Abstract
Objective
This study examined the individual and combined impact of N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity cardiac troponin T (hs-cTnT), interleukin-6 (IL-6), and high-sensitivity C-reactive protein (hs-CRP) on the prediction of heart failure incidence or progression in patients with type 2 diabetes.
Research Design and Methods
A nested case-cohort study was conducted in 3,098 participants with type 2 diabetes in the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial.
Results
A higher value of each biomarker was significantly associated with a higher risk of heart failure incidence or progression, after adjustment for major risk factors. The hazard ratios per 1-SD increase were 3.06 (95% CI 2.37-3.96) for NT-proBNP, 1.50 (1.27-1.77) for hs-cTnT, 1.48 (1.27-1.72) for IL-6, and 1.32 (1.12-1.55) for hs-CRP. Addition of NT-proBNP to the model including conventional risk factors meaningfully improved 5-year risk predictive performance (c-statistic 0.8162 to 0.8800; continuous net reclassification improvement [NRI] 73.1%; categorical NRI [<5%, 5-10%, >10% 5-year risk] 24.2%). In contrast, addition of hs-cTnT, IL-6 or hs-CRP did not improve the prediction metrics consistently either in combination or when added to NT-proBNP.
Conclusions
Only NT-proBNP, strongly and consistently improved prediction of heart failure in patients with type 2 diabetes beyond a wide range of clinical risk factors and biomarkers.
Keywords: Biomarkers, Heart failure, Diabetes mellitus, Natriuretic peptide, Risk assessment, Risk factors, Troponin T
The number of people with heart failure has been increasing, most likely the result of ageing and the increasing prevalence of hypertension, diabetes, obesity and atherosclerotic disease (1). Heart failure increases mortality, hospitalization and decreases health-related quality of life and functional status, and also results in increasing medical costs (1). Therefore, prevention and management of heart failure is an important global public health problem.
Diabetes is one of the major risk factors for heart failure, being associated with over 50% increase in risk (2), and has strong adverse effects on the prognosis of heart failure (3). Heart failure has also been noted to be the second most common first presentation of CVD in patients with type 2 diabetes, and more common than myocardial infarction (2). Yet insufficient emphasis has been placed on the prevention and treatment of heart failure in the clinical management of diabetes (4).
Recently, several circulating biomarkers, such as C-reactive protein (CRP) (5), interleukin-6 (IL-6) (6), N-terminal pro-B-type natriuretic peptide (NT-proBNP) (7,8), and high-sensitivity cardiac troponin T (hs-cTnT) (9), have been shown to be associated with the incidence of CVD. It has also been suggested that these biomarkers may be useful in predicting the risk of heart failure. However, few studies have examined the association between these biomarkers and the risk of heart failure in patients with diabetes, and it is uncertain how well these biomarkers can classify the risk of heart failure in such patients. Given limited medical resources and costs, efficient identification of high risk patients for subsequent precise evaluation and intervention is crucial.
The objective of the present study was thus to examine the association of circulating cardiac stress (NT-proBNP for myocardial stretch and volume overload and hs-cTnT for myocardial damage) and inflammatory (high-sensitivity C-reactive protein [hs-CRP] and IL-6) markers with the risk of heart failure and their additional risk predictive ability beyond that from traditional clinical risk factors in patients with type 2 diabetes.
Research Design and Methods
Study sample
We conducted a nested case-cohort study to examine the association between cardiac stress biomarkers and inflammatory markers and heart failure in patients with type 2 diabetes who participated in Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) study (ClinicalTrials.gov number NCT00145925). The design and results of ADVANCE have been published in detail previously (10–12). Briefly, 11,140 patients with type 2 diabetes at high risk of cardiovascular events were enrolled from 215 centers in 20 countries, and randomly assigned to either a gliclazide (modified release)-based intensive glucose control strategy (target HbA1c ≤6.5 %) or standard glucose control strategy based on local guidelines and to either a fixed-dose combination of perindopril (4 mg) and indapamide (1.25 mg) or matching placebo, after a 6-week active run-in period. Approval for the study was obtained from each center’s institutional review board. All participants provided written informed consent.
Baseline data included demographic and clinical information. Weight, height, blood pressure, hemoglobin A1c (HbA1c), fasting lipid levels, urinary albumin-to-creatinine ratio (ACR) and serum creatinine were measured. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (13). Twelve-lead electrocardiograms (ECGs) were obtained at baseline for the presence of atrial fibrillation, left ventricular hypertrophy, and pathological Q-waves. Atrial fibrillation was considered present when it was identified by the investigator on the baseline ECG or when atrial fibrillation confirmed by ECG had been previously diagnosed.
