Abstract
Background
The integrase strand transfer inhibitor dolutegravir (DTG) is being introduced into low- and middle-income countries (LMICs) as an alternative to first-line treatment with non-nucleoside reverse transcriptase inhibitors. However, DTG is not yet widely recommended for use in pregnant women. The aim of this systematic review was to analyse all available data on birth outcomes and congenital anomalies in the infants of pregnant women treated with DTG.
Methods
A PubMed and Embase search was conducted using the terms “dolutegravir” or “DTG” and “pregnancy” or “pregnant” from the earliest available date on the database to 26 July 2017. Any reports involving women who were pregnant, HIV positive and taking DTG were included. The percentage of pregnant women with adverse birth outcomes or congenital anomalies in their infants after taking dolutegravir was compared with five historical control databases.
Results
There were six databases included in the main analysis of birth outcomes and congenital anomalies, with a total of 1200 pregnant women. The percentage of pregnant women taking DTG with adverse birth outcomes and congenital abnormalities was similar to results from historical control studies of HIV-positive women. However, there was significant heterogeneity among the six databases – the percentage of infants with congenital anomalies ranged from 0.0% in Botswana (0/116 infants) to 13.3% in IMPAACT P1026S (2/15 infants).
Conclusions
Up to 15 million people could be on treatment with DTG in LMICs within the next 5 years, of whom a substantial percentage is likely to be women of child-bearing potential. In many countries with large HIV epidemics, unplanned pregnancies are common and access to antenatal clinic facilities may be limited. Continued pharmacovigilance is essential, but it is reassuring that no clear safety signals have been detected, to date, for pregnant women treated with DTG in terms of birth outcomes or congenital anomalies.
Introduction
The integrase strand transfer inhibitor (INSTI) dolutegravir (DTG) is recommended as an alternative first-line HIV treatment to efavirenz (EFV) in the current World Health Organization (WHO) consolidated antiretroviral (ARV) guidelines [1], and is widely recommended in other international treatment guidelines [2–4]. The efficacy of DTG has been established in studies of naive and pre-treated patients [5–8]. In particular, DTG has shown an improved safety profile compared to the non-nucleoside reverse transcriptase inhibitor (NNRTI) EFV as first-line treatment [5].
Generic versions of DTG have already become available as a single tablet regimen [9]. A generic fixed-dose combination of tenofovir, lamivudine and dolutegravir (TDF/3TC/DTG) is now becoming available in some low- and middle-income countries (LMICs) at a median price of US$75 per person-year, making a DTG-containing regimen more affordable than first-line EFV-containing regimens [10].
As of November 2017, almost 60 LMICs have adopted or are planning to incorporate DTG into national treatment guidelines. Brazil, Botswana, Kenya and Uganda have already started treating patients with DTG [11]. The President's Emergency Programme on AIDS Research (PEPFAR) has recommended the rapid introduction of DTG in its key target countries. It has been estimated that approximately 15 million people will be taking DTG by 2025 and that it will replace first-line EFV-based regimens [9,11].
The risks of adverse birth outcomes with in utero exposure to DTG should be evaluated before widespread introduction of DTG into national treatment programmes in LMICs, where women of childbearing age represent a large proportion of the HIV-positive population. Animal studies of DTG on rats and rabbits revealed an absence of infertility or harm to the fetus, even at high doses [12,13]. There is evidence from ex vivo animal studies that DTG penetrates the placenta [14], and as has been reported for other INSTIs, two case reports of infants exposed to DTG in utero have demonstrated cord blood drug concentrations higher than maternal plasma concentrations, suggesting significant fetal exposure [15]. Furthermore, the plasma half-life of DTG has been estimated to be twice as long in neonates as in adults [16,17]. DTG has also been shown to transfer into breast milk, resulting in significant plasma concentrations in the infant [18].
Randomised clinical trials assessing DTG in pregnancy, compared with other antiretrovirals, are in progress, but results will not be available until 2019–2020 [11]. Notably, in utero exposure to the first in-class INSTI raltegravir (RAL) has not been associated with birth defects, based on a substantial number of reported exposures to date (over 400 first trimester exposures have been reported to the Antiretroviral Pregnancy Registry [19] and nearly 500 exposures in the French Perinatal Cohort, of which 42% were in the first trimester [20]).
