Abstract
Accumulating evidence suggests that oxidation-specific epitopes (OSEs) constitute a novel class of damage-associated molecular patterns (DAMPs) generated during high oxidative stress but also in the physiological process of apoptosis. To deal with the potentially harmful consequences of such epitopes, the immune system has developed several mechanisms to protect from OSEs and to orchestrate their clearance, including IgM natural antibodies and both cellular and membrane-bound receptors. Here, we focus on malondialdehyde (MDA) epitopes as prominent examples of OSEs that trigger both innate and adaptive immune responses. First, we review the mechanism of MDA generation, the different types of adducts on various biomolecules and provide relevant examples for physiological carriers of MDA such as apoptotic cells, microvesicles (MV) or oxidized low-density lipoproteins (LDL). Based on recent insights, we argue that MDA epitopes contribute to the maintenance of homeostatic functions by acting as markers of elevated oxidative stress and tissue damage. We discuss multiple lines of evidence that MDA epitopes are pro-inflammatory and thus important targets of innate and adaptive immune responses. Finally, we illustrate the relevance of MDA epitopes in human pathologies by describing their capacity to drive inflammatory processes in atherosclerosis and highlighting protective mechanisms of immunity that could be exploited for therapeutic purposes.
Keywords: inflammation, atherosclerosis, oxidative stress, immunity, malondialdehyde, oxidized low density lipoprotein, oxidation-specific epitopes, damage-associated molecular pattern
1. Overview
Cellular stress, senescence, and cell death are tightly associated with oxidative stress. A major consequence of increased oxidative stress is the peroxidation of membrane lipids resulting in the generation of various oxidation specific epitopes (OSEs). OSEs and the immune responses targeting them have been implicated in many acute and chronic inflammatory diseases, most prominently atherosclerosis. Studies of the biological activities of oxidized LDL (OxLDL), which is a key pathogenic driver of atherosclerosis, have helped identify OSE as a novel class of damage-associated molecular patterns (DAMPs). In this chapter we will particularly focus on a certain group of OSEs, namely malondialdehyde (MDA) epitopes. MDA epitopes have been documented on the surface of dying cells and in damaged tissues. Recent studies have identified them as major targets of various immune responses that modulate homeostatic processes, e.g. the clearance of apoptotic cells. In atherosclerosis, which is characterized by impaired resolution and chronic inflammation, MDA epitopes have been identified as mediators of inflammation and therefore serve as interesting potential targets for immunological therapeutic interventions in CVDs.
2. Biochemistry and generation of MDA in vitro and in vivo
Oxygen is a fundamental prerequisite for energy production by cellular respiration in aerobic organism. However, this also results in the constant generation of reactive oxygen species (ROS) as potentially damaging by-products, which are produced endogenously in mitochondria, peroxisomes, the endoplasmic reticulum and even in the plasma membrane of cells, but can also be induced exogenously by UV light, heat, bacterial and environmental agents, such as tobacco smoke and ionizing radiation (Bae, Oh, Rhee, & Yoo, 2011; Nathan & Cunningham-Bussel, 2013). Newly generated ROS can attack membrane lipids containing carbon-carbon double bonds (e. g. poly-unsaturated fatty acids (PUFAs) of phospholipids), and damage them by a process called lipid peroxidation. Lipid peroxidation of free fatty acids occurs through both enzymatic and non-enzymatic mechanisms. If not efficiently controlled this emanates in the perturbed integrity of different cellular structures potentially leading to cellular death (Nathan & Cunningham-Bussel, 2013). Enzymatic mechanisms involve the activation of lipoxygenases, myeloperoxidases, cyclooxygenases, and cytochrome P450 (Niki, 2009). After the enzymatic removal of hydrogen from the double allylic-activated CH2 group of PUFAs, oxygen is added, generating a peroxydienyl radical. This is then transformed into an anion and the reaction is terminated by back-transfer of the proton generated in the first reaction step, resulting in the formation of a lipid-hydroperoxide molecule (LOOH). Non-enzymatic mechanisms are mediated by free radicals, which can be indirectly generated by nicotinamide adenine dinucleotide phosphate (NADPH) oxidases and nitric-oxide synthases (Niki, 2009). In turn, free radicals are able to remove hydrogen from a CH2 group of PUFAs, resulting in the generation of lipid-hydroperoxide molecules (LOOH) and new dienyl radicals, which propagate this chain reaction. LOOHs that are generated by both reactions then decompose and during their degradation a great variety of secondary products such as MDA, 4-hydroxynonenal (4-HNE) and the remaining core aldehyde of oxidized phospholipids, such as 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC), are produced (Yin, Xu, & Porter, 2011). These end products of lipid peroxidation are highly reactive aldehydes, which can further propagate oxidative damage and are therefore considered to be downstream mediators of oxidative stress. Under normal conditions, lipid peroxidation is inhibited enzymatically by the activities of glutathione peroxidases, superoxide dismutases and catalases, or non-enzymatically by antioxidants, such as Vitamin C and Vitamin E (Niki, 2009). It has been extensively shown that an imbalance between oxidative stress and antioxidant mechanisms results in pathological oxidation (Finkel & Holbrook, 2000; Nathan & Cunningham-Bussel, 2013). Therefore, total levels of end products of lipid peroxidation, especially of free and adducted MDA, are widely used as indicators of oxidative stress in higher eukaryotic organisms and have been shown to correlate with the extent of tissue damage in acute injury and chronic diseases (Imai et al., 2008; Pamplona et al., 2005; Weismann et al., 2011; Yla-Herttuala et al., 1989).
Here we will mainly focus on the biology of one of these reactive terminal degradation products: malondialdehyde. Malondialdehyde (Malonic aldehyde; Propanedial; 1,3-Propanedial, MDA) is an aldehyde with the chemical formula C3H4O2 that at physiological pH mainly exists in its enol form (Nair, O'Neil, & Wang, 2001). The chemical structure of MDA is given in Figure 1.
Figure 1.
Chemical structure of malondialdehyde. Malondialdehyde (Malonic aldehyde; Propanedial; 1,3-Propanedial, MDA) is an aldehyde with the chemical formula C3H4O2. At physiological pH it exists mainly in its enol form (left structure). MDA has a molar mass of 72.06 g/mol, a density of 0.991 g/ml and its melting point and boiling point are at 72°C and 108°C, respectively. It is produced by acid hydrolysis of 1,1,3,3-tetramethoxypropane at room temperature in vitro (Nair et al., 2001), and due to its high reactivity usually not observed in a pure form.
Under physiological conditions newly generated MDA can modify free amino acids, proteins, nucleotides and phospholipids creating stable covalent epitopes. Major in vivo carriers of MDA epitopes identified so far are apoptotic/necrotic cells (Amir et al., 2012; Chang et al., 1999; Chang et al., 2004; Chou et al., 2009; Weismann et al., 2011), microvesicles (MV) (Huber et al., 2002; Liu, Scalia, Mehta, & Williams, 2012; Tsiantoulas et al., 2015) and oxidized lipoprotein particles (Palinski et al., 1989; Shao, Tang, Heinecke, & Oram, 2010).
The main substrates for the generation of MDA in vivo are arachidonic acid (AA, 20:4) and docosahexaenoic acid (DHA, 22:6) present in membrane phospholipids. Already in the 1970’s Pryor et al (Pryor & Stanley, 1975) proposed possible mechanisms of MDA generation from these two PUFAs. He suggested the formation of intermediates that are bicyclo-endoperoxides, which are than broken down by thermal or acid catalyzed reactions giving rise to free MDA. Additionally, it was observed that certain amounts of MDA can be generated as a side product of Thromboxane A2 synthesis. The enzymes cyclooxygenase 1 (COX-1) or COX-2 metabolize AA to prostaglandin H2 (PGH2), and newly formed PGH2 is then metabolized by the thromboxane synthase, to Thromboxane A2, 12-hydroxyheptadecatrienoic acid and MDA (Hecker & Ullrich, 1989). Several reports suggested that MDA can also formed by oxidation of spermine (Quash et al., 1987), UV-irradiation of the skin surface lipid squalene (Dennis & Shibamoto, 1989) or by gamma irradiation of carbohydrates (Cheeseman, Beavis, & Esterbauer, 1988).
3. Generation of MDA epitopes
Free MDA exists as a bi-functional electrophile and its reactivity is pH-dependent. At physiological pH MDA is present in the enolate ion form. MDA reactivity can be increased by lowering the pH, which favors the formation of beta-hydroxyacrolein, thereby increasing its affinity towards nucleophilic molecules in the vicinity (Esterbauer, Schaur, & Zollner, 1991). In addition, it has been demonstrated that at high concentrations of MDA in an acidic milieu (pH range 4-7) long oligomers of MDA are formed, which results in hydrolytic cleavage of newly formed MDA oligomers, generating MDA and acetaldehyde (AcA)(Gomez-Sanchez A 1990). This observation is very important, as AcA and MDA can react together and create a more complex form of immunogenic epitopes, malondialdehyde-acetaldehyde (MAA). Due to their chemical nature free AcA and MDA possess the ability to create epitopes on major cellular macromolecules in different tissues serving as mediators of oxidative stress (Figure 2). Forming stable epitopes with biomolecules has been suggested to increase the half-life of MDA in vivo (Siu & Draper, 1982). Furthermore, MDA modifications of lipids, nucleotides, free amino acids and proteins may result in their loss of function, loss of structural integrity, and may lead to altered cellular responses (Fogelman et al., 1980; Hyvarinen, Uchida, Varjosalo, Jokela, & Jokiranta, 2014; Wallberg, Bergquist, Achour, Breij, & Harris, 2007). Modifications of each type of macromolecules will be discussed separately.
Figure 2.
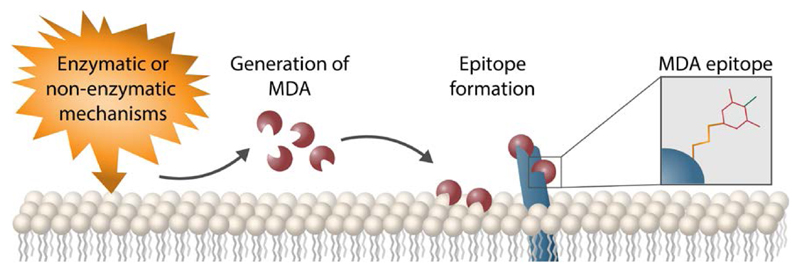
Mechanism of MDA epitope formation. Lipid peroxidation of poly-unsaturated fatty acids by enzymatic or non-enzymatic mechanisms results in the generation of highly reactive aldehydes such as MDA, acting as mediators of oxidative stress. Covalent attachment of MDA to amino groups present in e.g. Lys of biomolecules nearby results in the formation of MDA epitopes.
3.1. MDA modification of amino acids and proteins
MDA-modified biomolecules such as free amino acids or proteins have been identified in different tissues and body fluids in humans and animals in vivo (Akalin, Baltacioglu, Alver, & Karabulut, 2007; Gonenc, Ozkan, Torun, & Simsek, 2001; Imai et al., 2008; Pamplona et al., 2005; Weismann et al., 2011). Indeed, at neutral pH in the presence of free lysine (Lys) and arginine (Arg), MDA can hydrolyze and form epitopes on their side chains. Additionally, at lower pH MDA has also been shown to form epitopes on another group of amino acids, including glycine (Gly), leucine (Leu), valine (Val), histidine (His), arginine (Arg), tryptophan (Trp), tyrosine (Tyr), serine (Ser), and cysteine (Cys) (Esterbauer et al., 1991; Hadley & Draper, 1988; Nair et al., 2001). Proteins are typically modified by MDA on the ε-amino groups of their side chains forming various epitopes by Schiff base reaction (Slatter, Bolton, & Bailey, 2000; Watson, Preedy, & Zibadi, 2012). These include preferentially side chains of Lys, but modifications of His, Tyr, Arg, Methionines (Met), Glutamines (Gln) and Cys have also been observed (Esterbauer et al., 1991; Gurbuz & Heinonen, 2015; Watson et al., 2012). Moreover, it has been demonstrated that both glycosylation as well as the acetylation of proteins render them more susceptible for MDA modification, possibly as a result of slight alterations in the tertiary structure of the protein that facilitate access for MDA (Mooradian, Lung, & Pinnas, 1996; Tuma, Thiele, Xu, Klassen, & Sorrell, 1996).
In human and animal models of disease MDA epitopes have been detected on proteins found in serum and urine, as well as on proteins of the extracellular matrix, and many intracellular ones (Akalin et al., 2007; Draper, Csallany, & Hadley, 2000; Gonenc et al., 2001; Imai et al., 2008; Weismann et al., 2011; Yla-Herttuala et al., 1989). Based on available literature we have generated an extensive list of endogenous proteins that had been reported to be modified by MDA, and these are presented in Table 2. In 4 different species, MDA modifications of a total of 107 proteins have been reported, of which 5 were found in at least 2 different organisms. These 5 proteins are: ATP synthase (subunit β), NADH dehydrogenase Fe-S protein 2, Cytochrome b-c1 complex (subunit 1), Albumin, and Actin. Interestingly, the majority of the proteins have mitochondrial origin, strengthening the notion that mitochondria, being major reservoirs of ROS, could be one site within the cell where MDA epitopes are preferentially generated.
Table 2.
List of identified MDA-modified proteins.
| Organism | Tissue | Protein | NCBI (GI) | Detection method | Ref. |
|---|---|---|---|---|---|
| Homo sapiens | Retina | Tubulin, beta chain | 5174739 | 2D gel electrophoresis, immunoblot with anti-MDA antibody | (Schutt, Bergmann, Holz, & Kopitz, 2003) |
| Tubulin, alpha-1 chain | 135395 | ||||
| ATP synthase beta chain | 1145449 | ||||
| Ubiquinol-cytochrome c reductase core protein I | 4507841 | ||||
| Pyruvate kinase, muscle | 14750405 | ||||
| ATP synthase, alpha subunit | 4757810 | ||||
| Enolase 1, alpha | 4503571 | ||||
| S-arrestin | 14737493 | ||||
| NADH dehydrogenase Fe-S protein 2 | 4758786 | ||||
| Guanine nucleotide-binding protein, beta polypeptide 2 | 4885283 | ||||
| Pyruvate dehydrogenase | 2144337 | ||||
| Annexin A2 | 16306978 | ||||
| Glyceraldehyde-3-phosphate dehydrogenase | 31645 | ||||
| Porin 31HM (anion channel 1) | 238427 | ||||
| Voltage-dependent anion channel 2 | 4507881 | ||||
| H119n carbonic anhydrase Ii | 2554664 | ||||
| Annexin V (Lipocortin V) | 999937 | ||||
| Prohibitin | 4505773 | ||||
| ATP synthase, subunit d | 5453559 | ||||
| Calmodulin 2 (phosphorylase kinase, delta) | 14250065 | ||||
| Cytochrome c oxidase subunit Va precursor | 4758038 | ||||
| Peroxiredoxin 2 | 13631440 | ||||
| Crystallin, alpha A | 4503055 | ||||
| Crystallin, alpha B | 4503057 | ||||
| Atherosclerotic lesion | ApoA-I | 4557321 | Detection with MDA2 on lesional HDL | ||
| Atherosclerotic lesion | Apo B-100 | 105990532 | Western blot on lesional LDL | (Palinski et al., 1989) | |
| Frontal cortex | Superoxide dismutase 2 | 119568015 | 2D gel electrophoresis/MS | (Dalfo et al., 2005) | |
| α-synuclein | 80475099 | ||||
| Brain cortex | Neurofilament light polypeptide | 62511894 | 2D gel electrophoresis/MS | (Pamplona et al., 2005) | |
| Vimentin | 55977767 | ||||
| Tubulin beta-2 | 55977480 | ||||
| Tubulin alpha-1B chain | 55977471 | ||||
| Tubulin alpha-4A | 55977476 | ||||
| Tubulin alpha-1C chain | 20455322 | ||||
| Actin, cytoplasmic 1 | 46397316 | ||||
| Actin, cytoplasmic 2 | 54036665 | ||||
| Glial fibrillary acidic protein | 121135 | ||||
| Gamma-enolase | 20981682 | ||||
| Alpha-enolase | 119339 | ||||
| Cytochrome b-c1 complex subunit 1 | 92090651 | ||||
| ATP synthase subunit beta | 114549 | ||||
| Creatine kinase B-type | 125294 | ||||
| Glutamine synthetase | 1169929 | ||||
| Glutamate dehydrogenase 1 | 118541 | ||||
| Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 | 51317300 | ||||
| 60 kDa heat shock protein | 129379 | ||||
| Dihydropyrimidinase-related protein 2 | 3122051 | ||||
| Plasma | Albumin | 4502027 | LC/MS, WB | (Odhiambo et al., 2007) | |
| Aortic tissue | Elastin | Fluorescence of elastin fraction | (Yamamoto et al., 2002) | ||
| Mus musculus | Broncho-alveolar lavage | Surfactant protein D | 6677921 | Immunopreciptation and Western Blotting | (McCaskill et al., 2011) |
| Blood | Haemoglobin | Sodium borohydrate reduction of MDA adducts | (Kautiainen, Tornqvist, Svensson, & Osterman-Golkar, 1989) | ||
| Heart and skeletal muscle mitochondria | Aconitase | 18079339 | SDS-PAGE/MS of MDA positive bands | (Yarian, Rebrin, & Sohal, 2005) | |
| Acyl coenzyme A dehydrogenase | 23956084 | ||||
| Albumin | 163310765 | ||||
| ATP synthase | 31980648 | ||||
| α-ketoglutarate dehydrogenase | 21313536 | ||||
| Kidney mitochondria | NADH dehydrogenase (ubiquinone) Fe-S protein 1 | 148667767 | 2D gel electrophoresis/MS | (Choksi, Nuss, Boylston, Rabek, & Papaconstantinou, 2007) | |
| ATP synthase subunit alpha | 6680748 | ||||
| ATP synthase subunit beta | 31980648 | ||||
| NADH dehydrogenase (ubiquinone) Fe-S protein 2 | 16359270 | ||||
| NADH dehydrogenase [ubiquinone] iron-sulfur protein 4 | 281485615 | ||||
| NADH dehydrogenase [ubiquinone] 1 subunit C2 | 18859597 | ||||
| Succinate dehydrogenase [ubiquinone] flavoprotein subunit | 54607098 | ||||
| Gamma-glutamyltranspeptidase 1 | 807201132 | ||||
| Isocitrate dehydrogenase 2 (NADP+) | 37748684 | ||||
| Catalase | 157951741 | ||||
| Cytochrome b-c1 complex subunit 1 | 46593021 | ||||
| Cytochrome b-c1 complex subunit 2, | 22267442 | ||||
| Cytochrome b-c1 complex subunit Rieske | 13385168 | ||||
| Peroxisomal acyl-coenzyme A oxidase 1 isoform 1 | 66793429 | ||||
| Long-chain specific acyl-CoA dehydrogenase | 31982520 | ||||
| Cytochrome c oxidase subunit II | 34538601 | ||||
| Rattus norvegic | Limb and heart muscles | Elongation factor 2 | 8393296 | Immunoprecipitation with anti-MDA antibody, EF2 western | (Arguelles, Cano, Machado, & Ayala, 2011) |
| Gastrocnemius | Beta-enolase | 122065177 | (Marin-Corral et al., 2010) | ||
| Creatine kinase M-type | 124056470 | ||||
| Carbonic anhydrase 3 | 5921194 | ||||
| Actin, alpha skeletal muscle | 61217738 | ||||
| Tropomyosin alpha-1 chain | 92090646 | ||||
| ATP synthase subunit beta, mitochondrial | 114562 | ||||
| Tibialis anterior | Beta-enolase | 122065177 | |||
| Fructose biphosphate aldolase A | 113609 | ||||
| Creatine kinase M-type | 124056470 | ||||
| Actin, alpha skeletal muscle | 61217738 | ||||
| Tropomyosin alpha-1 chain | 92090646 | ||||
| Extensor digitorum longus | Beta-enolase | 122065177 | |||
| Fructose biphosphate aldolase A | 113609 | ||||
| Creatine kinase M-type | 124056470 | ||||
| Actin, alpha skeletal muscle | 61217738 | ||||
| ATP synthase subunit beta, mitochondrial | 114562 | ||||
| Soleus | Beta-enolase | 122065177 | |||
| Fructose biphosphate aldolase A | 113609 | ||||
| Creatine kinase M-type | 124056470 | ||||
| Carbonic anhydrase 3 | 5921194 | ||||
| Actin, alpha skeletal muscle | 61217738 | ||||
| Tropomyosin alpha-1 chain | 92090646 | ||||
| ATP synthase subunit beta, mitochondrial | 114562 | ||||
| Heart | Creatine kinase M-type | 124056470 | |||
| Actin, alpha cardiac muscle 1 | 54036667 | ||||
| Tropomyosin alpha-1 chain | 92090646 | ||||
| Myosin-6 | 127741 | ||||
| Myosin light chain | 127151 | ||||
| Vacuolar proton pump subunit C1 | 81882798 | ||||
| ATP synthase subunit beta, mitochondrial | 114562 | ||||
| NADH-ubiquinone oxidoreductase 75 kDa subunit mitochondrial | 53850628 | ||||
| Aldehyde dehydrogenase mitochondrial | 14192933 | ||||
| Kidney | Type IV Collagen | 157821889 | IHC, WB | (Neale et al., 1994)Neale TJ, J Clin Invest,1994 | |
| Oryctolagus cuniculus | Type II Collagen | 2190238 | WB | (Tiku, Allison, Naik, & Karry, 2003) | |
Structurally different types of epitopes between MDA and primary amines have been reported, including fluorescent and non-fluorescent ones as well as cross-linking or non-cross-linking ones. Important examples are presented in Figure 3.
