Abstract
Accumulating data support a role for bioactive lipids as mediators of lipotixicity in cardiomyocytes. One class of these, the ceramides, constitutes a family of molecules that differ in structure and are synthesized by distinct enzymes, ceramide synthase (CerS)1–CerS6. Data support that specific ceramides and the enzymes that catalyze their formation play distinct roles in cell function. In a mouse model of diabetic cardiomyopathy, sphingolipid profiling revealed increases in not only the CerS5-derived ceramides but also in very long chain (VLC) ceramides derived from CerS2. Overexpression of CerS2 elevated VLC ceramides caused insulin resistance, oxidative stress, mitochondrial dysfunction, and mitophagy. Palmitate induced CerS2 and oxidative stress, mitophagy, and apoptosis, which were prevented by depletion of CerS2. Neither overexpression nor knockdown of CerS5 had any function in these processes, suggesting a chain-length dependent impact of ceramides on mitochondrial function. This concept was also supported by the observation that synthetic mitochondria–targeted ceramides led to mitophagy in a manner proportional to N-acyl chain length. Finally, blocking mitophagy exacerbated cell death. Taken together, our results support a model by which CerS2 and VLC ceramides have a distinct role in lipotoxicity, leading to mitochondrial damage, which results in subsequent adaptive mitophagy. Our data reveal a novel lipotoxic pathway through CerS2.—Law, B. A., Liao, X., Moore, K. S., Southard, A., Roddy, P., Ji, R., Szulc, Z., Bielawska, A., Schulze, P. C., Cowart, L. A. Lipotoxic very-long-chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes.
Keywords: mitophagy, sphingolipid, diabetic cardiomyopathy, cardiovascular disease
In metabolic disease, bioactive lipids in the myocardium are thought to contribute to cardiac dysfunction by perturbation of crucial cellular pathways in a process generally termed lipotocixity. Ceramide, a lipotoxic sphingolipid intermediate, has been implicated in cardiomyocyte autophagy, apoptosis, diabetic cardiomyopathy, and the development of congestive heart failure, suggesting a role for ceramide in cardiomyocyte homeostasis and cardiac pathophysiology. Indeed, pharmacological and genetic studies in mice have pointed to a specific role for ceramide in several mouse models of cardiac pathophysiology because global inhibition of ceramide synthesis could rescue pathologic changes (1, 2). Ceramide biosynthesis occurs through 6 distinct ceramide synthase (CerS) isoforms (CerS1–CerS6), and although they catalyze the same basic reaction (N-acylation of a sphingoid base using an acyl-CoA donor), each has specific preferences for the acyl-CoA used and thus produces specific ceramide species. Lipidomic strategies have enabled the identification and quantification of these distinct species; subsequently, it has increasingly become appreciated that the N-acyl chain length of ceramide is a major determinant of its functional activity (2–4), suggesting that specific targeting of individual enzymes could ameliorate CerS isoform–specific outcomes. However, little information is available on specific CerS involved in cardiomyocyte sphingolipid metabolism under healthy or disease conditions, and mechanisms by which ceramides promote cardiac dysfunction are incompletely understood.
In mammals, ceramides contain an array of N-acyl chain lengths, including medium (C12–14), long (C16–C18), very long (C20–C24), and ultralong (C26 and above). We recently demonstrated that CerS5, which synthesizes medium- and long-chain (LC) ceramides, mediated macroautophagy and cardiomyocyte hypertrophy in the context of lipotoxicity (2). In a mouse model of diabetic cardiomyopathy, based on an obesogenic high-saturated-fat diet (HFD), mice showed left ventricular hypertrophy and loss of both diastolic and systolic function (2). Although that study addressed specifically the medium-chain and LC ceramides generated by CerS5, sphingolipid profiling of HFD-fed mice revealed that very long chain (VLC) ceramides, which arise from CerS2, also increased (2). We therefore speculated that the pool of VLC ceramides may also contribute to the cardiomyocyte pathology observed in the diabetic cardiomyopathy model and/or in cultured cells. In this study, we identified distinct contributions of VLC ceramides and CerS2 to abnormal cardiac metabolism and dysfunction. Our findings support a role for CerS2 in cardiomyocytes in the lipotoxic context, including mitochondrial damage, oxidative stress, and apoptosis, and subsequent induction of mitophagy, which appears to be protective in this context.
MATERIALS AND METHODS
Human myocardial samples
Myocardial samples were collected from the left ventricle of patients with advanced heart failure (HF) during the placement of a left ventricular assist device and from nondiseased control hearts not used for heart transplantation and donated to research. All protocols were reviewed and approved by the Institutional Review Board at Columbia University (IRB AAA2296).
Animal model
Animals were maintained and fed an HFD as described in Russo et al. (2).
Cell culture
AC16 cells (5), a human cardiomyocyte-like cell line, were maintained in DMEM with 1 g/L glucose (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA, USA) and 1% penicillin/streptomycin (Thermo Fisher Scientific) in a humidified environment at 37°C with 5% CO2. Cells were used through passage 10. Cells from rat neonatal cardiomyocyte cell line (H9c2; American Type Culture Collection, Manassas, VA, USA) were cultured similarly in DMEM with 0.45 g/L glucose (ATCC) supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C with 5% CO2. Cells were used through passage 10.
Mitochondrial fractionation
After treatments, mitochondrial-enriched fractions were obtained from cells using the Mitochondrial Isolation Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions.
Plasmids
The full length of human CerS2 constructed into a p3XFLAG-CMV-7 vector bacterial alkaline phosphatase (MilliporeSigma, St. Louis, MO, USA) was used as a control plasmid. Two CRISPR-CAS9 plasmids targeting rat CerS2 at the N-terminus (AGAAGTAGTCATACAAGGT) and the putative active site (GAACAGATCATCCACCATG) (GenScript, Nanjing, China) were used to produce a stable knockdown of CerS2. Plasmids were transfected using Lipofectamine 2000 or 3000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. For studies using the green fluorescent protein (GFP)-light chain 3B construct, cells were transfected or cotransfected with pSelect-GFP-light chain 3 (Invivogen, San Diego, CA, USA). All plasmids were cultured for at least 24 h as indicated.
Palmitate and compound treatment
Cell medium was supplemented with 20 mg/ml bovine serum albumin (BSA) and 0.5 mM palmitate (Matreya, State College, PA, USA) or vehicle (ethanol). Solutions were then sonicated at 60°C for 5 min, cooled to 37°C, and incubated with cells for the timepoints indicated. In certain experiments, myriocin (MilliporeSigma) at 1 μM, l-glutathione (Sigma-Aldrich) at 10 mM, and mitochondrial division inhibitor (mdivi-1) at 10 μM were pretreated 2 h before beginning the experimental treatments.
Lipidomic analysis
Cells and tissues were lipid extracted by methanol/KOH/CHCl3, and product ceramide species were detected by ultra-performance liquid chromatography with internal controls. Lipidomic profiling was performed by liquid chromatography/mass spectrometry by the Lipidomics Core Facility at Medical University of South Carolina as previously described (6).
Ceramide synthase 2 enzyme activity
Incorporation of (2S,3R)-2-amino-18-{(7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)amino}octadecane-1,3-diol (NBD)-labeled dihydrosphingosine and nervonyl (C24:1)-CoA by CerS2 in homogenized cell pellets of normal H9c2 cells and cells knocked down for CerS2 was used to assay CerS2 activity as previously described (7).
Sphingolipid analogs
All sphingolipid analogs were synthesized by previously reported methods (8, 9). Analogs were solubilized in 100% ethanol and supplemented in cell medium at 10 μM.
Measurement of cellular oxygen consumption rate
Oxygen consumption rate was determined using an XF24 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA, USA). AC16 cells were plated on XF24 microplates (Seahorse Bioscience) at 5.0 × 104 cells/well in DMEM supplemented with 10% FBS and kept at 37°C in a 5% CO2 humidified atmosphere 24 h before CerS2 overexpression. Intact cellular respiration was assayed 24 or 48 h after transfection under basal conditions (10 mM d-glucose, 10 mM pyruvate, 0% serum) and after the administration of various drugs as follows: mitochondrial inhibitor oligomycin (oligo) (1 μM), mitochondrial uncoupler carbonylcyanide p-trifluoromethoxyphenylhydrazone (1 μM), respiratory chain inhibitor antimycin A (AA) (1 μM), and rotenone (1 μM). The XF24 microplate was loaded into the Seahorse XF24 analyzer following the manufacturer’s instructions. All experiments were carried out at 37°C (n = 20).
Respiratory parameters were quantified by subtracting respiration rates at times before and after the addition of electron transport chain inhibitors according to Seahorse Bioscience: basal respiration, baseline respiration minus AA-dependent respiration; ATP turnover, baseline respiration minus oligo-dependent respiration; H+ leak, oligo-dependent respiration minus AA-dependent respiration; and respiratory capacity, carbonylcyanide p-trifluoromethoxyphenylhydrazone–dependent respiration minus AA-dependent respiration. Values were calculated for each well and averaged for each condition.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
After treatment with pyridinium ceramide analogs, H9c2 cells were treated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Thermo Fisher Scientific) for 1.5 h and read at AB 570 nm to determine MTT reduction. This assay was repeated twice with at least 3 replicates per treatment.
Glucose uptake
Glucose transport was examined using [14C] l-glucose (PerkinElmer, Waltham, MA, USA). AC16 cells (4 × 105 cell/well) were seeded in the 24-well plates and incubated in the growth medium overnight. After overexpression of CerS2 24 or 48 h, the cells were starved in HBSS for 30 min and then treated with insulin (100 nM) for 10 min. At 5 min after treatment with 1 µCi [14C] l-glucose, uptake was terminated by removing the uptake solution followed by washing 3 times with ice-cold HBSS. Cells were solubilized with 0.1 N NaOH, and radioactivity was measured by liquid scintillation spectrometry.
