Abstract
Alterations in Ca2+ homeostasis affect neuronal survival. However, the identity of Ca2+ channels and the mechanisms underlying neurotoxin-induced neuronal degeneration are not well understood. In this study, the dopaminergic neurotoxins 6-hydroxydopamine (6-OHDA) and 1-methyl-4-phenylpyridium ions (MPP+)/1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which mimic Parkinson’s disease (PD), induced neuronal degeneration by decreasing store-mediated Ca2+ entry. The function of the transient receptor potential canonical (TRPC)-1 channel was decreased upon exposure to the neurotoxins, followed by a decrease in TRPC1 expression. Similar to neurotoxins, samples from patients with PD exhibited attenuated TRPC1 expression, which was accompanied by a decrease in autophagic markers and a subsequent increase in apoptosis markers. Furthermore, exposure to neurotoxins attenuated PKC phosphorylation, decreased expression of autophagic markers, and increased apoptosis in SHSY-5Y neuroblastoma cells, which was again dependent on TRPC1. Prolonged neurotoxin treatment attenuated the binding of NF-κB to the TRPC1 promoter, which resulted in a decrease in TRPC1 expression, thereby attenuating autophagy and activating cell death. Restoration of TRPC1 expression rescued the effects of the dopaminergic neurotoxins in neuroblastoma cells by increasing Ca2+ entry, restoring NF-κB activity, and promoting autophagy. Overall, these results suggest that dopaminergic neurotoxins initially decreased Ca2+ entry, which inhibited the binding of NF-κB to the TRPC1 promoter, thereby inhibiting TRPC1 expression and resulting in cell death by preventing autophagy.—Sukumaran, P., Sun, Y., Antonson, N., Singh, B. B. Dopaminergic neurotoxins induce cell death by attenuating NF-κB–mediated regulation of TRPC1 expression and autophagy.
Keywords: calcium, SOCE, apoptosis, PD, calcium channels
Parkinson’s disease (PD) is the second most common neurodegenerative disorder (1, 2). Loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc) region underlies the motor symptoms observed in PD (3). Preventing the degeneration of DA neurons has been identified as a possible therapeutic mechanism to prevent or treat PD (4). In recent years changes in Ca2+ homeostasis have been suggested to play a key role in the degeneration of DA neurons (5). Changes in Ca2+ homeostasis, especially in the storage organelles such as mitochondria and the endoplasmic reticulum (ER), have been shown to affect neuronal survival and are closely associated with PD (6). Furthermore, Ca2+ is an important regulator of cell survival and death processes (7, 8) and could play an important role in modulating the neurodegeneration observed in PD.
6-Hydroxydopamine (6-OHDA) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) are potent neurotoxins that selectively destroy DA neurons in humans, subhuman primates, and lower animals, and they mimic PD-like symptoms (9, 10). MPTP is metabolized by the enzyme MAO-B to 1-methyl-4-phenyl-2, 3-dihydropyridium, which is deprotonated to generate the pyridium species, the 1-methyl-4-phenylpyridinium ion (MPP+) (11). Treatment of cells with MPP+ also causes mitochondrial dysfunction and disturbs Ca2+ homeostasis in the ER (5, 12). Similarly, 6-OHDA induces loss of DA neurons via reactive oxygen species (ROS) (13, 14); however, its relationship with Ca2+ is not well studied. MPP+ has been shown to activate the ROS-dependent cascade during dopaminergic cell death (5, 14). Evidence shows that ROS-induced dysfunction is often preceded by an alteration of intracellular (cytosolic) Ca2+ concentration ([Ca2+]i) (15), which could serve as an important second messenger to trigger apoptosis and cell death. In addition, Ca2+ entry has been shown to inhibit apoptosis by inducing autophagy in both neuronal and nonneuronal cells (7, 16, 17). When cells encounter stressful situations, they can either try to survive under these conditions via a very beneficial process called autophagy or experience cell death via apoptosis. Although autophagy and apoptosis are mechanistically different cellular processes, there are some common regulatory proteins, such as Bcl-2 and Bcl-xL, which, along with Ca2+ signaling, can intervene in both of these processes. One study has shown a positive role of Ca2+ in the induction of autophagy, suggesting that loss of cytosolic Ca2+ could inhibit autophagy and induce cell death (18).
Mitochondrial, ER, lysosomal, and cytosolic Ca2+ levels are regulated by Ca2+-permeable ion channels localized either on the membranes of the intracellular organelles or on the plasma membrane (19). The Ca2+-permeable channels, including families of transient receptor potential canonical (TRPC) channels, calcium release–activated calcium channel proteins (ORAIs), voltage-gated Ca2+ channels, 2-pore Ca2+ channels, mitochondrial Ca2+ uniporters, IP3, and ryanodine receptors have all been shown to contribute to changes in [Ca2+]i (19, 20). In addition, TRPC channels are involved in several Ca2+-dependent processes ranging from cell proliferation to contractility to apoptosis (20).
TRPC-1 is present in the plasma and activated upon ER store depletion, suggesting that it is the store-operated Ca2+ channel. Furthermore, we have shown that TRPC1 is essential for neuronal survival and that the neurotoxin MPP+ attenuates TRPC1 expression (6). However, the mechanism for this attenuation of TRPC1 expression is unknown. Herein, we report that neurotoxins have both short- and long-term effects on TRPC1 function and expression. The addition of neurotoxins initially decreases the TRPC1-mediated Ca2+ entry that reduces NF-κB activity. This further affects TRPC1 expression directly, thereby prolonging the effect of the neurotoxins. Restoration of TRPC1 channels rescues the effects of the neurotoxins by restoring Ca2+ entry and promoting autophagy. We have used mouse models, differentiated neuroblastoma cells, and samples from patients with PD to show that expression of TRPC1 is specifically decreased by neurotoxins that mimic PD. Overall, these results suggest that neurotoxin-induced cell degeneration via inhibition of NF-κB activity attenuates the expression of TRPC1 channels, leading to altered Ca2+ homeostasis, thereby inhibiting the autophagy that leads to apoptosis of DA neurons.
MATERIALS AND METHODS
Cell culture reagents and overexpression of TRPC1
SHSY-5Y neuroblastoma cells were cultured in the DMEM, F-12 medium along with various supplements (21). For rescue experiments, small hairpin RNA (shRNA) targeting the noncoding sequence of human TRPC1 was used, followed by expression of a TRPC1 plasmid lacking the noncoding region. For overexpression experiments, green fluorescent protein (GFP)-tagged TRPC1 or light chain (LC)-3 plasmids were used (5). Cells were transfected with individual small interfering (si)/shRNA (50 nM) and plasmids using Lipofectamine 2000 in Opti-MEM medium, as per the supplier’s instructions (Thermo Fisher Scientific, Waltham, MA, USA), and assayed after 48 h. All other reagents used were of molecular biology grade and obtained from Millipore-Sigma (Billerica, MA, USA). unless mentioned otherwise.
Cell viability assays
Cells were seeded in 96-well plates at a density of 0.5 × 105 cells/well. The cultures were grown for 24 h followed by addition of fresh medium before the experiment. Cell viability was measured by the MTT method. MTT reagent (20 µl of 5 mg/ml MTT in PBS) was added to each well and incubated in a CO2 incubator for 4 h. The resulting formazan dye was extracted with 100 µl of 0.01 N HCl in isopropanol, and the absorbance was measured on a microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 570 and 650 nm. A Vybrant Apoptosis Assay Kit (Thermo Fisher Scientific) was used to assess apoptosis as per the manufacturer’s instructions. The total number of apoptotic cells was counted by flow cytometry (using the LSR II; BD Biosciences, San Jose, CA, USA), and the percentages of cells exhibiting apoptosis were calculated (22).