DTG is indicated for use in pregnancy when the benefits outweigh the risks [12]. The WHO currently lists DTG as an alternative, rather than a preferred option, for first-line HIV treatment, partly due to the limited safety and effectiveness data available in pregnant women [1]. In October 2017, the US Department of Health and Human Services (DHHS) guidelines noted that there was sufficient data to recommend routine use of DTG-containing regimens for antiretroviral-naive pregnant women as an alternative agent for antiretroviral-naive women [19].
Botswana is currently the only LMIC where DTG is being widely used in pregnant women [11]. There is an ongoing research project in Botswana to assess birth outcomes and congenital anomalies in the infants of pregnant women treated with DTG, as part of a wider research programme to assess the safety of antiretrovirals in pregnancy [21]. In North America and Europe, where women take DTG during pregnancy, there are observational studies and research projects under way to evaluate birth outcomes, congenital anomalies and pharmacokinetics.
This systematic review was conducted to assess the prevalence of specific pregnancy outcomes and birth defects, and pharmacokinetics for pregnant women living with HIV who are taking DTG.
Methods
For this systematic review, a PubMed and Embase search was conducted using the terms “dolutegravir” or “DTG” and “pregnancy” or “pregnant” from the earliest available date on the database to 26 July 2017. This was cross referenced with a search of the clinical trials database, www.clinicaltrials.gov, and conference abstracts from the International AIDS Society (IAS) Conference in July 2017.
During the original clinical trials programme for DTG, women were advised to use contraception, and any women who became pregnant were discontinued from treatment with DTG. These measures, although typical for early clinical development studies, have resulted in a paucity of data regarding treatment outcome in pregnant women. Therefore, since randomised trials have not yet been completed, the main source of information on DTG in pregnancy is from non-randomised observational studies.
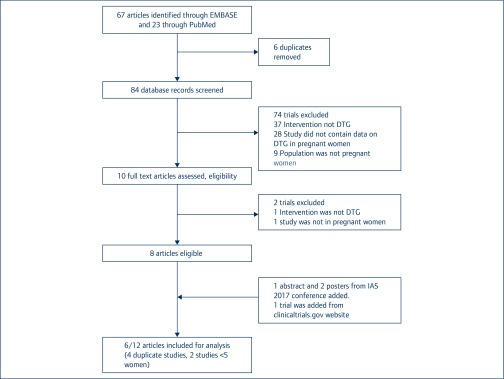
Any reports or databases involving women who were pregnant, living with HIV and taking DTG were included. In case of duplicate reports from the same research group, the most recent publication was used. Studies were excluded if their data had been reported in other larger research programmes, or if the number of pregnant women treated was less than five (Figure 1).
Figure 1.
Outcome of the systematic review.
In terms of the eligible databases, participant baseline characteristics collected included ethnicity and trimester of exposure. Effectiveness was defined as the number of children with an HIV-1 negative status. Pharmacokinetic information was identified from: drug cord blood to maternal plasma ratio; area under the curve geometric mean; and Cmin in the third trimester. Pregnancy outcomes included mean birth weight, stillbirth, preterm birth (<37 weeks) and small for gestational age (SGA) (<10th percentile). Birth defect information was collected on any congenital abnormality or anomaly reported in a trial, study or case report meeting eligibility criteria.
Data analysis
The main analysis concentrated on birth outcomes and congenital anomalies. This analysis included the six largest databases, shown in Table 1. The prevalence of birth outcomes and congenital anomalies was compared to prevalence seen globally and in five historical control studies of pregnant women living with HIV [22–26]. Pharmacokinetic data were recorded where evaluated.
Table 1.