Figure 3.
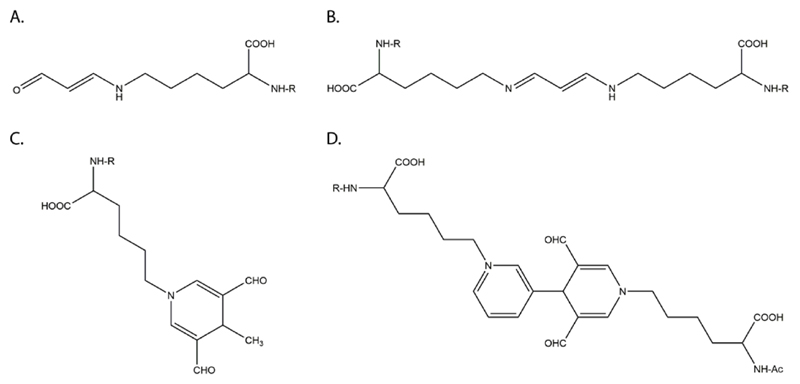
Chemical structures of MDA epitopes found on Lys side chains of protein (Gonen et al., 2014; Uchida, 2000). R = protein backbone. (A) N-ε-(2-propenal)Lys. (B) 1-Amino-3-iminopropene. (C) 4-methyl-1,4-dihydropyridine-3,5-dicarbaldehyde derivative (D) Pyridium-dihydropyridine.
The most common type of adduct is N-ε-(2-propenal) lysine (Fig 3A), which is a linear non-fluorescent adduct without the ability to generate cross-links and forms in the absence of AcA at pH 7 (Uchida, Sakai, Itakura, Osawa, & Toyokuni, 1997). It is the major form of endogenous MDA excreted in rat and human urine using ion exchange and high-performance liquid chromatography (Draper, McGirr, & Hadley, 1986; Piche, Cole, Hadley, van den Bergh, & Draper, 1988) and has been detected on in vitro MDA-modified keyhole limpet hemocyanin (KLH), MDA-modified LDL, and MDA-modified Apoprotein A-I (ApoAI), as well as copper-oxidized LDL (CuOx-LDL)(Shao et al., 2010). More complex MAA epitopes on ε-amino groups of lysines are formed by MDA and AcA in a 2:1 ratio (Tuma et al., 1996). The two major MAA epitopes are a 4-methyl-1,4-dihydropyridine-3,5-dicarbaldehyde derivative (MDHDC adduct) and the 2-formyl-3-(alkylamino) butanal derivative (FAAB adduct). In contrast to FAAB, MDHDC is a fluorescent adduct with ʎ ex=395nm and ʎ em=485nm. It requires the close proximity of two intermediates, including an FAAB adduct that is generated when one molecule of MDA and one of AcA react with free amino groups of a protein side chain, and an MDA Schiff base (MDA-enamine) generated by the reaction of MDA with another free amino group. After the generation of these two intermediates, the FAAB moiety is transferred to the nitrogen of the MDA-enamine followed by ring closure yielding the MDHDC adduct (Fig 3C) (Tuma et al., 2001). The formation of MDHDC epitopes has been demonstrated to be favored by acidic conditions (pH 4). Using NMR spectroscopy (Kearley, Patel, Chien, & Tuma, 1999) and performing chemical analysis (Xu et al., 1997) it has been shown that MAA epitopes are different from those epitopes formed when both aldehydes are allowed to react alone. Several lines of evidence suggest that this type of MAA epitopes possesses potent biological activity (Tuma et al., 1996; Uchida et al., 1997), although N-ε-(2-propenal) lysine epitopes have also been shown to be highly immunogenic (Gonen et al., 2014). Moreover, epitopes with the ability to form cross-links between different Lys residues in vitro have been found as well. These epitopes from cross-links within the same domain of a protein or domains in close proximity. In these reactions one lysine residue forms a Schiff base with MDA, which is stabilized by equilibration to an enolate, and reacts then with a second lysine residue to form a diimine cross-link (Requena et al., 1996). Both simple MDA cross-links, such as 1-amino-3-iminopropenes (Fig 3B), and more complex ones that are also fluorescent, such as pyridium–dihydropyridine epitopes have been described (Fig 3D) (Itakura, Uchida, & Osawa, 1996). Stable cross-linking MDA epitopes were first demonstrated under semi-physiological conditions using NMR and EI-MS (Slatter, Murray, & Bailey, 1998). Such epitopes have been proposed to play a role by affecting structural proteins, such as collagen in the vessel wall where this could contribute to vascular stiffening and development of vasculopathies. In addition, in vitro MDA cross-links distant Lys residues of ApoAI (Shao et al., 2010) as well as purified ApoE3 and ApoE4 molecules (Montine et al., 1996), and cross-links apoprotein(a) to ApoB100 (Haberland, Fogelman, & Edwards, 1982). Finally, endogenous cross-linking MDA epitopes of proteins are formed on platelet proteins upon platelet activation and have been found to be increased in diseases associated with platelet activation, such as metabolic syndrome and sickle cell anemia (Zagol-Ikapite et al., 2015).
3.2. MDA modification of nucleotides and nucleic acids
Free MDA has been shown to form covalent epitopes with nucleotides, such as deoxy-guanosine, deoxy-adenosine and deoxy-cytidine, in a reaction that is enhanced in the acidic milieu. The major epitopes that can be formed by these reactions are pyrimido-[1,2-α]purin-10(3H)-one deoxyribose (M1G), N6-(3-oxo-propenyl)deoxyadenosine (M1A) and N4-(3-oxopropenyl)deoxycytidine (M1C) (Marnett, 1999). Of these M1G are five times more abundant than M1A adducts in the liver, and the amounts of M1C are generally very low (Chaudhary, Reddy, Blair, & Marnett, 1996). MDA is considered to be a potent mutagen (Esterbauer et al., 1991), and MDA-modifications of nucleotide bases of DNA have been shown to induce genotoxicity by various mechanisms. MDA epitopes on nucleotides can cause template changes (Maddukuri et al., 2010; VanderVeen, Hashim, Shyr, & Marnett, 2003), result in the formation of interstrand cross-links (Niedernhofer, Daniels, Rouzer, Greene, & Marnett, 2003), or create DNA-protein cross links (Voitkun & Zhitkovich, 1999). Interestingly, it has been shown that DNA isolated from human and rat liver exposed to enhanced lipid peroxidation contains increased amounts of MDA-epitopes on DNA (Chaudhary et al., 1994). Nucleotide excision repair has been suggested to be the main mechanism of repair for M1G-caused damage in DNA (Marnett, 1999). Moreover, mitochondrial DNA has been found to have a higher abundance of MDA-DNA epitopes compared to nuclear DNA. This increased amount of MDA-epitopes in mitochondrial DNA is thought to be due to the lack of nucleotide excision repair in mitochondria (Cline, Lodeiro, Marnett, Cameron, & Arnold, 2010). Interestingly, increased levels of MDA-adducted nucleotides are found in the serum and urine of patients suffering from cancer as well as diseases associated with increased ROS production (Gungor et al., 2010; Munnia et al., 2006; Peluso et al., 2011).
3.3. MDA modification of phospholipids
In contrast to MDA modifications of proteins or nucleotides, only little data are available on MDA modification of phospholipids, which can be modified as well. Indeed, aminophospholipids, such as phosphatidylserine (PS) and phosphatidylethanolamine (PE), have been found to form epitopes with MDA by enaminal derivatization. MDA-modified PS and PE have been identified in aging erythrocytes possibly as a consequence of peroxidative lipid damage (Gil, Farinas, Casado, & Lopez-Fernandez, 2002; Jain, 1988) and as metabolic waste products excreted in urine (Draper et al., 2000; Uchida et al., 1997).
3.4. Metabolism of MDA epitopes in vivo
Not much is known about metabolism of MDA epitopes in vivo. Marnett LJ et al (Marnett, Buck, Tuttle, Basu, & Bull, 1985) injected intraperitoneally 14C-labeled MDA into male and female Swiss Webster mice, and found rapid and uniform distribution of labeled MDA throughout the body. Moreover, it was observed that conversion of MDA to CO2 is completed in 4 hours. In another study urine samples obtained from rats challenged with oxidative stress by treatement with iron nitrilotriacetate or carbon tetrachloride or vitamin E deficiency were found to have MDA present on proteins, nucleic acid bases, and phospholipids as measured by HPLC (Draper et al., 2000) A similar excretion pattern, albeit in much smaller amounts, was observed in urine samples obtained from humans (Draper et al., 2000). Additionally, it has been shown that MDA levels in human urine can be modulated by diet, physical exercise, and smoking (Hadley, Visser, & Vander Steen, 2009; Mellor, Hamer, Smyth, Atkin, & Courts, 2010).
4. Carriers of MDA epitopes
Tissue stress and subsequent cellular damage result in the generation of dying cells, the release of microvesicles (MV) and the accumulation of cellular debris. A number of studies have documented the presence of MDA epitopes on apoptotic cells and MV. For example, we and others could show that MDA-specific antibodies bind to serum-deprived apoptotic endothelial cells, dexamethasone-treated or UV-irradiated apoptotic T cells, as well as to necrotic cells and apoptotic blebs from retinal pigment epithelial cells (Amir et al., 2012; Aredo et al., 2015; Chang et al., 1999; Chou et al., 2009; Weismann et al., 2011)(see Table 1 for an overview). Furthermore, MDA epitopes have been on the membranes of oxidized MV released from endothelial cells (Huber et al., 2002), and human monocytic cells treated with unesterified cholesterol (Liu et al., 2012), as well as on in vitro generated platelet-derived MV and on circulating MV collected from human blood (Gil et al., 2002; Tsiantoulas et al., 2015). In addition, MDA has been detected in ageing erythrocytes also suggesting the presence of MDA epitopes in their membranes.
Table 1.
Cellular and subcellular carriers of MDA epitopes.
| Carrier type | Induction | Method of Detection | References |
|---|---|---|---|
| Microvesicles (MV) | |||
| Circulating MV | Flow cytometry | (Tsiantoulas et al., 2015) | |
| Platelet MV | Ionomycin | Flow cytometry | (Tsiantoulas et al., 2015) |
| Endothelial MV | tert-butyl hydroperoxide, Fe2+ | Flow cytometry | (Huber et al., 2002) |
| Monocytic MV | Cholesterol | Flow cytometry | (Liu et al., 2012) |
| Activated or dying cells | |||
| Retinal pigment epithelial cells | Heat | Immunofluorescence | (Weismann et al., 2011) |
| Thymocytes | PMA | Flow cytometry, Immunofluorescence | (Chou et al., 2009) |
| Jurkat T cells | UV | Flow cytometry | (Amir et al., 2012) |
| Endothelial cells | Serum deprivation | Flow cytometry, Immunofluorescence | (Chang et al., 1999) |
| Thymocytes | Dexamethasone | Flow cytometry, Immunofluorescence | (Chang et al., 1999) |
| Jurkat T cells | UV | Flow cytometry, Immunofluorescence | (Tuominen et al., 2006) |
| THP-1 cells | Cholesterol | Immunofluorescence | (Liu et al., 2012) |
| Erythrocytes | Ageing | HPLC | (Gil et al., 2002) |
| Platelet | Metabolic syndrome, sickle cell anemia | Mass Spectrometry | (Zagol-Ikapite et al., 2015) |
MDA epitopes have been identified on plasma lipoproteins and proteins. Among the lipoproteins, oxidized HDL and LDL eluted from the lesional tissues have been shown to carry MDA epitopes (Haberland, Fong, & Cheng, 1988; Palinski et al., 1989; Shao et al., 2010; Yla-Herttuala et al., 1989). In addition to lipoproteins, number of other proteins modified by MDA have been detected in body fluids and tissues of different organisms listed in Table 2.
5. Targets of immune response
As described above, MDA-modifications of endogenous molecules are typically associated with structural changes and subsequently functional alterations of these macromolecules. Moreover, MDA-modifications occur as a consequence of increased oxidative stress and therefore signify tissue damage. Thus, for the host to deal with potentially harmful effects of altered self-molecules it needs to recognize them and respond to their accumulation, e.g. by mounting specific immune responses. Already in the 1980s Witztum and colleagues have shown that injection of guinea pigs with glucosylated autologous LDL or glucosylated autologous albumin resulted in the generation of antisera recognizing the glucosylation itself but not the proteins that were modified (Witztum, Steinbrecher, Fisher, & Kesaniemi, 1983).
In a follow-up study, specific antisera could also be generated with differently modified autologous LDL, including methylated, ethylated, acetylated and carbamylated LDL (Steinbrecher, Fisher, Witztum, & Curtiss, 1984). These pioneering studies demonstrated that even the smallest modifications of autologous proteins can result in the haptenization of self-proteins leading to the formation of neo-self antigens that are recognized by the immune system. This concept has provided insights into the mechanisms by which oxidation of LDL renders it immunogenic, as this process generates several different OSE that modify oxidized LDL particles and are recognized by the immune system in a hapten-specific manner. Many different OSEs have been characterized, including 4-hydroxynonenal (4-HNE), phosphocholine (PC) of oxidized phospholipids, and MDA (Hartvigsen et al., 2009). There is now ample evidence that OSE are recognized by both innate and adaptive immunity.
6. Innate immune responses towards OSEs
The innate immune system is instantaneously capable of recognizing a broad range of structures via pattern-recognition receptors (PRRs). Depending on whether the ligands originate from a foreign source (e.g. microbes) or from within the host, they have been collectively named pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs), respectively. Tissue-resident macrophages expressing PRRs on their surfaces are among the first cells of the innate immune system to sense PAMPs or DAMPs and initiate an inflammatory response to ultimately remove pathogens or the triggering agent, respectively. Several classes of PAMPs, such as bacterial lipopolysaccharide (LPS), bacterial flagellin, lipoteichoic acid from Gram positive bacteria, double stranded RNA, unmethylated CpG motifs and DAMPs, such as high mobility group box 1 protein (HMGB1), heat shock proteins (HSP), ATP and uric acid have been described (G. Y. Chen & Nunez, 2010; Krysko et al., 2012). DAMPs are typically intracellular components that are secluded from the recognition by the immune system and can be released into extracellular environment upon cellular damage. Because DAMPs convey a message of danger to other cells employing specific cellular receptors and triggering innate immunity response, OSE, incl. MDA, which fulfill these criteria can be considered a novel class of DAMPs (Figure 4) (Miller et al., 2011).
Figure 4.