Calcium measurement and electrophysiology
Cells were incubated with 2 μM Fura-2 (Thermo Fisher Scientific) for 45 min, washed twice with Ca2+ free standard external solution [SES; with 10 mM HEPES, 120 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, and 10 mM glucose (pH 7.4)] buffer. For fluorescence measurements, the fluorescence intensity of Fura-2-loaded control cells was monitored as previously described. The fluorescence traces shown represent [Ca2+]i values that are averages of at least 30–40 cells and representative of results obtained from at least 3–4 individual experiments. For electrophysiology, the cells were transferred to the recording chamber and perfused with an external Ringer’s solution composed of the following (in mM): NaCl, 145; KCl, 5; MgCl2, 1; CaCl2, 1; HEPES, 10; and glucose, 10 (pH 7.4) (NaOH). The patch pipette had resistances of between 3 and 5 mΩ after being filled with the standard intracellular solution containing the following (mM): cesium methanesulfonate, 145; NaCl, 8; MgCl2, 10; HEPES, 10; and EGTA, 10; pH 7.2 (CsOH). With a holding potential 0 mV, voltage ramps ranging from −80 to + 80 mV were delivered for 100-ms durations at 2-s intervals after the whole-cell configuration was formed. All electrophysiological experiments were filtered at 2 kHz, digitized at 10 kHz, acquired, and analyzed using pClamp 10 software (Molecular Devices).
Membrane preparations and Western blot analysis
Cells were harvested, and crude lysates were prepared (23). Protein concentrations were determined using the Bradford reagent (Bio-Rad, Hercules, CA, USA), and 25–50 μg proteins was resolved on 4–12% SDS-Bis-Tris gels, transferred to PVDF membranes, and probed with the respective antibodies. The proteins were detected with an Enhanced Chemiluminescent Detection (Pierce Super Signal West Pico; Thermo Fisher Scientific). All information concerning the antibodies used is provided in Supplemental Table 1. Densitometric analysis was performed by using ImageJ analysis, and results were corrected for protein loading by normalization to β-actin (Cell Signaling Technology, Danvers, MA, USA) expression.
NF-κB and caspase 3 activity
NF-κB activity was measured using the Abcam NF-κB p65 Transcription Factor Assay Kit (Abcam, Cambridge, United Kingdom). A nuclear extract taken from 1 million cells was used to analyze NF-κB activity according to the manufacturer’s instructions. Caspase 3 activity was measured using a Caspase 3 Assay Kit (Abcam). One million cells were lysed and used to analyze caspase 3 activity according to the manufacturer’s instructions. The sample absorbances were measured at 405 nm and plotted.
Chromatin immunoprecipitation and RT-PCR
Cells were crosslinked with formaldehyde for 10 min at room temperature, followed by addition of 125 mM glycine for 3–5 min at 4°C to quench the formaldehyde. Cells were washed with ice-cold PBS and lysed by sonication using previously determined optimal cycle conditions to generate 250- to 150-bp DNA fragments using a sonicator (Covaris, Woburn, MA, USA). Immunoprecipitation was performed with the appropriate antibodies, and IgG was used as a control. Precipitated DNA fragments were detected by PCR with specific primers. The sequences of the specific primers used for NF-κB binding sites in 1 NF-κB1 were: (sense) 5′-GATCTGCATCT-CTCACCTTATACA-3′ and (antisense) 5′-GCTCTTTCATTTAATCAAGTCCCA-3′, NF-κB2 (sense) 5′-CACAAATGG-CCAACAAACATATGAA-3′ and (antisense) 5′-CCATAGTGGTGTACTAGTTTACAGTC-3′; NF-κB3 (sense) 5′-AACAGTGTGGA-GATTCCTTAGAG-3′ and (antisense) 5′-TCTTTATCCACTCATTGATTCATGG-3′; and nonbinding NF-κB site (sense) 5′-GGCACCTAATGTAAGGAGGTATG-3′ and (antisense) 5′-TCAGCATCAAAGGAG-TCCAG-3′. Real-time PCR analysis of the respective immunoprecipitated DNA, as well as the TRPC1 and -3 transcripts, was performed with SYBR green (24).
Animal surgery
For MPTP treatment, animals were subdivided into 2 groups (n = 6–10), and the C57BL/6 mouse strain was used in the study. Group I served as a control and received equivalent intraperitoneal injections of saline. Group II mice received 25 mg/kg of MPTP (i.p.) for 5 consecutive days. The animals were killed 7 d after the last MPTP injection, and midbrain regions were used. The mice received a unilateral injection of 6-OHDA into the substantia nigra (5). They were euthanized 7 d after the last 6-OHDA injection, and the midbrain region was removed.
Human brain samples
Frozen blocks of postmortem human substantia nigra samples from controls and patients with PD patients (stage 3–4 based on the Hoehn and Yahr scale) were used. A total of 4–9 samples were evaluated for expression of individual proteins (25).
Immunohistochemistry
Animals were anesthetized after 7 d of MPTP or 6-OHDA treatment and then perfused with PBS followed by paraformaldehyde (4%, w/v). The fixed tissue was serially sectioned at 40 μm throughout the entire midbrain region and used for immunohistochemical analysis of tyrosine hydroxylase (TH). A Vector ABC Elite Kit from Vector Laboratories (Burlingame, CA, USA) was used along with 3′,4′-diaminobenzoic acid as a chromogen to develop the reaction in the presence of H2O2. Measurements from 4 to 6 sections were analyzed.
Statistics
Data analysis was performed using Origin 7.0 (OriginLab) and GraphPad Prism 6.0 software (GraphPad, La Jolla, CA, USA). Statistical comparisons were made using 1-way ANOVA. Experimental values are expressed as means ± sem or sd. Differences in the mean values were considered to be significant at P < 0.05, P < 0.01, or P < 0.001, respectively.
RESULTS
Neurotoxin induces cell death in dopaminergic neurons and cells
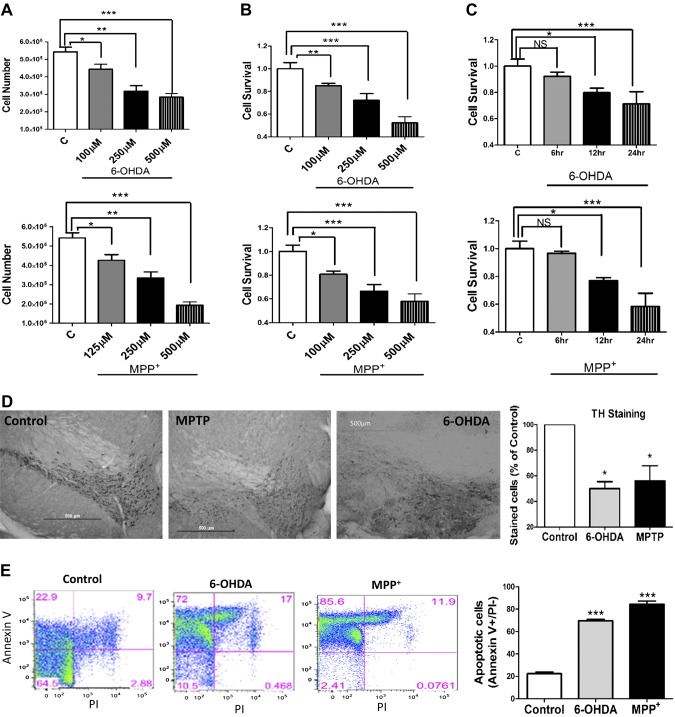
Dopaminergic neurotoxins are known to induce cell death (5, 26). Thus, we used 2 different neurotoxins for our studies. The human neuroblastoma cell line SHSY-5Y was pretreated with 6-OHDA and MPP+, which attenuated cell survival in a dose-dependent manner (Fig. 1A). Consistent with previous studies (13, 27), the number of cells and cell survival were attenuated with 6-OHDA and MPP+ treatment (Fig. 1A, B). We next evaluated whether the duration of neurotoxin treatment was critical. Our data showed that early time points (up to 6 h) failed to show any significant cell death, and a neurotoxin-induced decrease in cell survival was observed only after 12 h of neurotoxin treatment (Fig. 1C). To validate this cell culture model, we next evaluated survival of dopaminergic neurons in the SNpc region in control or neurotoxin-treated (MPTP or 6-OHDA) mice. Both MPTP and 6-OHDA treatment significantly decreased TH staining (a marker for dopaminergic neurons), suggesting that addition of neurotoxins leads to the loss of dopaminergic neurons in the SNpc region (Fig. 1D). To further confirm these results and to define the mode of cell death, we performed fluorescence-activated cell sorting analysis on cells treated with MPP+ or 6-OHDA. Consistent with the results presented above, the addition of MPP+ or 6-OHDA resulted in a significant increase in annexin V staining (a marker for apoptosis) without any change in PI staining (a marker for necrosis) (Fig. 1E). Together, these results suggest that prolonged neurotoxin treatment induces significant apoptosis that could lead to neuronal loss.