Plasma samples were obtained from all study participants at baseline and stored at -80°C for a median of 7.8 years. Samples were available from all countries involved in ADVANCE, except China and India, giving a total population of 7,376. For the nested case-cohort study (14), a random subcohort of 3,500 participants was selected from this base population, plus 131 additional participants who had experienced a heart failure event during 5-year follow-up (Supplemental Figure 1).
High-sensitivity IL-6 levels was assayed by ELISA (R&D Systems, Oxford, U.K.) and high-sensitivity CRP by immunonephelometry (ProSpec; Dade Behring, Milton Keynes, U.K.) (15). NT-proBNP and hs-cTnT were assayed by electrochemiluminescence immunoassays performed on a Roche Elecsys 2010 automated platform (Roche Diagnostics, Burgess Hill, U.K.) (16,17). A detailed description of the measurement of stored samples has been published previously (15–17).
Study Outcome
In this project, the study outcome was incidence or progression of heart failure (death due to heart failure, hospitalization due to heart failure, or worsening New York Heart Association class).
Statistical analysis
Categorical data are presented as number (percentage) and continuous data as mean (SD), where approximately symmetrically distributed, or median (interquartile range), where skewed. Differences in the mean values or proportions of the baseline characteristics of the patients according to outcome status were tested by chi-square test, unpaired t-test or Wilcoxon test, as appropriate. Hazard ratios (HRs) for incidence or progression of heart failure were calculated by weighted Cox regression models for case-cohort analyses using groups defined by the fifths and for a one standard deviation (SD) increase in each of IL-6, hs-CRP, hs-cTnT, and NT-proBNP, after log transformation. Three models, with different potential confounding variables, were fitted for each biomarker-heart failure combination: model 1 with age, sex, randomized blood pressure-lowering intervention, and randomized glucose control intervention; model 2 with, additionally, duration of diabetes mellitus, current smoking, history of myocardial infarction, history of hospitalization for heart failure, BMI, systolic blood pressure, heart rate, current or previous atrial fibrillation, pathological Q-wave on ECG, left ventricular hypertrophy on ECG, aspirin or other antiplatelet agent use, β-blocker use, calcium-channel blocker use, diuretics use, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers use, total cholesterol, HDL cholesterol, triglyceride, statin or other lipid-lowering agent, HbA1c, thiazolidinedione use, insulin use, urinary ACR and eGFR; and model 3 with, additionally to model 2, the other three biomarkers. Pre-defined subgroup analyses, using model 2, were performed by baseline history of heart failure, history of myocardial infarction, sex, age (split at its median), and duration of diabetes (also split at its median).
Prediction metrics for heart failure were calculated in the random subcohort. Discrimination was evaluated by c statistics for 5-year risk, accounting for censoring (18), and compared between model 2 and model 2 plus each biomarker individually and in combination. In addition, the ability of each biomarker to better classify the 5-year risk for incidence or progression of heart failure, compared to model 2, was evaluated by the integrated discrimination index (IDI) and the net reclassification improvement (NRI), using methods suitable for survival data (14). NRI was calculated by a continuous model for changes in risk classification and a categorical model based on <5%, 5-10%, and >10% 5-year risk.
In addition, sensitivity analyses were conducted after excluding 1) patients with a history of hospitalization for heart failure (n = 136) and 2) patients with NT-proBNP levels >400 pg/mL (n = 391). All analyses were performed using SAS Enterprise Guide 7.11 (SAS Institute Inc., Cary, NC) or Stata software (release 13; StataCorp, College Station, TX, USA). A two-sided P <0.05 was considered to be statistically significant in all analyses.
Results
A total of 3,631 patients comprised the entire case-cohort study. After a number of exclusions, shown in Supplemental Figure 1 (283 patients with insufficient stored plasma for measurement of biomarkers and 250 with missing values for covariates), the remaining 3,098 patients were included in the present analysis. During a median follow-up of 5.0 years, 237 experienced a heart failure event. Table 1 shows the baseline characteristics of study participants. 40% of the cohort was female, and the mean age was 67 years. IL-6, hs-CRP, hs-cTnT and NT-proBNP levels were significantly higher in patients who experienced a heart failure event.
Table 1.