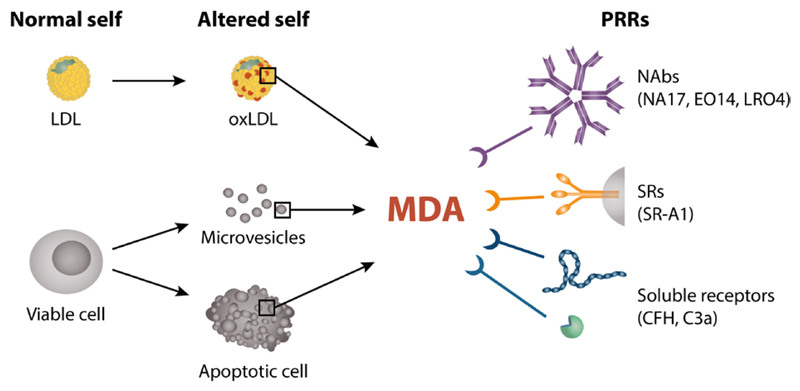
Overview of MDA epitopes and their interaction with different arcs of innate immunity. MDA epitopes are danger signals generated on biomolecules such as LDL in atherosclerotic lesions due to oxidative stress and on the surface of cells, cellular debris or microvesicles during apoptosis and cell activation. Both soluble and membrane-bound factors of the innate immune system bind to MDA epitopes presented on OxLDL or cell membranes including natural antibodies such as E014 and NA-17, the scavenger receptor SR-A1 and two members of the complement system, CFH and C3a. Also other OSEs are recognized by innate immunity (not shown here).
Indeed, OSEs are recognized by PRRs present on macrophages, such as the heterogeneous family of scavenger receptors (SRs), which bind and internalize (scavenge) oxidized but not native LDL (Canton, Neculai, & Grinstein, 2013). Among SRs, CD36 and SR-A1 are the two most relevant receptors for the uptake of OxLDL, which leads to the formation of lipid-laden foam cells – a hallmark of atherosclerotic lesions. Importantly, some OSEs are not merely taken up by SRs, but can also serve as inflammatory mediators alarming the immune system about the damage inflicted by atherogenic lipoproteins. Another important PRR involved in clearing damaged lipoproteins are toll-like receptors (TLRs), which bind to a vast variety of different PAMPs and DAMPs, including OSEs. Certain TLRs have been shown to bind - in part as part of a multimeric complex - to oxidized phospholipids, OxLDL and other OSEs, thereby mediating pro-inflammatory signals. For example, recognition of OxLDL by CD36 on macrophages enables the formation of a heterotrimeric complex consisting of CD36, TLR4 and TLR6, which mediates inflammatory responses resulting in nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) pathway activation and expression of chemokines including chemokine (C-X-C motif) ligand 1 (Cxcl1), Cxcl2, chemokine (C-C motif) ligand 5 (Ccl5) and Ccl9 (Stewart et al., 2010). Macrophages lacking any of the three receptors fail to respond to OxLDL stimulation, while TLR2 deficiency does not have any effect. TLR2, however, seems to play a role when macrophages subjected to ER stress are stimulated with several oxidized phospholipids, saturated fatty acids and OxLDL, which results in a TLR2/CD36-dependent induction of apoptosis (Seimon et al., 2010).
Thus, a number of PRRs recognize OSEs and act as sensors of oxidative stress. Upon ligation of OSEs with their corresponding PRRs, signal transduction events are initiated that culminate in sterile inflammatory responses characterized by chemokine secretion, leukocyte recruitment or tissue repair. There is accumulating evidence that certain PRRs and PRPs are also involved in sensing of MDA epitopes as DAMPs and in the following section we will review published work both on the recognition by innate immunity and on the pro-inflammatory effects of MDA epitopes.
6.1. Cellular receptors of MDA epitopes
In line with initial studies showing that modified but not native LDL is recognized by macrophages via SRs (Goldstein, Ho, Basu, & Brown, 1979; Henriksen, Mahoney, & Steinberg, 1981), Shechter et al. demonstrated that both acetyl-LDL and fucoidin, a known SR-A1 ligand, inhibited the uptake of MDA-modified LDL (MDA-LDL) by human monocyte-macrophages (Shechter et al., 1981), indicating that MDA-LDL is recognized and degraded also via a SR-dependent mechanisms. Similarly, Lipoprotein (a) (Lp(a)) is recognized by human monocyte-macrophages via SRs only upon modification with MDA, as MDA-LDL, CuOx-LDL and polyinosinic acid inhibited binding and degradation of MDA-Lp(a) (Haberland, Fless, Scanu, & Fogelman, 1992). In addition, binding of LDL isolated from rabbit atherosclerotic lesions to mouse peritoneal macrophages was competed for by MDA-LDL or polyinosinic acid, suggesting SR-A1-mediated uptake (Yla-Herttuala et al., 1989). In light of this, MDA-epitopes present on altered self-molecules or on the surface of dying cells could be sensed and identified by SRs as waste markers and signals of disturbed homeostasis that are required to be scavenged, i.e. removed from the system.
More recently, additional groups have confirmed that SR-A1 is involved in binding to MDA-modified proteins in a number of different cell types, including J774 cells (Willis, Klassen, Carlson, Brouse, & Thiele, 2004), bovine or human bronchial epithelial cells (Berger et al., 2014; Wyatt, Kharbanda, Tuma, Sisson, & Spurzem, 2005), murine bone-marrow-derived macrophages (Wallberg et al., 2007), mouse peritoneal macrophages and SR-A1-expressing human embryonic kidney cells (Nikolic, Cholewa, Gass, Gong, & Post, 2007), corroborating prior data that SR-A1 is one but not necessarily the only receptor binding to MDA-epitopes. Indeed, Zhu et al accidentally found that CD16, an Fcγ receptor (FcγR) included in the study as a negative control, inhibited the binding of J774 cells to MDA-LDL-coated wells to a larger extent than soluble CD36, which was the original target of interest (Zhu et al., 2014). Similar to SRs, FcγRs have been shown to identify and eliminate microbial pathogens. Binding to the Fc portion of antibodies attached directly to pathogens or to the surface of infected cells allows enhanced uptake and clearance by cells equipped with an FcγR. Four groups of FcγRs exist in mice: FcγRI (CD64), FcγRIIB (CD32), FcγRIII (CD16) and FcγRIV (CD16-2) of which all but FcγRIIB are activating receptors containing an immunoreceptor tyrosine-based activation motif (ITAM) in their intracytoplasmic domain. In the study, the inhibition of MDA-LDL binding occurred in a dose-dependent manner and was competed for by immune complexes, known ligands of CD16. Of note, CD16 did not bind to OxLDL. The authors also observed decreased MDA-LDL and immune complex binding to macrophages after silencing or knocking out CD16, while OxLDL binding was unchanged between wild-type and CD16-/- macrophages. Lack of CD16 resulted also in reduced secretion of TNF-α, monocyte chemoattractant protein 1 (MCP-1) and RANTES upon MDA-LDL stimulation, which was dependent on Syk phosphorylation. Together, the authors showed that CD16 might be an additional receptor for MDA epitopes next to SR-A1, possibly implying that a redundancy of uptake and clearance mechanisms could be beneficial for an organism to protect from consequences of oxidative stress.
6.2. Pro-inflammatory effects of MDA epitopes
Inflammation is a physiological process caused by the body’s own immune mechanisms that have evolved to sense and handle the undesirable presence of both altered endogenous and exogenous stimuli. Resolution of an inflammatory response can only occur when the inflammatory stimuli are cleared and no longer cause any harm. Impaired resolution and/or continuous generation of inflammatory agents might result in the establishment of a chronic inflammation accompanied by persistent tissue damage. Various studies have addressed the pro-inflammatory capacity of MDA-modified proteins in different cell types including primary cells and cell lines from different animal models. Stimulation of cells derived from rat heart or liver such as endothelial (Duryee et al., 2008; Hill et al., 1998; Thiele et al., 2004) and stellate cells (Kharbanda, Todero, Shubert, Sorrell, & Tuma, 2001) results in the release of tumor necrosis factor alpha (TNF-α), MCP-1, macrophage inflammatory protein 2-alpha (MIP2α) and fibronectin or the expression of intercellular adhesion molecule 1 (ICAM-1), major histocompatibility complex (MHC) class II, and vascular cell adhesion molecule (VCAM-1), MCP-1 and MIP-2. In bovine bronchial epithelial cells MDA-epitopes induce interleukin (IL-8) and urokinase-type plasminogen activator (uPA) secretion via protein kinase C (PKC) activation (Kharbanda, Shubert, Wyatt, Sorrell, & Tuma, 2002; Wyatt, Kharbanda, Tuma, & Sisson, 2001). Indeed, several studies demonstrate that the pro-inflammatory effects induced by MDA epitopes involve the activation of PKC (Kharbanda et al., 2002; Wyatt et al., 2012; Wyatt et al., 2001) but also PI3-kinase, Src-kinase (Nikolic et al., 2007), PL-C/IP3 (Cai et al., 2009) and NF-kB (Raghavan, Subramaniyam, & Shanmugam, 2012; Shanmugam et al., 2008) have been implicated. Interestingly, in analogy to cholesterol crystals, MDA epitopes were shown to induce lysosomal rupture upon uptake (Willis et al., 2004) when stimulating J774 macrophages with MDA-modified hen egg lysozyme (MDA-HEL). The authors found that binding of MDA-HEL to the cells occurs in a SR-A1-dependent manner and results in lysosomal rupture followed by cell death. Other studies have shown that treatment of a human hepatic stellate cell line with MDA-modified horse-serum albumin induces activation of ERK1/2 and NFkB (Kwon et al., 2014). The same authors also demonstrated that murine Kupffer cells stimulated with MAA-modified human serum albumin secrete interleukin 6 after 2 h in the presence of LPS, building on an earlier observation that MAA-BSA-stimulated Kupffer cells exhibited an synergistically enhanced cytokine response in the presence of LPS (Duryee et al., 2004). In contrast, no synergistic effect of the TLR2 ligand Pam3CSK4 with MDA-BSA in macrophages could be observed (Saeed et al., 2014).
Additional validation of the pro-inflammatory capacity of MDA epitopes in vitro is provided in an unbiased approach, where Shanmugam et al (Shanmugam et al., 2008) analyzed conditioned media of THP-1 cells treated with MDA-modified Lys using hybridization-based cytokine arrays. MDA-Lys was found to increase the levels of 20 cytokines, including C-C motif chemokine 11 (CCL11), chemokine (C-C motif) ligand 18 (CCl18), C-C motif chemokine 28 (CCL28), tumor necrosis factor superfamily member 14 (TNFSF14), MCP-1 and MCP-2. Further in silico analysis of the networks involved using a pathway analysis software revealed that treatment with MDA-Lys induces targets involved in networks such as immune response, cellular movement and cell-to-cell signaling. Using reporter gene assays or chemical inhibitors, the authors showed that MDA-Lys induced activation of NF-kB and p38 MAPK pathways. Furthermore, they observed that incubation of monocytes with MDA-Lys increases their adhesion to smooth muscle or endothelial cells. Together, they demonstrated that MDA-Lys impacts inflammatory gene expression, activation and motility of monocytes. This was also observed in a separate study using Jurkat T cells stimulated with free MDA (Raghavan et al., 2012).
Next to the expression of cytokines and adhesion molecules several groups have also investigated motility, morphological changes and effects on cell viability caused by MDA epitopes. For example, MDA-BSA-coated plates incubated with SR-A1-expressing human embryonic kidney (HEK) cells results a spread morphology within 10 minutes, accompanied by an increase in cell surface area and the formation of membrane extensions after 2 hours in a phosphoinositide 3-kinase (PI3)-kinase- and Src-kinase-dependent manner(Nikolic et al., 2007). Morphological changes were also observed in rat heart endothelial cells stimulated with MAA-BSA (Hill et al., 1998) or in J774 macrophages (Willis et al., 2004) and in some cases these morphological alterations are followed by detachment and cell death (Thiele et al., 2004; Willis et al., 2004; Willis, Klassen, Tuma, & Thiele, 2002). In contrast, MDA-LDL only weakly induced the expression of collagen I, collagen III and fibronectin in human hepatic stellate cells (Schneiderhan et al., 2001), while the cell morphology was found to be unchanged. Also THP-1 cells stimulated with MDA-LDL displayed even increased cell proliferation in one study (Suzuki, Sasaki, Kumagai, Sakaguchi, & Nagata, 2010) and other researchers found that free MDA induces migration in PBMCs and BMDMs after 1 hour of incubation (Geiger-Maor et al., 2012) while rat neurons were damaged by free MDA via induction of Ca2+ influx (Cai et al., 2009) or via a MAPK pathway in a dose- and time-dependent manner (Cheng, Wang, Yu, Wu, & Chen, 2011). These apparent inconsistent results may be due to the different cell types used in these studies. Nevertheless, there is little doubt about the pro-inflammatory properties of MDA epitopes in vitro.
However, there are only few studies investigating the in vivo effect of MDA epitopes. One study by us showed that MAA-BSA induces a pro-inflammatory response in the retinal pigment epithelium of mice upon intravitreal injection characterized by a robust increase in KC expression (Weismann et al., 2011). In another murine model of lung injury, MAA-BSA or MAA-modified surfactant D (SPD) was instilled into the lungs of wild-type mice upon which elevated levels of neutrophils and KC were detected in the lung lavage fluid (Wyatt et al., 2012). Even though the amount of in vivo data is scarce it clearly corroborates the in vitro data demonstrating that MDA epitopes are DAMPs capable of alerting the immune system of high oxidative stress, inflammation and potential tissue damage by inducing secretion of cytokines.
6.3. Innate recognition by IgM natural antibodies and complement
Natural antibodies (NAbs) are predominantly of the IgM class and constitute an important component of humoral innate immunity. In addition to their key role in the first line defense against microbial infections, NAbs also play a vital role in the removal of apoptotic cells and metabolic waste to maintain tissue homeostasis (Manson, Mauri, & Ehrenstein, 2005). They are naturally occurring antibodies that can bind microbial structures and stress-induced self-antigens through an evolutionary conserved repertoire of variable regions made out of non-mutated germline genes. In mice IgM NAbs are secreted by B-1 cells, which constitute a subset of primordial B cells with different surface marker expression, activation requirements, and a distinct anatomical location (Baumgarth, 2011). NAbs are present at birth and detectable already at the fetal stage. They can be found in germ-free mice, indicating that their occurrence is independent of external antigenic stimuli. Nevertheless, their titers can be enhanced by positive antigenic stimulation (Baumgarth, 2011; Baumgarth, Tung, & Herzenberg, 2005).
First insights into the concept that IgM NAbs bind OSEs came from studies in which spleens of 9 months old Apolipoprotein E (Apoe)-/- mice fed a high fat high cholesterol diet for 7 months, which exhibit high titers of OSE-specific IgM, were used to generate hybridoma cell lines (Palinski et al., 1996). About 30% of the hybridoma cell lines producing IgM antibodies were recognizing epitopes of OxLDL. Interestingly, a large part of the clones identified were found to have specificity for MDA-LDL. Detailed characterization of one of the clones termed E06 that specifically binds to PC of oxidized phospholipids of OxLDL demonstrated its CDR3 region to be encoded by the canonical rearrangement of the identical V-D-J germ line genes that encode a previously characterized IgA natural antibody, called T15 (Shaw et al., 2000). This suggested that many more OSE-specific IgM could be NAbs. Indeed, we could subsequently show that plasma of mice that were kept under complete germ free conditions contained IgM antibodies with specificity for several OSEs, including MDA-type epitopes (Chou et al., 2009), demonstrating their natural occurrence in the absence of exposure to foreign antigens. Notably, colonization of the gut of these mice with microbiota of conventionally housed mice resulted in the expansion of some, but not all, OSE-specific IgM. The latter is consistent with the fact that many IgM NAbs possess dual reactivity for microbial and self-antigens (Baumgarth, 2011; Racine & Winslow, 2009). Moreover, we could also demonstrate that IgM antibodies in human umbilical cord blood of newborns contained high titers of IgM with specificity for OxLDL and MDA-LDL. In contrast to IgG, IgM are not transported across the placenta. Therefore, IgM in umbilical cord blood are exclusively of fetal origin and therefore can be regarded as human NAbs. Interestingly, compared to the matched maternal plasma of the newborns the IgM titers to OxLDL and MDA-LDL were significantly enriched in the umbilical cord plasma. Key evidence for the existence of OSE-specific and in particular MDA-specific IgM NAbs came from characterization of the binding specificity of IgM derived from murine B-1 cells, isolated from the peritoneal cavities of wild type mice. Both supernatants of purified B-1 cells stimulated with LPS or IL-5 as well as plasma of recombination-activating gene 1 deficient mice (Rag1-/-) (that do not have any functional B- or T cells) reconstituted with purified B-1 cells contained IgM with specificity for MDA-epitopes. Importantly, ELISpot studies for the frequencies of IgM-secreting cells in the spleens and antigen absorption studies of the plasma IgM of B-1 cell reconstituted mice revealed that ca. 15% of all B-1 cell derived IgM NAbs have specificity for MDA-epitopes, as well as more complex MAA-epitopes, which constitute the majority of OSE-specific IgM NAbs that represent 30% of the NAb repertoire. Finally, sequence analyses of the variable region of a newly cloned anti-MDA IgM mAb, termed NA17, derived from the spleens of these mice, did not reveal nucleotide variation to germline genes in the VH rearrangements, and only 1 nucleotide insertion between VL and JL germline gene segments (Chou et al., 2009). Similarly, previously cloned anti-MDA IgM NAbs (E014, LR04) have also been found to express unmutated germline variable genes (Lichtman, Binder, Tsimikas, & Witztum, 2013). Thus, several lines of evidence identify MDA-epitopes as major targets of IgM Nabs.
The high prevalence of MDA-specific IgM NAbs is consistent with an important role in homeostatic house-keeping functions. Indeed, as described above apoptotic cells as well as MV carry MDA-epitopes and are recognized by MDA-specific IgM. NAbs have been shown to play a critical role in the clearance of apoptotic cells via complement-dependent mechanisms (Y. Chen, Park, Patel, & Silverman, 2009; Ogden, Kowalewski, Peng, Montenegro, & Elkon, 2005). We could also demonstrate that the MDA-specific IgM NAb NA17 binds apoptotic thymocytes, but not viable cells. Moreover, when injected together with apoptotic thymocytes into the peritoneal cavity of mice, NA17 enhances their uptake by peritoneal macrophages in vivo (Chou et al., 2009). It has been suggested that MDA epitopes exposed on apoptotic cells guide the immunosilent and anti-inflammatory clearance of apoptotic cells by recruitment of MDA specific IgMs and co-recruitment of C1q (Y. Chen et al., 2009). Thus, IgM NAbs with specificity for MDA have the capacity to engage certain functions of complement for host homeostasis.