Live cell imaging
MitoTracker Red and MitoTracker Green (Thermo Fisher Scientific) were used to detect mitochondria and mitochondrial membrane potential in live cells. LysoTracker Red (Thermo Fisher Scientific) was used to detect lysosomes in a similar manner. MitoSox Red (Thermo Fisher Scientific) was used to detect formation of superoxide in live cultures. In certain experiments, DAPI was used to detect cell nuclei. All reagents were used according to the manufacturer’s instructions, and live cells were imaged using an FV10i microscope (Olympus, Tokyo, Japan).
CellRox Deep Red reagent (Thermo Fisher Scientific) was used for detection of oxidative stress in cells according to the manufacturer’s instructions. CellRoxDeep Red Reagent (5 μM) was added into medium after treatment. After incubation at 37°C for 30 min, the cells were washed 3 times with PBS and stripped with 0.05% trypsin/EDTA. Cells were analyzed with FACS.
Antibodies
Light chain 3B, caspase 3, poly(ADP-ribose) polymerase (PARP), protein kinase B (AKT), P-AKT (Ser473), glycogen synthase kinase 3 (GSK3), GSK3 (Ser9) (Cell Signaling Technology, Danvers, MA, USA), Tom20, Gapdh, CerS2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and p62 (Abcam, Cambridge, United Kingdom) were used at 1:1000 (1:500 for CerS2) with 1% BSA in Tris-buffered saline with 0.1% Tween 20 (TBS-T) overnight at 4°C to detect protein expression by Western blot. Appropriate HRP-conjugated secondary anti-sera (anti-goat, anti-mouse, and anti-rabbit) were incubated with 1% BSA in TBS-T at 1:5000 for 30 min at room temperature. ECL (Bio-Rad, Hercules, CA, USA) was used to detect bands. Blots were imaged with a Bio-Rad chemiluminescent imager. Annexin V and 4-hydroxynoneal (Abcam) antibodies were used for immunocytochemistry. Cells were fixed with 4% buffered neutral paraformaldehyde and incubated with 1:50 primary antibody in 2% BSA–supplemented TBS-T overnight at 4°C. Alexa Fluor 488–conjugated secondary antisera (Thermo Fisher Scientific) was incubated in the dark at room temperature for 2 h. Cells were imaged using an Olympus FV10i microscope.
Statistical analyses
A Student’s t test and 1-way ANOVA were used to compare experimental conditions. A value of P < 0.05 between groups was considered significant. Results are displayed as ± sem.
RESULTS
High-fat feeding increased long- and very long-chain ceramides in mouse myocardium
Previous studies in mouse models have supported that in obese and diabetic hearts, increased myocardial ceramides are correlated with the development of cardiac dysfunction (10–15). Moreover, reduction of ceramide synthesis has been shown to improve cardiac function (1, 10, 16, 17). Ceramides have largely been studied in this context as a mixed group, although distinct ceramide species are synthesized through specific enzymes. We previously identified a role for medium-chain and/or LC ceramides and CerS5 in macroautophagy in the mouse model and in vitro in primary cardiomyocytes (2). However, cardiomyocytes express several CerS isoforms, which we speculated may also contribute to the ceramide pools and may or may not mediate distinct cell processes.
C57bl/6J mice were placed on an HFD for 18 wk, which we previously showed to cause a diabetic cardiomyopathy–like phenotype (e.g., left ventricular hypertrophy, reduced ejection fraction, and reduced mitral annular velocity). One group of HFD-fed mice was injected with myriocin, a global sphingolipid synthesis inhibitor. Sphingolipid profiling of myocardium of these animals was performed to assess the effects of the HFD on cardiomyocyte ceramide profiles, and, consistent with previous work (2), we observed changes in LC ceramides, which were partially attenuated with myriocin. However, VLC ceramides also increased under high-fat feeding, and these were more robustly inhibited because treatment restored these species to baseline levels in animals treated with myriocin (Table 1). This raised the hypothesis that distinct aspects of the sphingolipid-dependent pathophysiology may arise via medium-chain and lLC ceramides and from VLC ceramides, which in the heart are likely derived from CerS2.
TABLE 1.
Day 18 ceramide mouse heart high-fat diet lipidomics (pM/mg)
| N-acyl chain | Control | Control + myriocin | Milk-fat diet | Milk-fat diet + myriocin |
|---|---|---|---|---|
| Total | 104.7 ± 24.0 | 134.8 ± 23.8 | 191.2* ± 21.9 | 128.5 ± 16.3 |
| C14 | 0.2 ± 0.1 | 0.4 ± 0.1 | 1.5 ± 0.7 | 1.2 ± 0.6 |
| C16 | 5.2 ± 1.2 | 7.2 ± 1.9 | 15.8 ± 6.2 | 9.7 ± 2.4 |
| C16:1 | 16.0 ± 3.3 | 21.1 ± 4.5 | 34.8 ± 5.5 | 26.6 ± 2.3 |
| C18 | 4.1 ± 0.9 | 5.3 ± 1.1 | 12.0* ± 2.2 | 8.1 ± 0.8 |
| C18:1 | 29.4 ± 7.0 | 36.6 ± 8.0 | 41.7** ± 3.3 | 27.8 ± 4.4 |
| C20 | 11.0 ± 2.6 | 12.5 ± 2.6 | 17.5 ± 2.6 | 10.1 ± 1.8 |
| C20:1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.2 |
| C22 | 12.4 ± 2.7 | 16.8 ± 2.0 | 23.3* ± 2.5 | 13.7^ ± 2.4 |
| C22:1 | 7.6 ± 1.8 | 7.6 ± 1.1 | 12.5 ± 1.1 | 7.5 ± 1.4 |
| C24 | 6.8 ± 1.6 | 10.0 ± 0.9 | 13.5* ± 1.9 | 8.8 ± 1.3 |
| C24:1 | 11.9 ± 3.1 | 17.1 ± 2.5 | 18.5 ± 2.7 | 14.6 ± 2.5 |
| C26 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 |
| C26:1 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 |
Data are means ± SEM; n = 5 for all groups.
In addition to these canonical ceramides containing the classic 18-carbon sphingoid base (d18-sphingosine), we previously demonstrated that in this model of diabetic cardiomyopathy, the atypical 16-carbon sphingoid bases increased, as did the ceramides that generate them (2). In contrast to the d18-based ceramides, there were virtually no medium-chain or LC d16-ceramides detectable (data not shown); on the other hand, VLC ceramides, which are products of CerS2, increased with HFD feeding and were partially to completely restored to basal levels in myriocin-treated mice (Table 2). Together, these data suggest that both CerS5 and CerS2 generate classic d18 ceramides of LC and VLC lengths, respectively, but only CerS2 generates d16 ceramides. This is intriguing because we previously reported that d16-sphingolipids, but not d18-sphingolipids, promoted apoptosis in cardiomyocytes, indicating distinct functions of these lipids dependent not only on N-acyl chain length but also on the length of the sphingoid base.
TABLE 2.
Day 16 ceramide mouse heart high-fat diet lipidomics (pM/mg)
| N-acyl chain | Control | Control + myriocin | Milk-fat diet | Milk-fat diet + myriocin |
|---|---|---|---|---|
| Total | 0.5 ± 0.3 | 1.5 ± 0.5 | 4.2*** ± 0.6 | 2.4 ± 0.5 |
| C14 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| C16 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| C18 | 0.1 ± 0.1 | 0.4 ± 0.1 | 1.4*** ± 0.3 | 1.0** ± 0.2 |
| C18:1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| C20 | 0.3 ± 0.1 | 0.7 ± 0.2 | 1.1** ± 0.2 | 0.7 ± 0.1 |
| C20:1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| C22 | 0.0 ± 0.0 | 0.2 ± 0.2 | 1.3*** ± 0.2 | 0.4** ± 0.2 |
| C22:1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| C24 | 0.0 ± 0.0 | 0.2 ± 0.1 | 0.5** ± 0.1 | 0.3 ± 0.1 |
| C24:1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| C26 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| C26:1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Data are means ± SEM; n = 5 for all groups
Ceramide synthase 2 overexpression induces mitochondrial dysfunction and apoptosis
Previous work has described mitochondrial dysfunction and cardiomyocyte death to be hallmarks of various cardiomyopathies (18–23). To test cell outcomes resulting from increased VLC ceramides, CerS2 was overexpressed in a human cardiomyocyte-like cell line (AC16). This resulted in an overall increase in ceramides (Table 3), which was similar to the increase in ceramides detected after treatment with palmitate (Table 4). Although most species were significantly elevated, the most abundant ceramides included C16, a product of CerS5 [consistent with our previous study (2)] and C24 and 24:1, which are specific CerS2 products. This was similar to the ceramide profiles in human HF (Supplemental Tables 2 and 3), consistent with the notion that CerS2 may contribute to changes in ceramides in humans.
TABLE 3.
Day 18 ceramide AC16 CerS2 overexpression lipidomics (pM/mg)
| N-acyl chain | Control | CerS2 overexpression |
|---|---|---|
| Total | 211.7 ± 9.4 | 659.7*** ± 29.8 |
| C14 | 5.8 ± 0.2 | 17.2*** ± 0.4 |
| C16 | 61.0 ± 3.4 | 324.6*** ± 21.4 |
| C16:1 | 1.1 ± 0.1 | 4.3*** ± 0.3 |
| C18 | 3.3 ± 0.1 | 28.5*** ± 1.2 |
| C18:1 | 0.4 ± 0.0 | 2.1*** ± 0.1 |
| C20 | 0.1 ± 0.0 | 1.0*** ± 0.1 |
| C20:1 | 0.0 ± 0.0 | 0.4*** ± 0.0 |
| C22 | 5.0 ± 0.3 | 17.4*** ± 1.2 |
| C22:1 | 0.4 ± 0.0 | 2.5*** ± 0.2 |
| C24 | 89.2 ± 3.5 | 156.0*** ± 5.4 |
| C24:1 | 21.8 ± 1.1 | 78.1*** ± 3.8 |
| C26 | 12.1 ± 0.9 | 10.2 ± 0.4 |
| C26:1 | 11.3 ± 0.7 | 17.3*** ± 0.2 |
Data are means ± SEM; n = 6 for all groups
TABLE 4.