Baseline characteristics according to outcome status
| Heart failure event | Overall | ||
|---|---|---|---|
| Variables | Yes n=237 |
No n=2,861 |
n=3,098 |
| Female (%) | 83 (35) | 1,162 (41) | 1,245 (40) |
| Age (years) | 70 (7)* | 66 (7) | 67 (7) |
| Duration of diabetes mellitus (years) | 10.0 (7.6)* | 7.5 (6.2) | 7.7 (6.3) |
| Current smoking (%) | 30 (13) | 426 (15) | 456 (15) |
| History of myocardial infarction (%) | 61 (26)* | 98 (3) | 159 (5) |
| History of hospitalization for heart failure (%) | 38 (16)* | 98 (3) | 136 (4) |
| BMI (kg/m2) | 30.7 (5.6) | 30.0 (5.2) | 30.0 (5.2) |
| SBP (mmHg) | 149 (23) | 147 (21) | 147 (21) |
| DBP (mmHg) | 80 (12)* | 82 (11) | 82 (11) |
| Heart rate (bpm) | 74 (13) | 73 (12) | 73 (12) |
| Current or previous atrial fibrillation (%) | 49 (21)* | 275 (10) | 324 (10) |
| Pathological Q-wave on ECG (%) | 51 (22)* | 307 (11) | 358 (12) |
| LVH on ECG (%) | 37 (16)* | 222 (8) | 259 (8) |
| Aspirin or other antiplatelet agent (%) | 143 (60)* | 1,378 (48) | 1,521 (49) |
| β-blocker (%) | 71 (30) | 856 (30) | 927 (30) |
| Calcium-channel blocker (%) | 106 (45)* | 815 (28) | 921 (30) |
| Diuretics ¶ (%) | 119 (50)* | 823 (29) | 942 (30) |
| ACE inhibitors ¶ or ARB (%) | 175 (74)* | 1,628 (57) | 1,803 (58) |
| Total cholesterol (mmol/l) | 5.03 (1.13) | 5.16 (1.17) | 5.15 (1.17) |
| HDL cholesterol (mmol/l) | 1.17 (0.28)* | 1.23 (0.33) | 1.23 (0.33) |
| Triglyceride (mmol/l) | 1.60 (1.2 2, 2.30) | 1.70 (1.2 0, 2.34) | 1.70 (1.2 0, 2.33) |
| Statin or other cholesterol-lowering agent (%) | 101 (43) | 1,265 (44) | 1,366 (44) |
| Hemoglobin A1c (%) | 7.8 (1.5)* | 7.4 (1.4) | 7.4 (1.4) |
| Hemoglobin A1c (mmol/mol) | 61.3 (16.0)* | 56.9 (15.1) | 57.2 (15.2) |
| Thiazolidinedione (%) | 7 (3) | 126 (4) | 133 (4) |
| Other oral antidiabetic agents (%) | 219 (92) | 2,562 (90) | 2,781 (90) |
| Insulin (%) | 4 (2) | 37 (1) | 41 (1.3) |
| Urinary ACR (µg/mg) | 30.9 (10.4, 91.1)* | 12.9 (6. 2, 32.7) | 13.5 (6. 2, 36.2) |
| eGFR (ml/min/1.73m2) | 63 (18)* | 73 (16) | 72 (17) |
| IL-6 (pg/mL) | 3.05 (2.13, 4.49)* | 2.19 (1.5 7, 3.21) | 2.26 (1.6 1, 3.33) |
| hs-CRP (mg/L) | 2.35 (1.19, 5.89)* | 1.75 (0.84, 3.91) | 1.80 (0.86, 4.03) |
| hs-cTnT (ng/L) | 12.0 (6.0, 20.0)* | 5.0 (1.5, 10.0) | 5.0 (1.5, 10.0) |
| NT-proBNP (pg/mL) | 353.0 (131.0, 819.0)* | 75.0 (31.0, 172.0) | 84.0 (33.0, 203.0) |
Values are mean (standard deviation) for continuous variables (except for triglycerides, urinary albumin-creatinine ratio, IL-6, hs-CRP, hs-cTnT, and NT-proBNP), median (interquartile range) for triglycerides, urinary albumin-creatinine ratio, IL-6, hs-CRP, hs-cTnT, and NT-proBNP, and number (%) for categorical variables.
Abbreviations: ACE, Angiotensin-converting enzyme; ACR, albumin-creatinine ratio; ARB, angiotensin II receptor blocker; DBP, diastolic blood pressure; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein; hs-cTnT, high-sensitivity cardiac troponin T; IL-6, interleukin-6; LVH, left ventricular hypertrophy; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SBP, systolic blood pressure.
P <0.05 vs patients without heart failure event.
Randomized BP-lowering treatment with perindopril-indapamide was not included.