The complement system represents another humoral component of innate immunity. It is a complex system consisting of about 40 different proteins organized in three major pathways: the classical, the lectin and the alternative pathway (Ricklin, Hajishengallis, Yang, & Lambris, 2010). Major functions of complement involve the protection of the host from invading pathogens, the orchestration of innate and adaptive immune responses, and house-keeping functions in promoting clearance of apoptotic cells. Immune complexes deposited on pathogenic surfaces initiate the classical pathway, a range of different carbohydrates exposed on pathogens initiate the lectin pathway, and the alternative pathway has a low grade of constitutive activation. Although each complement pathway has a unique way of initiation, they all converge at the step of C3 cleavage, a central component of the complement, by C3 convertases into smaller C3a and a larger C3b fragment. Downstream of C3, all pathways are merged into one resulting in the generation of the terminal complement complex (TCC, MAC). Clearly, the proteolytic cascade of complement needs to be tightly controlled at several levels to prevent deleterious consequences for the host, which is achieved by a group of proteins called regulators of complement activity (RCA). RCA family members are divided into two major subgroups, soluble ones, such as complement factor H (CFH) and C4 binding protein (C4BP), and membrane-bound ones, including CD46, CD55, CR1, CR2, CR3, and CR4 (Zipfel & Skerka, 2009).
There is now increasing evidence that MDA-epitopes also directly trigger specific aspects of complement. Using an unbiased approach to identify MDA-reactive plasma proteins in pull-down assays using MDA-modified polystyrene beads and plasma from Rag-/-Ldlr-/- mice, we identified CFH as a major binding protein for MDA-epitopes (Weismann et al., 2011). CFH is a glycoprotein in plasma with a concentration of 500 µg/ml and acts as major regulator of the alternative complement pathway. Soluble and attached to host cell surfaces CFH plays an important complement inhibitory role. CFH regulates complement in three different ways: it disables the formation of the C3 convertase, facilitates its decay and enhances proteolytic cleavage of C3b to generate inactive iC3b. Employing different ELISAs we showed that CFH binds to MDA-epitopes directly and independently of their protein carrier, but not to PC- or 4-HNE-epitopes (Weismann et al., 2011). Furthermore, this binding seems to require the presence of advanced MAA-epitopes such as MDHDC and is Ca2+-and Mg2+-independent. When CFH is bound to MAA-modified BSA, its complement regulatory activity in factor-I-mediated C3 cleavage, is retained, as demonstrated by the fact that MAA-bound CFH had the capacity to cleave C3b into iC3b in the presence of factor I. This is important as opsonization of apoptotic cells with iC3b has been shown to mediate their anti-inflammatory clearance by macrophages, and we have demonstrated that binding of CFH to dying cells and blebs is in part mediated by MDA-epitopes. CFH is a multi-domain molecule made out of 20 domains named short consensus repeats (SCRs) arranged in a ‘beads on the string’ fashion. Using recombinant constructs of CFH, in which different numbers of SCRs had been deleted, we could demonstrate that only SCR7 and SCR19/20 were able to mediate binding of CFH to MAA epitopes. Notably, SCR7 and SCR20 have been described as clustering sites for mutations in CFH that are associated with many complement-related diseases, such as age-related macular degeneration (AMD), atypical hemolytic uremic syndrome (aHUS), and C3 glomerulopathies (Ricklin & Lambris, 2013). In this regard we could demonstrate that CFH of carriers of the common CFH SNP rs1061170 that results in an H to Y exchange on position 402 (H402Y variant) exhibits severely impaired binding to MAA in a gene-dosage-dependent manner with >60% decreased binding for homozygous carriers compared to homozygous controls. These findings provided important insights into the strong association of rs1061170 with the risk to develop AMD – a chronic inflammatory disease of the retina that is associated with increased oxidative stress and the most common cause of blindness in the elderly. One may speculate that binding of MAA on apoptotic and necrotic cells by CFH may allow it to inhibit pro-inflammatory effects of MAA and regulate complement activation on these surfaces. Indeed, we could show that CFH at physiological concentrations has the capacity to inhibit MAA-induced IL-8 secretion by the human monocytic THP-1 cell line. Moreover, the induction of KC expression in the retinal pigment epithelium of mice injected intravitreally with MAA-BSA was completely abolished when CFH was co-injected. Thus, CFH has been shown to scavenge pro-inflammatory properties of MAA in vitro and in vivo, and to mediate important co-factor activity on MAA-carrying surfaces. Both properties would favor an anti-inflammatory removal of potentially harmful dying cells. Recently, Aredo et al generated transgenic CFH mice carrying human CFH sequence for SCR6–8 (with either 402Y or 402H), flanked by the mouse sequence for SCR1–5 and SCR9–20 (Aredo et al., 2015). Aged mice transgenic for the human CFH H402 variant, in contrast to wild-type mice, had increased accumulation of MDA-modified proteins, increased microglial/macrophage activation, and induction of pro-inflammatory gene expression in the retina. Similar results were observed when 11 months old mice of both groups where subjected to another model of chronic oxidative stress using hydroquinone diet and exposure to white fluorescent lights for 8 weeks (Aredo et al., 2015). These findings support the notion that CFH binding to MDA may play an important role in AMD pathogenesis by modulating oxidative stress and inflammation in the retinal pigment epithelium.
Further insights on the potential importance of CFH binding to MDA was provided by studies investigating the effect of SNPs in SCR19-20 of CFH that predispose to the development of atypical hemolytic uremic syndrome (aHUS) (Hyvarinen et al., 2014). This disease is characterized by the development of microangiopathic hemolytic anemia and thrombocytopenia leading to renal failure (Nester et al., 2015). In this study, recombinant CFH SCR19-20 constructs harboring various SNPs were tested for their ability to bind MDA-BSA. Eight out of twelve SNPs were found to alter binding to MDA-BSA. The authors suggested that impaired MDA binding observed in these CFH variants may be involved in pathogenesis of aHUS. In addition, CFH SCR19–20 fragments were found to bind to MDHDC epitopes as it has been shown for full length CFH and SCR7 containing fragments (Hyvarinen et al., 2014; Weismann et al., 2011). Furthermore, the authors suggested that binding of SCR6-8 to MDA-BSA has a different nature of interaction, in contrast to the binding of SCR19-20, as the SCR6-8 could not be inhibited with increasing concentrations of NaCl (Hyvarinen et al., 2014). Although CFH is a single chain molecule with SCRs organized in a “beads on the string” fashion, structural analyses suggest that when CFH is bound to e.g. C3b on cellular surface it is bent and SCR4 and SCR9 are brought together in close proximity (Kajander et al., 2011; J. Wu et al., 2009). It is not known how the 3D structure of CFH looks when it is bound to MDA, but it can be speculated that both domains (SCR7 and SCR19/20) cooperate in mediating binding to MDA, although both SCRs may bind to MDA epitopes in a different manner. The binding affinity of CFH to MAA-BSA, which actually represents the avidity of the whole molecule, was found to be ∼62nM (Weismann et al., 2011).
In addition to SCR-containing complement proteins, it has also been shown that recombinant human C3a, a pro-inflammatory anaphylatoxin, binds specifically to MDA- and MAA-modified LDL or BSA (Veneskoski et al., 2011). The same authors also showed that MAA and MDA modified LDL co-migrated with C3a in gel shift assays and facilitated scavenging of C3a by J744A1 macrophages. Using ELISA they demonstrated that C3a was binding to MDA and MAA modified proteins (HDL and LDL) but not to phosphorylcholine (PC) modified proteins, suggesting that MAA /MDA epitopes on OxLDL mediate binding to C3a. Additionally, we also tested the binding of plasma purified C3 to MAA-BSA by ELISA, but could not observe any binding. These data suggest that only upon cleavage of C3, the C3a fragment can bind to MDA (Weismann et al., 2011).
Thus, certain complement regulators and complement effectors bind to MDA and MAA epitopes in particular, which are present on OxLDL, apoptotic cells, and MV (Veneskoski et al., 2011; Weismann et al., 2011). While CFH has been shown to limit MAA-induced inflammation, future studies need to explore the effects MAA-decorated surfaces on complement activation in general. Because MDA/MAA-epitopes are major targets of IgM NAbs as well as CFH they may represent an important hub on dying cells and oxidized lipoproteins allowing complement to mediate critical house-keeping functions to maintain host homeostasis. The fact that MDA/MAA-epitopes are recognized by membrane PRR, such as SR-A1, as well as soluble pattern recognition proteins, such as CFH and IgM NAbs, identifies them as potent regulators of tissue homeostasis and as novel DAMPs of innate immunity (Figure 4).
7. Adaptive immunity responses on MDA epitopes
In contrast to innate immunity, adaptive immunity is acquired throughout life following exposure to specific antigens. This results in the generation of a nearly unlimited repertoire of receptors with high antigen-specificity and the generation of immune memory, which is carried out by various subsets of CD4+ Th and CD8+ Tc as well as B cells and the antibodies they secrete (Lichtman et al., 2013). Different subsets of Th cells, including IFNγ and TNFα secreting Th1 cells; IL-4, IL-5, IL-10, and IL-13 secreting Th2 cells; and IL-17 secreting Th17 cells have been described.
A multitude of studies exists demonstrating that active immunization with MDA-modified proteins or lipoproteins triggers adaptive immune responses. These characterizations were mainly done in atherosclerosis-prone rabbits and mice. Moreover, adaptive IgG antibodies with specificity for MDA have been documented in humans and several animal models of atherosclerosis as well as models of ethanol-induced chronic inflammatory tissue injury (Tuma et al., 1996; Xu et al., 1997). Based on this, Witztum and colleagues initiated studies to demonstrate the possibility to induce hapten-specific immune response against MDA-epitopes. Both antisera (MAL-2) and monoclonal IgG antibodies (MDA2) could be generated in guinea pigs and mice immunized with MDA-modified homologous LDL (Palinski et al., 1989; Palinski et al., 1990). These data suggested that the robust production of hapten-specific IgG antibodies occurs in a T cell dependent manner. We could show that immunization of mice with homologous MDA-LDL induced the robust production of T cell dependent IgG antibody titers with specificity for MDA-epitopes. Moreover, this immunization in which MDA-LDL was emulsified in complete Freund’s adjuvant for the primary and incomplete Freund’s adjuvant for the boosting injections resulted in a preferential production of Th2 dependent IgG1, whereas Th1 dependent IgG2a/c antibodies were only moderately induced. Antigen stimulation assays of splenocytes of immunized mice led to a secretion of primarily IL-5, IL-10, IL-13 and to a much lower degree IFNγ following stimulation with MDA-LDL, but not native LDL, consistent with a Th2-biased immune response (Binder et al., 2004; Gonen et al., 2014). The studies also demonstrated the induction of T cells specific for MDA-epitopes, which likely are recognized by T cells in the context of a specific peptide sequence. Indeed, Wuttge et al showed that subcutaneous immunization of nude mice that lack T cells with MDA-modified autologous albumin fail to mount specific IgG responses (Wuttge, Bruzelius, & Stemme, 1999). We have confirmed the requirement of T cells for IgG responses triggered by MDA-LDL, as immunization of MHC class II-/- or T-cell receptor β-/- mice with MDA-LDL failed to induce robust IgG antibody titers (Binder et al., 2004). In addition, immunization of CD4-/- Apoe-/- mice with MDA-LDL results in reduced IgG1, IgG2a/c, and IgM antibody titers to MDA-LDL (Zhou, Robertson, Rudling, Parini, & Hansson, 2005). Notably, the remarkable Th2 dominated immune response to MDA-LDL induced by immunization, occurs independent of thecarrier and can be observed also when other proteins, e.g. mouse serum albumin, modified with other adducts, such as MAA and propanal are used as antigens (Gonen et al., 2014). Nevertheless, MAA-modified proteins have been found to induce the strongest antibody responses. The strong immunogenicity of MAA-epitopes in particular has been demonstrated before in several immunization studies of various organisms (bovine, human, rabbit and mice), as immunization with MAA-modified homologous albumin induced specific antibodies even in the absence of any adjuvant (Thiele et al., 1998). Of considerable interest is the fact that immunization of SR-A1-/- mice with MAA-BSA resulted in a reduced generation of anti-MAA-BSA antibodies compared to control wild type, suggesting that SR-A1-mediated uptake of MDA-modified proteins by antigen-presenting cells is critical (Duryee et al., 2005). Thus, both endogenous and exogenous MDA/MAA haptens have the capacity to induce robust adaptive immune responses, which are characterized by antibody production and induction of specific Th cells.
8. MDA epitopes in diseases
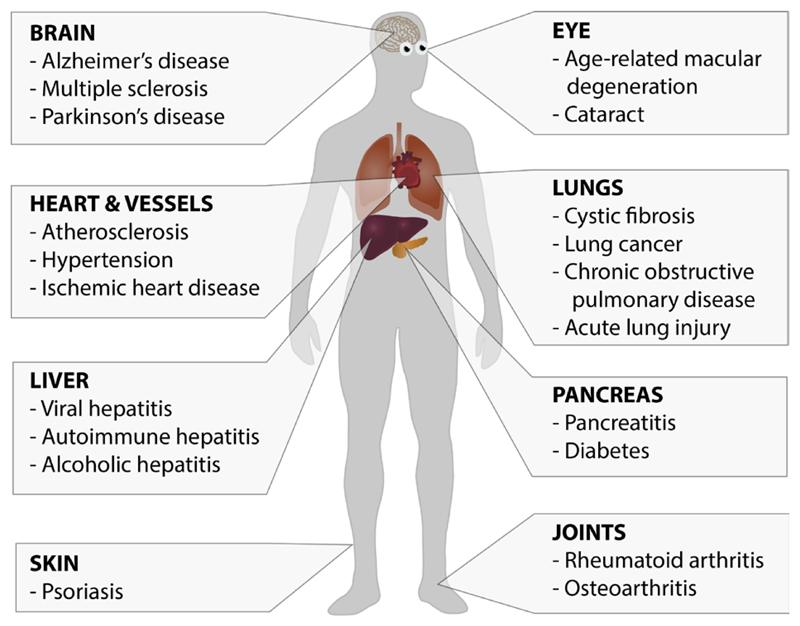
There is a growing list of diseases in which increased levels of MDA have been detected using various methods (Bhuyan, Bhuyan, & Podos, 1986; Brown & Kelly, 1994; Dei et al., 2002; Dexter et al., 1989; Gonenc et al., 2001; Grigolo, Roseti, Fiorini, & Facchini, 2003; Haider et al., 2011; Imai et al., 2008; Jain, McVie, Duett, & Herbst, 1989; Odhiambo et al., 2007; Pemberton et al., 2004; Schoenberg et al., 1995; Shimizu et al., 2001; Sikar Akturk et al., 2012; Tuma et al., 1996; Valles, Aznar, Santos, & Fernandez, 1982; Wade, Jackson, Highton, & van Rij, 1987; Weismann et al., 2011; Yla-Herttuala et al., 1989). MDA has been documented in both chronic and acute diseases associated with high levels of oxidative stress, such as cardiovascular, neurodegenerative, metabolic, and communicable diseases (Figure 5). However, the levels of MDA determined in the plasma of healthy individuals have shown great variability, in part due to the method of blood drawing and sample preparation (Del Rio, Stewart, & Pellegrini, 2005). Moreover, the detection methods for MDA still possess a number of limitations. For example, the most commonly used method for MDA detection, the TBARS assay, has long been known to be non-specific (Esterbauer et al., 1991). Although more reliable methods for detecting MDA levels with high specificity exist including MDA-specific antibodies or assays based on mass spectrometry (Zagol-Ikapite et al., 2015), it still remains unclear if the detected changes of MDA are reliable biomarkers for all these diseases.
Figure 5.
MDA epitopes in diseases. MDA epitopes can be found in many pathological settings affecting multiple organs including neurodegenerative diseases, metabolic diseases, cancers and also infectious diseases.
9. Relevance of oxidation-specific epitopes in cardiovascular disease
CVDs are among the most studied diseases with respect to a role for MDA-epitopes. CVDs constitute the major cause of mortality and disability worldwide (Mendis et al., 2011). Generally, CVDs can be divided into two major sub-groups, CVDs that arise due to atherosclerosis (stroke, heart attack, hypertension and peripheral vascular disease) or CVDs that are independent of atherosclerotic process (congenital and rheumatic heart diseases, cardiomyopathies and arrhythmias). According to WHO CVDs with atherogenic origin are responsible for 80% of CVD deaths.
Atherosclerosis is a chronic inflammatory disease of large and medium-sized arteries and the underlying cause for heart attacks and strokes. Several non-modifiable and modifiable risk factors, such as hypercholesterolemia, hypertension, diabetes, smoking, gender and age have been identified. Atherosclerotic lesion formation is sustained by high levels of plasma LDL cholesterol that are deposited in the artery wall, where an inflammatory reaction is triggered. These initial fatty streak lesions can progress to more complex plaques with many inflammatory infiltrates and large acellular necrotic areas. Once these advanced plaques become unstable they are prone to rupture, which triggers a local thrombotic event that can ultimately lead to a heart attack or stroke.