Day 18 ceramide H9c2 palmitate treatment (pM/mg)
| N-acyl chain | Control | Palmitate |
|---|---|---|
| C14-Cer | 0.6 ± 0.1 | 2.9*** ± 0.0 |
| C16-Cer | 5.4 ± 0.5 | 57.5*** ± 2.9 |
| C18-Cer | 1.9 ± 0.2 | 16.4*** ± 1.2 |
| C18:1-Cer | 0.5 ± 0.1 | 2.1** ± 0.2 |
| C20-Cer | 1.1 ± 0.2 | 7.5*** ± 0.3 |
| C20:1-Cer | 0.4 ± 0.1 | 0.8*** ± 0.0 |
| C20:4-Cer | 0.0 ± 0.0 | 0.0 ± 0.0 |
| C22-Cer | 3.5 ± 0.6 | 19.2*** ± 1.3 |
| C22:1-Cer | 1.0 ± 0.1 | 3.4*** ± 0.1 |
| C24-Cer | 6.5 ± 0.8 | 25.8*** ± 1.6 |
| C24:1-Cer | 9.6 ± 1.4 | 28.6*** ± 1.4 |
| C26-Cer | 0.1 ± 0.1 | 0.3* ± 0.0 |
| C26:1-Cer | 0.2 ± 0.1 | 0.5* ± 0.1 |
Data are means ± SEM; n = 3 for all groups
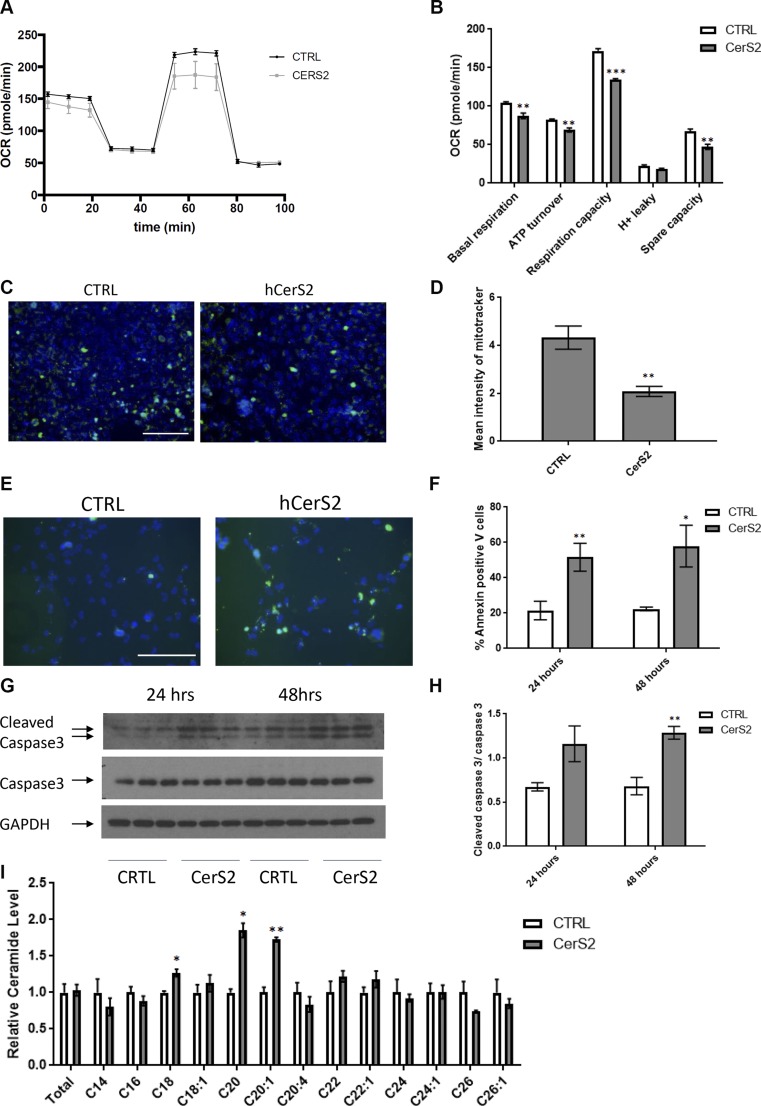
In human HF there is a loss of mitochondrial ATP production, although whether this results from defects in mitochondria per se remains controversial. Therefore, to test whether CerS2 may compromise mitochondrial function, we assessed mitochondrial respiration in cells overexpressing CerS2. Indeed, these cells showed loss of mitochondrial integrity relative to control cells, as evidenced by their diminished mitochondrial basal respiration, ATP turnover, respiration capacity, membrane proton leakiness, and spare capacity (Fig. 1A, B). Overexpression of CerS2 also decreased mitochondrial membrane potential relative to controls (Fig. 1C, D). Furthermore, increased membrane exposure of phosphatidylserine was detected in cells overexpressing CerS2 (Fig. 1E, F). Similarly, these cells showed increased cleavage of caspase 3 (Fig. 1G, H). Although an overall increase of ceramide occurred after CerS2 overexpression, only LC and VLC ceramides were increased in the mitochondria (Fig. 1I). Together these data indicated enhanced apoptotic cell death in these cells compared with controls. In summary, these results suggest that CerS2 increased VLC ceramides, decreased mitochondrial performance, and increased apoptosis in cardiomyocytes.
Figure 1.
A, B) Overexpression of CerS2 results in cardiomyocyte mitochondrial dysfunction and apoptosis. AC16 cardiomyocytes ovexpressing CerS2 exhibited reduced basal oxygen consumption, ATP turnover, and respiration capacity. C, D) Decreased mitochondrial membrane potential was detected with MitoTracker green (mitochondria) and DAPI (nuclei) in cells with overexpressed CerS2. E, F) Cardiomyocytes overexpressing CerS2 showed increased Annexin V detection after 24 and 48 h transfection: Annexin V, green; DAPI, blue (E) and quantification (F). G, H) Cells overexpressing CerS2 showed increased cleavage of caspase 3 after 24 and 48 h. I) An increase in LC ceramides in the mitochondrial enriched fraction was observed in cells overexpressing CerS2 for 48 h. Student’s t test (n = 3–5). Scale bars, 40 µm. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control (ctrl).
Cardiomyocyte insulin resistance is caused by overexpression of ceramide synthase 2
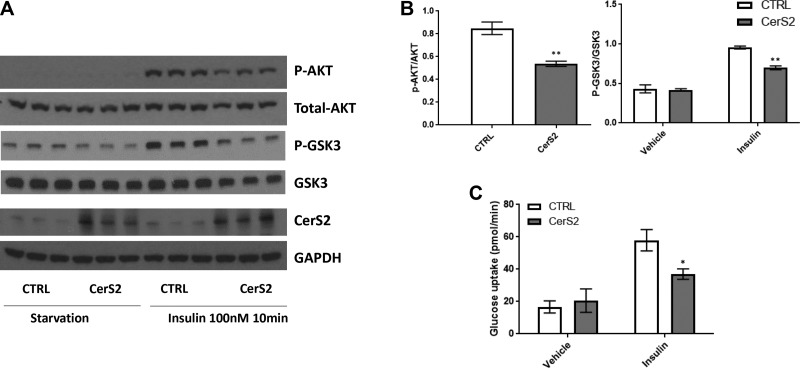
It is well recognized that insulin sensitivity is impaired in HF (13, 24–26). Moreover, insulin resistance occurs after myocardial infarction and may predict poor outcomes. Ceramides promote insulin resistance in many organs, and thus it is possible that CerS2-derived ceramides contribute to insulin resistance after myocardial infarction. Human CerS2 overexpression in cardiomyocytes decreased insulin-dependent phosphorylation of AKT and GSK3 compared with controls (Fig. 2A, B). Moreover, although basal glucose uptake was not affected by CerS2 overexpression, insulin-dependent glucose uptake was decreased compared with empty vector–treated cardiomyocytes (Fig. 2C). Taken together, these results suggest a role for CerS2 in driving insulin resistance in cardiomyocytes.
Figure 2.
CerS2 overexpression induces increased insulin resistance in AC16 cardiomyocytes. A, B) CerS2 overexpression caused decreased insulin-induced phosphorylation of AKT compared with vehicle plasmid controls. C) Although CerS2 overexpression had no effect on basal glucose uptake, it disrupted insulin-dependent glucose uptake. Student’s t test (n = 3–6). *P < 0.05, **P < 0.01 compared with control (ctrl).