Figure 1.
Cell death induced by neurotoxins 6-OHDA and MPP+ in SHSY-5Y cells. A) Cell viability (determined via MTT assay) in SHSY-5Y cells pretreated with different concentrations of 6-OHDA and MPP+ is shown as a bar graph. B) Bar diagram showing the viable number of SHSY-5Y cells pretreated with different concentrations of 6-OHDA and MPP+. Each bar gives the mean ± sem of 4 separate experiments. C) Cell viability assay in SHSY-5Y cells pretreated at given time points with 250 µM of 6-OHDA and MPP+. D) TH immunoreactivity in the SNpc region of mice that received saline (control), MPTP, or 6-OHDA treatment. The bar graph indicates the quantitative analysis of TH-immunopositive neurons. E) Representative flow cytometry plots show the results of 3 independent experiments. The cells were pretreated with 250 µM of 6-OHDA or MPP+ for 24 h. The quantification of the number of apoptotic cells is indicated by the total number of cells in the annexin V+ quadrant that were negative for propidium iodide (PI) staining. NS, nonsignificant vs. control. *P < 0.05, **P < 0.01, ***P < 0.001.
MPP+- and 6-OHDA-treatment decreases calcium entry mainly via the loss of TRPC1 expression
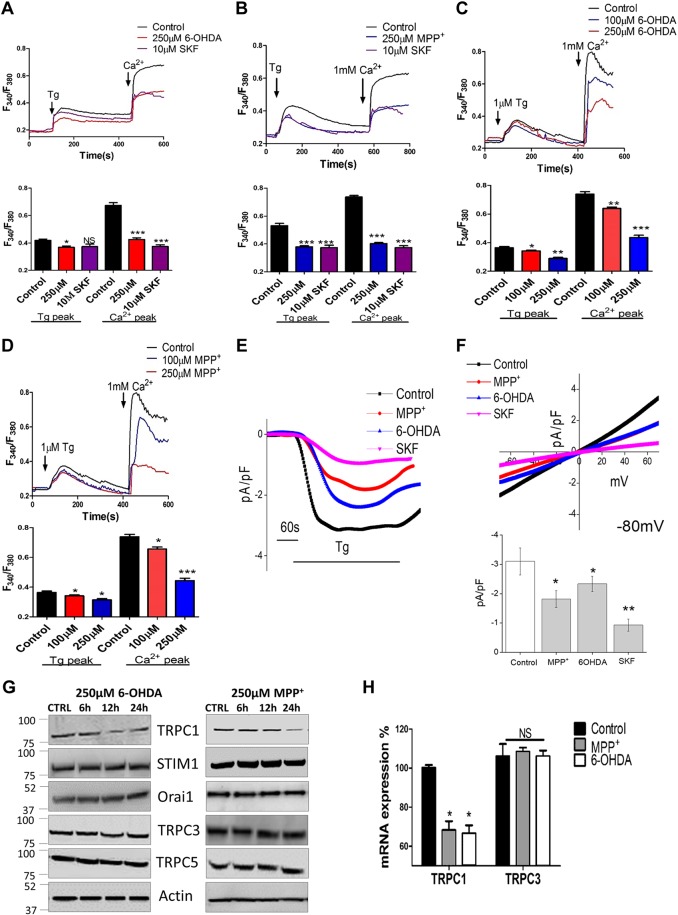
The data presented above suggest that long-term neurotoxin treatments induce apoptosis; however, the mechanism is still not well established. Ca2+ is a common factor in cell survival and cell death; thus, we evaluated the intracellular Ca2+ levels in SHSY-5Y cells treated with 6-OHDA or MPP+. To evaluate Ca2+ entry, ER Ca2+ stores were depleted by the addition of thapsigargin [a SERCA pump blocker (Tg; 2 μM)]. In the absence of extracellular Ca2+, the Tg-evoked increased in [Ca2+]i (first peak) was significantly attenuated in both 6-OHDA- and MPP+- treated cells (Fig. 2A, B), when compared with untreated control cells (6 h treatment). Similar results were obtained when cells were exposed to 6-OHDA and MPP+ for 1 h (data not shown). Ca2+ entry [addition of external Ca2+ (1 mM)] was still significantly decreased in both 6-OHDA- and MPP+-treated cells (Fig. 2A, B). Pretreatment of cells with the Ca2+ channel blocker SKF96365 (SKF) further attenuated Tg-induced Ca2+ entry (Fig. 2A, B). Although lower doses of neurotoxins decreased Ca2+ entry, a more prominent decrease in [Ca2+]i was observed at higher concentrations of 6-OHDA and MPP+ (Fig. 2C, D). These results suggest that exposure to the neurotoxins (6-OHDA and MPP+) attenuates Ca2+ entry in these cells, which could lead to apoptosis (as previously observed).
Figure 2.
Neurotoxin attenuates Ca2+ entry mainly via TRPC1 expression. A, B) Representative traces show that Tg-evoked Ca2+ entry and Ca2+-evoked [Ca2+]i changes were attenuated in SHSY-5Y cells pretreated with 6-OHDA, MPP+, or 10 µM SKF for 6 h. Bar diagrams of the fluorescence ratio (340:380) from a mean of 80–100 cells are shown below the traces. C, D) Representative traces and graph showing cytosolic calcium levels in SHSY-5Y cells pretreated with different concentrations of 6-OHDA or MPP+, respectively. Each bar is the mean ± sem of 5–6 separate experiments. *P < 0.05, ** P < 0.01, ***P < 0.001 vs. control. E) Bath application of Tg (1 μM) induced inward currents at a holding potential of −80 mV in control-, 250 µM MPP+-, 250 µM 6OHDA-, and 10 µMSKF-treated SHSY-5Y cells. F) Respective current–voltage curves and the mean (8–10 recordings) current intensity under various conditions. *P < 0.05, **P < 0.01 vs. control. G) SHSY-5Y cells were pretreated with 250 µM 6-OHDA or MPP+. Cell lysates were resolved, and the expression of various components of SOCE was analyzed by Western blot. H) The mRNA expression of TRPC1 and -3. Each bar represents 3 different experiments individually normalized to GAPDH expression. NS, nonsignificant vs. control. *P < 0.05.