LDL belongs to a group of lipoprotein particles ranging in size from 18-25 nm with the major purpose to transport cholesterol in the circulation. It contains a single ApolipoproteinB100 (ApoB100) molecule (with 4,536 amino acids, of which 356 are Lys) and a lipid part consisting of phospholipid and cholesterol molecules. Atherogenesis is initiated by endothelial cell dysfunction and intimal retention of LDL. Once LDL is trapped in the subendothelial space of the arteries it undergoes several types of modifications, most prominently enzymatic and non-enzymatic oxidation leading to the generation of OxLDL. OxLDL itself triggers pro-inflammatory responses in endothelial cells and enhanced recruitment of monocytes into the intima of the artery wall (Gerrity, 1981; Quinn, Parthasarathy, Fong, & Steinberg, 1987). Recruited monocytes then differentiate into macrophages that further propagate the oxidation of LDL through 12/15-lipoxygenase (12/15-LO) and myeloperoxidase (MPO) pathway, thus enhancing the pro-atherogenic inflammatory cascade. OxLDL is then taken up by macrophages using an array of scavenger receptors expressed on their surface, such as CD36 and SR-A1 (Greaves & Gordon, 2009; Moore, Sheedy, & Fisher, 2013). An impaired balance between cellular uptake and efflux of cholesterol results in the transformation of lesional macrophages into lipid-laden foam cells - hallmark cells of atherosclerosis. Consequently, intracellular accumulation of OxLDL results in the formation of cholesterol crystals that trigger lysosomal rupture and activation of the inflammasome. In addition, intracellular accumulation of free cholesterol can enhance ER stress and promote an unfolded protein response that triggers apoptosis foam cell macrophages (Feng et al., 2003). Usually, lesional apoptotic cells are silently cleared by professional phagocytes, but once the efferocytotic mechanism becomes overloaded either with a multitude of apoptotic cells or excess of OxLDL emanates in impaired clearance of apoptotic cells. Uncleared apoptotic cells then undergo secondary necrosis, lose membrane integrity, and release cellular debris and DAMPs further propagating the inflammatory response. Thus, OxLDL, apoptotic cells, and cellular debris accumulate in atherosclerotic lesions and sustain the inflammatory process (Glass & Witztum, 2001; Hartvigsen et al., 2009; Tabas, 2010; Tsiantoulas, Diehl, Witztum, & Binder, 2014). Moreover, in addition to innate immune responses adaptive immunity also plays a role in atherogenesis (Lichtman et al., 2013). T cells – most prominently IFNγ-secreting Th1 cells - have been found in lesions and were demonstrated to promote atherogenesis. Although B cells are rarely found in lesions, their modulatory roles on atherogenic process have recently gained much attention.
10. MDA epitopes in atherosclerosis
During the oxidative modification of LDL the phospholipid and cholesterol moiety of LDL undergoes lipid peroxidation, which leads to the generation of many different OSEs, including MDA epitopes. OxLDL has been shown to be pro-inflammatory and immunogenic, suggesting that it is a major driver of the inflammatory process during atherogenesis. Indeed, the characterization of OSEs as novel class of DAMPs has provided critical insights into the sterile inflammatory process of atherosclerosis. Accumulating evidence suggests that MDA-epitopes represent critical mediators of inflammation in atherosclerosis.
The fact that atherosclerotic lesions contain MDA epitopes has been known for more than 30 years. Using a monoclonal antibody raised against MDA-Lys residues the presence of MDA epitopes was demonstrated in atherosclerotic lesions of WHHL rabbits (Haberland et al., 1988), and later Ylä-Herttuala (Yla-Herttuala et al., 1989) has shown that LDL in rabbit and human lesions is in fact oxidized and a major carrier of MDA-epitopes. MDA-modified LDL - in contrast to native LDL – possesses a strong chemotactic potential and can be engulfed more readily by macrophages (Yla-Herttuala et al., 1989). Consistent with findings in rabbits and humans, lesions from Apoe-/- mice were shown to contain MDA epitopes in regions rich in macrophages as well as in the necrotic core, which is an area replete with apoptotic cells (Palinski et al., 1994). Likely, accumulating apoptotic cells prominently contribute to MDA epitopes in atherosclerotic lesions. Indeed, we have recently shown that plasma MV isolated from the culprit lesion site of patients suffering a myocardial infarction are increased and enriched in MDA-epitopes compared to plasma MV of the same patients obtained from the periphery (Tsiantoulas et al., 2015). Additionally, it has been shown that HDL isolated from human atherosclerotic tissues is enriched in MDA epitopes compared to HDL from plasma of healthy donors. Modification of ApoAI, the major protein moiety of HDL, with MDA in vitro results in dysfunctional HDL. MDA-epitopes on ApoAI cause structural and conformational changes and thereby impairing its ability to interact with ABCA1 and promoting cholesterol efflux onto HDL, which is a homeostatic mechanism to remove access cholesterol from macrophages. Thus, oxidative modification of ApoAI by MDA impairs an important cardioprotective function of HDL, which may also contribute to atherosclerosis development (Shao et al., 2010).
Inflammation in vascular lesions is considered a key driver of atherogenesis. Major carriers of MDA epitopes, such as OxLDL, MV, dying cells have been shown to possess robust pro-inflammatory properties, and there is accumulating evidence that MDA epitopes themselves are mediators of these effects. For example, the cytotoxic effect of OxLDL on endothelial cells can be blocked by a polyclonal antibody against MDA but not by a polyclonal antibody against ApoB100. Moreover, it was shown that MDA-epitope mediated OxLDL cytotoxicity was partially dependent on Akt pathway activity (Yang et al., 2014). Additionally, the direct pro-inflammatory nature of MDA epitopes has been demonstrated by several groups. As discussed above, treatment of THP1 cells with MDA-Lys, resulted in strong activation of NFκB activity as well as the activation of signaling pathways related to inflammation, cellular motility, and cell-to-cell signaling. Furthermore, MDA-Lys also increased monocyte binding to vascular smooth muscle and endothelial cells (Shanmugam et al., 2008). Additionally, MAA-BSA induced expression of pro-inflammatory cytokines IL-8, IL-1β, TNF-α and IL-12β in THP-1 cells (Weismann et al., 2011). All these cytokines have been shown to play an important role in atherosclerotic lesion formation (Ait-Oufella, Taleb, Mallat, & Tedgui, 2011). For example, IL-8 or CXCL1 promotes leukocyte recruitment to the vascular wall and atherosclerosis-prone mice deficient in either Cxcl1 or its receptor Cxcr1 develop significantly reduced atherosclerosis. Interestingly, also MDA-carrying MV were able to induce IL-8 secretion in human monocytes, and this effect could be neutralized in the presence of the MAA-specific IgM NAb LR04, suggesting that MAA-epitopes are in part responsible for the pro-inflammatory effect of MV (Tsiantoulas et al., 2015). All these data strongly support the fact that MDA epitopes act as DAMPs that modulate lesional inflammation, and have a fundamentally important role in the process of atherogenesis.
10.1. MDA immunization protects from atherosclerosis
The importance of MDA in atherogenesis has been demonstrated in a large number of immunization studies, which showed induction of atheroprotective immunity by immunization with MDA-antigens. These studies indicate that endogenously generated MDA is relevant for atherogenesis, as the immune response against MDA specifically mediates protection. For example, immunization of WHHL rabbits with MDA-modified homologous LDL resulted in increased antibody titers to MDA-epitopes and atheroprotection, in contrast to immunization with keyhole limpet hemocyanine (KLH) (Palinski, Miller, & Witztum, 1995). Similar atheroprotective effects as well as increased antibody levels were observed in immunization studies of atherosclerosis-prone Apoe-/- and Ldlr-/- mice (Binder et al., 2004; Fredrikson et al., 2003; Freigang, Horkko, Miller, Witztum, & Palinski, 1998; George et al., 1998; Gonen et al., 2014; Zhou, Caligiuri, Hamsten, Lefvert, & Hansson, 2001; Zhou et al., 2005). Apoe-/- mice immunized with homologous MDA-LDL develop high titers of anti-MDA-LDL IgG antibodies and had 50% less lesional area at the aortic sinus compared to control mice injected with PBS (George et al., 1998). Additionally, Ldlr-/- mice immunized with homologous MDA-LDL or native LDL developed high IgM and IgG titers to MDA-LDL and had attenuated atherosclerosis. Interestingly, immunization with homologous native LDL also protected from atherosclerosis without inducing high titer of antibodies, suggesting a different mechanism for atheroprotection with native LDL (Freigang et al., 1998). Subsequently, seven peptides within human ApoB100 were shown to be immunogenic and were recognized by IgM and IgG autoantibodies in human serum (Fredrikson et al., 2003). In a follow up study, immunization of Apoe-/- mice with a mixture of two peptides of human ApoB100 (peptides 142 and 210 with 85-90% similarity to the mouse ApoB100 sequence) resulted in a reduction of plaque area by 60%, in contrast to control immunized mice. Immunization with ApoB100 peptides increased IgG titers against MDA modified peptides, suggesting that the peptides used as antigens became modified with MDA in vivo (Fredrikson et al., 2003). The importance of MDA and MAA as a key antigen has been demonstrated by the fact that immunization of Apoe-/- mice with plaque homogenates from Apoe-/- mice triggers MDA-specific antibodies and reduces lesion formation. More precisely, immunization with MDA-LDL or plaque homogenates led an increase T-cell dependent IgG antibodies recognizing MDA-LDL, the titers of which were found to negatively correlate with plaque size or serum cholesterol levels, respectively (Zhou et al., 2001). In an attempt to identify the exact requirements for the atheroprotective effect of immunization with MDA-LDL, extensive immunization studies with different types of related antigens have been performed. In these studies, Ldlr-/- mice were immunized with MDA-LDL (MDA-LDL), MDA modified murine serum albumin (MDA-MSA), MAA-MSA, propanal (PA) modified MSA, native MSA, and PBS, and then fed an atherogenic diet for 28 weeks. The most robust reduction in aortic lesion area (40%) compared to corresponding PBS control was observed when MAA-MSA was used as an immunogen. Despite similar induction of hapten-specific immune responses with the other antigens, only mice immunized with MDA-LDL and MDA-MSA had a significant but lesser reduction of lesion area, compared to potent effect of immunization with MAA-MSA. Notably, immunization with MDA-LDL also induced antibodies that bound to MAA-modified peptides. This observation led to the conclusion that MAA epitopes are the immunodominant epitopes inducing atheroprotective immune responses. Thus, MAA hapten-specific immunity mediates the protective effect of immunization with MDA-LDL, independent of the carrier protein.
Several studies tried to characterize the mechanisms responsible for the protective effect of immunization. For example, as discussed above, we could show that immunization of both wild type and atherosclerosis-prone mice with homologous MDA-LDL and MAA-MSA induces a robust Th2 biased immune response that is dominated by MDA-specific IgG1 antibodies (Binder et al., 2004). High titers of antibodies may mediate atheroprotection, as infusion of IEI-E3, a human IgG1 antibody that was selected against MDA modified peptides of human ApoB100, into Apoe-/- mice for 4 weeks resulted in decreased atherosclerotic lesion formation compared to infusion of an isotype control (Schiopu et al., 2007). Moreover, infusion of another human recombinant IgG1 (2D03) recognizing the MDA modifications on the same human ApoB100 peptide also reduced atherosclerosis and constrictive injury–induced remodeling in the carotid artery of Ldlr-/- mice (Strom et al., 2007). Using the human-derived anti-OxLDL IgG Fab IK17 specific for MDA epitopes it was demonstrated that IK17 inhibits the uptake of OxLDL by macrophages in vitro (Shaw et al., 2001). In addition, infusion of an adenoviral construct overexpressing recombinant IK17 scFv into cholesterol-fed Ldlr-/-Rag-1-/- mice resulted in decreased peritoneal foam-cell formation and reduced atherosclerosis (Tsimikas et al., 2011). These antibodies have been suggested to block foam cell formation and protect from atherosclerosis. On the other hand, Th2 cytokines, such as IL-5 and IL-13, induced by MDA-immunization possess important atheroprotective properties. For example, IL-5 stimulates B-1 cells to secrete IgM NAb in a non-cognate manner. A large part of IgM NAb have specificity for OSE (Chou et al., 2009), and atherosclerosis-prone mice unable to secrete IgM antibodies develop accelerated atherosclerosis (Lewis et al., 2009). Hematopoetic IL-5 deficiency in Ldlr-/- mice leads to increased atherosclerosis and reduced levels of specific atheroprotective IgM NAb, T15/E06. Furthermore, we also showed that IL-13 protects mice from atherosclerotic lesion formation by promoting alternative activation of lesional macrophages that are more efficient in removal of OxLDL (Cardilo-Reis et al., 2012). Moreover, it has been suggested that immunization with MDA-LDL also induces regulatory T cells, which could suppress the secretion of the pro-atherogenic cytokine IFNγ by specific Th1 cells. Nevertheless, Zhou et al showed that immunization of CD4-/-Apoe-/- mice that lack Th cells with homologous MDA-LDL still induced atheroprotection (Zhou et al., 2005). On the other hand, adoptive transfer of CD4+ T cells from MDA-LDL immunized mice into Apoe-/- SCID mice resulted in accelerated atherosclerosis compared to transfer of T cells from control immunized mice. Thus, the true effects of Th dependent immunity to MDA-LDL remains to be elucidated in detail. Clearly, a feature common to all immunization studies is the production of MDA-specific antibodies at much higher levels than the ones occurring naturally and in the course of diet-feeding.
In the past 25 years, many epidemiological studies assessed associations of IgG and IgM antibodies to OxLDL and MDA-LDL epitopes with the development of coronary, carotid and peripheral artery disease (Salonen et al., 1992; Tsimikas et al., 2003; Tsimikas, Witztum, et al., 2004; R. Wu et al., 1997). These studies have often been very inconsistent due to lack of standardized antigens or assays, and because tested cohorts of patients were simply too small cohorts or not followed prospectively (Tsimikas et al., 2012). Some of these inconsistencies could be overcome with the use of recombinant peptide mimotopes of MAA that serve as standardized antigens for MAA-specific antibodies (Amir et al., 2012). Nevertheless, studies in humans have provided insights into the role of these immune responses in CVD. While the role of IgG antibodies to MDA-LDL is less clear, there is now substantial evidence that IgM antibodies to MDA-LDL are inversely associated with atherosclerosis or clinical manifestations thereof (Gounopoulos, Merki, Hansen, Choi, & Tsimikas, 2007; Tsiantoulas et al., 2014). These data suggest that MDA-LDL specific IgM antibodies may mediate protective functions in atherosclerosis. Interestingly, MDA-specific IgM antibodies have been found to be higher in people younger than 65, females, and non–diabetics (Fraley et al., 2009; Tsimikas, Lau, et al., 2004). Moreover, many if not most IgM antibodies with specificity for MDA-LDL may be IgM natural antibodies.
10.2. MDA specific natural immunity protects from atherosclerosis
As discussed above IgM NAbs are important mediators of MDA-specific immunity, and several potential mechanisms on how they can mediate atheroprotective effects have been suggested. First, IgM with specificity for MDA/MAA have been shown to act anti-inflammatory. We could demonstrate that the MAA-specific IgM NAb LR04 signficantly decreased the ability of platelet-derived MV to induce IL-8 secretion by THP-1 cells and primary human monocytes (Tsiantoulas et al., 2015). IL-8 is a key mediator of leukocyte recruitment to the vascular wall and chief pro-atherogenic chemokine (Ait-Oufella et al., 2011). Thus, inhibition of MDA/MAA-induced IL-8 secretion by endothelial cells and monocytes may have an important modulatory function in atherogenesis. Furthermore, MDA-specific IgM may also protect by promoting clearance of dying cells. Indeed, defective phagocytic clearance of dying cells in atherosclerotic lesions has also been implicated in the progression of atherosclerosis (Tabas, 2010; Van Vre, Ait-Oufella, Tedgui, & Mallat, 2012). For example, Ldlr-/- mice reconstituted with bone marrow of mice deficient in the milk fat globule-EGF factor 8 protein (Mfge8), which facilitates PS-mediated clearance of apoptotic cells, developed increased lesion size with more accumulating apoptotic cells an increased necrotic areas (Ait-Oufella et al., 2007). A similar effect has been observed when tyrosine kinase defective Mertk receptor was crossed with Apoe-/- mice (Thorp, Cui, Schrijvers, Kuriakose, & Tabas, 2008). The ability of MDA-specific IgM to modulate efferocytosis has been addressed in the studies by Chou et al and Chen et al (Y. Chen et al., 2009; Chou et al., 2009). For example, incubation of apoptotic thymocytes with wild type mouse serum in the presence of MDA-BSA resulted in diminished deposition of IgM and C1q on apoptotic cell surfaces and consequently led to decrease in complement-mediated phagocytosis by dendritic cells (Y. Chen et al., 2009). To explain this, the authors suggested that MDA-BSA competed for the binding to MDA epitopes on apoptotic cells, on which they may act as hubs for activation of complement-mediated efferocytosis via IgM and C1q axis. We could directly demonstrate a role for MDA-specific IgM NAbs in apoptotic cell clearance in an in vivo clearance assay in Rag1-/- mice, deficient in B and T cell populations. Intraperitoneal injection of apoptotic thymocytes preincubated with the IgM NAb NA-17 resulted in significantly enhanced phagocytosis by macrophages compared to apoptotic thymocytes preincubated with control IgM (Chou et al., 2009). The importance of this mechanism is further supported by the fact that soluble IgM deficiency (Lewis et al., 2009) as well as C1q deficiency aggravates atherosclerosis in atherosclerosis-prone mice (Bhatia et al., 2007). Thus, MDA epitopes may act as marker of metabolic waste and dying cells in atherosclerotic lesions, allowing the immune system to mediate house- keeping functions (Chang et al., 1999; Y. Chen et al., 2009; Chou et al., 2009; Weismann et al., 2011).
Not only IgMs can protect from MDA induced inflammation. Additionally, CFH that co-localizes in human coronary lesions with MDA epitopes has been shown to neutralize MDA-induced expression of pro- inflammatory genes, including IL-8. Interestingly, a few studies have shown an association of the 402H allele, which reduces MDA-binding of CFH (Weismann et al., 2011), with increased cardiovascular risk. However, a meta-analysis of eight different study populations failed to find a significant association of this gene variant with CVDs (Sofat et al., 2010). Nevertheless, additional SNPs of CFH that can affect its capacity to bind MDA have been identified and only functional data on MDA-binding of CFH in plasma may provide insights into a potential association of this CFH function with CVD. In support of this, we could demonstrate that CFH bound on MDA-decorated surfaces still can mediate co-factor activity for Factor I to inactivate C3b into iC3b. In turn, freshly deposited inactive iC3b promotes anti-inflammatory clearance of opsonized particles (Weismann et al., 2011). Thus, CFH may protect from atherosclerosis by binding to MDA epitopes, thereby reducing inflammation and additionally increasing opsonization with iC3b, which facilitates efferocytosis. Studies in animal models of atherosclerosis may provide critical insights into these functions.