Lipid overload induces sphingolipid-dependent oxidative stress
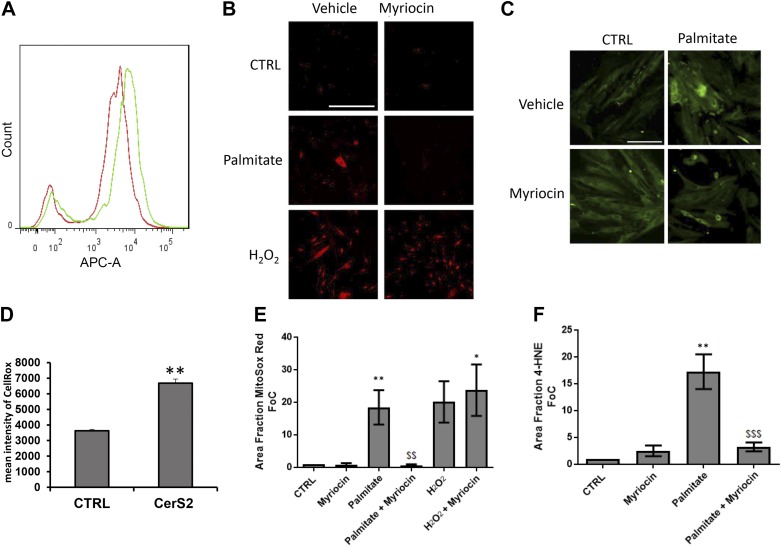
Over the past several years, laboratory and clinical studies have demonstrated that elevations in the production of reactive oxygen species (ROS), or oxidative stress, occur in HF and contribute to disease progression (27–33). In HF there is an acute increase in circulating levels of fatty acids due to insulin resistance and increased peripheral lipolysis (34), which oversupplies cardiomyocytes with substrate for mitochondrial oxidation and increases ROS. The mitochondrial dysfunction observed with overexpression of CerS2 suggested that ceramide may link excess fatty acids to an increase in ROS. Indeed, overexpression of CerS2 alone increased ROS compared with controls, which was exacerbated by providing excess mitochondrial substrate (i.e., palmitate) to cells (Fig. 3A, B). Similarly, palmitate treatment elevated superoxide in cardiomyocytes, and this was prevented by inhibition of sphingolipid synthesis (Fig. 3C). On the other hand, inhibition of sphingolipid synthesis had no effect on ROS induced by treatment with H2O2, placing ceramide upstream from oxidative stress (Fig. 3D). Palmitate treatment increased lipid peroxidation as evidenced by increased 4-hydroxynonenal treatment, and this is also sphingolipid dependent (Fig. 3E, F). Together, these data demonstrate a specific role for sphingolipid synthesis in ROS production in response to increased lipid supply.
Figure 3.
Lipid overload induces sphingolipid-dependent oxidative stress in a manner similar to CerS2 overexpression. A, B) Elevated ROS was detected in AC16 cells overexpressing CerS2 compared with control cells by CellROX detection of superoxide. Superoxide formation (red) was induced by palmitate (16 h) in cardiomyocytes (H9c2) and was rescued by myriocin cotreatment. C, D) H2O2 served as a positive control for superoxide formation, and myriocin exhibited no protective effect in cardiomyocytes. Similarly, increased lipid peroxidation (detected by 4-hydroxynoneal formation; green) in cardiomyocytes (H9c2) was induced by palmitate (16 h). E, F) Coincubation with sphingolipid inhibitor myriocin rescued from this increase. Student’s t test and 1-way ANOVA. *P < 0.05, **P < 0.01 compared with control (ctrl), $$$P < 0.05 compared with palmitate (n = 3–7). Scale bars, 40 µm (A); 100 µm (B).
Ceramide synthase S2 overexpression and lipid overload induce autophagy
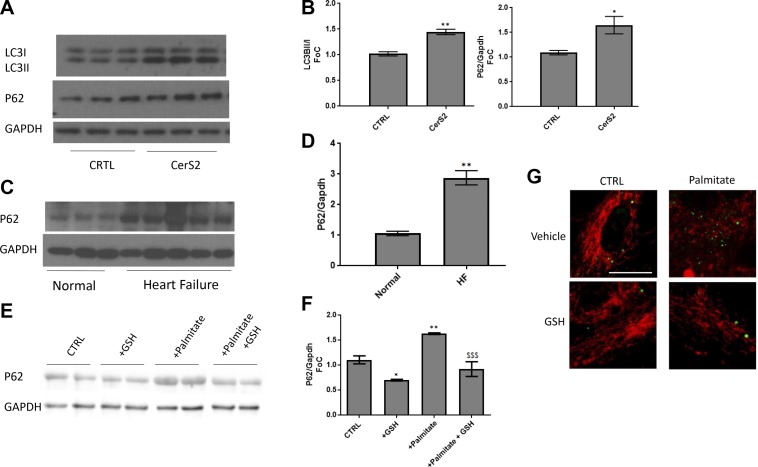
Autophagy is maintained at low levels in the normal functioning heart, and defects in this process have been demonstrated to cause cardiac dysfunction and eventual HF (35). Additionally, elevated autophagy has been observed in various cardiomyopathies (36–42). Because we have previously identified a role for CerS5-driven autophagy in cardiomyocytes in a model of diabetic cardiomyopathy (2, 43), we sought to investigate a potential role for CerS2 and palmitate overload in regulating cardiomyocyte autophagy. Overexpression of CerS2 increased both lipidation of LC3BI and levels of autophagic adaptor protein p62 (Fig. 4A, B), which also occurred in human HF (Fig. 4C, D). Moreover, palmitate-induced p62 was prevented by glutathione, as was positive LC3BII staining in palmitate-treated cells (Fig. 4E, G). Together, these data suggested that lipid oversupply was driving the observed autophagy through oxidative stress, thus implicating mitochondrial-directed autophagy (mitophagy) in this system.
Figure 4.
CerS2 overexpression and lipid overload induce autophaghy in cardiomyocytes. A, B) Increased autophagic flux (detected by ratio of lipidated to nonlipidated LC3B) and autophagy adaptor protein P62 occurred in CerS2 overexpressing AC16 cardiomyocytes. C, D) P62 was also elevated in human heart failure samples compared with normal control cells. E–G) Cardiomyocytes (H9c2) exposed to palmitate (16 h) exhibited increased autophagy protein P62 (E, F) and LC3-GFP labeled puncta (red = MitoTracker Red) (G). This increase was rescued by scavenging of free radicals by antioxidant glutathione. Student’s t test and 1-way ANOVA. *P < 0.05, **P < 0.01 compared with control (ctrl), $$$P < 0.05 compared with palmitate (n = 3–5). Scale bar, 40 µm.
Lipid overload results in increased sphingolipid-dependent cardiomyocyte mitophagy
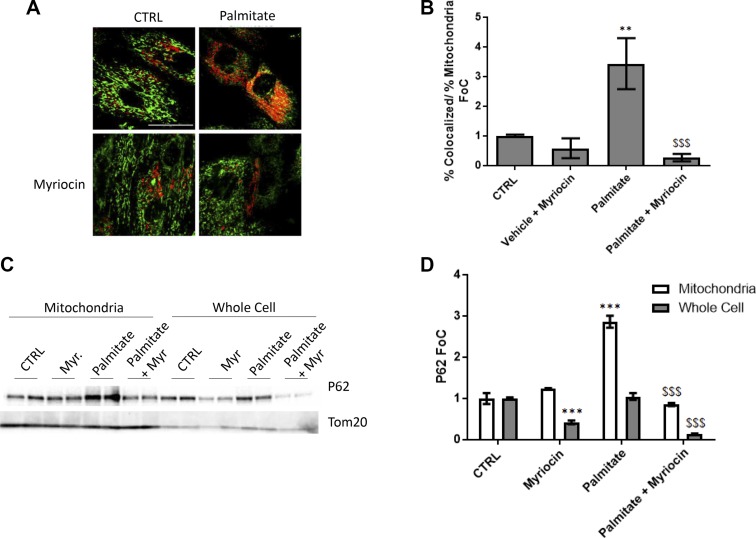
The observation of ceramide-induced cardiac mitochondrial dysfunction coupled with oxidative stress–dependent autophagy suggested that mitophagy may occur via CerS2. To test this, cells were treated with palmitate, which increased lysosomal acidification and colocalization of mitochondria with lysosomes in a sphingolipid-dependent manner (Fig. 5A, B). Palmitate also increased p62, a protein necessary for mitophagy, in total cell lysates and to a greater extent in the mitochondria-enriched fraction after treatment with palmitate (Fig. 5C, D). Cotreatment with myriocin prevented p62 accumulation in mitochondria and notably reduced p62 levels under both control and palmitate-treated conditions, suggesting a role of sphingolipids in constitutive as well as lipotoxic mitophagy.
Figure 5.
Lipid overload results in increased cardiomyocyte mitophagy, and CerS2 knockdown inhibits palmitate-induced mitophagy. A) Increased colocalization of mitochondria (MitoTracker Green) to lyosomes (LysoTracker Red) was observed in cardiomyocytes (H9c2) treated with palmitate (2 h). A, B) This increase was attenuated by inhibition of sphingolipid synthesis by myriocin. C, D) Increased localization of autophagy adaptor protein P62 to the mitochondrial-enriched fraction occurred in cardiomyocytes (H9c2) treated with palmitate (16 h; C). Myriocin blocked this increase (C, D). One-way ANOVA. **P < 0.01, ***P < 0.001 compared with control (ctrl), $$$P < 0.05 compared with palmitate (n = 3–7). Scale bar, 40 µm.
Ceramide synthase S2 overexpression increases mitophagy, whereas knockdown rescues from mitophagy-induced by lipid overload
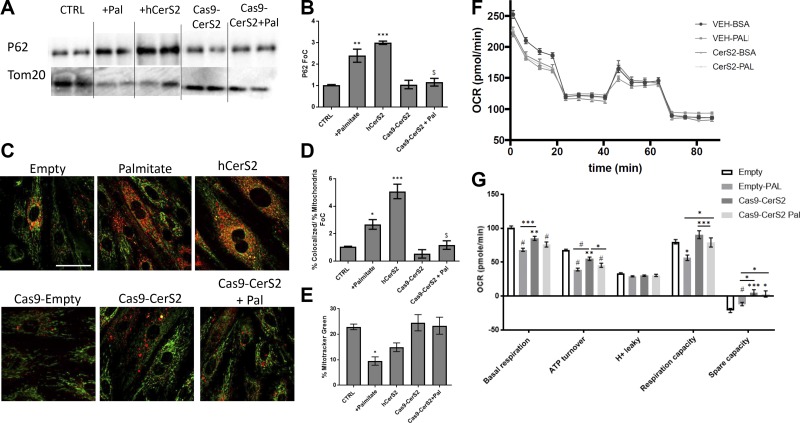
Because CerS2 overexpression damaged mitochondria, it seemed plausible that CerS2-mediated mitochondrial damage may lead to mitophagy. Similar to what was observed with palmitate treatment, overexpression of CerS2 increased p62 localization in the mitochondrial fraction, which was completely prevented by depletion of CerS2 function using the CRISPR/Cas9 system to remove CerS2 from the genome (Fig. 6A, B). Additionally, the same relationship was observed using a read-out of colocalization of lysosomes with mitochondria, further supporting a role for CerS2 in mitophagy (Fig. 6C, D). CerS2 knockdown rescued loss of mitochondrial membrane potential caused by palmitate (Fig. 6E). Knockdown of CerS2 also rescued cardiomyocytes from palmitate-induced loss of mitochondrial reserve capacity (Fig. 6F, G), parallel to the mitochondrial dysfunction observed with CerS2 overexpression (Fig. 1A, B).