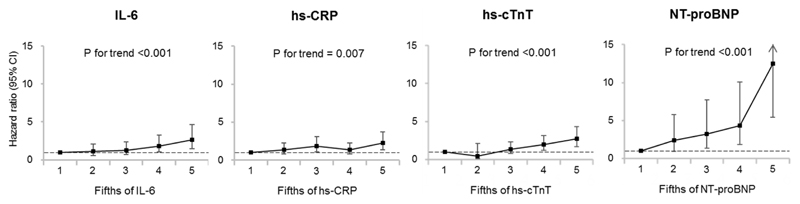
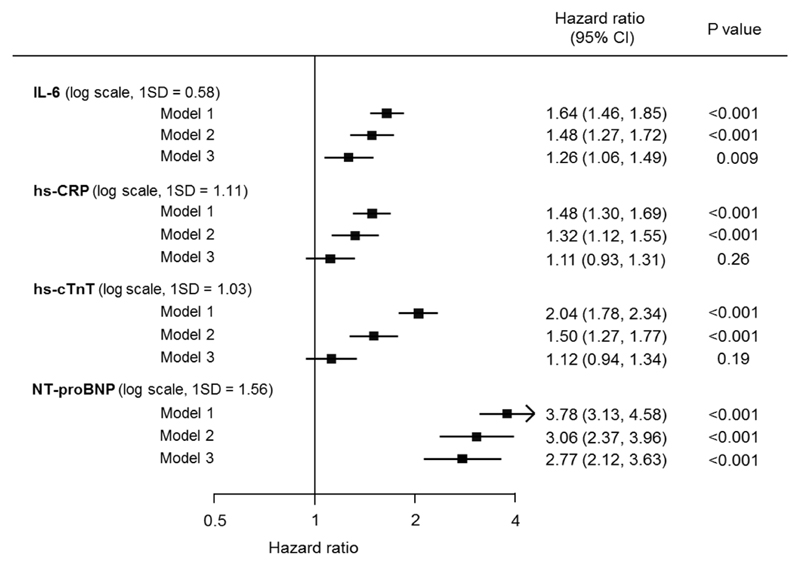
The HRs and 95% CIs for heart failure according to fifths of each biomarker are depicted in Figure 1. The risk of heart failure increased significantly with increasing levels of all the biomarkers after adjustment for age, sex, randomized blood pressure-lowering and glucose control interventions, and clinical risk factors (all P for trend <0.01 in model 2). Multivariable-adjusted HRs (95% CIs) for the highest fifths compared with the lowest fifths were 2.62 (1.48-4.63) for IL-6, 2.22 (1.33-3.72) for hs-CRP, 2.70 (1.68-4.34) for hs-cTnT, and 12.53 (5.41-29.02) for NT-proBNP. Figure 2 shows the HRs and 95% CIs for heart failure according to a 1 SD increment in each biomarker. Higher values of all four biomarkers were significantly associated with higher risk of heart failure after adjusting for clinical risk factors (Model 2, all P <0.001). After further adjustment for the other biomarkers (Model 3), associations were attenuated and became non-significant for hs-CRP and hs-cTnT. In all adjustment sets, NT-proBNP showed the strongest association with heart failure (HR 2.77 [95% CI 2.12-3.63] in model 3). Broadly similar findings were observed in the sensitivity analyses after excluding patients with past history of hospitalization for heart failure (Supplemental Figure 2) or those with NT-proBNP levels >400 pg/mL (Supplemental Figure 3), though the associations were slightly attenuated when patients with NT-proBNP levels >400 pg/mL were excluded. There was no evidence of effect modification in the association between heart failure and NT-proBNP by sex, age, duration of diabetes, or history of hospitalization for heart failure (model 2, Supplemental Figure 4). Although significant heterogeneity (P = 0.004) was observed in the association in those with (HR=2.28 [95% CI 1.08-4.81]) and without (HR=3.52 [95% CI 2.66-4.66]) a history of myocardial infarction, the direction of the association was the same in both groups.
Figure 1. Adjusted hazard ratios and 95% CIs for heart failure according to fifths of the biomarker.
Abbreviations: hs-CRP, high-sensitivity C-reactive protein; hs-cTnT, high-sensitivity cardiac troponin T; IL-6, interleukin-6; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Each biomarker was categorized into 5 groups according to the fifths. The ranges of IL-6 were 0.19-1.48, 1.49-1.95, 1.96-2.58, 2.59-3.68, and 3.69-16.13 pg/mL. The ranges of hs-CRP were 0.08-0.73, 0.74-1.33, 1.34-2.45, 2.46-4.75, and 4.78-130.00 mg/L. The ranges of hs-cTnT were 1.5, 3.0, 4.0-6.0, 7.0-12.0, and 13.0-751.0 ng/L. The ranges of NT-proBNP were 2.5-24.0, 25.0-59.0, 60.0-116.0, 117.0-255.0, and 256.0-35000.0 pg/mL.