Studies included in the systematic review
| Study [ref] | Study design | n | Location | Trimester exposure |
|---|---|---|---|---|
| Included studies | ||||
| BOTSWANA [26] | Cohort study | 845 | Africa | 2nd (median) |
| APR [27] | Prospective report | 142 | North America, Europe, South America and Australia | 1st: 88
2nd–3rd: 54 |
| EPPICC+PANNA+NEAT-ID [29,30] | Pooled analysis of prospective observational studies | 101 | Europe | 1st: 58
2nd: 21 3rd: 18 |
| DTG post-marketing surveillance [11,31] | ViiV safety database | 67 | International | No data |
| DTG Phase 3 trials [11,31] | ViiV safety database | 30 | International | No data |
| IMPAACT P1026s [32] | Pharmacokinetic study | 15 | US | 2nd and 3rd |
aReported with EPPICC; bReported in APR
Results
Literature search
The search identified 23 published articles from PubMed and 67 conference presentations from Embase. From this set, eight contained data on DTG in pregnancy (shown in Table 1). Three further abstracts were then added from the International AIDS Society Conference in Paris, July 2017, and one additional study was identified from the clinical trials registry www.clinicaltrials.gov. It led to a total of 12 articles or conference presentations for analysis [27–36]. Of these, two were pharmacokinetic studies, three were prospective cohort studies, five were case reports, one was the Antiretroviral Pregnancy Registry (APR) and one was pregnancy outcome data from the pharmaceutical company ViiV, which developed DTG. Six of these articles were then excluded: two studies were already reported in the EPPIIC study [33,34]; two were already reported in the Antiretroviral Pregnancy Registry [15,17]; and two (one in four mothers [35], one for a single mother [36]) were too small for inclusion (Figure 1).
There were six studies that were included in the main analysis of birth outcomes and congenital anomalies, with a total of 1200 pregnant women:
-
1.
Botswana [27]
This ongoing prospective cohort study in Botswana consisted of 845 women initiating treatment with DTG during pregnancy and 4593 women initiating treatment with EFV during pregnancy. Adverse pregnancy outcomes recorded were: stillbirth, preterm birth (<37 weeks), very preterm birth (<32 weeks), SGA (<10th percentile), very SGA (<3rd percentile) and neonatal death. Congenital anomalies were assessed in both live and stillbirths by nurse surface exam at the time of birth, photographed (with maternal consent) and reviewed by a medical geneticist, for 116 mothers taking DTG in the first trimester.
-
2.
Antiretroviral Pregnancy Registry (APR) [28]
The APR is a voluntary reporting system that includes outcomes for only a minority of births at the national level and does not contain the outcomes of all births with first-trimester exposure to DTG. The APR data consisted of 142 women (140 HIV positive, two HIV negative) reported to be taking DTG during pregnancy. Of these, 88 women were exposed to DTG in the first trimester and 54 in the second or third trimesters. The women were mainly from treatment centres in the USA (92%). Data were collected prospectively. Adverse pregnancy outcome measures included stillbirths, spontaneous abortions, SGA and low birthweight. Congenital anomalies were reported from only the live births in this cohort. In the APR, results from individual drugs are normally compared with other drugs after outcomes are available for at least 200 mothers. This threshold has not yet been reached, but current results are shown in Table 2, compared to other reference cohorts.
-
3.
EPPICC, PANNA and NEAT-ID [29]
Table 2.
Prevalence of pregnancy outcomes and birth defects in mothers taking ARVs, from six studies of dolutegravir and four control databases
| Trial location and year [ref] | n | Congenital anomalies (%) | Still birth (%) | Preterm birth <37 weeks (%) | Small for gestational age (%) |
|---|---|---|---|---|---|
| Control databases | |||||
| France 2017 [22] | 13,272 | 4.4 | 0.7 | 14.0 | |
| Botswana 2012 [23] | 9504 | 2.3 | 4.6 | 23.7 | 18.4 |
| SA/Zambia 2014 [24] | 600 | 6.2 | 2.0 | 24.0 | – |
| Zambia 2012 [25] | 1229 | – | 2.6 | 16.3 | – |
| UK 2017 [26] | 6073 | 2.9 | - | 10.4 | – |
| DTG studies [ref] | |||||
| APR [28] | 142 | 3.0 | 0.0 | 10.9 | 11.8 |
| EPPICC/NEAT/PANNA [30] | 81 | 4.9 | 1.0 | 13.9 | 18.7 |
| Botswana [27] | 845 | 0.0% | 2.1 | 17.8 | 18.7 |
| DTG post-marketing surveillance [11,31] | 67 | 7.5 | – | – | – |
| DTG Phase 3 trials [11,31] | 30 | 3.3 | – | – | – |
| IMPAACT
P1026S [32] |
15 | 13.3 | – | – | – |
The study includes 101 pregnancies from seven countries in Europe. It has included data collected from the European Pregnancy and Paediatric HIV Cohort Collaboration (EPPICC), NEAT-ID network and PANNA (Pharmacokinetics of newly developed ANtiretroviral agents in HIV-infected pregNAnt women). All sources of data were collected prospectively. Adverse pregnancy outcomes collected included stillbirth, spontaneous abortion, SGA and low birthweight. Congenital anomaly data were available for 81 of the 84 live births [28].