Figure 6.
A, B) Overexpression of CerS2 resulted in increased P62 in the mitochondrial fraction of cardiomyocytes (H9c2) with and without addition of palmitate (16 h). In contrast, knockdown of CerS2 protected cardiomyocytes from mitophagy in response to palmitate treatment (16 h). C, D) Increased colocalization of mitochondria to lysosomes was observed with overexpression of CerS2. Knocking down CerS2 with Crispr-Cas9 technology resulted in protection from mitochondria and lysosome colocalization due to palmitate treatment (2 h). E) Significant loss of mitochondrial membrane potential (mitotracker green) occurred after palmitate treatment, whereas CerS2 knockdown prevented this. F, G) Palmitate (16 h) reduced basal oxygen consumption, ATP turnover, and respiration capacity, but knockdown of CerS2 rescued respiration capacity. One-way ANOVA. *P < 0.05 compared with compared with control (ctrl), $P < 0.05 compared with palmitate, #P < 0.0001 (n = 3–7). Scale bar, 40 µm.
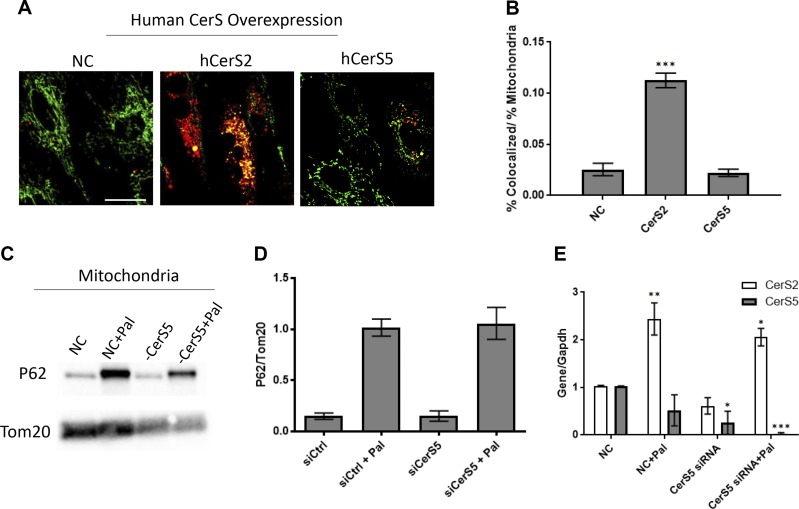
Because we previously reported that CerS5 mediated autophagy in cardiomyocytes, we speculated that in fact this may have been mitophagy because experimental approaches used in that study would not have distinguished between the two. Thus, it was also possible that ceramides in general could drive mitophagy. Therefore, we tested specificity of CerS2 vs. CerS5 in mitophagy. Surprisingly, in contrast to CerS2, overexpression of CerS5 did not increase mitochondria–lysosome colocalization (Fig. 7A, B), suggesting a distinct role for CerS2 in this process. Further supporting this, in contrast to CerS2, knockdown of CerS5 only very slightly attenuated accumulation of p62 in the mitochondrial fraction in response to palmitate treatment (Fig. 7C, D). In fact, knockdown of CerS5 may have exacerbated mitophagy, as indicated by reduction in TOM20 (Fig. 7C). Intriguingly, palmitate robustly increased message for CerS2 but not CerS5 (Fig. 7E). Together, these data suggest that CerS2 has a specific and distinct function as compared with CerS5. Specifically, the data demonstrate specificity of CerS2 and its products, the VLC ceramides, in mitophagy in the context of metabolic disruption in caridiomyocytes.
Figure 7.
Other cardiomyocyte CerS isoforms do not induce mitophagy or increase after palmitate treatment in H9c2 cardiomyocytes. A, B) Overexpression of human CerS2 increases mitophagy, but overexpression of CerS5 does not. C, D) siRNA knockdown of CerS5 exacerbates mitochondrial loss and does not prevent accumulation of P62 in the mitochondrial fraction. E) Palmitate increases CerS2 but not CerS5 message; knockdown of CerS5 followed by palmitate treatment also increases message of CerS2. One-way ANOVA. *P < 0.05 compared with control (ctrl), $P < 0.05 compared with palmitate (n = 3). Scale bar, 40 µm.
Inhibition of mitophagy exacerbates cell death caused by ceramide analogs
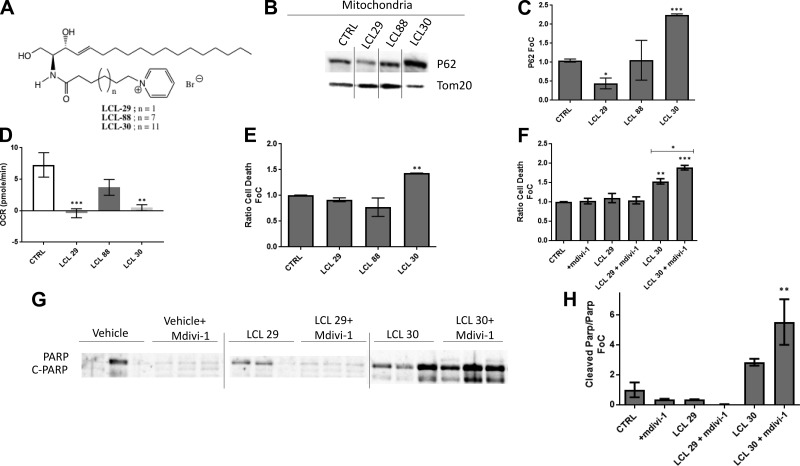
The observation that mitophagy depended on CerS2, which generates VLC ceramides, in contrast to CerS5, which generates LC ceramides, suggested that the N-acyl chain length of ceramide may be a major determinant of whether mitophagy occurs. Moreover, a potential direct role of ceramides in mitochondrial damage was suggested by lipidomics profiling of the mitochondrial fraction in cells overexpressing CerS2, which indicated enrichment of the CerS2-dependent species C20 and C20:1 in this organelle (Fig. 1I). Additionally, a recent report indicated that CerS2 localized to mitochondria in a rat model of type 1 diabetes (1). Therefore, to test the concept that mitochondrial ceramides directly lead to mitophagy in a manner depending on N-acyl chain length, we treated cells with soluble, mitochondria-targeted ceramide analogs (8) with varied chain lengths to identify whether chain length, per se, is important for these processes (Fig. 8A). Consistent with a role for ceramide chain length in mitophagy, increased N-acyl chain length in ceramide analogs resulted in increased p62 in the mitochondrial fraction (Fig. 8B, C). Similar to the apoptotic effects of overexpression of CerS2 in cardiomyocytes, these analogs also increased cell death in a chain length–dependent manner (Fig. 8E). Mitophagy has been reported to promote cell death in some contexts (44) and to protect from cell death in other contexts. To test whether ceramide-dependent mitophagy was deleterious or protective, mitophagy was blocked by inhibiting mitochondrial division in the presence of the VLC ceramide analog LCL30. Although LCL30 increased cell death, inhibition of mitophagy exacerbated this (Fig. 8F). Moreover, LCL30 increased PARP cleavage as compared with vehicle controls, and this was exacerbated by inhibition of mitophagy (Fig. 8G, H). Interestingly, both LCL29 and LCL30 resulted in decreased mitochondrial respiration capacity (Fig. 8D). Taken together, these results suggest that in cardiomyocytes, mitophagy is initiated as a protective process that results in cell death in response to elevations of ceramide in mitochondria.
Figure 8.
Inhibition of mitophagy exacerbates apoptosis induced by a VLC mitochondrially targeted ceramide analog in H9c2 cardiomyocytes. A) Positively charged pyridinium ceramides were used of medium (LCL29), long (LCL88), and very long (LCL30) acyl-chain lengths. B, C) LCL30 increased localization of P62 to the mitochondrially enriched fraction. D) Mitochondrial reserve capacity was significantly decreased after LCL29 and LCL30 treatment. E) Only LCL30 reduced cardiomyocyte viability. G) PARP cleavage was induced by addition of LCL30. F–H) Increased PARP cleavage occurred after treatment with LCL-30 and drp-1 mitochondrial division inhibitor mdivi-1, which resulted in greater cardiomyocyte (H9c2) apoptosis. One-way ANOVA. *P < 0.05 compared with control (ctrl) (n = 3).
DISCUSSION
Previous work has shown ceramides to be increased in the myocardium in various models of cardiomyopathy (1, 2, 12, 14, 45, 46), and although a role for ceramide in general in mediating cardiac dysfunction is strongly supported, specific metabolic pathways and lipid species that lead to cardiac dysfunction have not been widely investigated. Additionally, most of these studies, including ours, specifically addressed roles for ceramide in regulation of cardiomyocyte cell functions in the specific context of lipotoxicity (2), and whether sphingolipid metabolism may contribute to cardiac pathology in diverse etiological contexts has not been widely addressed.