Hazard ratios were adjusted for age, sex, randomised blood pressure-lowering intervention, randomised glucose control intervention, duration of diabetes mellitus, current smoking, history of myocardial infarction, history of hospitalization for heart failure, BMI, systolic blood pressure, heart rate, current or previous atrial fibrillation, pathological Q-wave on ECG, left ventricular hypertrophy on ECG, aspirin or other antiplatelet agent use, β-blocker use, calcium-channel blocker use, diuretics use, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers use, total cholesterol, HDL cholesterol, triglyceride, statin or other lipid-lowering agent, hemoglobin A1c, thiazolidinedione use, insulin use, urinary albumin-creatinine ratio, and estimated glomerular filtration rate.
Figure 2. Adjusted hazard ratios and 95% CIs for heart failure according to a one standard deviation increment in the biomarker.
Abbreviations: ECG, electrocardiogram; HR, hazard ratio; hs-CRP, high-sensitivity C-reactive protein; hs-cTnT, high-sensitivity cardiac troponin T; IL-6, interleukin-6; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SD, standard deviation.
Model 1 was adjusted for age, sex, randomised blood pressure-lowering intervention, and randomised glucose control intervention.
Model 2 was additionally adjusted for duration of diabetes mellitus, current smoking, history of myocardial infarction, history of hospitalization for heart failure, BMI, systolic blood pressure, heart rate, current or previous atrial fibrillation, pathological Q-wave on ECG, left ventricular hypertrophy on ECG, aspirin or other antiplatelet agent use, β-blocker use, calcium-channel blocker use, diuretics use, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers use, total cholesterol, HDL cholesterol, triglyceride, statin or other lipid-lowering agent, hemoglobin A1c, thiazolidinedione use, insulin use, urinary albumin-creatinine ratio, and estimated glomerular filtration rate.
Model 3 was additionally adjusted for the other biomarkers.
Addition of NT-proBNP to a model including conventional risk factors (model 2) greatly improved discrimination and classification of the 5-year risk of heart failure (c-statistic: 0.8162 to 0.8800, P <0.001; IDI: 0.107, P <0.001; continuous NRI: 0.731, P <0.001, categorical NRI: 0.242, P <0.001) (Table 3). On the other hand, when any of IL-6, hs-CRP, or hs-cTnT was added to model 2, the improvements were not uniformly significant. NT-proBNP alone showed comparable predictive ability compared with a comprehensive set of conventional risk factors (c-statistic: 0.8239 vs. 0.8162, P = 0.74).
Addition of NT-proBNP to model 2 plus IL-6, hs-CRP, and hs-cTnT significantly improved the c-statistic (0.8384 to 0.8816, P <0.001) and classification of outcomes (IDI: 0.081, P <0.001; continuous NRI: 0.664, P <0.001; categorical NRI: 0.191, P <0.001). On the other hand, addition of a combination of IL-6, hs-CRP, and hs-cTnT to model 2 plus NT-proBNP improved classification (IDI: 0.029, P = 0.002; continuous NRI: 0.304, P = 0.002; categorical NRI: 0.010, P = 0.56), but did not improve discrimination (c-statistic: 0.8800 to 0.8816, P = 0.65). Almost identical results were obtained when patients with past history of hospitalization for heart failure or those with NT-proBNP levels >400 pg/mL were excluded (Supplemental Tables 1 and 2).
Conclusions
To the best of our knowledge, this is the first study to examine the improvement in the risk predictive ability for future heart failure by adding biomarkers to conventional risk factors in patients with type 2 diabetes, using a comprehensive set of prediction metrics. Higher values of IL-6, hs-CRP, hs-cTnT, and NT-proBNP were significantly associated with a higher risk of incidence or progression of heart failure in patients with type 2 diabetes. These associations persisted after adjusting for a comprehensive set of conventional CVD risk factors. In addition, incorporation of NT-proBNP into a prediction model greatly improved discrimination and classification of the 5-year risk of heart failure beyond conventional use of risk factors. In contrast, none of IL-6, hs-CRP, and hs-cTnT provided clinically useful incremental information. These data were broadly similar in those with no previous history of heart failure hospitalization and when those with NT-proBNP levels >400 pg/mL were excluded.
A number of studies have reported that the usefulness of newly identified biomarkers such as NT-proBNP, hs-cTnT, hs-CRP, and IL-6 to predict incident heart failure (19,20). However, few studies have investigated prognostic ability for heart failure in patients with diabetes. A sub-analysis of the Steno-2 study found that NT-proBNP levels above the median of the study population were associated with an increased risk of a composite of cardiovascular mortality and hospitalization for congestive heart failure in 160 microalbuminuric patients with type 2 diabetes (21). Another observational study from the SAVOR-TIMI 53 randomized trial, among 12,301 patients with type 2 diabetes, also showed a stepwise increased risk of hospitalization for heart failure with increasing levels of NT-proBNP, with no evidence of heterogeneity among those taking saxagliptin treatment or placebo (22). The addition of NT-proBNP to the model with clinical variables increased the c-statistic from 0.81 to 0.85 (22). The present study adds to these prior studies by providing evidence that NT-proBNP considerably improved risk prediction for heart failure beyond that derived from a wide range of clinical risk factors, using a comprehensive set of discrimination and reclassification metrics. Addition of NT-proBNP to the model with clinical risk factors increased the c-statistic and improved IDI, continuous NRI, and categorical NRI. These findings suggest that assessment of NT-proBNP will help to identify those at high risk who should go on to further investigation, such as undergoing an echocardiogram, and intervention.