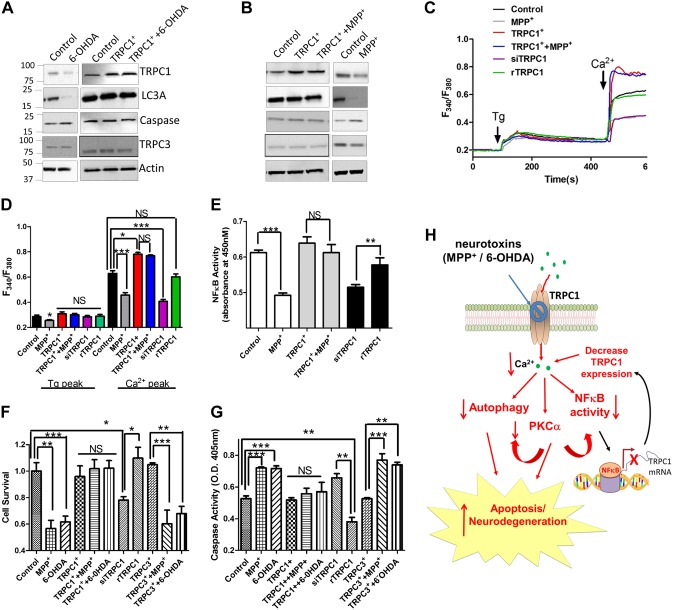
The electrophysiological data further support these results, and both 6-OHDA and MPP+ treatment significantly decreased Ca2+ entry (Fig. 2E, F). The channel properties were similar to those previously observed in TRPC1 channels (28), which had a reverse potential of ∼0 mV, and a nonselective current was observed (Fig. 2F). These results suggest that TRPC1 could contribute to the endogenous Ca2+ entry channel in these cells. Hence, we studied the expression of various Ca2+ entry components in neurotoxin-treated cells. Only TRPC1 expression was significantly decreased upon neurotoxin treatment. Furthermore, the loss of TRPC1 expression was observed only after 12 h of neurotoxin treatment (Fig. 2G and Supplemental Fig. 1A, B), even though Ca2+ entry was decreased at early neurotoxin-treatment time points. In contrast, no changes in the expression of TRPC3, TRPC5,ORAI1, or stromal interaction molecule (STIM)-1 was observed in the neurotoxin-treated cells. Furthermore, the mRNA level of TRPC1, but not -3, was significantly decreased after 24 h of neurotoxin treatment (Fig. 2H and Supplemental Fig. 1C). These results suggest that neurotoxin specifically decreases TRPC1 levels, which could be related to the loss of transcription rather than TRPC1 degradation, per se. These results further suggest that neurotoxins such as MPP+ and 6-OHDA initially attenuate intracellular Ca2+, which leads to a reduction in TRPC1 expression that could have a prolonged effect and contribute to dopaminergic cell death.
Effects of TRPC1 expression in a mouse model and PD
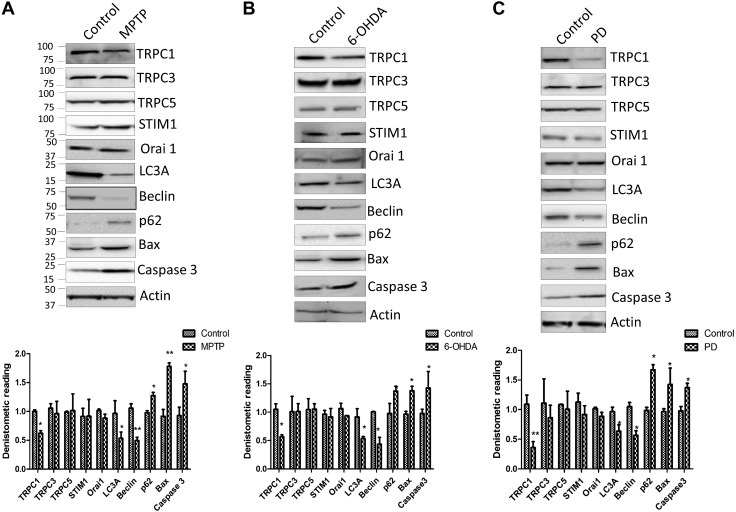
Our neurotoxin-induced mouse model and patients with PD exhibited attenuated TRPC1 expression that inhibits autophagy and induces apoptosis. To further define the role of Ca2+ channels in neurotoxin models, animals were injected with either MPTP or 6-OHDA (5). Midbrain samples were collected, and Western blot analysis was performed. Among the key store-operated Ca2+ channel components, only TRPC1 expression was attenuated in MPTP- and 6-OHDA-treated animal models (Fig. 3A, B). Again, no changes in the expression of TRPC3, TRPC5, ORAI1, or STIM1 were observed in the MPTP- or 6-OHDA-treated samples. Moreover, protein samples form the SNpc region of postmortem human PD samples showed a decrease in the expression of TRPC1 compared with age-matched controls, without any changes in other TRPC/ORAI channels (Fig. 3C). Previous studies from our lab have shown that TRPC1 may be important for autophagy (23), and recent studies have shown that dysfunction in autophagy could induce apoptosis, which could play a vital role in neurodegenerative diseases (2, 16). Hence, we evaluated the expression of autophagic (LC3A; Beclin, p62) and apoptotic (caspase 3 and Bax) markers in both 6-OHDA- and MPTP-treated mouse models. A significant decrease in the expression of autophagic markers was observed in samples obtained from patients with PD and from MPTP- or 6-OHDA-treated regions of the SNpc (Fig. 3). In contrast, an increase in caspase 3 expression was observed in patients with PD and neurotoxin-treated samples (Fig. 3). Caspase-dependent pathways have been shown to induce neuronal degeneration after neurotoxin treatment (26). A comparative analysis of the mouse SNpc and human SNpc from postmortem PD samples was performed and showed that TRPC1 expression was reduced in both PD samples and neurotoxin-treated samples, suggesting that a common mechanism might be involved (Supplemental Fig. 2A). Together, these results suggest that neurotoxin-induced loss of TRPC1 could lead to a decrease in autophagy, resulting in apoptosis in dopaminergic neurons.
Figure 3.
TRPC1 expression is decreased in neurotoxin-treated and PD samples. A, B) Western blots showing the expression of various Ca2+ channels, its modulator STIM1, and markers of autophagy and apoptosis in control mice (PBS treated) or mice that received an injection of MPTP or 6-OHDA. C) Western blots from the SNpc region of postmortem human PD samples and age-matched controls showing the expression of various Ca2+ entry channels and markers of autophagy and apoptosis. Densitometric values (normalized to the control) of each Western blot is shown below. Each blot is a representative of at least 3 independent experiments performed in duplicate. *P < 0.05, **P < 0.01 vs. control.
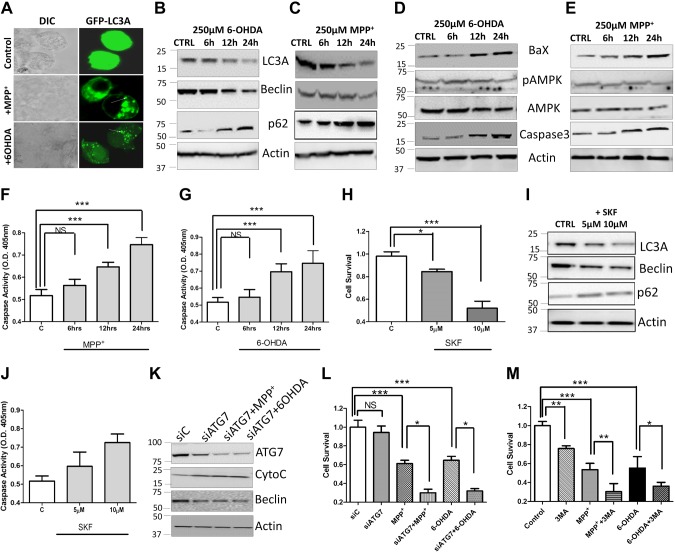
To further establish that neurotoxin treatment indeed induces apoptosis via decreasing autophagy, we studied the expression of individual proteins involved in autophagy and apoptosis in neurotoxin-treated cells. Cells transfected with GFP-LC3 and treated with either 6-OHDA or MPP+ for 24 h showed a decrease in LC3 staining, along with an increase in larger vesicles (Fig. 4A). However, a gradual decrease in autophagic markers (LC3A and Beclin) was observed in cells treated with either 6-OHDA or MPP+. A significant loss of both LC3A and LC3A/B conjugate as well as Beclin was observed upon neurotoxin treatment (Fig. 4B, C and Supplemental Fig. 3A–C). A loss of p62 expression has been reported when cells undergo autophagy (29, 30). Thus, we evaluated the expression of p62, which was significantly increased in 6-OHDA- and MPP+-treated cells (Fig. 4B, C and Supplemental Fig. 3A, B). Autophagy is promoted by AMPK, which is a key energy sensor that regulates cellular metabolism to maintain energy homeostasis (31, 32). However, no change in the phosphorylation of a conserved threonine residue (T172), which is a prerequisite for the activity of AMPK, was observed upon neurotoxin treatment (Fig. 4D, E and Supplemental Fig. 3D, E). In contrast, an increase in the expression of caspase 3 was observed upon neurotoxin treatment (Fig. 4D, E and Supplemental Fig. 3D, E). Similarly, a significant increase in caspase activity was observed after 6–12 h of neurotoxin treatment (Fig. 4F, G). To establish that the decrease in autophagy and increase in apoptosis is indeed due to the loss of Ca2+ entry, we inhibited TRPC1 function using SKF, a store-operated calcium entry (SOCE) inhibitor. Cells treated with SKF showed a gradual decrease in cell survival, with more cell death observed at higher SKF concentrations (Fig. 4H). The addition of SKF alone (without neurotoxin) resulted in a decrease in autophagic markers (Fig. 4I and Supplemental Fig. 3F). Similarly, an increase in caspase activity was observed upon SKF treatment (Fig. 4J). Knockdown of the key autophagy gene ATG7 (siATG7) (Fig. 4K and Supplemental Fig. 3G) or autophagy inhibitor 3-MA enhanced the neurotoxic effect induced by 6-OHDA or MPP+ (Fig. 4K–M), and more cell death and loss of autophagy was observed under these conditions. Together, these results suggest that autophagy is essential for inhibiting neurotoxin-induced cell death, which is dependent on Ca2+ entry.