Within this set of studies, PANNA [30] included analysis of pharmacokinetics of DTG during the third trimester and postpartum.
-
4.
DTG Phase 3 trials [11, 31]
This database includes adverse events reported in ViiV-sponsored Phase 2 and Phase 3 trials of DTG, either using a 50-mg tablet or in combination with abacavir and lamivudine. This database included information on congenital anomalies. Pregnancy was an exclusion criterion for most of these clinical trials. However, women who became pregnant after starting randomly allocated treatment were then followed up to monitor the pregnancy outcome.
-
5.
DTG post-marketing surveillance [11,31]
This database includes reports of congenital anomalies and birth outcomes sent from clinicians to ViiV, for patients taking DTG in pregnancy. This reporting of pregnancy outcomes is not mandated by the originator company.
-
6.
IMPAACT P1026s [32]
IMPAACT P1026s is an ongoing, non-randomised, parallel group pharmacokinetic study evaluating ARVs in pregnancy. In this study, 15 women were taking DTG. In addition, pregnancy outcomes and birth defects were evaluated by the investigators.
Pharmacokinetics of DTG in pregnancy
In the IMPAACT P1026S study, DTG area under the curve (AUC) exposures were 25–30% lower in the second or third trimesters, compared to postpartum [32]. In the PANNA study, DTG AUC exposures were a median 50% lower in the third trimester of pregnancy, compared with postpartum. However, DTG Cmin levels remained above minimum effective concentrations. The median DTG exposures in cord blood were 40% higher than in maternal blood, suggesting that DTG crosses the placenta efficiently [30]. Three studies reported the cord blood to maternal plasma ratio with a mean of 1.33 [30].
Pregnancy outcomes
There were no reports of vertical HIV transmission in any of the studies, but data were only available for 42 infants in four studies at the time of the analyses.
The data from Botswana (Table 3) are reported separately due to it being the only study with a control group. The study found no significant difference in birth outcomes between DTG and EFV, in multivariate analysis adjusted for maternal age, gravidity and education.
Table 3.
Pregnancy outcomes from the Botswana cohort
| Botswana | DTG/TDF/FTC (n=845) | EFV/TDF/FTC (n=4593) | ||
|---|---|---|---|---|
| Stillbirth | 18 | 2.1% | 105 | 2.3% |
| Neonatal death | 11 | 1.3% | 60 | 1.3% |
| Preterm birth (<37 weeks) | 149 | 17.6% | 844 | 18.4% |
| Preterm birth (<32 weeks) | 35 | 4.1% | 160 | 3.5% |
| Small for gestational age (<10th percentile) | 156 | 18.5% | 838 | 18.3% |
| Small for gestational age (<3rd percentile) | 51 | 6.0% | 302 | 6.6% |
| Congenital anomalies | 0/116 | 0.0% | 1/396 | 0.3% |
Table 2 displays the pregnancy outcomes for the six studies included in the main analysis, compared with results from comparator control studies in HIV-positive pregnant women in a range of countries. There were no clear differences in the risk of stillbirth, preterm birth (<37 weeks) or SGA between the studies of DTG-treated women and the historical control studies.
Congenital anomalies
For the six main studies, there were 442 live births with information available on congenital anomalies (Table 4). There were 16 infants with congenital anomalies reported. The most common anomaly in the babies born to mothers who took DTG was polydactyly, with five cases. Polydactyly is also a very common anomaly in babies unexposed to HIV, seen in more than 1% of births to women of African descent [37]. In some of the studies, polydactyly was classified as a normal variant and not included in the final results (for example in the IMPAACT P1026s study).
Table 4.