There is only one prior study examining the association between CRP and heart failure in patients with diabetes, and none examining associations for IL-6 and hs-cTnT. In a subgroup analysis from the Strong Heart Study, elevated CRP levels were associated with incident heart failure in American Indians with diabetes (23). Our findings are consistent and extend to patients with diabetes from a range of countries across Australasia, Europe and North America. In addition, our study adds new information on the relationship between the risk of heart failure and IL-6 and hs-cTnT levels, with findings showing an elevated risk of heart failure with increasing levels in these markers but no improvements in prediction metrics when added to a model with traditional clinical risk factors.
Only addition of NT-proBNP to the model with clinical risk factors and the other three biomarkers strongly improved prognostic ability. On the other hand, addition of a combination of IL-6, hs-CRP and hs-cTnT to the model with clinical risk factors and NT-proBNP had no such benefit. This suggests that NT-proBNP adds to predictive capacity for the incidence of heart failure and could be added to routine assessment of the risk.
Recently, the EMPAREG-REG OUTCOME trial reported that patients with type 2 diabetes who received empagliflozin, an inhibitor of sodium-glucose cotransporter 2, had a significantly lower risk of hospitalization for heart failure than those in the placebo group (24). This association may be partly driven by osmotic diuresis and changes in plasma volume and sodium excretion with modulation of the cardio-renal axis mediated by empagliflozin (25,26). NT-proBNP is a cardiac hormone secreted by cardiac myocytes in response to ventricular wall stresses secondary to volume and pressure overload (27). Thus risk prediction for heart failure using NT-proBNP could more accurately identify those patients who may benefit most from this kind of drug. Future interventional studies are required to ascertain the utility of this possible strategy.
The strengths of the present study include its large sample size, international recruitment in a well-characterized trial population, which was closely monitored and treated uniformly, completeness of follow-up, and adjustment for a variety of risk factors. In addition, this is the first study to examine the additional predictive ability of biomarkers beyond conventional risk factors, using a comprehensive range of prediction statistics, in patients with type 2 diabetes. Some limitations of our study should be discussed. First, a single measurement of levels of biomarkers may not accurately represent the true status of the participants. However, this would bias our results toward the null hypothesis of no association. Therefore, the true association may be stronger than that observed in the present study. Second, the participants in this study were those eligible for the clinical trial. Therefore, applicability of the present findings to the general populations of patients with diabetes may not be justified, although the characteristics of the ADVANCE cohort of baseline were similar to those reported by a number of community based epidemiological studies (28). Finally, there may be other possible confounders besides those used in the current study, leading to bias by residual confounding.
In conclusion, we found that IL-6, hs-CRP, hs-cTnT, and NT-proBNP were independent predictors of incidence of heart failure in patients with type 2 diabetes. However, only the addition of NT-proBNP materially improved the predictive performance for heart failure beyond that from conventional clinical risk factors. Further studies are needed to validate our findings.
Supplementary Material
Table 2.