Figure 4.
6-OHDA and MPP+ attenuate the autophagy that induces apoptosis. A) Confocal image of SHSY-5Y cells transfected with GFP-LC3 and treated with 250 µM 6-OHDA or MPP+ for 24 h. B–E) SH-SY5Y cells were pretreated with 250 µM 6-OHDA (B, D) or 250 µM MPP+ (C, E). The cell lysates were resolved, and the expressions of various autophagy and apoptosis markers were analyzed by Western blot. F, G) The relative caspase 3 activity in SH-SY5Y cells pretreated with 250 µM 6-OHDA (F) or 250 µM MPP+ (G) at various time points. H) Cell viability (MTT assay) in SHSY-5Y cells pretreated with different concentrations of SKF-96365 (SKF). I) Representative Western blot images show the expression of autophagy markers in cells pretreated with 10 µM SKF for 24 h. J) The relative caspase 3 activity in cells pretreated with different concentrations of the calcium channel blocker SKF for 24 h. K) Representative Western blot shows protein expression of siATG7 in cells pretreated with 250 µM of 6-OHDA and MPP+ for 24 h. L) Cell survival (determined via MTT assay) in control and ATG silenced cells (siATG7) pretreated with 250 µM 6-OHDA or 250 µM MPP+. M) Survival (determined via MTT assay) of the cells pretreated with 250 µM 6-OHDA or 250 µM MPP+ and 1 mM of the autophagy inhibitor 3-MA. In all graphs, each bar represents the mean ± sem of 4 separate experiments. NS, nonsignificant. *P < 0.05, **P < 0.01, ***P < 0.001.
Effects of reduction in [Ca2+]i on PKC/PKA on phosphorylation and NF-κB activity
Previous studies have suggested the involvement of PKC in neurotoxin-mediated cell death in PD (10, 33). Hence, we studied the phosphorylation of PKC, which requires Ca2+ for its activation. Supporting the previous studies, we observed a reduction in the expression of phosphorylated PKC-α in cells treated with 6-OHDA or MPP+ (Fig. 5A and Supplemental Fig. 4A). Attenuation of PKC phosphorylation has been shown to reduce phosphorylation of protein kinase B (AKT) (34). Thus, we examined AKT phosphorylation, which was reduced upon neurotoxin treatment (Fig. 5B and Supplemental Fig. 4B). Moreover, inhibition of TRPC1 activity (10 µM SKF treatment) resulted in a decrease in AKT phosphorylation (data not shown).
Figure 5.
Reduced PKC phosphorylation after neurotoxin treatment results in reduced NF-κB activity and attenuates TRPC1 expression. A) Phosphorylation of calcium-dependent classic PKC-α, which was attenuated in cells pretreated with 250 µM 6-OHDA or 250 µM MPP+. B) Expression of p-AKT (Thr 308) and p-AKT (Ser 473) in cells pretreated with 250 µM 6-OHDA and 250 µM MPP+. C) Expression of pNF-κB and total NF-κB expression in SHSY-5Y cells pretreated with 250 µM 6-OHDA, 250 µM MPP+, or 10 µM GȮ6893 for 24 h. D) NF-κB activity in the various conditions. E) ChIP analysis input percentage of 3 different NF-κB binding sites in the TRPC1 gene in cells pretreated with 250 µM MPP+ or 250 µM 6-OHDA. Each bar represents the mean ± sem of 3 separate experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control. F) Confocal image of SHSY-5Y cells transfected with GFP-LC3 and pretreated with 5 µM SKF and 10 µM GȮ6893 for 24 h.
To understand specifically why TRPC1 mRNA is decreased upon neurotoxin treatment, we focused on the promoter region of TRPC1. The TRPC1 promoter has 3 putative NF-κB binding sites that are essential for its expression (35). Thus, we evaluated the phosphorylation and activation of NF-κB. NF-κB phosphorylation was decreased in 6-OHDA- or MPP+-treated cells or cells treated with the PKC inhibitor GȮ6983 (Fig. 5C and Supplemental Fig. 4C). Moreover, NF-κB activity was significantly decreased in neurotoxin- or PKC inhibitor–treated cells (Fig. 5D). To establish whether the decrease in NF-κB phosphorylation leads to the loss of TRPC1 expression, ChIP experiments were performed. Although 3 predictive NF-κB sites are present on the TRPC1 promoter, pretreatment with MPP+ or 6-OHDA resulted in a loss of only NF-κB binding at the second promoter site (Fig. 5E). Confocal images of the cells transfected with GFP-LC3 and treated with the PKC inhibitor GȮ6983 or the Ca2+ channel inhibitor SKF showed an attenuation of autophagy, further supporting these results (Fig. 5F). Ca2+ entry has been shown to modulate NF-κB activity (36), which explains the long-term effect of the loss of Ca2+ entry on TRPC1 expression that contributes to cell death. The data presented thus far suggest that neurotoxin treatment decreases Ca2+ entry, which inhibits PKC phosphorylation and activation of NF-κB. This effect results in attenuated expression of TRPC1, which decreases autophagy to induce apoptosis in neuronal cells.
TRPC1 overexpression rescues SHSY-5Y cells from MPP+- and 6OHDA-induced cell death
To establish the significance of TRPC1 in neurotoxin-induced cell death, we altered TRPC1 expression in these cells. Overexpression of TRPC1 inhibited both the MPP+- and 6-OHDA-induced loss of the autophagic marker LC3A (Fig. 6A, B and Supplemental Fig. 5A, B). Similarly, the neurotoxin-induced increase in caspase 3 expression was decreased upon TRPC1 overexpression. Furthermore, overexpression of TRPC1 restored the neurotoxin-induced decrease in Ca2+ entry. In contrast, silencing of TRPC1 (TRPC1 siRNA) attenuated Ca2+ entry, which was rescued by overexpressing a TRPC1 plasmid (rTRPC1) that was resistant to the siRNA. (Fig. 6C, D and Supplemental Fig. 5C). Overexpression of TRPC1 led to increased NF-κB activity and attenuated the neurotoxin-induced decrease in NF-κB activity (Fig. 6E). In contrast, TRPC1 silencing attenuated NF-κB activity, which was again rescued by restoring TRPC1 expression (Fig. 6E). Furthermore, the neurotoxin-induced decrease in cell survival, along with the increase in caspase activity was reversed in TRPC1- but not TRPC3-overexpressing cells (Fig. 6F, G). In contrast, silencing of TRPC1 resulted in decreased cell survival and increased caspase activity, which was again rescued by overexpressing TRPC1 (Fig. 6F, G). These results suggest that restoration of TRPC1 expression not only maintains Ca2+ homeostasis but also induces the autophagy that inhibits apoptosis, thereby protecting against neuronal loss (Fig. 6H).