The present study addressed this by first testing whether ceramides change in the failing heart and then identifying specific ceramide species that may change. This allowed deduction of the specific CerS enzymes involved. Specifically, VLC ceramides increased in human heart failure, providing a rationale for investigating CerS2, which produces these species (47). Indeed, CerS2-specific species were increased in the myocardium of HFD-fed mice, and overexpression of CerS2 in cultured cells also increased CerS2-derived species (Tables 1–3). Moreover, depletion of CerS2 prevented pathologic outcomes in response to lipid oversupply, which mimics the acute increase in lipid uptake and utilization in the failing heart.
In addition to the VLC ceramides, C16:0 ceramide, a product of CerS5, increased in human HF samples and in cells overexpressing CerS2. In fact, several reports indicate that CerS5 and CerS2 directly interact and that these interactions may augment catalytic activity (2, 3, 48). Moreover, these enzymes and their respective ceramide products have been identified in mitochondria (1, 4, 49). Our previous studies demonstrated that loss of function of CerS5 led to cell hypertrophy (2), which we did not observe in CerS2 knockdown in this study (data not shown), suggesting that each of these enzymes directly regulates distinct cell processes.
Overexpression of CerS2, but not CerS5, drove mitophagy, strongly supporting that CerS2 mediates mitophagy in a manner not limited by endogenous levels of CerS5. Similarly, knockdown of CerS2, but not CerS5, prevented palmitate-induced mitophagy, further supporting that CerS2 is required for mitophagy. Based on these data, CerS2 is necessary and sufficient to drive mitophagy, and this is independent of CerS5. Measurements of mitochondrial P62 accumulation and colocalization of mitochondria with lysosomes are indicative of mitophagy initiation but not necessarily of mitochondrial loss. However, cardiomyocytes supplemented with palmitate showed decreased expression of mitochondrial marker Tom20 (Fig. 6A) and decreased mitochondrial membrane potential (Fig. 6E), which was prevented by knockdown of CerS2. These results, along with increased apoptosis observed with VLC, led us to conclude that CerS2-dependent mitophagy is being initiated and mitochondria lost.
CerS2 generates ceramides ranging in N-acyl chain length from C20 to C24. Many of these ceramides were increased in whole cell lysates. However, in contrast to whole cell lysates, only C20 and C20:1 were enriched in the mitochondrial fraction, and there was no increase of C24 or 24:1 ceramide. This finding suggests some compartmentalization of specific species, a concept that is increasingly appreciated.
An increase in CerS2 and its ceramides was linked with mitochondrial dysfunction in human cardiomyocytes (Fig. 1A–D). There have been a multitude of effects reported for ceramide in mitochondria, including alteration of calcium homeostasis, ATP depletion, increased generation of ROS, inhibition and/or enhancement of different complexes in the electron transport chain, and release of proteins from the intermembrane space (50–61). In many experimental models, increased ceramide in the mitochondria has been associated with apoptosis (60, 62, 63), and this was also observed in isolated cardiomyoctyes in response to overexpression of CerS2 (Fig. 1G–J). Furthermore, in the context of lipid treatment, depletion of CerS2 prevented mitophagy, a known response to mitochondrial damage.
In this study, mitophagy occurred in a manner dependent on the N-acyl chain length of the soluble, mitochondrial-targeted analogs used for treatment, with only the longer-chain species mediating mitophagy. This is consistent with a role for CerS2-derived products in mitophagy (Fig. 8A–C). It is possible that localization of CerS2-derived ceramides to mitochondria results in mitochondrial dysfunction, activation of mitophagy, and eventual apoptosis. Interestingly, pharmacological inhibition of mitophagy in the presence of these agents increased apoptosis (Fig. 8E–G). Thus, although some evidence supports a deleterious role for mitophagy in inducing cell death (44), these data suggest that the mitophagy observed here is similar to what others have described (i.e., a process that protects the cell by clearing the damaged mitochondria and reducing ROS) (64). Thus, the role of mitophagy likely depends on cell type and experimental context. Intriguingly, others have demonstrated lysosomal dysfunction and subsequent inhibition of autophagy in cardiomyocytes in the presence of palmitate through the diacylglycerol/protein kinase C pathway (65). In contrast, our results demonstrate that blocking CerS activity in the presence of palmitate is sufficient to prevent mitophagy. These findings are consistent with specificity for bioactive lipid pathways in mediating distinct functions. Further studies are required to determine how each of these pathways is regulated in the context of lipid treatment.
Ceramides have been shown in other cell models to promote insulin resistance, particularly by attenuating AKT signaling (66, 67). In parallel, reduced AKT and GSK3 phosphorylation was observed in cells overexpressing CerS2 (Fig. 2A, B). Although no direct link was made between mitochondrial damage and cardiomyocyte insulin resistance in this study, other researchers have suggested that insulin resistance is downstream of mitochondrial dysfunction in models of diabetic cardiomyopathy (68, 69).
Recent studies demonstrated an inverse relationship in hepatocytes between CerS2 expression and expression of CerS6, which produces LC ceramides (48, 70, 71). Our data demonstrate a role for CerS2 in insulin desensitization in cardiomyocytes, suggesting that CerS differentially regulate insulin signaling in distinct tissues. Moreover, these findings underscore the complexity of ceramide regulation and function and provide a strong rationale for further study to identify the most suitable therapeutic targets for specific disease contexts.
These studies demonstrated an increase in CerS2-derived ceramides in human heart failure of unknown (and likely diverse) etiology, a mouse model of diabetic cardiomyopathy, and in cell culture models of lipotoxic cardiomyopathy (i.e., palmitate treatment). The role of CerS2 in these diverse experimental systems suggests that CerS2-dependent mitochondrial damage may serve as a common theme in cardiac pathology, specifically where mitochondrial dysfunction, insulin resistance, oxidative stress, increased autophagy and mitophagy, and ultimately cell death occur. Our data support a model in which CerS2-derived ceramides underlie mitochondrial dysfunction that ultimately leads to protective mitophagy. Therefore, CerS2 may serve as a therapeutic target in human HF.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health, National Heart, Lung, and Blood Institute (Grant 1R01HL117233), the U.S. Department of Veteran’s Affairs, and the American Heart Association. The authors declare no conflicts of interest.
Glossary
- AA
antimycin A
- AKT
protein kinase B
- BSA
bovine serum albumin
- CerS
ceramide synthase
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- GSK3
glycogen synthase kinase 3
- HF
heart failure
- HFD
high-saturated-fat diet
- LC
long chain
- LCL30
d-erythro-2-N-[16′-(1″-pyridinium)-hexadecanoyl]-sphingosine bromide
- oligo
oligomycin
- PARP
poly(ADP-ribose) polymerase
- ROS
reactive oxygen species
- TBS-T
Tris-buffered saline with 0.1% Tween 20
- VLC
very long chain
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
B. A. Law designed the research, conducted the research, generated the figures, and wrote the paper; X. Liao designed and conducted the research; K. S. Moore conducted the research; A. Southard conducted the research; P. Roddy conducted the research; R. Ji conducted the research; Z. Szulc contributed new reagents and advisement; A. Bielawska contributed new reagents and advisement; P. C. Schulze designed the research and wrote the paper; and L. A. Cowart designed the research and wrote the paper.