Congenital anomalies reported for infants born from DTG-treated mothers
| Studies [ref] | Congenital anomalies (N) | Percentage (%) (95% confidence interval) |
|---|---|---|
| Botswana [27] | ||
| No major anomalies reported | 0/116 | 0%(0.0–3.1%) |
| APR [28] | ||
| Polydactyly | 2/133 | |
| Hypoglossia | 1/133 | |
| Down's syndrome | 1/133 | |
| Total | 4/133 | 3.0%(0.8–7.5%) |
| EPPICC+PANNA+NEAT-ID [29] | ||
| Patent foramen ovale | 1/81 | |
| Bilateral hexadactyly hands(polydactyly) | 1/81 | |
| Hypospadias | ||
| Ankyloglossia | 1/81 | |
| Hyperpigmentation | 1/81 | |
| Total | 4/81 | 4.9%(1.4–12.2%) |
| DTG post-marketing surveillance [31] | ||
| Polydactyly | 3/67 | |
| Intracranial calcifications and growth retardation | 1/67 | |
| Bilateral hydroureter, hydronephrosis and pyelocaliectasis | 1/67 | |
| Total | 5/67 | 7.5%(2.5–16.6%) |
| DTG Phase 3 trials [31] | ||
| Ventricular septal defect | 1/30 | 3.3%(0.1–17.2%) |
| IMPAACT P1026s [32] | ||
| Multicystic dysplastic right kidney | 1/15 | |
| Cyst in left kidney | 1/15 | |
| Total | 2/15 | 13.3%(1.7–40.5%) |
The percentage of infants with congenital anomalies varied between the studies, ranging from 0/116 infants in the Botswana study (0%) to 2/15 infants in the IMPAACT P1026S study (13.3%) (Table 4). In IMPAACT P1026S, five of the 15 DTG-exposed babies were reported to have congenital anomalies; there were two other babies whose anomalies were judged to be ‘normal variants’. The investigators judged that, based on the nature of the anomalies and the timing of first exposure in pregnancy, the association of DTG with these anomalies could be ruled out for all but two of the anomalies (renal cysts, shown in Table 4). Owing to the gestational age at which DTG was started and the nature of the renal cysts, the investigators also considered it unlikely that these were related to exposure to DTG. There was no clear pattern of specific congenital anomalies recorded across all the studies. Detailed evaluation of the potential causality for these anomalies would require more information on the timing of initiation of DTG in each pregnant mother – this information is not available for all studies in the systematic review.
Discussion
This systematic review of DTG use in HIV-positive pregnant women shows no evidence for increased risks of stillbirth, preterm birth, SGA or congenital anomalies, compared to historical control studies of ARV-treated pregnant women. The largest observational study in Botswana shows no evidence for increased risk of adverse birth outcomes for women treated with DTG compared with EFV, which has shown a favourable safety profile in a recent analysis [27]. There were no cases of perinatal transmission of HIV reported. Pharmacokinetic studies show lower DTG exposures during the third trimester of pregnancy compared to postpartum. However, this pharmacological effect has also been seen for other antiretrovirals, and all women evaluated maintained DTG exposures above minimum effective levels. In addition, there was evidence for transplacental passage – median DTG concentrations were 40% higher in cord blood compared to maternal blood.
There are several limitations to this systematic review. Two databases of publications or conference presentations in English were searched – there may be additional reports in other languages. There were only six publications identified, including 1200 women, with data on outcomes from 442 live births. Publications with clear evidence for duplicate reporting were excluded. However, it is possible that data from the studies included in the main analysis could have been reported in the APR as well. We believe that this was unlikely, given that 92% of women in the current APR were from the USA, whereas the other five studies in this review were enrolled outside the USA.
None of the studies included was a randomised clinical trial evaluating outcomes for mothers taking DTG versus other antiretrovirals. Several randomised trials of DTG have been conducted over the past 5 years, but pregnant women have been excluded and so outcome data are not yet available. There are four ongoing randomised trials of DTG in pregnancy (Table 5). Three of these include a control arm of EFV, while one includes atazanavir/ritonavir. DOLPHIN-1 is a randomised comparison of TDF/FTC plus either DTG or EFV given in the third trimester of pregnancy in Uganda, in 60 women. This study has been fully recruited and final results are expected in mid-2018. There is a follow up study – DOLPHIN-2 – with a similar design but a larger sample size of 250 women. This study is currently recruiting. The VESTED study includes three arms: TAF/FTC/DTG, TDF/FTC/DTG and TDF/FTC/EFV in 550 pregnant women. This trial is also recruiting, with initial results expected in mid-2019. Before the combined results of the DOLPHIN and VESTED studies become available, it will be necessary to make clinical decisions about the safety and efficacy of DTG in pregnancy from non-randomised studies, which could be prone to bias.