Figure 6.
Overexpression of TRPC1 rescues the effect of neurotoxins. A, B) Expression of TRPC1, autophagy marker LC3A, and apoptotic marker caspase 3 in cells overexpressing TRPC1 and control cells pretreated with 250 µM 6-OHDA and 250 µM MPP+, respectively. C) Tg-evoked calcium entry and calcium-evoked [Ca2+]i changes in various cells, as mentioned in the figure, pretreated with MPP+ for 6 h. D) The fluorescence ratios of an average of 40–60 cells of the previously mentioned traces are shown. E) The NF-κB activity in the various conditions, with comparisons as labeled. F, G) Survival (determined via MTT assay) of cells (F) and caspase activity (G), in the various conditions, with group comparisons as indicated by brackets. H) Neurodegeneration and apoptosis process: the dopaminergic neurotoxin-induced loss of Ca2+ entry (short-term effect) inhibits PKC activation and autophagy and induces cell death. Moreover, a decrease in cytosolic calcium decreases the binding of NF-κB to the TRPC1 promoter, thereby inhibiting TRPC1 expression (long-term effect) to further decrease cytosolic calcium levels that increase cell death by preventing autophagy. NS, nonsignificant. *P < 0.05, **P < 0.01, ***P< 0.001.
DISCUSSION
Ca2+ signaling plays a vital role in neuronal signaling, and altered Ca2+ homeostasis has been implicated in many neuronal diseases including PD (12). Treatment with neurotoxins such as MPTP/MPP+ and 6-OHDA in neuronal cells are an established in vivo model for mimicking PD (5). When injected into the SNpc, 6-OHDA rapidly produces a complete lesion in the nigrostriatal pathway, making it an excellent model for PD; however, the mechanism of how it induces cell death is not fully established. Data presented herein show that both 6-OHDA and MPTP/MPP+ affect the Ca2+ signaling that precedes cell loss. It has been shown that the depletion of ER Ca2+ stores is toxic to dopaminergic cells and that intracellular Ca2+ chelators increase cell death (37). These studies are consistent with our results and imply that loss of Ca2+ entry is perhaps the major cause of cell death in dopaminergic cells.
Previous studies have shown that Ca2+ homeostasis is affected by preincubation with the neurotoxin MPP+. Ca2+ entry, especially upon ER store depletion, is a mechanism devised by cells to ensure optimal refilling of internal Ca2+ stores to maintain the Ca2+ homeostasis that is necessary for the activation of Ca2+-dependent kinases. TRPC1 is an important Ca2+ entry channel that is activated upon store depletion (20). In addition, loss of Ca2+ entry and a decrease in TRPC1 expression have been observed in PD models (5). However, the mechanism that underlies the progressive loss of TRPC1 has not been established. We propose a model in which the dopaminergic neurotoxins have a biphasic effect. Early exposure to the neurotoxins (within 6 h) initially decreases Ca2+ entry by inhibiting TRPC1-mediated SOCE. The expression of STIM1 and ORAI1 channels (other important components of SOCE) was not altered by neurotoxin treatment, suggesting that neurotoxins specifically inhibit TRPC1 without altering other Ca2+ channels or their regulators. Previous studies have shown that STIM1 and ORAI1 appear to be necessary for the generation of Ca2+ release-activated Ca2+ channels (CRACs) as well as SOCE channels. Although ORAI1+STIM1 appears to be sufficient for CRACs, TRPC1, ORAI1, and STIM1 concertedly generate SOC channels (38), which could play an essential role in neuronal survival. However, specific loss of Ca2+ entry inhibits NF-κB activity, which is essential for TRPC1 expression, thereby providing a long-term effect that is essential for inhibiting autophagy and inducing apoptosis, consistent with our results, given that decreased TRPC1 expression (both protein and mRNA) was observed only after 12 h of neurotoxin treatment. NF-κB is an important transcription factor that is retained in the cytoplasm of cells by the inhibitor subunit IκB. Stimulation of NF-κB results in the sequential phosphorylation and degradation of IκB, which is dependent on Ca2+. This exposes a nuclear localization signal on NF-κB, allowing it to translocate to the nucleus to promote gene transcription. The loss of NF-κB activity is important for the prolonged effect of these neurotoxins, as overexpression of TRPC1 under a CMV promoter was able to overcome neurotoxin-mediated loss and promote cell survival. Other studies have shown that the binding sites of NF-κB are located in the 5′ regulatory region of the TRPC1 promoter (39), which may be the reason that TRPC1 expression, specifically, was decreased upon neurotoxin treatment. Our data further identify the NF-κB binding site (second site), that is important for TRPC1 expression, which is necessary for cell survival. In addition pretreatment of SHSY-5Y cells with neurotoxins results in a decrease in PKC activity, which has been shown to attenuate the phosphorylation of IκB that could again decrease the localization of NF-κB in the nucleus (40) and could result in a decrease in TRPC1 expression. More research is needed to explore this possibility.
Ca2+ influx via TRPC channels plays a critical role in regulating cell survival. Previous studies have suggested the involvement of PKC in neurotoxin-mediated cell death (10, 33). The classic form of PKC, PKCα, is known to be activated by Ca2+ entry, and the TRPC channel has been shown to be phosphorylated by PKC (41). However, the roles of PKCα in the regulation of Ca2+ entry and TRPC1 channels in neuronal cells are still unclear. Furthermore, results of studies using several cell types have been contradictory (41, 42). The decrease in PKC activity upon neurotoxin treatment (13, 43) could affect TRPC1 expression and cell survival by inhibiting autophagy. Although our data suggest that autophagy is important for the TRPC1-mediated protection of dopaminergic cells, other studies have shown that autophagy is harmful and could be a mechanism for several neurodegenerative diseases (44). A possible explanation could be that different stressors promote alternative pathways that could overcome autophagy and proceed to apoptosis. Alternatively, activation of autophagy by Ca2+ entry could be protective, whereas activation by other methods could be harmful. One caveat in our results is that the expression of LC3 was decreased upon neurotoxin treatment. LC3 expression is dependent on the activation of several transcription factors (such as GATA binding protein-1, forkhead box-O3, and Jun proto-oncogene), which are dependent on Ca2+. However, additional experiments are needed to fully identify the mechanism of decreased LC3 expression. In addition, treatment of cells with MPP+ could cause mitochondrial dysfunction and disturb ER Ca2+ homeostasis (5, 12), which may not need autophagy. The decrease in mitochondrial function not only increases reactive oxygen species but also decreases ATP levels, which is important for cellular function. The decrease in ATP along with a subsequent increase in AMP is known to activate AMPK, which initiates autophagy (31). In our studies, we did not observe an increase in AMPK activation, suggesting that the induction of autophagy was not due to the loss of ATP levels. Nevertheless, our results imply that TRPC1 activity is important for the induction of autophagy, which protects SH-SY5Y cells from the neurotoxins MPP+ and 6-OHDA, because blocking Ca2+ entry increased cell death and inhibited autophagy (Fig. 6H).