Discrimination and reclassification statistics (95% CIs) for 5-year risk of heart failure after addition of biomarkers to a model containing clinical risk factors
| NRI |
|||||
|---|---|---|---|---|---|
| C-statistic | IDI | Relative IDI (%) | Continuous | Categorical† | |
| Base model* | 0.8162 (0.7785, 0.8540) | ||||
| Base model plus IL-6 | 0.8264 (0.7904, 0.8624) P = 0.052 |
0.029 (0.008, 0.050) P = 0.006 |
8.73 (2.38, 15.98) | 0.393 (0.210, 0.569) P <0.001 |
0.030 (-0.053, 0.108) P = 0.45 |
| Base model plus hs-CRP | 0.8261 (0.7900, 0.8621) P = 0.11 |
0.018 (0.003, 0.034) P = 0.02 |
5.50 (0.91, 10.45) | 0.215 (0.036, 0.387) P = 0.03 |
0.092 (0.024, 0.160) P = 0.008 |
| Base model plus hs-cTnT | 0.8253 (0.7888, 0.8618) P = 0.22 |
0.020 (0.004, 0.038) P = 0.01 |
6.08 (1.19, 11.70) | 0.403 (0.223, 0.583) P <0.001 |
0.065 (-0.008, 0.140) P = 0.07 |
| Base model plus NT-proBNP | 0.8800 (0.8529, 0.9072) P <0.001 |
0.107 (0.064, 0.154) P <0.001 |
32.2 (18.2, 49.3) | 0.731 (0.564, 0.892) P <0.001 |
0.242 (0.145, 0.342) P <0.001 |
| Base model plus IL-6, hs-CRP, and hs-cTnT | 0.8384 (0.8040, 0.8729) | ||||
| Addition of NT-proBNP to base model plus IL-6, hs-CRP, and hs-cTnT | 0.8816 (0.8546, 0.9085) P <0.001 |
0.081 (0.043, 0.123) P <0.001 |
20.8 (10.4, 32.6) | 0.664 (0.492, 0.828) P <0.001 |
0.191 (0.105, 0.287) P <0.001 |
| Base model plus NT-proBNP | 0.8800 (0.8529, 0.9072) | ||||
| Addition of IL-6, hs-CRP, and hs-cTnT to base model plus NT-proBNP | 0.8816 (0.8546, 0.9085) P = 0.65 |
0.029 (0.010, 0.049) P = 0.002 |
6.57 (2.21, 11.2) | 0.304 (0.117, 0.497) P = 0.002 |
0.010 (-0.040, 0.059) P = 0.56 |
Results were derived from the random subcohort (n = 2,989).
Biomarkers were log transformed.
Base model included age, sex, randomised blood pressure-lowering intervention, randomised glucose control intervention, duration of diabetes mellitus, current smoking, history of myocardial infarction, history of hospitalization for heart failure, BMI, systolic blood pressure, heart rate, current or previous atrial fibrillation, pathological Q-wave on ECG, left ventricular hypertrophy on ECG, aspirin or other antiplatelet agent use, β-blocker use, calcium-channel blocker use, diuretics use, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers use, total cholesterol, HDL cholesterol, triglyceride, statin or other lipid-lowering agent, hemoglobin A1c, thiazolidinedione use, insulin use, urinary albumin-creatinine ratio, and estimated glomerular filtration rate.
Using cutoff points of 5% and 10% 5-year risk.
Abbreviations: IDI, integrated discrimination improvement; HR, hazard ratio; hs-CRP, high-sensitivity C-reactive protein; hs-cTnT, high-sensitivity cardiac troponin T; IL-6, interleukin-6; NRI, net reclassification improvement; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Funding
The ADVANCE trial was funded by a grant from the National Health and Medical Research Council (NHMRC) of Australia. T.O. holds the JSPS Postdoctoral Fellowships for Research Abroad. M.W. is a National Health and Medical Research Council of Australia Principal Research Fellow (1080206). S.Z. is a National Health and Medical Research Council of Australia Senior Research Fellow (1081328). J.E.S is a National Health and Medical Research Council of Australia Senior Research Fellow (1079438).
Abbreviations
- ACR
albumin-to-creatinine ratio
- ADVANCE
Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation
- ECG
electrocardiogram
- eGFR
estimated glomerular filtration rate
- HR
hazard ratio
- HbA1c
hemoglobin A1c
- hs-CRP
high-sensitivity C-reactive protein
- hs-cTnT
high-sensitivity cardiac troponin T
- IDI
integrated discrimination index
- IL-6
interleukin-6
- NRI
net reclassification improvement
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- SD
standard deviation
Footnotes
Duality of Interest
M.W. reports consultancy fees from Servier. S.Z. reports past participation in advisory boards and/or receiving honoraria from Amgen Australia, AstraZeneca/Bristol-Myers Squibb Australia, Janssen-Cilag, Merck Sharp & Dohme (Australia), Novartis Australia, Sanofi, Servier Laboratories and Takeda Australia. M.E.C has received lecturing fees from Servier. P.H. ireports being a consultant to Servier. G.M. reports personal fees from Servier, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, Novartis, Menarini International, Recordati, and Takeda. B.W. reports personal fees from Servier, Novartis, Boerhinger Ingelheim, and MSD. J.E.S. reports past participation in advisory boards and/or receiving honoraria from AstraZeneca, Janssen-Cilag, Merck Sharp & Dohme (Australia), Novartis Australia, Sanofi, and Mylan. J.C. reports grants from Servier, administered through the University of Sydney as Co-Principal investigator for ADVANCE and ADVANCE-ON and personal fees from Servier. No other potential conflicts of interest relevant to this article were reported.
Author contributions
T.O., M.J., M.W., and J.C. contributed to the concept and rationale for the study and interpretation of the results, and drafted the manuscript. T.O. conducted statistical analysis. S.Z., M.E.C, D.E.G., P.H., G.M., B.W., P.W., N.S., J.E.S., and K.R. contributed to discussion and reviewed and edited the manuscript. J.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Clinical trial reg. no. NCT00145925, clinicaltrials.gov.