Table 5.
Ongoing randomised clinical trials of DTG in pregnant women [11]
| Clinical trial | Treatment arms | Sample size | Inclusion | Time expected first results |
|---|---|---|---|---|
| DOLPHIN-1 | TDF/FTC/EFV 600
TDF/FTC/DTG |
60 | Pregnant women
Uganda, South Africa |
2Q2018 |
| DOLPHIN-2 | TDF/3TC/EFV 600
TDF/3TC/DTG |
250 | Pregnant women
Uganda, South Africa |
2Q2019 |
| VESTED | TDF/FTC/EFV 600
TDF/FTC/DTG TAF/FTC/DTG |
550 | Pregnant women
International |
2Q2019 |
| ING20026 | TDF/FTC/ATV/r
TDF/FTC/DTG |
25 | Pregnant women
International Sub-study of ARIA |
2020 |
Data from non-randomised trials have been used widely in the assessment of safety of ARVs in pregnancy. There are potential biases with this approach. For example, if the collection or reporting of results is not prospective, there is the potential for over-reporting of adverse outcomes. Reports sent to the originator company, ViiV, as part of post-marketing surveillance is voluntary and may not represent a random sample of treated pregnant mothers with DTG and may not provide an accurate picture of the safety profile of DTG in pregnancy. Other studies where pregnant women are enrolled into trials and studied systematically and prospectively (such as in Botswana) are less prone to this reporting bias. In the Botswana study, the nurse examination of the infant after birth might miss congenital anomalies; longer-term follow-up would be beneficial.
The most standardised mechanism for evaluating the safety of antiretrovirals in pregnancy has been by analysis of the APR, after data has been collected from at least 200 mothers treated with the antiretroviral under investigation during the first trimester. DTG is not yet at this stage of evaluation, with only 88 women exposed in the first trimester (71 live births), by the most recent analysis in January 2017. The speed of introduction of DTG in LMICs has necessitated a review of safety before mature results become available from the APR. Therefore our analysis should be repeated in 6–12 months, when more results become available.
The studies included in this systematic review have used a range of methods to ascertain and define congenital anomalies. In the Botswana study, the method was based on visual inspection of babies just after birth and only major congenital anomalies were reported (defined as having clinical, surgical or cosmetic significance) with no major congenital anomalies detected among the 116 babies born to mothers treated with DTG in the first trimester. The other studies included in the review had longer follow-up of the babies and were able to use imaging and genetic testing to evaluate birth defects. However, these studies reported all anomalies, including some that would not normally be classified as birth defects and some that occur among women without first-trimester exposure to DTG. This may have resulted in higher estimates of anomaly rates, for example in the IMPAACT P1026s study, where there were two anomalies detected among 15 births (13.3%). Some of the estimates presented are also limited by the small denominators, with a single additional anomaly potentially resulting in a large percentage difference. Although methodologies differ between these studies included in this review, there were no common severe malformations or a pattern of multiple malformations seen in more than 400 births.
Up to 15 million people could be on treatment with DTG in LMICs within the next 5 years [9,11], and among these a substantial percentage are likely to be women of child-bearing potential. In many countries with large HIV epidemics, unplanned pregnancies are common and access to antenatal clinic facilities may be limited. Given these issues, continued pharmacovigilance is essential. However, it is reassuring that no clear safety signals have been detected, to date, for pregnant women treated with DTG in terms of birth outcomes or congenital anomalies.
References
- 1. World Health Organization Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. 2nd edn 2016. Available at: www.who.int/hiv/pub/arv/arv-2016/en ( accessed March 2018). [PubMed]
- 2. Günthard HF, Saag MS, Benson CA et al. . Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society–USA Panel. JAMA 2016; 316: 191– 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. AIDSinfo Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV; 2017. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf ( accessed March 2018).