Consistent with the cell culture studies, our in vivo mouse models exhibited decreased TRPC1 expression. Although other TRPCs and ORAI channels are expressed in neuronal cells, their expression was not decreased upon neurotoxin treatment, suggesting that these channels were not sufficient to rescue the loss of TRPC1. Restoration of TRPC3 failed to rescue the neurotoxin-mediated loss of cell survival. The decrease in TRPC1 not only inhibited autophagy but also led to the induction of apoptosis, suggesting a vital role of TRPC1 in the protection of dopaminergic neurons. Although much of the effects of neurotoxins could be attributed to the loss of Ca2+ homeostasis, it is clear that TRPC1 is the main Ca2+ channel in dopaminergic cells. Together, our results suggest that neurotoxins alter the Ca2+ homeostasis that decreases phosphorylation of PKC and AKT, along with inhibiting the binding of NF-κB to the TRPC1 promoter. This loss of NF-κB binding further decreases TRPC1 expression, which promotes cell survival by activating autophagy and inhibiting apoptosis.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank the Confocal Facility at the University of North Dakota, which is partially supported by the U.S. National Institutes of Health (NIH) Centers of Biomedical Research Excellence (COBRE; Grant P30GM103329). This work was supported by the NIH, National Institute of Dental and Craniofacial Research (Grants R01DE017102, R01DE022765, and R21DE024300), and the NIH National Institute of General Medical Sciences (NIGMS; Grant P20GM113123 to B.B.S.). The Flow Cytometer Core is supported by NIH/NIGMS Grants P20GM103442 and P20GM113123. The authors declare no conflicts of interest.
Glossary
- 6-OHDA
6-hydroxy dopamine
- [Ca2+]i
intracellular (cytosolic) Ca2+ concentration
- AKT
protein kinase B
- DA
dopaminergic
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- LC
light chain
- MPP+
1-methyl-4-phenylpyridium ion
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- ORAI
calcium release–activated calcium channel protein
- PD
Parkinson’s disease
- PM
plasma membrane
- ROS
reactive oxygen species
- SERCA
sacro/endoplasmic reticulum Ca2+-ATPase
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- SNpc
substantia nigra pars compacta
- SOCE
store-operated calcium entry
- STIM
stromal interaction molecule
- Tg
thapsigargin
- TH
tyrosine hydrolase
- TRPC
transient receptor potential canonical
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
P. Sukumaran designed and performed the experiments and wrote the manuscript; Y. Sun performed the electrophysiological experiments and wrote the manuscript; N. Antonson performed the animal experiments and reviewed the manuscript; and B. B. Singh designed the experiments, evaluated the results, and wrote the manuscript.
REFERENCES
- 1.Badger J. L., Cordero-Llana O., Hartfield E. M., Wade-Martins R. (2014) Parkinson's disease in a dish: using stem cells as a molecular tool. Neuropharmacology 76, 88–96 [DOI] [PubMed] [Google Scholar]
- 2.Ghavami S., Shojaei S., Yeganeh B., Ande S. R., Jangamreddy J. R., Mehrpour M., Christoffersson J., Chaabane W., Moghadam A. R., Kashani H. H., Hashemi M., Owji A. A., Łos M. J. (2014) Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 112, 24–49https://doi.org/10.1016/j.pneurobio.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 3.Venderova K., Park D. S. (2012) Programmed cell death in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2, a009365 https://doi.org/10.1101/cshperspect.a009365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schober A. (2004) Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res. 318, 215–224https://doi.org/10.1007/s00441-004-0938-y [DOI] [PubMed] [Google Scholar]
- 5.Selvaraj S., Watt J. A., Singh B. B. (2009) TRPC1 inhibits apoptotic cell degeneration induced by dopaminergic neurotoxin MPTP/MPP(+). Cell Calcium 46, 209–218https://doi.org/10.1016/j.ceca.2009.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvaraj S., Sun Y., Watt J. A., Wang S., Lei S., Birnbaumer L., Singh B. B. (2012) Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. J. Clin. Invest. 122, 1354–1367https://doi.org/10.1172/JCI61332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kania E., Pająk B., Orzechowski A. (2015) Calcium homeostasis and ER stress in control of autophagy in cancer cells. Biomed Res. Int. 2015, 352794.https://doi.org/10.1155/2015/352794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marks A. R. (1997) Intracellular calcium-release channels: regulators of cell life and death. Am. J. Physiol. 272, H597–H605 [DOI] [PubMed] [Google Scholar]
- 9.Burns R. S., Chiueh C. C., Markey S. P., Ebert M. H., Jacobowitz D. M., Kopin I. J. (1983) A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc. Natl. Acad. Sci. USA 80, 4546–4550https://doi.org/10.1073/pnas.80.14.4546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latchoumycandane C., Anantharam V., Jin H., Kanthasamy A., Kanthasamy A. (2011) Dopaminergic neurotoxicant 6-OHDA induces oxidative damage through proteolytic activation of PKCδ in cell culture and animal models of Parkinson’s disease. Toxicol. Appl. Pharmacol. 256, 314–323https://doi.org/10.1016/j.taap.2011.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitahama K., Denney R. M., Maeda T., Jouvet M. (1991) Distribution of type B monoamine oxidase immunoreactivity in the cat brain with reference to enzyme histochemistry. Neuroscience 44, 185–204https://doi.org/10.1016/0306-4522(91)90260-U [DOI] [PubMed] [Google Scholar]
- 12.Lotharius J., Dugan L. L., O’Malley K. L. (1999) Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons. J. Neurosci. 19, 1284–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanrott K., Gudmunsen L., O’Neill M. J., Wonnacott S. (2006) 6-Hydroxydopamine-induced apoptosis is mediated via extracellular auto-oxidation and caspase 3-dependent activation of protein kinase Cdelta. J. Biol. Chem. 281, 5373–5382https://doi.org/10.1074/jbc.M511560200 [DOI] [PubMed] [Google Scholar]
- 14.Choi W. S., Yoon S. Y., Oh T. H., Choi E. J., O’Malley K. L., Oh Y. J. (1999) Two distinct mechanisms are involved in 6-hydroxydopamine- and MPP+-induced dopaminergic neuronal cell death: role of caspases, ROS, and JNK. J. Neurosci. Res. 57, 86–94https://doi.org/10.1002/(SICI)1097-4547(19990701)57:1%3c86::AID-JNR9%3e3.0.CO;2-E [DOI] [PubMed] [Google Scholar]
- 15.Thanan R., Oikawa S., Hiraku Y., Ohnishi S., Ma N., Pinlaor S., Yongvanit P., Kawanishi S., Murata M. (2014) Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int. J. Mol. Sci. 16, 193–217https://doi.org/10.3390/ijms16010193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen S., Kepp O., Michaud M., Martins I., Minoux H., Métivier D., Maiuri M. C., Kroemer R. T., Kroemer G. (2011) Association and dissociation of autophagy, apoptosis and necrosis by systematic chemical study. Oncogene 30, 4544–4556https://doi.org/10.1038/onc.2011.168 [DOI] [PubMed] [Google Scholar]
- 17.Yap Y. W., Llanos R. M., La Fontaine S., Cater M. A., Beart P. M., Cheung N. S. (2016) Comparative microarray analysis identifies commonalities in neuronal injury: evidence for oxidative stress, dysfunction of calcium signalling, and inhibition of autophagy-lysosomal pathway. Neurochem. Res. 41, 554–567https://doi.org/10.1007/s11064-015-1666-2 [DOI] [PubMed] [Google Scholar]