11. MDA epitopes as therapeutic targets in CVD
Current therapies in CVD mainly aim at lowering LDL plasma levels by increasing the availability of the LDL receptor at the cell surface using for example statins or PCSK9 antibodies. Nevertheless, an important contribution of the innate and adaptive immunity to the development of atherosclerosis has become evident. With two ongoing clinical studies, the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) and the Cardiovascular Inflammation Reduction Trial (CIRT), we are currently witnessing a premiere in regard to clinical trials addressing the role of inflammation in CVD. CANTOS and CIRT are the first studies which aim at preventing recurrent cardiovascular events in patients by reducing inflammation without affecting plasma cholesterol levels using low dose methotrexate or the neutralizing anti-IL-1β antibody canakinumab, respectively. Other potentially interesting though pre-clinical immunomodulatory approaches for therapeutic intervention in atherosclerosis have been reviewed elsewhere (Amir & Binder, 2010; Lichtman et al., 2013; Miller & Tsimikas, 2013; Witztum & Lichtman, 2014).
As OSEs have been implicated as potential antigens triggering innate and adaptive immune responses in atherosclerosis, targeting these structures by active or passive immunization in order to reduce the inflammatory burden could provide a novel therapeutic approach to treat atherosclerosis. Indeed, several lines of evidence point towards a protective effect of increasing antibody titers against OSEs. Interestingly, active immunization using a single OSE, MDA-LDL as an immunogen is sufficient to protect from atherosclerosis as demonstrated by a number of studies in animals (Binder et al., 2004; Freigang et al., 1998; George et al., 1998; Gonen et al., 2014; Palinski et al., 1995; Zhou et al., 2001). Even more intriguing is the fact that this protective effect seems to be independent of the carrier presenting MDA epitopes on its surface as several studies showed that immunization using various carrier proteins all modified with MDA epitopes results in less atherosclerosis, including MDA-modified ApoB100-peptides (Fredrikson et al., 2003), MDA-modified laminin (Duner et al., 2010) and fibronectin (Duner et al., 2011) or killed Porphyromonas gingivalis (Turunen et al., 2012), a pathogen carrying surface structures that mimic MDA epitopes. Therefore, the identification of MDA epitopes as critical structures conveying atheroprotection through immunization could allow for the development of more standardized immunogens benefiting from lower variability and thus higher reproducibility. Accordingly, using phage display libraries we have identified an MDA mimotope, the P2 peptide, and demonstrated that immunization with P2 induces MDA-specific antibodies, which bind to human atherosclerotic lesions (Amir et al., 2012). On the other hand, several studies reported that passive immunization by therapeutic infusion of anti-MDA antibodies also had beneficial effects by reducing plaque inflammation and regression of atherosclerotic lesions (Schiopu et al., 2007; Tsimikas et al., 2011). Even though animal studies have convincingly shown that vaccination using MDA epitopes is protective in atherosclerosis, translation to human settings has proven to be difficult. Not only do critical differences between rodents and humans exist in terms of lipoprotein metabolism during atherogenesis, which advises against a direct implementation of experimental setups to the clinics, but the ubiquitous presence of MDA epitopes in the body also complicates any human vaccination study because the generation of antibodies against continuously formed MDA epitopes could lead to unwanted side effects of the vaccination in the long term given that we still lack a deeper understanding of the mechanisms involved.
Meanwhile, other approaches offering protection from pathologically elevated levels of MDA epitopes might be worth of consideration. Several studies demonstrated that endogenous production of natural IgMs can be boosted by treatment with IL-18 (Kinoshita et al., 2006), IL-25 (Mantani et al., 2015) and IL-5 (Binder et al., 2004), by genetic defciency of sialic acid binding Ig-like lectin G (Siglec G), a negative regulator of the B cell receptor present also on B1a cells (Hoffmann et al., 2007), and potentially by additional strategies yet to be elucidated. While many of these approaches are not specific or even potentially harmful, identification of pathways that specifically regulate IgM NAb production may provide novel therapeutic opportunities. In addition, the MDA-neutralizing properties of CFH could be harnessed for therapeutic purposes as we have shown that CFH protects from MDA-induced inflammation in a mouse model of wet AMD by binding to MDA epitopes (Weismann et al., 2011). Treatment of patients possessing a CFH variant with impaired binding using CFH or truncated CFH constructs which contain only a fraction of the protein including the critical MDA-binding region SCR19/20 could help protecting from MDA-mediated inflammation. Indeed, such constructs, termed mini CFH, have demonstrated their therapeutic potential in mouse models of C3 glomerulopathy (Nichols et al., 2015) and we speculate that their neutralizing capabilities could be also beneficial in atherosclerosis. Regardless of the successes in animal models, the feasibility of these approaches in human patients has to be taken with caution as the treatment of a chronic disease, which develops over decades might result in long-term complications. Nevertheless, in cases where only short-term treatment is required or for individuals at high risk the benefits could outweigh the disadvantages.
12. Conclusions
Lipid peroxidation of cellular membranes results in the generation of various OSEs that act as downstream mediators of oxidative stress in tissues. In this chapter, we have discussed MDA epitopes as a prominent and important example. This includes evidence that they: 1) are pro-inflammatory danger signals for a wide range of cell types, 2) can be recognized by multiple arcs of innate immunity with critical roles in house keeping functions, and 3) are potent immunogens, which elicit protective immune response in mouse models of atherosclerosis. We speculate that MDA epitopes present on dying cells and biomolecules damaged by oxidative stress serve as waste markers for the immune system, flagging their carriers for complement-mediated rapid and silent clearance by professional phagocytes. However, excessive production of MDA epitopes and/or impaired clearance capabilities result in their non-physiological accumulation and subsequently elicits pro-inflammatory responses, which are sustained as long as the carriers of MDA epitopes cannot be neutralized or scavenged - a condition most likely occurring in many if not all chronic inflammatory diseases that are associated with impaired resolution, such as atherosclerosis.
References
- Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, et al. Mallat Z. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115(16):2168–2177. doi: 10.1161/CIRCULATIONAHA.106.662080. [DOI] [PubMed] [Google Scholar]
- Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(5):969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- Akalin FA, Baltacioglu E, Alver A, Karabulut E. Lipid peroxidation levels and total oxidant status in serum, saliva and gingival crevicular fluid in patients with chronic periodontitis. J Clin Periodontol. 2007;34(7):558–565. doi: 10.1111/j.1600-051X.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- Amir S, Binder CJ. Experimental immunotherapeutic approaches for atherosclerosis. Clin Immunol. 2010;134(1):66–79. doi: 10.1016/j.clim.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir S, Hartvigsen K, Gonen A, Leibundgut G, Que X, Jensen-Jarolim E, et al. Binder CJ. Peptide mimotopes of malondialdehyde epitopes for clinical applications in cardiovascular disease. J Lipid Res. 2012;53(7):1316–1326. doi: 10.1194/jlr.M025445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aredo B, Li T, Chen X, Zhang K, Wang CX, Gou D, et al. Ufret-Vincenty RL. A chimeric Cfh transgene leads to increased retinal oxidative stress, inflammation, and accumulation of activated subretinal microglia in mice. Invest Ophthalmol Vis Sci. 2015;56(6):3427–3440. doi: 10.1167/iovs.14-16089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguelles S, Cano M, Machado A, Ayala A. Effect of aging and oxidative stress on elongation factor-2 in hypothalamus and hypophysis. Mech Ageing Dev. 2011;132(1–2):55–64. doi: 10.1016/j.mad.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Bae YS, Oh H, Rhee SG, Yoo YD. Regulation of reactive oxygen species generation in cell signaling. Mol Cells. 2011;32(6):491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11(1):34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26(4):347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- Berger JP, Simet SM, DeVasure JM, Boten JA, Sweeter JM, Kharbanda KK, et al. Wyatt TA. Malondialdehyde-acetaldehyde (MAA) adducted proteins bind to scavenger receptor A in airway epithelial cells. Alcohol. 2014;48(5):493–500. doi: 10.1016/j.alcohol.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia VK, Yun S, Leung V, Grimsditch DC, Benson GM, Botto MB, et al. Haskard DO. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol. 2007;170(1):416–426. doi: 10.2353/ajpath.2007.060406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuyan KC, Bhuyan DK, Podos SM. Lipid peroxidation in cataract of the human. Life Sci. 1986;38(16):1463–1471. doi: 10.1016/0024-3205(86)90559-x. [DOI] [PubMed] [Google Scholar]
- Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, et al. Witztum JL. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114(3):427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RK, Kelly FJ. Evidence for increased oxidative damage in patients with cystic fibrosis. Pediatr Res. 1994;36(4):487–493. doi: 10.1203/00006450-199410000-00013. [DOI] [PubMed] [Google Scholar]
- Cai J, Chen J, He H, Yin Z, Zhu Z, Yin D. Carbonyl stress: malondialdehyde induces damage on rat hippocampal neurons by disturbance of Ca(2+) homeostasis. Cell Biol Toxicol. 2009;25(5):435–445. doi: 10.1007/s10565-008-9097-3. [DOI] [PubMed] [Google Scholar]
- Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol. 2013;13(9):621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- Cardilo-Reis L, Gruber S, Schreier SM, Drechsler M, Papac-Milicevic N, Weber C, et al. Binder CJ. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med. 2012;4(10):1072–1086. doi: 10.1002/emmm.201201374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MK, Bergmark C, Laurila A, Horkko S, Han KH, Friedman P, et al. Witztum JL. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci U S A. 1999;96(11):6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, Witztum JL. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med. 2004;200(11):1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary AK, Nokubo M, Reddy GR, Yeola SN, Morrow JD, Blair IA, Marnett LJ. Detection of endogenous malondialdehyde-deoxyguanosine adducts in human liver. Science. 1994;265(5178):1580–1582. doi: 10.1126/science.8079172. [DOI] [PubMed] [Google Scholar]
- Chaudhary AK, Reddy GR, Blair IA, Marnett LJ. Characterization of an N6- oxopropenyl-2'-deoxyadenosine adduct in malondialdehyde-modified DNA using liquid chromatography/electrospray ionization tandem mass spectrometry. Carcinogenesis. 1996;17(5):1167–1170. doi: 10.1093/carcin/17.5.1167. [DOI] [PubMed] [Google Scholar]
- Cheeseman KH, Beavis A, Esterbauer H. Hydroxyl-radical-induced iron-catalysed degradation of 2-deoxyribose. Quantitative determination of malondialdehyde. Biochem J. 1988;252(3):649–653. doi: 10.1042/bj2520649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Park YB, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2009;182(10):6031–6043. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Wang F, Yu DF, Wu PF, Chen JG. The cytotoxic mechanism of malondialdehyde and protective effect of carnosine via protein cross-linking/mitochondrial dysfunction/reactive oxygen species/MAPK pathway in neurons. Eur J Pharmacol. 2011;650(1):184–194. doi: 10.1016/j.ejphar.2010.09.033. [DOI] [PubMed] [Google Scholar]
- Choksi KB, Nuss JE, Boylston WH, Rabek JP, Papaconstantinou J. Age-related increases in oxidatively damaged proteins of mouse kidney mitochondrial electron transport chain complexes. Free Radic Biol Med. 2007;43(10):1423–1438. doi: 10.1016/j.freeradbiomed.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, et al. Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119(5):1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline SD, Lodeiro MF, Marnett LJ, Cameron CE, Arnold JJ. Arrest of human mitochondrial RNA polymerase transcription by the biological aldehyde adduct of DNA, M1dG. Nucleic Acids Res. 2010;38(21):7546–7557. doi: 10.1093/nar/gkq656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalfo E, Portero-Otin M, Ayala V, Martinez A, Pamplona R, Ferrer I. Evidence of oxidative stress in the neocortex in incidental Lewy body disease. J Neuropathol Exp Neurol. 2005;64(9):816–830. doi: 10.1097/01.jnen.0000179050.54522.5a. [DOI] [PubMed] [Google Scholar]
- Dei R, Takeda A, Niwa H, Li M, Nakagomi Y, Watanabe M, et al. Sobue G. Lipid peroxidation and advanced glycation end products in the brain in normal aging and in Alzheimer's disease. Acta Neuropathol. 2002;104(2):113–122. doi: 10.1007/s00401-002-0523-y. [DOI] [PubMed] [Google Scholar]
- Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15(4):316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Dennis KJ, Shibamoto T. Production of malonaldehyde from squalene, a major skin surface lipid, during UV-irradiation. Photochem Photobiol. 1989;49(5):711–716. doi: 10.1111/j.1751-1097.1989.tb08445.x. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, et al. Marsden CD. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem. 1989;52(2):381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- Draper HH, Csallany AS, Hadley M. Urinary aldehydes as indicators of lipid peroxidation in vivo. Free Radic Biol Med. 2000;29(11):1071–1077. doi: 10.1016/s0891-5849(00)00367-1. [DOI] [PubMed] [Google Scholar]
- Draper HH, McGirr LG, Hadley M. The metabolism of malondialdehyde. Lipids. 1986;21(4):305–307. doi: 10.1007/BF02536418. [DOI] [PubMed] [Google Scholar]
- Duner P, To F, Beckmann K, Bjorkbacka H, Fredrikson GN, Nilsson J, Bengtsson E. Immunization of apoE-/- mice with aldehyde-modified fibronectin inhibits the development of atherosclerosis. Cardiovasc Res. 2011;91(3):528–536. doi: 10.1093/cvr/cvr101. [DOI] [PubMed] [Google Scholar]
- Duner P, To F, Berg K, Alm R, Bjorkbacka H, Engelbertsen D, et al. Bengtsson E. Immune responses against aldehyde-modified laminin accelerate atherosclerosis in Apoe-/- mice. Atherosclerosis. 2010;212(2):457–465. doi: 10.1016/j.atherosclerosis.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Duryee MJ, Freeman TL, Willis MS, Hunter CD, Hamilton BC, 3rd, Suzuki H, et al. Thiele GM. Scavenger receptors on sinusoidal liver endothelial cells are involved in the uptake of aldehyde-modified proteins. Mol Pharmacol. 2005;68(5):1423–1430. doi: 10.1124/mol.105.016121. [DOI] [PubMed] [Google Scholar]
- Duryee MJ, Klassen LW, Freeman TL, Willis MS, Tuma DJ, Thiele GM. Lipopolysaccharide is a cofactor for malondialdehyde-acetaldehyde adduct-mediated cytokine/chemokine release by rat sinusoidal liver endothelial and Kupffer cells. Alcohol Clin Exp Res. 2004;28(12):1931–1938. doi: 10.1097/01.alc.0000148115.90045.c5. [DOI] [PubMed] [Google Scholar]
- Duryee MJ, Klassen LW, Jones BL, Willis MS, Tuma DJ, Thiele GM. Increased immunogenicity to P815 cells modified with malondialdehyde and acetaldehyde. Int Immunopharmacol. 2008;8(8):1112–1118. doi: 10.1016/j.intimp.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, et al. Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5(9):781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Fogelman AM, Shechter I, Seager J, Hokom M, Child JS, Edwards PA. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc Natl Acad Sci U S A. 1980;77(4):2214–2218. doi: 10.1073/pnas.77.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley AE, Schwartz GG, Olsson AG, Kinlay S, Szarek M, Rifai N, et al. Investigators MS Relationship of oxidized phospholipids and biomarkers of oxidized low-density lipoprotein with cardiovascular risk factors, inflammatory biomarkers, and effect of statin therapy in patients with acute coronary syndromes: Results from the MIRACL (Myocardial Ischemia Reduction With Aggressive Cholesterol Lowering) trial. J Am Coll Cardiol. 2009;53(23):2186–2196. doi: 10.1016/j.jacc.2009.02.041. [DOI] [PubMed] [Google Scholar]
- Fredrikson GN, Soderberg I, Lindholm M, Dimayuga P, Chyu KY, Shah PK, Nilsson J. Inhibition of atherosclerosis in apoE-null mice by immunization with apoB-100 peptide sequences. Arterioscler Thromb Vasc Biol. 2003;23(5):879–884. doi: 10.1161/01.ATV.0000067937.93716.DB. [DOI] [PubMed] [Google Scholar]
- Freigang S, Horkko S, Miller E, Witztum JL, Palinski W. Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler Thromb Vasc Biol. 1998;18(12):1972–1982. doi: 10.1161/01.atv.18.12.1972. [DOI] [PubMed] [Google Scholar]
- Geiger-Maor A, Levi I, Even-Ram S, Smith Y, Bowdish DM, Nussbaum G, Rachmilewitz J. Cells exposed to sublethal oxidative stress selectively attract monocytes/macrophages via scavenger receptors and MyD88-mediated signaling. J Immunol. 2012;188(3):1234–1244. doi: 10.4049/jimmunol.1101740. [DOI] [PubMed] [Google Scholar]