REFERENCES
- 1.Park T. S., Hu Y., Noh H. L., Drosatos K., Okajima K., Buchanan J., Tuinei J., Homma S., Jiang X. C., Abel E. D., Goldberg I. J. (2008) Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 49, 2101–2112https://doi.org/10.1194/jlr.M800147-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo S. B., Baicu C. F., Van Laer A., Geng T., Kasiganesan H., Zile M. R., Cowart L. A. (2012) Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J. Clin. Invest. 122, 3919–3930https://doi.org/10.1172/JCI63888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartmann D., Lucks J., Fuchs S., Schiffmann S., Schreiber Y., Ferreirós N., Merkens J., Marschalek R., Geisslinger G., Grösch S. (2012) Long chain ceramides and very long chain ceramides have opposite effects on human breast and colon cancer cell growth. Int. J. Biochem. Cell Biol. 44, 620–628https://doi.org/10.1016/j.biocel.2011.12.019 [DOI] [PubMed] [Google Scholar]
- 4.Senkal C. E., Ponnusamy S., Bielawski J., Hannun Y. A., Ogretmen B. (2010) Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 24, 296–308https://doi.org/10.1096/fj.09-135087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson M. M., Nesti C., Palenzuela L., Walker W. F., Hernandez E., Protas L., Hirano M., Isaac N. D. (2005) Novel cell lines derived from adult human ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 39, 133–147https://doi.org/10.1016/j.yjmcc.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 6.Bielawski J., Szulc Z. M., Hannun Y. A., Bielawska A. (2006) Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods 39, 82–91https://doi.org/10.1016/j.ymeth.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 7.Kim H. J., Qiao Q., Toop H. D., Morris J. C., Don A. S. (2012) A fluorescent assay for ceramide synthase activity. J. Lipid Res. 53, 1701–1707https://doi.org/10.1194/jlr.D025627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szulc Z. M., Mayroo N., Bai A., Bielawski J., Liu X., Norris J. S., Hannun Y. A., Bielawska A. (2008) Novel analogs of D-e-MAPP and B13. Part 1: synthesis and evaluation as potential anticancer agents. Bioorg. Med. Chem. 16, 1015–1031https://doi.org/10.1016/j.bmc.2007.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szulc Z. M., Bielawski J., Gracz H., Gustilo M., Mayroo N., Hannun Y. A., Obeid L. M., Bielawska A. (2006) Tailoring structure-function and targeting properties of ceramides by site-specific cationization. Bioorg. Med. Chem. 14, 7083–7104https://doi.org/10.1016/j.bmc.2006.07.016 [DOI] [PubMed] [Google Scholar]
- 10.Basu R., Oudit G. Y., Wang X., Zhang L., Ussher J. R., Lopaschuk G. D., Kassiri Z. (2009) Type 1 diabetic cardiomyopathy in the Akita (Ins2WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. Am. J. Physiol. Heart Circ. Physiol. 297, H2096–H2108https://doi.org/10.1152/ajpheart.00452.2009 [DOI] [PubMed] [Google Scholar]
- 11.Chiu H. C., Kovacs A., Blanton R. M., Han X., Courtois M., Weinheimer C. J., Yamada K. A., Brunet S., Xu H., Nerbonne J. M., Welch M. J., Fettig N. M., Sharp T. L., Sambandam N., Olson K. M., Ory D. S., Schaffer J. E. (2005) Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ. Res. 96, 225–233https://doi.org/10.1161/01.RES.0000154079.20681.B9 [DOI] [PubMed] [Google Scholar]
- 12.Chiu H. C., Kovacs A., Ford D. A., Hsu F. F., Garcia R., Herrero P., Saffitz J. E., Schaffer J. E. (2001) A novel mouse model of lipotoxic cardiomyopathy. J. Clin. Invest. 107, 813–822https://doi.org/10.1172/JCI10947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chokshi A., Drosatos K., Cheema F. H., Ji R., Khawaja T., Yu S., Kato T., Khan R., Takayama H., Knöll R., Milting H., Chung C. S., Jorde U., Naka Y., Mancini D. M., Goldberg I. J., Schulze P. C. (2012) Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation 125, 2844–2853https://doi.org/10.1161/CIRCULATIONAHA.111.060889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Son N. H., Park T. S., Yamashita H., Yokoyama M., Huggins L. A., Okajima K., Homma S., Szabolcs M. J., Huang L. S., Goldberg I. J. (2007) Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J. Clin. Invest. 117, 2791–2801https://doi.org/10.1172/JCI30335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yagyu H., Chen G., Yokoyama M., Hirata K., Augustus A., Kako Y., Seo T., Hu Y., Lutz E. P., Merkel M., Bensadoun A., Homma S., Goldberg I. J. (2003) Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J. Clin. Invest. 111, 419–426https://doi.org/10.1172/JCI16751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L., Shi X., Bharadwaj K. G., Ikeda S., Yamashita H., Yagyu H., Schaffer J. E., Yu Y. H., Goldberg I. J. (2009) DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J. Biol. Chem. 284, 36312–36323https://doi.org/10.1074/jbc.M109.049817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y. T., Grayburn P., Karim A., Shimabukuro M., Higa M., Baetens D., Orci L., Unger R. H. (2000) Lipotoxic heart disease in obese rats: implications for human obesity. Proc. Natl. Acad. Sci. USA 97, 1784–1789https://doi.org/10.1073/pnas.97.4.1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo T. H., Moore K. H., Giacomelli F., Wiener J. (1983) Defective oxidative metabolism of heart mitochondria from genetically diabetic mice. Diabetes 32, 781–787https://doi.org/10.2337/diab.32.9.781 [DOI] [PubMed] [Google Scholar]
- 19.Lesnefsky E. J., Moghaddas S., Tandler B., Kerner J., Hoppel C. L. (2001) Mitochondrial dysfunction in cardiac disease: ischemia--reperfusion, aging, and heart failure. J. Mol. Cell. Cardiol. 33, 1065–1089https://doi.org/10.1006/jmcc.2001.1378 [DOI] [PubMed] [Google Scholar]
- 20.Narula J., Kolodgie F. D., Virmani R. (2000) Apoptosis and cardiomyopathy. Curr. Opin. Cardiol. 15, 183–188https://doi.org/10.1097/00001573-200005000-00011 [DOI] [PubMed] [Google Scholar]
- 21.Russell L. K., Finck B. N., Kelly D. P. (2005) Mouse models of mitochondrial dysfunction and heart failure. J. Mol. Cell. Cardiol. 38, 81–91https://doi.org/10.1016/j.yjmcc.2004.10.010 [DOI] [PubMed] [Google Scholar]
- 22.Shen X., Zheng S., Thongboonkerd V., Xu M., Pierce W. M. Jr., Klein J. B., Epstein P. N. (2004) Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. Am. J. Physiol. Endocrinol. Metab. 287, E896–E905https://doi.org/10.1152/ajpendo.00047.2004 [DOI] [PubMed] [Google Scholar]
- 23.Yaoita H., Maruyama Y. (2008) Intervention for apoptosis in cardiomyopathy. Heart Fail. Rev. 13, 181–191https://doi.org/10.1007/s10741-007-9074-6 [DOI] [PubMed] [Google Scholar]
- 24.Banerjee D., Biggs M. L., Mercer L., Mukamal K., Kaplan R., Barzilay J., Kuller L., Kizer J. R., Djousse L., Tracy R., Zieman S., Lloyd-Jones D., Siscovick D., Carnethon M. (2013) Insulin resistance and risk of incident heart failure: cardiovascular health study. Circ. Heart Fail. 6, 364–370https://doi.org/10.1161/CIRCHEARTFAILURE.112.000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coughlin S. S., Tefft M. C. (1994) The epidemiology of idiopathic dilated cardiomyopathy in women: the Washington DC dilated cardiomyopathy study. Epidemiology 5, 449–455https://doi.org/10.1097/00001648-199407000-00012 [DOI] [PubMed] [Google Scholar]
- 26.Witteles R. M., Tang W. H., Jamali A. H., Chu J. W., Reaven G. M., Fowler M. B. (2004) Insulin resistance in idiopathic dilated cardiomyopathy: a possible etiologic link. J. Am. Coll. Cardiol. 44, 78–81https://doi.org/10.1016/j.jacc.2004.03.037 [DOI] [PubMed] [Google Scholar]
- 27.Belch J. J., Bridges A. B., Scott N., Chopra M. (1991) Oxygen free radicals and congestive heart failure. Br. Heart J. 65, 245–248https://doi.org/10.1136/hrt.65.5.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campolo J., Caruso R., De Maria R., Parolini M., Oliva F., Roubina E., Cighetti G., Frigerio M., Vitali E., Parodi O. (2007) Aminothiol redox alterations in patients with chronic heart failure of ischaemic or non-ischaemic origin. J. Cardiovasc. Med. (Hagerstown) 8, 1024–1028https://doi.org/10.2459/JCM.0b013e3281053a63 [DOI] [PubMed] [Google Scholar]
- 29.Canton M., Menazza S., Sheeran F. L., Polverino de Laureto P., Di Lisa F., Pepe S. (2011) Oxidation of myofibrillar proteins in human heart failure. J. Am. Coll. Cardiol. 57, 300–309https://doi.org/10.1016/j.jacc.2010.06.058 [DOI] [PubMed] [Google Scholar]
- 30.Hill M. F., Singal P. K. (1997) Right and left myocardial antioxidant responses during heart failure subsequent to myocardial infarction. Circulation 96, 2414–2420https://doi.org/10.1161/01.CIR.96.7.2414 [DOI] [PubMed] [Google Scholar]
- 31.Hill M. F., Singal P. K. (1996) Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am. J. Pathol. 148, 291–300 [PMC free article] [PubMed] [Google Scholar]
- 32.Mallat Z., Philip I., Lebret M., Chatel D., Maclouf J., Tedgui A. (1998) Elevated levels of 8-iso-prostaglandin F2alpha in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation 97, 1536–1539https://doi.org/10.1161/01.CIR.97.16.1536 [DOI] [PubMed] [Google Scholar]
- 33.Watanabe E., Matsuda N., Shiga T., Kajimoto K., Ajiro Y., Kawarai H., Kasanuki H., Kawana M. (2006) Significance of 8-hydroxy-2′-deoxyguanosine levels in patients with idiopathic dilated cardiomyopathy. J. Card. Fail. 12, 527–532https://doi.org/10.1016/j.cardfail.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 34.Ashrafian H., Frenneaux M. P., Opie L. H. (2007) Metabolic mechanisms in heart failure. Circulation 116, 434–448https://doi.org/10.1161/CIRCULATIONAHA.107.702795 [DOI] [PubMed] [Google Scholar]
- 35.Nakai A., Yamaguchi O., Takeda T., Higuchi Y., Hikoso S., Taniike M., Omiya S., Mizote I., Matsumura Y., Asahi M., Nishida K., Hori M., Mizushima N., Otsu K. (2007) The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat. Med. 13, 619–624https://doi.org/10.1038/nm1574 [DOI] [PubMed] [Google Scholar]
- 36.Knaapen M. W., Davies M. J., De Bie M., Haven A. J., Martinet W., Kockx M. M. (2001) Apoptotic versus autophagic cell death in heart failure. Cardiovasc. Res. 51, 304–312https://doi.org/10.1016/S0008-6363(01)00290-5 [DOI] [PubMed] [Google Scholar]