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 2.Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105–113. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pocock SJ, Wang D, Pfeffer MA, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014;2:843–851. doi: 10.1016/S2213-8587(14)70031-2. [DOI] [PubMed] [Google Scholar]
- 5.Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danesh J, Kaptoge S, Mann AG, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wannamethee SG, Welsh P, Lowe GD, et al. N-terminal pro-brain natriuretic Peptide is a more useful predictor of cardiovascular disease risk than C-reactive protein in older men with and without pre-existing cardiovascular disease. J Am Coll Cardiol. 2011;58:56–64. doi: 10.1016/j.jacc.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 8.Welsh P, Doolin O, Willeit P, et al. N-terminal pro-B-type natriuretic peptide and the prediction of primary cardiovascular events: results from 15-year follow-up of WOSCOPS. Eur Heart J. 2013;34:443–450. doi: 10.1093/eurheartj/ehs239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang TJ, Wollert KC, Larson MG, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ADVANCE Management Committee. Study rationale and design of ADVANCE: Action in Diabetes and Vascular disease --preterax and diamicron MR controlled evaluation. Diabetologia. 2001;44:1118–1120. doi: 10.1007/s001250100612. [DOI] [PubMed] [Google Scholar]
- 11.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 12.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodward M. Epidemiology: Study Design and Data Analysis. Boca Raton, Florida: Chapman & Hall/CRC; 2014. [Google Scholar]
- 15.Lowe G, Woodward M, Hillis G, et al. Circulating inflammatory markers and the risk of vascular complications and mortality in people with type 2 diabetes and cardiovascular disease or risk factors: the ADVANCE study. Diabetes. 2014;63:1115–1123. doi: 10.2337/db12-1625. [DOI] [PubMed] [Google Scholar]
- 16.Hillis GS, Welsh P, Chalmers J, et al. The relative and combined ability of high-sensitivity cardiac troponin T and N-terminal pro-B-type natriuretic peptide to predict cardiovascular events and death in patients with type 2 diabetes. Diabetes Care. 2014;37:295–303. doi: 10.2337/dc13-1165. [DOI] [PubMed] [Google Scholar]
- 17.Welsh P, Woodward M, Hillis GS, et al. Do cardiac biomarkers NT-proBNP and hsTnT predict microvascular events in patients with type 2 diabetes? Results from the ADVANCE trial. Diabetes Care. 2014;37:2202–2210. doi: 10.2337/dc13-2625. [DOI] [PubMed] [Google Scholar]
- 18.Newson R. Comparing the predictive power of survival models using Harrell's C or Somers’ D. Stata J. 2010;10:339–358. [Google Scholar]
- 19.Echouffo-Tcheugui JB, Greene SJ, Papadimitriou L, et al. Population risk prediction models for incident heart failure: a systematic review. Circ Heart Fail. 2015;8:438–447. doi: 10.1161/CIRCHEARTFAILURE.114.001896. [DOI] [PubMed] [Google Scholar]
- 20.Rahimi K, Bennett D, Conrad N, et al. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail. 2014;2:440–446. doi: 10.1016/j.jchf.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Gaede P, Hildebrandt P, Hess G, Parving HH, Pedersen O. Plasma N-terminal pro-brain natriuretic peptide as a major risk marker for cardiovascular disease in patients with type 2 diabetes and microalbuminuria. Diabetologia. 2005;48:156–163. doi: 10.1007/s00125-004-1607-0. [DOI] [PubMed] [Google Scholar]
- 22.Scirica BM, Braunwald E, Raz I, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–1588. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 23.Barac A, Wang H, Shara NM, et al. Markers of inflammation, metabolic risk factors, and incident heart failure in American Indians: the Strong Heart Study. J Clin Hypertens. 2012;14:13–19. doi: 10.1111/j.1751-7176.2011.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 25.Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016;37:1526–1534. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sattar N, McLaren J, Kristensen SL, Preiss D, McMurray JJ. SGLT2 Inhibition and cardiovascular events: why did EMPA-REG Outcomes surprise and what were the likely mechanisms? Diabetologia. 2016;59:1333–1339. doi: 10.1007/s00125-016-3956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghashghaei R, Arbit B, Maisel AS. Current and novel biomarkers in heart failure: bench to bedside. Curr Opin Cardiol. 2016;31:191–195. doi: 10.1097/HCO.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 28.Chalmers J, Arima H. Importance of blood pressure lowering in type 2 diabetes: focus on ADVANCE. J Cardiovasc Pharmacol. 2010;55:340–347. doi: 10.1097/fjc.0b013e3181d26469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.