- George J, Afek A, Gilburd B, Levkovitz H, Shaish A, Goldberg I, et al. Harats D. Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis. 1998;138(1):147–152. doi: 10.1016/s0021-9150(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Gerrity RG. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981;103(2):191–200. [PMC free article] [PubMed] [Google Scholar]
- Gil P, Farinas F, Casado A, Lopez-Fernandez E. Malondialdehyde: A possible marker of ageing. Gerontology. 2002;48(4):209–214. doi: 10.1159/000058352. [DOI] [PubMed] [Google Scholar]
- Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104(4):503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez A, H I, Maya I. Cleavage and oligomerization of malondialdehyde under physiological conditions. Tetrahedron Letters. 1990;31(28):4077–4080. [Google Scholar]
- Gonen A, Hansen LF, Turner WW, Montano EN, Que X, Rafia A, et al. Hartvigsen K. Atheroprotective immunization with malondialdehyde-modified LDL is hapten specific and dependent on advanced MDA adducts: implications for development of an atheroprotective vaccine. J Lipid Res. 2014;55(10):2137–2155. doi: 10.1194/jlr.M053256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonenc A, Ozkan Y, Torun M, Simsek B. Plasma malondialdehyde (MDA) levels in breast and lung cancer patients. J Clin Pharm Ther. 2001;26(2):141–144. doi: 10.1046/j.1365-2710.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- Gounopoulos P, Merki E, Hansen LF, Choi SH, Tsimikas S. Antibodies to oxidized low density lipoprotein: epidemiological studies and potential clinical applications in cardiovascular disease. Minerva Cardioangiol. 2007;55(6):821–837. [PubMed] [Google Scholar]
- Greaves DR, Gordon S. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J Lipid Res. 2009;50(Suppl):S282–286. doi: 10.1194/jlr.R800066-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigolo B, Roseti L, Fiorini M, Facchini A. Enhanced lipid peroxidation in synoviocytes from patients with osteoarthritis. J Rheumatol. 2003;30(2):345–347. [PubMed] [Google Scholar]
- Gungor N, Pennings JL, Knaapen AM, Chiu RK, Peluso M, Godschalk RW, Van Schooten FJ. Transcriptional profiling of the acute pulmonary inflammatory response induced by LPS: role of neutrophils. Respir Res. 2010;11:24. doi: 10.1186/1465-9921-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurbuz G, Heinonen M. LC-MS investigations on interactions between isolated beta-lactoglobulin peptides and lipid oxidation product malondialdehyde. Food Chem. 2015;175:300–305. doi: 10.1016/j.foodchem.2014.11.154. [DOI] [PubMed] [Google Scholar]
- Haberland ME, Fless GM, Scanu AM, Fogelman AM. Malondialdehyde modification of lipoprotein(a) produces avid uptake by human monocyte-macrophages. J Biol Chem. 1992;267(6):4143–4151. [PubMed] [Google Scholar]
- Haberland ME, Fogelman AM, Edwards PA. Specificity of receptor-mediated recognition of malondialdehyde-modified low density lipoproteins. Proc Natl Acad Sci U S A. 1982;79(6):1712–1716. doi: 10.1073/pnas.79.6.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland ME, Fong D, Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Hadley M, Draper HH. Identification of N-(2-propenal) serine as a urinary metabolite of malondialdehyde. FASEB J. 1988;2(2):138–140. doi: 10.1096/fasebj.2.2.3125082. [DOI] [PubMed] [Google Scholar]
- Hadley M, Visser MF, Vander Steen T. Antioxidant effect on urinary excretion of malondialdehyde in non-athletes during aerobic training. Int J Vitam Nutr Res. 2009;79(1):5–13. doi: 10.1024/0300-9831.79.1.5. [DOI] [PubMed] [Google Scholar]
- Haider L, Fischer MT, Frischer JM, Bauer J, Hoftberger R, Botond G, et al. Lassmann H. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134(Pt 7):1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartvigsen K, Chou MY, Hansen LF, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. The role of innate immunity in atherogenesis. J Lipid Res. 2009;50(Suppl):S388–393. doi: 10.1194/jlr.R800100-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M, Ullrich V. On the mechanism of prostacyclin and thromboxane A2 biosynthesis. J Biol Chem. 1989;264(1):141–150. [PubMed] [Google Scholar]
- Henriksen T, Mahoney EM, Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A. 1981;78(10):6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill GE, Miller JA, Baxter BT, Klassen LW, Duryee MJ, Tuma DJ, Thiele GM. Association of malondialdehyde-acetaldehyde (MAA) adducted proteins with atherosclerotic-induced vascular inflammatory injury. Atherosclerosis. 1998;141(1):107–116. doi: 10.1016/s0021-9150(98)00153-1. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Kerr S, Jellusova J, Zhang J, Weisel F, Wellmann U, et al. Nitschke L. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol. 2007;8(7):695–704. doi: 10.1038/ni1480. [DOI] [PubMed] [Google Scholar]
- Huber J, Vales A, Mitulovic G, Blumer M, Schmid R, Witztum JL, et al. Leitinger N. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler Thromb Vasc Biol. 2002;22(1):101–107. doi: 10.1161/hq0102.101525. [DOI] [PubMed] [Google Scholar]
- Hyvarinen S, Uchida K, Varjosalo M, Jokela R, Jokiranta TS. Recognition of malondialdehyde-modified proteins by the C terminus of complement factor H is mediated via the polyanion binding site and impaired by mutations found in atypical hemolytic uremic syndrome. J Biol Chem. 2014;289(7):4295–4306. doi: 10.1074/jbc.M113.527416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, et al. Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura K, Uchida K, Osawa T. A novel fluorescent malondialdehyde-lysine adduct. Chemistry and Physics of Lipids. 1996;84(1):75–79. doi: 10.1016/S0009-3084(96)02624-2. [DOI] [Google Scholar]
- Jain SK. Evidence for membrane lipid peroxidation during the in vivo aging of human erythrocytes. Biochim Biophys Acta. 1988;937(2):205–210. doi: 10.1016/0005-2736(88)90242-8. [DOI] [PubMed] [Google Scholar]
- Jain SK, McVie R, Duett J, Herbst JJ. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes. 1989;38(12):1539–1543. doi: 10.2337/diab.38.12.1539. [DOI] [PubMed] [Google Scholar]
- Kajander T, Lehtinen MJ, Hyvarinen S, Bhattacharjee A, Leung E, Isenman DE, et al. Jokiranta TS. Dual interaction of factor H with C3d and glycosaminoglycans in host-nonhost discrimination by complement. Proc Natl Acad Sci U S A. 2011;108(7):2897–2902. doi: 10.1073/pnas.1017087108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautiainen A, Tornqvist M, Svensson K, Osterman-Golkar S. Adducts of malonaldehyde and a few other aldehydes to hemoglobin. Carcinogenesis. 1989;10(11):2123–2130. doi: 10.1093/carcin/10.11.2123. [DOI] [PubMed] [Google Scholar]
- Kearley ML, Patel A, Chien J, Tuma DJ. Observation of a new nonfluorescent malondialdehyde-acetaldehyde-protein adduct by 13C NMR spectroscopy. Chem Res Toxicol. 1999;12(1):100–105. doi: 10.1021/tx980132u. [DOI] [PubMed] [Google Scholar]
- Kharbanda KK, Shubert KA, Wyatt TA, Sorrell MF, Tuma DJ. Effect of malondialdehyde-acetaldehyde-protein adducts on the protein kinase C-dependent secretion of urokinase-type plasminogen activator in hepatic stellate cells. Biochem Pharmacol. 2002;63(3):553–562. doi: 10.1016/s0006-2952(01)00883-8. [DOI] [PubMed] [Google Scholar]
- Kharbanda KK, Todero SL, Shubert KA, Sorrell MF, Tuma DJ. Malondialdehyde-acetaldehyde-protein adducts increase secretion of chemokines by rat hepatic stellate cells. Alcohol. 2001;25(2):123–128. doi: 10.1016/s0741-8329(01)00174-4. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Shinomiya N, Ono S, Tsujimoto H, Kawabata T, Matsumoto A, et al. Seki S. Restoration of natural IgM production from liver B cells by exogenous IL-18 improves the survival of burn-injured mice infected with Pseudomonas aeruginosa. J Immunol. 2006;177(7):4627–4635. doi: 10.4049/jimmunol.177.7.4627. [DOI] [PubMed] [Google Scholar]
- Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Won YS, Park O, Chang B, Duryee MJ, Thiele GE, et al. Gao B. Aldehyde dehydrogenase 2 deficiency ameliorates alcoholic fatty liver but worsens liver inflammation and fibrosis in mice. Hepatology. 2014;60(1):146–157. doi: 10.1002/hep.27036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120(5):417–426. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Binder CJ, Tsimikas S, Witztum JL. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J Clin Invest. 2013;123(1):27–36. doi: 10.1172/JCI63108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ML, Scalia R, Mehta JL, Williams KJ. Cholesterol-induced membrane microvesicles as novel carriers of damage-associated molecular patterns: mechanisms of formation, action, and detoxification. Arterioscler Thromb Vasc Biol. 2012;32(9):2113–2121. doi: 10.1161/ATVBAHA.112.255471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddukuri L, Eoff RL, Choi JY, Rizzo CJ, Guengerich FP, Marnett LJ. In vitro bypass of the major malondialdehyde- and base propenal-derived DNA adduct by human Y-family DNA polymerases kappa, iota, and Rev1. Biochemistry. 2010;49(38):8415–8424. doi: 10.1021/bi1009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JJ, Mauri C, Ehrenstein MR. Natural serum IgM maintains immunological homeostasis and prevents autoimmunity. Springer Semin Immunopathol. 2005;26(4):425–432. doi: 10.1007/s00281-004-0187-x. [DOI] [PubMed] [Google Scholar]
- Mantani PT, Duner P, Bengtsson E, Alm R, Ljungcrantz I, Soderberg I, et al. Fredrikson GN. IL-25 inhibits atherosclerosis development in apolipoprotein E deficient mice. PLoS One. 2015;10(1):e0117255. doi: 10.1371/journal.pone.0117255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Corral J, Fontes CC, Pascual-Guardia S, Sanchez F, Olivan M, Argiles JM, et al. Barreiro E. Redox balance and carbonylated proteins in limb and heart muscles of cachectic rats. Antioxid Redox Signal. 2010;12(3):365–380. doi: 10.1089/ars.2009.2818. [DOI] [PubMed] [Google Scholar]
- Marnett LJ. Lipid peroxidation-DNA damage by malondialdehyde. Mutat Res. 1999;424(1–2):83–95. doi: 10.1016/s0027-5107(99)00010-x. [DOI] [PubMed] [Google Scholar]
- Marnett LJ, Buck J, Tuttle MA, Basu AK, Bull AW. Distribution and oxidation of malondialdehyde in mice. Prostaglandins. 1985;30(2):241–254. doi: 10.1016/0090-6980(85)90188-1. [DOI] [PubMed] [Google Scholar]
- McCaskill ML, Kharbanda KK, Tuma DJ, Reynolds JD, DeVasure JM, Sisson JH, Wyatt TA. Hybrid malondialdehyde and acetaldehyde protein adducts form in the lungs of mice exposed to alcohol and cigarette smoke. Alcohol Clin Exp Res. 2011;35(6):1106–1113. doi: 10.1111/j.1530-0277.2011.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor DD, Hamer H, Smyth S, Atkin SL, Courts FL. Antioxidant-rich spice added to hamburger meat during cooking results in reduced meat, plasma, and urine malondialdehyde concentrations. Am J Clin Nutr. 2010;92(4):996–997. doi: 10.3945/ajcn.2010.29976. author reply 997. [DOI] [PubMed] [Google Scholar]
- Mendis S, Puska P, Norrving B, Organization WH, Federation WH, Organization WS. Global Atlas on Cardiovascular Disease Prevention and Control. World Health Organization in collaboration with the World Heart Federation and the World Stroke Organization; 2011. [Google Scholar]
- Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, et al. Witztum JL. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108(2):235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller YI, Tsimikas S. Oxidation-specific epitopes as targets for biotheranostic applications in humans: biomarkers, molecular imaging and therapeutics. Curr Opin Lipidol. 2013;24(5):426–437. doi: 10.1097/MOL.0b013e328364e85a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Huang DY, Valentine WM, Amarnath V, Saunders A, Weisgraber KH, et al. Strittmatter WJ. Crosslinking of apolipoprotein E by products of lipid peroxidation. J Neuropathol Exp Neurol. 1996;55(2):202–210. doi: 10.1097/00005072-199602000-00009. [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Lung CC, Pinnas JL. Glycosylation enhances malondialdehyde binding to proteins. Free Radic Biol Med. 1996;21(5):699–701. doi: 10.1016/0891-5849(96)00127-x. [DOI] [PubMed] [Google Scholar]
- Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13(10):709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnia A, Bonassi S, Verna A, Quaglia R, Pelucco D, Ceppi M, et al. Peluso M. Bronchial malondialdehyde DNA adducts, tobacco smoking, and lung cancer. Free Radic Biol Med. 2006;41(9):1499–1505. doi: 10.1016/j.freeradbiomed.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Nair V, O'Neil CL, Wang PG. Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons, Ltd; 2001. Malondialdehyde. [Google Scholar]
- Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat Rev Immunol. 2013;13(5):349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale TJ, Ojha PP, Exner M, Poczewski H, Ruger B, Witztum JL, et al. Kerjaschki D. Proteinuria in passive Heymann nephritis is associated with lipid peroxidation and formation of adducts on type IV collagen. J Clin Invest. 1994;94(4):1577–1584. doi: 10.1172/JCI117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester CM, Barbour T, de Cordoba SR, Dragon-Durey MA, Fremeaux-Bacchi V, Goodship TH, et al. Smith RJ. Atypical aHUS: State of the art. Mol Immunol. 2015;67(1):31–42. doi: 10.1016/j.molimm.2015.03.246. [DOI] [PubMed] [Google Scholar]
- Nichols EM, Barbour TD, Pappworth IY, Wong EK, Palmer JM, Sheerin NS, et al. Marchbank KJ. An extended mini-complement factor H molecule ameliorates experimental C3 glomerulopathy. Kidney Int. 2015;88(6):1314–1322. doi: 10.1038/ki.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem. 2003;278(33):31426–31433. doi: 10.1074/jbc.M212549200. [DOI] [PubMed] [Google Scholar]
- Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med. 2009;47(5):469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Nikolic DM, Cholewa J, Gass C, Gong MC, Post SR. Class A scavenger receptor-mediated cell adhesion requires the sequential activation of Lyn and PI3-kinase. Am J Physiol Cell Physiol. 2007;292(4):C1450–1458. doi: 10.1152/ajpcell.00401.2006. [DOI] [PubMed] [Google Scholar]
- Odhiambo A, Perlman DH, Huang H, Costello CE, Farber HW, Steinberg MH, et al. Klings ES. Identification of oxidative post-translational modification of serum albumin in patients with idiopathic pulmonary arterial hypertension and pulmonary hypertension of sickle cell anemia. Rapid Commun Mass Spectrom. 2007;21(14):2195–2203. doi: 10.1002/rcm.3074. [DOI] [PubMed] [Google Scholar]
- Ogden CA, Kowalewski R, Peng Y, Montenegro V, Elkon KB. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38(4):259–264. doi: 10.1080/08916930500124452. [DOI] [PubMed] [Google Scholar]
- Palinski W, Horkko S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, Witztum JL. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98(3):800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci U S A. 1995;92(3):821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W, Ord VA, Plump AS, Breslow JL, Steinberg D, Witztum JL. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb. 1994;14(4):605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- Palinski W, Rosenfeld ME, Yla-Herttuala S, Gurtner GC, Socher SS, Butler SW, et al. Witztum JL. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W, Yla-Herttuala S, Rosenfeld ME, Butler SW, Socher SA, Parthasarathy S, et al. Witztum JL. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 1990;10(3):325–335. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Dalfo E, Ayala V, Bellmunt MJ, Prat J, Ferrer I, Portero-Otin M. Proteins in human brain cortex are modified by oxidation, glycoxidation, and lipoxidation. Effects of Alzheimer disease and identification of lipoxidation targets. J Biol Chem. 2005;280(22):21522–21530. doi: 10.1074/jbc.M502255200. [DOI] [PubMed] [Google Scholar]
- Peluso M, Munnia A, Risso GG, Catarzi S, Piro S, Ceppi M, et al. Brancato B. Breast fine-needle aspiration malondialdehyde deoxyguanosine adduct in breast cancer. Free Radic Res. 2011;45(4):477–482. doi: 10.3109/10715762.2010.549485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton PW, Aboutwerat A, Smith A, Burrows PC, McMahon RF, Warnes TW. Oxidant stress in type I autoimmune hepatitis: the link between necroinflammation and fibrogenesis? Biochim Biophys Acta. 2004;1689(3):182–189. doi: 10.1016/j.bbadis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Piche LA, Cole PD, Hadley M, van den Bergh R, Draper HH. Identification of N-epsilon-(2-propenal)lysine as the main form of malondialdehyde in food digesta. Carcinogenesis. 1988;9(3):473–477. doi: 10.1093/carcin/9.3.473. [DOI] [PubMed] [Google Scholar]
- Pryor WA, Stanley JP. Letter: A suggested mechanism for the production of malonaldehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymatic production of prostaglandin endoperoxides during autoxidation. J Org Chem. 1975;40(24):3615–3617. doi: 10.1021/jo00912a038. [DOI] [PubMed] [Google Scholar]
- Quash G, Ripoll H, Gazzolo L, Doutheau A, Saba A, Gore J. Malondialdehyde production from spermine by homogenates of normal and transformed cells. Biochimie. 1987;69(2):101–108. doi: 10.1016/0300-9084(87)90241-0. [DOI] [PubMed] [Google Scholar]
- Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine R, Winslow GM. IgM in microbial infections: taken for granted? Immunol Lett. 2009;125(2):79–85. doi: 10.1016/j.imlet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Subramaniyam G, Shanmugam N. Proinflammatory effects of malondialdehyde in lymphocytes. J Leukoc Biol. 2012;92(5):1055–1067. doi: 10.1189/jlb.1211617. [DOI] [PubMed] [Google Scholar]
- Requena JR, Fu MX, Ahmed MU, Jenkins AJ, Lyons TJ, Thorpe SR. Lipoxidation products as biomarkers of oxidative damage to proteins during lipid peroxidation reactions. Nephrol Dial Transplant. 1996;11(Suppl 5):48–53. doi: 10.1093/ndt/11.supp5.48. [DOI] [PubMed] [Google Scholar]
- Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J Immunol. 2013;190(8):3831–3838. doi: 10.4049/jimmunol.1203487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed AM, Duffort S, Ivanov D, Wang H, Laird JM, Salomon RG, et al. Perez VL. The oxidative stress product carboxyethylpyrrole potentiates TLR2/TLR1 inflammatory signaling in macrophages. PLoS One. 2014;9(9):e106421. doi: 10.1371/journal.pone.0106421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen JT, Yla-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, et al. Witztum JL. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992;339(8798):883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- Schiopu A, Frendeus B, Jansson B, Soderberg I, Ljungcrantz I, Araya Z, et al. Fredrikson GN. Recombinant antibodies to an oxidized low-density lipoprotein epitope induce rapid regression of atherosclerosis in apobec-1(-/-)/low-density lipoprotein receptor(-/-) mice. J Am Coll Cardiol. 2007;50(24):2313–2318. doi: 10.1016/j.jacc.2007.07.081. [DOI] [PubMed] [Google Scholar]
- Schneiderhan W, Schmid-Kotsas A, Zhao J, Grunert A, Nussler A, Weidenbach H, et al. Bachem MG. Oxidized low-density lipoproteins bind to the scavenger receptor, CD36, of hepatic stellate cells and stimulate extracellular matrix synthesis. Hepatology. 2001;34(4 Pt 1):729–737. doi: 10.1053/jhep.2001.27828. [DOI] [PubMed] [Google Scholar]
- Schoenberg MH, Buchler M, Pietrzyk C, Uhl W, Birk D, Eisele S, et al. Beger HG. Lipid peroxidation and glutathione metabolism in chronic pancreatitis. Pancreas. 1995;10(1):36–43. doi: 10.1097/00006676-199501000-00005. [DOI] [PubMed] [Google Scholar]
- Schutt F, Bergmann M, Holz FG, Kopitz J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44(8):3663–3668. doi: 10.1167/iovs.03-0172. [DOI] [PubMed] [Google Scholar]
- Seimon TA, Nadolski MJ, Liao X, Magallon J, Nguyen M, Feric NT, et al. Tabas I. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12(5):467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam N, Figarola JL, Li Y, Swiderski PM, Rahbar S, Natarajan R. Proinflammatory effects of advanced lipoxidation end products in monocytes. Diabetes. 2008;57(4):879–888. doi: 10.2337/db07-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao B, Tang C, Heinecke JW, Oram JF. Oxidation of apolipoprotein A-I by myeloperoxidase impairs the initial interactions with ABCA1 required for signaling and cholesterol export. J Lipid Res. 2010;51(7):1849–1858. doi: 10.1194/jlr.M004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PX, Horkko S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105(12):1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PX, Horkko S, Tsimikas S, Chang MK, Palinski W, Silverman GJ, et al. Witztum JL. Human-derived anti-oxidized LDL autoantibody blocks uptake of oxidized LDL by macrophages and localizes to atherosclerotic lesions in vivo. Arterioscler Thromb Vasc Biol. 2001;21(8):1333–1339. doi: 10.1161/hq0801.093587. [DOI] [PubMed] [Google Scholar]
- Shechter I, Fogelman AM, Haberland ME, Seager J, Hokom M, Edwards PA. The metabolism of native and malondialdehyde-altered low density lipoproteins by human monocyte-macrophages. J Lipid Res. 1981;22(1):63–71. [PubMed] [Google Scholar]
- Shimizu I, Inoue H, Yano M, Shinomiya H, Wada S, Tsuji Y, et al. Ito S. Estrogen receptor levels and lipid peroxidation in hepatocellular carcinoma with hepatitis C virus infection. Liver. 2001;21(5):342–349. doi: 10.1034/j.1600-0676.2001.210507.x. [DOI] [PubMed] [Google Scholar]
- Sikar Akturk A, Ozdogan HK, Bayramgurler D, Cekmen MB, Bilen N, Kiran R. Nitric oxide and malondialdehyde levels in plasma and tissue of psoriasis patients. J Eur Acad Dermatol Venereol. 2012;26(7):833–837. doi: 10.1111/j.1468-3083.2011.04164.x. [DOI] [PubMed] [Google Scholar]
- Siu GM, Draper HH. Metabolism of malonaldehyde in vivo and in vitro. Lipids. 1982;17(5):349–355. doi: 10.1007/BF02535193. [DOI] [PubMed] [Google Scholar]
- Slatter DA, Bolton CH, Bailey AJ. The importance of lipid-derived malondialdehyde in diabetes mellitus. Diabetologia. 2000;43(5):550–557. doi: 10.1007/s001250051342. [DOI] [PubMed] [Google Scholar]
- Slatter DA, Murray M, Bailey AJ. Formation of a dihydropyridine derivative as a potential cross-link derived from malondialdehyde in physiological systems. FEBS Lett. 1998;421(3):180–184. doi: 10.1016/s0014-5793(97)01554-8. [DOI] [PubMed] [Google Scholar]
- Sofat R, Casas JP, Kumari M, Talmud PJ, Ireland H, Kivimaki M, et al. Hingorani AD. Genetic variation in complement factor H and risk of coronary heart disease: eight new studies and a meta-analysis of around 48,000 individuals. Atherosclerosis. 2010;213(1):184–190. doi: 10.1016/j.atherosclerosis.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Steinbrecher UP, Fisher M, Witztum JL, Curtiss LK. Immunogenicity of homologous low density lipoprotein after methylation, ethylation, acetylation, or carbamylation: generation of antibodies specific for derivatized lysine. J Lipid Res. 1984;25(10):1109–1116. [PubMed] [Google Scholar]
- Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, et al. Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11(2):155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom A, Fredrikson GN, Schiopu A, Ljungcrantz I, Soderberg I, Jansson B, et al. Nilsson J. Inhibition of injury-induced arterial remodelling and carotid atherosclerosis by recombinant human antibodies against aldehyde-modified apoB-100. Atherosclerosis. 2007;190(2):298–305. doi: 10.1016/j.atherosclerosis.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sasaki T, Kumagai T, Sakaguchi S, Nagata K. Malondialdehyde-modified low density lipoprotein (MDA-LDL)-induced cell growth was suppressed by polycyclic aromatic hydrocarbons (PAHs) J Toxicol Sci. 2010;35(2):137–147. doi: 10.2131/jts.35.137. [DOI] [PubMed] [Google Scholar]
- Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10(1):36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele GM, Duryee MJ, Willis MS, Sorrell MF, Freeman TL, Tuma DJ, Klassen LW. Malondialdehyde-acetaldehyde (MAA) modified proteins induce pro-inflammatory and pro-fibrotic responses by liver endothelial cells. Comp Hepatol. 2004;3(Suppl 1):S25. doi: 10.1186/1476-5926-2-S1-S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele GM, Tuma DJ, Willis MS, Miller JA, McDonald TL, Sorrell MF, Klassen LW. Soluble proteins modified with acetaldehyde and malondialdehyde are immunogenic in the absence of adjuvant. Alcohol Clin Exp Res. 1998;22(8):1731–1739. [PubMed] [Google Scholar]
- Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe-/- mice. Arterioscler Thromb Vasc Biol. 2008;28(8):1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiku ML, Allison GT, Naik K, Karry SK. Malondialdehyde oxidation of cartilage collagen by chondrocytes. Osteoarthritis Cartilage. 2003;11(3):159–166. doi: 10.1016/s1063-4584(02)00348-5. [DOI] [PubMed] [Google Scholar]
- Tsiantoulas D, Diehl CJ, Witztum JL, Binder CJ. B cells and humoral immunity in atherosclerosis. Circ Res. 2014;114(11):1743–1756. doi: 10.1161/CIRCRESAHA.113.301145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiantoulas D, Perkmann T, Afonyushkin T, Mangold A, Prohaska TA, Papac-Milicevic N, et al. Binder CJ. Circulating microparticles carry oxidation-specific epitopes and are recognized by natural IgM antibodies. J Lipid Res. 2015;56(2):440–448. doi: 10.1194/jlr.P054569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsimikas S, Bergmark C, Beyer RW, Patel R, Pattison J, Miller E, et al. Witztum JL. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J Am Coll Cardiol. 2003;41(3):360–370. doi: 10.1016/s0735-1097(02)02769-9. [DOI] [PubMed] [Google Scholar]
- Tsimikas S, Lau HK, Han KR, Shortal B, Miller ER, Segev A, et al. Strauss BH. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 2004;109(25):3164–3170. doi: 10.1161/01.CIR.0000130844.01174.55. [DOI] [PubMed] [Google Scholar]
- Tsimikas S, Miyanohara A, Hartvigsen K, Merki E, Shaw PX, Chou MY, et al. Witztum JL. Human oxidation-specific antibodies reduce foam cell formation and atherosclerosis progression. J Am Coll Cardiol. 2011;58(16):1715–1727. doi: 10.1016/j.jacc.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsimikas S, Willeit P, Willeit J, Santer P, Mayr M, Xu Q, et al. Kiechl S. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol. 2012;60(21):2218–2229. doi: 10.1016/j.jacc.2012.08.979. [DOI] [PubMed] [Google Scholar]
- Tsimikas S, Witztum JL, Miller ER, Sasiela WJ, Szarek M, Olsson AG, et al. Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering Study, I High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation. 2004;110(11):1406–1412. doi: 10.1161/01.CIR.0000141728.23033.B5. [DOI] [PubMed] [Google Scholar]
- Tuma DJ, Kearley ML, Thiele GM, Worrall S, Haver A, Klassen LW, Sorrell MF. Elucidation of reaction scheme describing malondialdehyde-acetaldehyde-protein adduct formation. Chem Res Toxicol. 2001;14(7):822–832. doi: 10.1021/tx000222a. [DOI] [PubMed] [Google Scholar]
- Tuma DJ, Thiele GM, Xu D, Klassen LW, Sorrell MF. Acetaldehyde and malondialdehyde react together to generate distinct protein adducts in the liver during long-term ethanol administration. Hepatology. 1996;23(4):872–880. doi: 10.1002/hep.510230431. [DOI] [PubMed] [Google Scholar]
- Tuominen A, Miller YI, Hansen LF, Kesaniemi YA, Witztum JL, Horkko S. A natural antibody to oxidized cardiolipin binds to oxidized low-density lipoprotein, apoptotic cells, and atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2006;26(9):2096–2102. doi: 10.1161/01.ATV.0000233333.07991.4a. [DOI] [PubMed] [Google Scholar]
- Turunen SP, Kummu O, Harila K, Veneskoski M, Soliymani R, Baumann M, et al. Horkko S. Recognition of Porphyromonas gingivalis gingipain epitopes by natural IgM binding to malondialdehyde modified low-density lipoprotein. PLoS One. 2012;7(4):e34910. doi: 10.1371/journal.pone.0034910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free Radic Biol Med. 2000;28(12):1685–1696. doi: 10.1016/s0891-5849(00)00226-4. [DOI] [PubMed] [Google Scholar]
- Uchida K, Sakai K, Itakura K, Osawa T, Toyokuni S. Protein modification by lipid peroxidation products: formation of malondialdehyde-derived N(epsilon)-(2-propenol)lysine in proteins. Arch Biochem Biophys. 1997;346(1):45–52. doi: 10.1006/abbi.1997.0266. [DOI] [PubMed] [Google Scholar]
- Valles J, Aznar J, Santos MT, Fernandez MA. Elevated lipid peroxide levels in platelets of chronic ischemic heart disease patients. Thromb Res. 1982;27(5):585–589. doi: 10.1016/0049-3848(82)90305-x. [DOI] [PubMed] [Google Scholar]
- Van Vre EA, Ait-Oufella H, Tedgui A, Mallat Z. Apoptotic cell death and efferocytosis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(4):887–893. doi: 10.1161/ATVBAHA.111.224873. [DOI] [PubMed] [Google Scholar]
- VanderVeen LA, Hashim MF, Shyr Y, Marnett LJ. Induction of frameshift and base pair substitution mutations by the major DNA adduct of the endogenous carcinogen malondialdehyde. Proc Natl Acad Sci U S A. 2003;100(24):14247–14252. doi: 10.1073/pnas.2332176100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneskoski M, Turunen SP, Kummu O, Nissinen A, Rannikko S, Levonen AL, Horkko S. Specific recognition of malondialdehyde and malondialdehyde acetaldehyde adducts on oxidized LDL and apoptotic cells by complement anaphylatoxin C3a. Free Radic Biol Med. 2011;51(4):834–843. doi: 10.1016/j.freeradbiomed.2011.05.029. [DOI] [PubMed] [Google Scholar]
- Voitkun V, Zhitkovich A. Analysis of DNA-protein crosslinking activity of malondialdehyde in vitro. Mutat Res. 1999;424(1–2):97–106. doi: 10.1016/s0027-5107(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Wade CR, Jackson PG, Highton J, van Rij AM. Lipid peroxidation and malondialdehyde in the synovial fluid and plasma of patients with rheumatoid arthritis. Clin Chim Acta. 1987;164(3):245–250. doi: 10.1016/0009-8981(87)90298-1. [DOI] [PubMed] [Google Scholar]
- Wallberg M, Bergquist J, Achour A, Breij E, Harris RA. Malondialdehyde modification of myelin oligodendrocyte glycoprotein leads to increased immunogenicity and encephalitogenicity. Eur J Immunol. 2007;37(7):1986–1995. doi: 10.1002/eji.200636912. [DOI] [PubMed] [Google Scholar]
- Watson RR, Preedy VR, Zibadi S. Alcohol, Nutrition, and Health Consequences. Humana Press; 2012. [Google Scholar]
- Weismann D, Hartvigsen K, Lauer N, Bennett KL, Scholl HP, Charbel Issa P, et al. Binder CJ. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478(7367):76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis MS, Klassen LW, Carlson DL, Brouse CF, Thiele GM. Malondialdehyde-acetaldehyde haptenated protein binds macrophage scavenger receptor(s) and induces lysosomal damage. Int Immunopharmacol. 2004;4(7):885–899. doi: 10.1016/j.intimp.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Willis MS, Klassen LW, Tuma DJ, Thiele GM. Malondialdehyde-acetaldehyde-haptenated protein induces cell death by induction of necrosis and apoptosis in immune cells. Int Immunopharmacol. 2002;2(4):519–535. doi: 10.1016/s1567-5769(01)00195-3. [DOI] [PubMed] [Google Scholar]
- Witztum JL, Lichtman AH. The influence of innate and adaptive immune responses on atherosclerosis. Annu Rev Pathol. 2014;9:73–102. doi: 10.1146/annurev-pathol-020712-163936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witztum JL, Steinbrecher UP, Fisher M, Kesaniemi A. Nonenzymatic glucosylation of homologous low density lipoprotein and albumin renders them immunogenic in the guinea pig. Proc Natl Acad Sci U S A. 1983;80(9):2757–2761. doi: 10.1073/pnas.80.9.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wu YQ, Ricklin D, Janssen BJ, Lambris JD, Gros P. Structure of complement fragment C3b-factor H and implications for host protection by complement regulators. Nat Immunol. 2009;10(7):728–733. doi: 10.1038/ni.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Nityanand S, Berglund L, Lithell H, Holm G, Lefvert AK. Antibodies against cardiolipin and oxidatively modified LDL in 50-year-old men predict myocardial infarction. Arterioscler Thromb Vasc Biol. 1997;17(11):3159–3163. doi: 10.1161/01.atv.17.11.3159. [DOI] [PubMed] [Google Scholar]
- Wuttge DM, Bruzelius M, Stemme S. T-cell recognition of lipid peroxidation products breaks tolerance to self proteins. Immunology. 1999;98(2):273–279. doi: 10.1046/j.1365-2567.1999.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TA, Kharbanda KK, McCaskill ML, Tuma DJ, Yanov D, DeVasure J, Sisson JH. Malondialdehyde-acetaldehyde-adducted protein inhalation causes lung injury. Alcohol. 2012;46(1):51–59. doi: 10.1016/j.alcohol.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TA, Kharbanda KK, Tuma DJ, Sisson JH. Malondialdehyde-acetaldehyde-adducted bovine serum albumin activates protein kinase C and stimulates interleukin-8 release in bovine bronchial epithelial cells. Alcohol. 2001;25(3):159–166. doi: 10.1016/s0741-8329(01)00177-x. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Kharbanda KK, Tuma DJ, Sisson JH, Spurzem JR. Malondialdehyde-acetaldehyde adducts decrease bronchial epithelial wound repair. Alcohol. 2005;36(1):31–40. doi: 10.1016/j.alcohol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Xu D, Thiele GM, Kearley ML, Haugen MD, Klassen LW, Sorrell MF, Tuma DJ. Epitope characterization of malondialdehyde-acetaldehyde adducts using an enzyme-linked immunosorbent assay. Chem Res Toxicol. 1997;10(9):978–986. doi: 10.1021/tx970069t. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Sakata N, Meng J, Sakamoto M, Noma A, Maeda I, et al. Takebayashi S. Possible involvement of increased glycoxidation and lipid peroxidation of elastin in atherogenesis in haemodialysis patients. Nephrol Dial Transplant. 2002;17(4):630–636. doi: 10.1093/ndt/17.4.630. [DOI] [PubMed] [Google Scholar]
- Yang TC, Chen YJ, Chang SF, Chen CH, Chang PY, Lu SC. Malondialdehyde mediates oxidized LDL-induced coronary toxicity through the Akt-FGF2 pathway via DNA methylation. J Biomed Sci. 2014;21:11. doi: 10.1186/1423-0127-21-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarian CS, Rebrin I, Sohal RS. Aconitase and ATP synthase are targets of malondialdehyde modification and undergo an age-related decrease in activity in mouse heart mitochondria. Biochem Biophys Res Commun. 2005;330(1):151–156. doi: 10.1016/j.bbrc.2005.02.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Xu L, Porter NA. Free radical lipid peroxidation: mechanisms and analysis. Chem Rev. 2011;111(10):5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- Yla-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, et al. Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagol-Ikapite I, Sosa IR, Oram D, Judd A, Amarnath K, Amarnath V, et al. Boutaud O. Modification of platelet proteins by malondialdehyde: prevention by dicarbonyl scavengers. J Lipid Res. 2015;56(11):2196–2205. doi: 10.1194/jlr.P063271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Caligiuri G, Hamsten A, Lefvert AK, Hansson GK. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21(1):108–114. doi: 10.1161/01.atv.21.1.108. [DOI] [PubMed] [Google Scholar]
- Zhou X, Robertson AK, Rudling M, Parini P, Hansson GK. Lesion development and response to immunization reveal a complex role for CD4 in atherosclerosis. Circ Res. 2005;96(4):427–434. doi: 10.1161/01.RES.0000156889.22364.f1. [DOI] [PubMed] [Google Scholar]
- Zhu X, Ng HP, Lai YC, Craigo JK, Nagilla PS, Raghani P, Nagarajan S. Scavenger receptor function of mouse Fcgamma receptor III contributes to progression of atherosclerosis in apolipoprotein E hyperlipidemic mice. J Immunol. 2014;193(5):2483–2495. doi: 10.4049/jimmunol.1303075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9(10):729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]