- 37.Kostin S., Pool L., Elsässer A., Hein S., Drexler H. C., Arnon E., Hayakawa Y., Zimmermann R., Bauer E., Klövekorn W. P., Schaper J. (2003) Myocytes die by multiple mechanisms in failing human hearts. Circ. Res. 92, 715–724https://doi.org/10.1161/01.RES.0000067471.95890.5C [DOI] [PubMed] [Google Scholar]
- 38.Miyata S., Takemura G., Kawase Y., Li Y., Okada H., Maruyama R., Ushikoshi H., Esaki M., Kanamori H., Li L., Misao Y., Tezuka A., Toyo-Oka T., Minatoguchi S., Fujiwara T., Fujiwara H. (2006) Autophagic cardiomyocyte death in cardiomyopathic hamsters and its prevention by granulocyte colony-stimulating factor. Am. J. Pathol. 168, 386–397https://doi.org/10.2353/ajpath.2006.050137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishino I., Fu J., Tanji K., Yamada T., Shimojo S., Koori T., Mora M., Riggs J. E., Oh S. J., Koga Y., Sue C. M., Yamamoto A., Murakami N., Shanske S., Byrne E., Bonilla E., Nonaka I., DiMauro S., Hirano M. (2000) Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease). Nature 406, 906–910https://doi.org/10.1038/35022604 [DOI] [PubMed] [Google Scholar]
- 40.Shimomura H., Terasaki F., Hayashi T., Kitaura Y., Isomura T., Suma H. (2001) Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Jpn. Circ. J. 65, 965–968https://doi.org/10.1253/jcj.65.965 [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto S., Sawada K., Shimomura H., Kawamura K., James T. N. (2000) On the nature of cell death during remodeling of hypertrophied human myocardium. J. Mol. Cell. Cardiol. 32, 161–175https://doi.org/10.1006/jmcc.1999.1064 [DOI] [PubMed] [Google Scholar]
- 42.Zhu H., Tannous P., Johnstone J. L., Kong Y., Shelton J. M., Richardson J. A., Le V., Levine B., Rothermel B. A., Hill J. A. (2007) Cardiac autophagy is a maladaptive response to hemodynamic stress. J. Clin. Invest. 117, 1782–1793https://doi.org/10.1172/JCI27523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russo S. B., Tidhar R., Futerman A. H., Cowart L. A. (2013) Myristate-derived d16:0 sphingolipids constitute a cardiac sphingolipid pool with distinct synthetic routes and functional properties. J. Biol. Chem. 288, 13397–13409https://doi.org/10.1074/jbc.M112.428185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sentelle R. D., Senkal C. E., Jiang W., Ponnusamy S., Gencer S., Selvam S. P., Ramshesh V. K., Peterson Y. K., Lemasters J. J., Szulc Z. M., Bielawski J., Ogretmen B. (2012) Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat. Chem. Biol. 8, 831–838https://doi.org/10.1038/nchembio.1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holland W. L., Brozinick J. T., Wang L. P., Hawkins E. D., Sargent K. M., Liu Y., Narra K., Hoehn K. L., Knotts T. A., Siesky A., Nelson D. H., Karathanasis S. K., Fontenot G. K., Birnbaum M. J., Summers S. A. (2007) Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5, 167–179https://doi.org/10.1016/j.cmet.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 46.Shimabukuro M., Zhou Y. T., Levi M., Unger R. H. (1998) Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc. Natl. Acad. Sci. USA 95, 2498–2502https://doi.org/10.1073/pnas.95.5.2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levy M., Futerman A. H. (2010) Mammalian ceramide synthases. IUBMB Life 62, 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laviad E. L., Kelly S., Merrill A. H. Jr., Futerman A. H. (2012) Modulation of ceramide synthase activity via dimerization. J. Biol. Chem. 287, 21025–21033https://doi.org/10.1074/jbc.M112.363580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Novgorodov S. A., Riley C. L., Yu J., Keffler J. A., Clarke C. J., Van Laer A. O., Baicu C. F., Zile M. R., Gudz T. I. (2016) Lactosylceramide contributes to mitochondrial dysfunction in diabetes. J. Lipid Res. 57, 546–562https://doi.org/10.1194/jlr.M060061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arora A. S., Jones B. J., Patel T. C., Bronk S. F., Gores G. J. (1997) Ceramide induces hepatocyte cell death through disruption of mitochondrial function in the rat. Hepatology 25, 958–963https://doi.org/10.1002/hep.510250428 [DOI] [PubMed] [Google Scholar]
- 51.Di Paola M., Zaccagnino P., Montedoro G., Cocco T., Lorusso M. (2004) Ceramide induces release of pro-apoptotic proteins from mitochondria by either a Ca2+-dependent or a Ca2+-independent mechanism. J. Bioenerg. Biomembr. 36, 165–170https://doi.org/10.1023/B:JOBB.0000023619.97392.0c [DOI] [PubMed] [Google Scholar]
- 52.Di Paola M., Cocco T., Lorusso M. (2000) Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry 39, 6660–6668https://doi.org/10.1021/bi9924415 [DOI] [PubMed] [Google Scholar]
- 53.France-Lanord V., Brugg B., Michel P. P., Agid Y., Ruberg M. (1997) Mitochondrial free radical signal in ceramide-dependent apoptosis: a putative mechanism for neuronal death in Parkinson’s disease. J. Neurochem. 69, 1612–1621https://doi.org/10.1046/j.1471-4159.1997.69041612.x [DOI] [PubMed] [Google Scholar]
- 54.García-Ruiz C., Colell A., Marí M., Morales A., Fernández-Checa J. C. (1997) Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J. Biol. Chem. 272, 11369–11377https://doi.org/10.1074/jbc.272.17.11369 [DOI] [PubMed] [Google Scholar]
- 55.Ghafourifar P., Klein S. D., Schucht O., Schenk U., Pruschy M., Rocha S., Richter C. (1999) Ceramide induces cytochrome c release from isolated mitochondria: importance of mitochondrial redox state. J. Biol. Chem. 274, 6080–6084https://doi.org/10.1074/jbc.274.10.6080 [DOI] [PubMed] [Google Scholar]
- 56.Gudz T. I., Tserng K. Y., Hoppel C. L. (1997) Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J. Biol. Chem. 272, 24154–24158https://doi.org/10.1074/jbc.272.39.24154 [DOI] [PubMed] [Google Scholar]
- 57.Muriel M. P., Lambeng N., Darios F., Michel P. P., Hirsch E. C., Agid Y., Ruberg M. (2000) Mitochondrial free calcium levels (Rhod-2 fluorescence) and ultrastructural alterations in neuronally differentiated PC12 cells during ceramide-dependent cell death. J. Comp. Neurol. 426, 297–315https://doi.org/10.1002/1096-9861(20001016)426:2%3c297::AID-CNE10%3e3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
- 58.Pinton P., Ferrari D., Rapizzi E., Di Virgilio F., Pozzan T., Rizzuto R. (2001) The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 20, 2690–2701https://doi.org/10.1093/emboj/20.11.2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quillet-Mary A., Jaffrézou J. P., Mansat V., Bordier C., Naval J., Laurent G. (1997) Implication of mitochondrial hydrogen peroxide generation in ceramide-induced apoptosis. J. Biol. Chem. 272, 21388–21395https://doi.org/10.1074/jbc.272.34.21388 [DOI] [PubMed] [Google Scholar]
- 60.Siskind L. J., Kolesnick R. N., Colombini M. (2002) Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J. Biol. Chem. 277, 26796–26803https://doi.org/10.1074/jbc.M200754200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zamzami N., Marchetti P., Castedo M., Decaudin D., Macho A., Hirsch T., Susin S. A., Petit P. X., Mignotte B., Kroemer G. (1995) Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J. Exp. Med. 182, 367–377https://doi.org/10.1084/jem.182.2.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deng X., Yin X., Allan R., Lu D. D., Maurer C. W., Haimovitz-Friedman A., Fuks Z., Shaham S., Kolesnick R. (2008) Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science 322, 110–115https://doi.org/10.1126/science.1158111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee H., Rotolo J. A., Mesicek J., Penate-Medina T., Rimner A., Liao W. C., Yin X., Ragupathi G., Ehleiter D., Gulbins E., Zhai D., Reed J. C., Haimovitz-Friedman A., Fuks Z., Kolesnick R. (2011) Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PLoS One 6, e19783 [Erratum] https://doi.org/10.1371/journal.pone.0019783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoshino A., Mita Y., Okawa Y., Ariyoshi M., Iwai-Kanai E., Ueyama T., Ikeda K., Ogata T., Matoba S. (2013) Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat. Commun. 4, 2308.https://doi.org/10.1038/ncomms3308 [DOI] [PubMed] [Google Scholar]
- 65.Jaishy B., Zhang Q., Chung H. S., Riehle C., Soto J., Jenkins S., Abel P., Cowart L. A., Van Eyk J. E., Abel E. D. (2015) Lipid-induced NOX2 activation inhibits autophagic flux by impairing lysosomal enzyme activity. J. Lipid Res. 56, 546–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dobrowsky R. T., Kamibayashi C., Mumby M. C., Hannun Y. A. (1993) Ceramide activates heterotrimeric protein phosphatase 2A. J. Biol. Chem. 268, 15523–15530 [PubMed] [Google Scholar]
- 67.Stratford S., DeWald D. B., Summers S. A. (2001) Ceramide dissociates 3′-phosphoinositide production from pleckstrin homology domain translocation. Biochem. J. 354, 359–368https://doi.org/10.1042/bj3540359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watanabe T., Saotome M., Nobuhara M., Sakamoto A., Urushida T., Katoh H., Satoh H., Funaki M., Hayashi H. (2014) Roles of mitochondrial fragmentation and reactive oxygen species in mitochondrial dysfunction and myocardial insulin resistance. Exp. Cell Res. 323, 314–325https://doi.org/10.1016/j.yexcr.2014.02.027 [DOI] [PubMed] [Google Scholar]
- 69.Westermeier F., Navarro-Marquez M., López-Crisosto C., Bravo-Sagua R., Quiroga C., Bustamante M., Verdejo H. E., Zalaquett R., Ibacache M., Parra V., Castro P. F., Rothermel B. A., Hill J. A., Lavandero S. (2015) Defective insulin signaling and mitochondrial dynamics in diabetic cardiomyopathy. Biochim. Biophys. Acta 1853, 1113–1118https://doi.org/10.1016/j.bbamcr.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mesicek J., Lee H., Feldman T., Jiang X., Skobeleva A., Berdyshev E. V., Haimovitz-Friedman A., Fuks Z., Kolesnick R. (2010) Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell. Signal. 22, 1300–1307https://doi.org/10.1016/j.cellsig.2010.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santana P., Peña L. A., Haimovitz-Friedman A., Martin S., Green D., McLoughlin M., Cordon-Cardo C., Schuchman E. H., Fuks Z., Kolesnick R. (1996) Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell 86, 189–199https://doi.org/10.1016/S0092-8674(00)80091-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.