- 4. European AIDS Clinical Society Guidelines 2017. Available at: www.eacsociety.org/files/guidelines_8.2-english.pdf ( accessed March 2018).
- 5. Walmsley S, Baumgarten A, Berenguer J et al. . Brief report: Dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naïve patients: week 96 and week 144 results from the SINGLE randomised clinical trial. J Acquir Immune Defic Syndr 2015; 70: 515– 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clotet B, Feinberg J, Lunzen J et al. . Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014; 383: 2222– 2231. [DOI] [PubMed] [Google Scholar]
- 7. Orrell C, Hagins D, Belonosova E et al. . Superior efficacy of dolutegravir/abacavir/lamivudine fixed dose combination compared with ritonavir-boosted atazanavir plus tenofovir/emtricitabine in treatment-naive women with HIV-1 infection (ARIA study). Australasian HIV and AIDS Conference. November 2016. Adelaide, Australia. Abstract 136.
- 8. Aboud M, Kaplan M, Lombaard J et al. . Superior efficacy of dolutegravir (DTG) plus 2 nucleoside reverse transcriptase inhibitors (NRTIs) compared with lopinavir/ritonavir (LPV/RTV) plus 2NRTIs in second-line treatment: interim data from the DAWNING study. International AIDS Society Conference on HIV Science. July 2017. Paris, France. Abstract TUAB0105LB.
- 9. Clinton Health Access Initiative ARV market report; 2017. Available at: https://clintonhealthaccess.org/content/uploads/2017/09/2017-ARV-Market-Report_Final-2.pdf ( accessed March 2018).
- 10. UNITAID New high-quality antiretroviral therapy to be launched in South Africa, Kenya and over 90 low- and middle-income countries at reduced price; 2017. Available at: https://unitaid.eu/news-blog/new-high-quality-antiretroviral-therapy-launched-south-africa-kenya-90-low-middle-income-countries-reduced-price/#en ( accessed March 2018).
- 11. World Health Organization Transition to new antiretrovirals in HIV programmes; 2017. Available at: http://apps.who.int/iris/bitstream/10665/255888/1/WHO-HIV-2017.20-eng.pdf?ua=1 ( accessed March 2018).
- 12. ViiV Healthcare Full prescribing information: dolutegravir; 2013. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2013/204790lbl.pdf ( accessed March 2018).
- 13. Kandel CE, Walmsley SL. Dolutegravir. A review of the pharmacology, efficacy, and safety in the treatment of HIV. Drug Des Devel Ther 2015; 9: 3547– 3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schalkwijk S, Greupink R, Colbers AP et al. . Placental transfer of the HIV integrase inhibitor dolutegravir in an ex vivo human cotyledon perfusion model. J Antimicrob Chemother 2016; 71: 480– 483. [DOI] [PubMed] [Google Scholar]
- 15. Lewis JM, Railton E, Riordan A et al. . Early experience of dolutegravir pharmacokinetics in pregnancy: high maternal levels and significant foetal exposure with twice-daily dosing. AIDS 2016; 30: 1313– 1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schalkwijk S, Feiterna-Sperling C, Weizsäcker K et al. . Substantially lowered dolutegravir exposure in a treatment-experienced perinatally HIV-1-infected pregnant woman. AIDS 2016; 30: 1999– 2001. [DOI] [PubMed] [Google Scholar]
- 17. Pain JB, Lê MP, Caseris M et al. . Pharmacokinetics of dolutegravir in a premature neonate after HIV treatment intensification during pregnancy. Antimicrob Agents Chemother 2015; 59: 3660– 3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kobbe R, Schalkwijk S, Dunay G et al. . Dolutegravir in breast milk and maternal and infant plasma during breastfeeding. AIDS 2016; 30: 2731– 2733. [DOI] [PubMed] [Google Scholar]
- 19. AIDSinfo Recommendations for the use of antiretroviral drugs in pregnant women with HIV infection and interventions to reduce perinatal HIV transmission in the United States; 2017. Available at: https://aidsinfo.nih.gov/guidelines/html/3/perinatal/0 ( accessed March 2018).
