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3238.0.55.001June%202011?OpenDocument ( accessed March 2018).
- 23. Directions ACT Needle and Syringe Program 2016. Available at: www.directionsact.com/needle-syringe-program ( accessed March 2018).
- 24. Government of Western Australia Where to find needle and syringe programs in WA; 2015. Available at: http://healthywa.wa.gov.au/Articles/U_Z/Where-to-find-needle-and-syringe-programs-in-WA ( accessed March 2018).
- 25. Northern Territory Aids and Hepatitis Council Harm Reduction Needle and Syringe Program; 2016. Available at: www.ntahc.org.au/programs/harm-reduction-needle-and-syringe-program/collection-and-disposal#accordion-0-0 ( accessed March 2018).
- 26. New South Wales Government NSW Needle and Syringe Program (NSP) Outlets; 2015. Available at: www.health.nsw.gov.au/hepatitis/Pages/nsp-outlets.aspx ( accessed March 2018).
- 27. Queensland Government Queensland Needle and Syringe Program; 2016. Available at: www.health.qld.gov.au/public-health/topics/atod/queensland-needle-syringe-program ( accessed March 2018).
- 28. Tasmanian Government Needle and Syringe Program; 2015. Available at: www.dhhs.tas.gov.au/publichealth/communicable_diseases_prevention_unit/infectious_diseases/needle_and_syringe_program ( accessed March 2018).
- 29. Victoria State Government Needle and Syringe Program; 2015. Available at: www2.health.vic.gov.au/alcohol-and-drugs/aod-treatment-services/aod-prevention-harm-reduction/needle-and-syringe-program ( accessed March 2018).
- 30. Government of South Australia South Australian prisons; 2010. Available at: www.corrections.sa.gov.au/prison ( accessed March 2018).
- 31. Northern Territory Government Prisons and probation; 2016. Available at: nt.gov.au/law/prisons ( accessed March 2018).
- 32. Queensland Government Prisons and detention centres; 2015. Available at: www.qld.gov.au/law/sentencing-prisons-and-probation/prisons-and-detention-centres/prison-locations ( accessed March 2018).
- 33. Tasmanian Government Visiting a prison; 2016. Available at: www.justice.tas.gov.au/prisonservice/visiting ( accessed March 2018).
- 34. State Government of Victoria Corrections, Prisons and Parole; 2016. Available at: www.corrections.vic.gov.au/home/prison ( accessed March 2018).
- 35. Australian Bureau of Statistics Australian Statistical Geography Standard (ASGS) correspondences; 2016. Available at: www.abs.gov.au/websitedbs/d3310114.nsf/home/correspondences ( accessed March 2018).
- 36. Scott N, Doyle JS, Wilson DP et al. . Reaching hepatitis C virus elimination targets requires health system interventions to enhance the care cascade. Int J Drug Policy 2017; 47: 107– 116. [DOI] [PubMed] [Google Scholar]
- 37. Grebely J, Oser M, Taylor LE, Dore GJ.. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. J Infect Dis 2013; 207( Suppl 1): S19– 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Australian Institute of Health and Welfare National Drug Strategy Household Survey 2016: detailed findings. Available at: www.aihw.gov.au/reports/illicit-use-of-drugs/ndshs-2016-detailed/contents/table-of-contents ( accessed March 2018).
- 39. McGrail MR. Spatial accessibility of primary health care utilising the two step floating catchment area method: an assessment of recent improvements. Int J Health Geogr 2012; 11: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Australian Institute of Health and Welfare Rural, regional and remote health: indicators of health status and determinants of health; 2008. Available at: www.aihw.gov.au/reports/rural-remote-australians/rural-regional-remote-health-indicators/contents/table-of-contents ( accessed March 2018).
- 41. Marshall AD, Cunningham EB, Nielsen S et al. . Restrictions for reimbursement of interferon-free direct-acting antiviral drugs for HCV infection in Europe. Lancet Gastroenterol Hepatol 2017; 3: 125– 133. [DOI] [PubMed] [Google Scholar]
- 42. Lazarus J, Safreed-Harmon K, Stumo S et al. . Restrictions on access to direct-acting antivirals for people who inject drugs: The European Hep-CORE study and the role of patient groups in monitoring national HCV responses. Int J Drug Policy 2017; 47: 47– 50. [DOI] [PubMed] [Google Scholar]
- 43. Thompson AJ. Australian recommendations for the management of hepatitis C virus infection: a consensus statement. Med J Aust 2016; 204: 268– 272. [DOI] [PubMed] [Google Scholar]
- 44. Gastroenterological Society of Australia: Hepatitis C Virus Infection Consensus Statement Working Group Australian recommendations for the management of hepatitis C virus infection: a consensus statement ( August 2017). Available at: www.asid.net.au/documents/item/1208 ( accessed March 2018).
- 45. Wade AJ, Veronese V, Hellard ME, Doyle JS.. A systematic review of community based hepatitis C treatment. BMC Infect Dis 2016; 16: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhou K, Fitzpatrick T, Walsh N et al. . Interventions to optimise the care continuum for chronic viral hepatitis: a systematic review and meta-analyses. Lancet Infect Dis 2016; 16: 1409– 1422. [DOI] [PubMed] [Google Scholar]
- 47. Bibra S, Doyle J, Higgs P et al. . Feasibility of recruiting people who inject drugs into a nurse-Led model of care trial: The Tap Study. J Hepatol 2016; 64: S817. [Google Scholar]
- 48. Morris L, Smirnov A, Kvassay A et al. . Initial outcomes of integrated community-based hepatitis C treatment for people who inject drugs: findings from the Queensland injectors' health network. Int J Drug Policy 2017; 47: 216– 20. [DOI] [PubMed] [Google Scholar]