- 20. Sibiude J, Warszawski J, Blanche S et al. . Evaluation of the risk of birth defects among children exposed to raltegravir in utero in the ANRS-French Perinatal Cohort EPF. International AIDS Society Conference on HIV Science. July 2018. Paris, France. Abstract MOAB0204.
- 21. Zash R, Jacobson DL, Diseko M et al. . Comparative safety of antiretroviral treatment regimens in pregnancy. JAMA Pediatr 2017; 171: e172222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uthman OA, Nachega JB, Anderson J et al. . Timing of initiation of antiretroviral therapy and adverse pregnancy outcomes: a systematic review and meta-analysis. Lancet HIV 2017; 4: e21– 30. [DOI] [PubMed] [Google Scholar]
- 23. Chen JY, Ribaudo HJ, Souda S et al. . Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis 2012; 206: 1695– 1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu KC, Farahani M, Mashamba T et al. . Pregnancy outcomes and birth defects from an antiretroviral drug safety study of women in South Africa and Zambia. AIDS 2014; 28: 2259– 2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim H-Y, Kasonde P, Mwiya M et al. . Pregnancy loss and role of infant HIV status on perinatal mortality among HIV-infected women. BMC Pediatr 2012; 12: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Favorato G, Townsend C, Bailey H et al. . Protease inhibitors and pre-term delivery another piece in the puzzle. AIDS 2018; 32: 243– 252. [DOI] [PubMed] [Google Scholar]
- 27. Zash R, Jacobson D, Mayondi G et al. . Dolutegravir/tenofovir/emtricitabine (DTG/TDF/FTC) started in pregnancy is as safe as efavirenz/tenofovir/emtricitabine (EFV/TDF/FTC) in nationwide birth outcomes surveillance in Botswana. International AIDS Society Conference on HIV Science. July 2017. Paris, France. Abstract MOAX0202LB.
- 28. Vannappagari V, Albano J, Ragone L et al. . Dolutegravir use during pregnancy and birth outcomes: data from the Antiretroviral Pregnancy Registry (APR). International AIDS Society Conference on HIV Science. July 2017. Paris, France. Abstract MOPEB0283.
- 29. Thorne C, Favarato G, Peters H et al. . Pregnancy and neonatal outcomes following prenatal exposure to dolutegravir. International AIDS Society Conference on HIV Science. July 2017. Paris, France. Abstract MOPEC0609.
- 30. Bollen P, Colbers A, Schalkwijk S et al. . A comparison of the pharmacokinetics of dolutegravir during pregnancy and postpartum. International Workshop on Clinical Pharmacology of Antiviral Therapy. July 2017. Chicago, USA. Abstract O_07.
- 31. Vitoria M, Ford N, Clayden P et al. . When could new antiretrovirals be recommended for national treatment programmes in low-income and middle-income countries: results of a WHO think tank. Curr Opin HIV AIDS 2017; 12: 414– 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mulligan N, Best B, Capparelli E et al. . Dolutegravir pharmacokinetics in HIV-infected pregnant and postpartum women. Conference on Retroviruses and Opportunistic Infections. February 2016. Boston, MA, USA. Abstract 438.
- 33. Simons R. Dolutegravir in pregnancy: a retrospective case review. HIV Med 2017; 18( Suppl 1): 27 (abstract P40). [Google Scholar]
- 34. Simons R. Dolutegravir use in 181 patients, 54 women and 9 pregnancies-a real life experience. HIV Med 2016; 17( Suppl 1): 17 (abstract P9). [Google Scholar]
- 35. Rahangdale L, Cates J, Potter J et al. . Integrase inhibitors in late pregnancy and rapid HIV viral load reduction. Am J Obstet Gynecol 2016; 214: 385.e1– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pinnetti C, Tintoni M, Ammassari A et al. . Successful prevention of HIV mother-to-child transmission with dolutegravir-based combination antiretroviral therapy in a vertically infected pregnant woman with multiclass highly drug-resistant HIV-1. AIDS 2015; 29: 2534– 2537. [DOI] [PubMed] [Google Scholar]
- 37. Watson BT, Hennrikus WL. Postaxial type-B polydactyly. Prevalence and treatment. J Bone Joint Surg Am 1997; 79: 65– 68. [DOI] [PubMed] [Google Scholar]