- 18.Kondratskyi A., Yassine M., Kondratska K., Skryma R., Slomianny C., Prevarskaya N. (2013) Calcium-permeable ion channels in control of autophagy and cancer. Front. Physiol. 4, 272.https://doi.org/10.3389/fphys.2013.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berridge M. J., Bootman M. D., Roderick H. L. (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529https://doi.org/10.1038/nrm1155 [DOI] [PubMed] [Google Scholar]
- 20.Abramowitz J., Birnbaumer L. (2009) Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 23, 297–328https://doi.org/10.1096/fj.08-119495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y., Sukumaran P., Varma A., Derry S., Sahmoun A. E., Singh B. B. (2014) Cholesterol-induced activation of TRPM7 regulate cell proliferation, migration, and viability of human prostate cells. Biochim. Biophys. Acta 1843, 1839–1850https://doi.org/10.1016/j.bbamcr.2014.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steichen A. L., Binstock B. J., Mishra B. B., Sharma J. (2013) C-type lectin receptor Clec4d plays a protective role in resolution of Gram-negative pneumonia. J. Leukoc. Biol. 94, 393–398https://doi.org/10.1189/jlb.1212622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sukumaran P., Sun Y., Vyas M., Singh B. B. (2015) TRPC1-mediated Ca2+ entry is essential for the regulation of hypoxia and nutrient depletion-dependent autophagy. Cell Death Dis. 6, e1674.https://doi.org/10.1038/cddis.2015.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chauhan A., Quenum F. Z., Abbas A., Bradley D. S., Nechaev S., Singh B. B., Sharma J., Mishra B. B. (2015) Epigenetic modulation of microglial inflammatory gene loci in helminth-induced immune suppression: implications for immune regulation in neurocysticercosis. ASN Neuro 7,1759091415592126https://doi.org/10.1177/1759091415592126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller J., Wenning G. K., Jellinger K., McKee A., Poewe W., Litvan I. (2000) Progression of Hoehn and Yahr stages in Parkinsonian disorders: a clinicopathologic study. Neurology 55, 888–891https://doi.org/10.1212/WNL.55.6.888 [DOI] [PubMed] [Google Scholar]
- 26.Han B. S., Hong H. S., Choi W. S., Markelonis G. J., Oh T. H., Oh Y. J. (2003) Caspase-dependent and -independent cell death pathways in primary cultures of mesencephalic dopaminergic neurons after neurotoxin treatment. J. Neurosci. 23, 5069–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi W. S., Canzoniero L. M., Sensi S. L., O’Malley K. L., Gwag B. J., Sohn S., Kim J. E., Oh T. H., Lee E. B., Oh Y. J. (1999) Characterization of MPP(+)-induced cell death in a dopaminergic neuronal cell line: role of macromolecule synthesis, cytosolic calcium, caspase, and Bcl-2-related proteins. Exp. Neurol. 159, 274–282https://doi.org/10.1006/exnr.1999.7133 [DOI] [PubMed] [Google Scholar]
- 28.Pani B., Liu X., Bollimuntha S., Cheng K. T., Niesman I. R., Zheng C., Achen V. R., Patel H. H., Ambudkar I. S., Singh B. B. (2013) Impairment of TRPC1-STIM1 channel assembly and AQP5 translocation compromise agonist-stimulated fluid secretion in mice lacking caveolin1. J. Cell Sci. 126, 667–675https://doi.org/10.1242/jcs.118943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parkhitko A., Myachina F., Morrison T. A., Hindi K. M., Auricchio N., Karbowniczek M., Wu J. J., Finkel T., Kwiatkowski D. J., Yu J. J., Henske E. P. (2011) Tumorigenesis in tuberous sclerosis complex is autophagy and p62/sequestosome 1 (SQSTM1)-dependent. Proc. Natl. Acad. Sci. USA 108, 12455–12460https://doi.org/10.1073/pnas.1104361108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen Y., Yang J., Zhao J., Xiao C., Xu C., Xiang Y. (2015) The switch from ER stress-induced apoptosis to autophagy via ROS-mediated JNK/p62 signals: a survival mechanism in methotrexate-resistant choriocarcinoma cells. Exp. Cell Res. 334, 207–218https://doi.org/10.1016/j.yexcr.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 31.Høyer-Hansen M., Jäättelä M. (2007) AMP-activated protein kinase: a universal regulator of autophagy? Autophagy 3, 381–383https://doi.org/10.4161/auto.4240 [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Chen C., Yao F., Su Q., Liu D., Xue R., Dai G., Fang R., Zeng J., Chen Y., Huang H., Ma Y., Li W., Zhang L., Liu C., Dong Y. (2014) AMPK inhibits cardiac hypertrophy by promoting autophagy via mTORC1. Arch. Biochem. Biophys. 558, 79–86https://doi.org/10.1016/j.abb.2014.06.023 [DOI] [PubMed] [Google Scholar]
- 33.Shao C. Y., Crary J. F., Rao C., Sacktor T. C., Mirra S. S. (2006) Atypical protein kinase C in neurodegenerative disease II: PKCiota/lambda in tauopathies and alpha-synucleinopathies. J. Neuropathol. Exp. Neurol. 65, 327–335https://doi.org/10.1097/01.jnen.0000218441.00040.82 [DOI] [PubMed] [Google Scholar]
- 34.Partovian C., Simons M. (2004) Regulation of protein kinase B/Akt activity and Ser473 phosphorylation by protein kinase Calpha in endothelial cells. Cell. Signal. 16, 951–957https://doi.org/10.1016/j.cellsig.2004.01.008 [DOI] [PubMed] [Google Scholar]
- 35.Yang L. X., Guo R. W., Liu B., Wang X. M., Qi F., Guo C. M., Shi Y. K., Wang H. (2009) Role of TRPC1 and NF-kappaB in mediating angiotensin II-induced Ca2+ entry and endothelial hyperpermeability. Peptides 30, 1368–1373https://doi.org/10.1016/j.peptides.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 36.Lilienbaum A., Israël A. (2003) From calcium to NF-kappa B signaling pathways in neurons. Mol. Cell. Biol. 23, 2680–2698https://doi.org/10.1128/MCB.23.8.2680-2698.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su J., Zhou L., Kong X., Yang X., Xiang X., Zhang Y., Li X., Sun L. (2013) Endoplasmic reticulum is at the crossroads of autophagy, inflammation, and apoptosis signaling pathways and participates in the pathogenesis of diabetes mellitus. J. Diabetes Res. 2013, 193461.https://doi.org/10.1155/2013/193461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng K. T., Liu X., Ong H. L., Ambudkar I. S. (2008) Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J. Biol. Chem. 283, 12935–12940https://doi.org/10.1074/jbc.C800008200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marasa B. S., Rao J. N., Zou T., Liu L., Keledjian K. M., Zhang A. H., Xiao L., Chen J., Turner D. J., Wang J. Y. (2006) Induced TRPC1 expression sensitizes intestinal epithelial cells to apoptosis by inhibiting NF-kappaB activation through Ca2+ influx. Biochem. J. 397, 77–87https://doi.org/10.1042/BJ20060124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mut M., Amos S., Hussaini I. M. (2010) PKC alpha phosphorylates cytosolic NF-kappaB/p65 and PKC delta delays nuclear translocation of NF-kappaB/p65 in U1242 glioblastoma cells. Turk Neurosurg. 20, 277–285 [DOI] [PubMed] [Google Scholar]
- 41.Ahmmed G. U., Mehta D., Vogel S., Holinstat M., Paria B. C., Tiruppathi C., Malik A. B. (2004) Protein kinase Calpha phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J. Biol. Chem. 279, 20941–20949https://doi.org/10.1074/jbc.M313975200 [DOI] [PubMed] [Google Scholar]
- 42.Kondo I. (2000) Protein kinase C potentiates capacitative Ca2+ entry that links to steroidogenesis in bovine adrenocortical cells. Jpn. J. Pharmacol. 82, 210–217https://doi.org/10.1254/jjp.82.210 [DOI] [PubMed] [Google Scholar]
- 43.Fan Y., Li J., Zhang Y. Q., Jiang L. H., Zhang Y. N., Yan C. Q. (2014) Protein kinase C delta mediated cytotoxicity of 6-hydroxydopamine via sustained extracellular signal-regulated kinase 1/2 activation in PC12 cells. Neurol. Res. 36, 53–64https://doi.org/10.1179/1743132813Y.0000000267 [DOI] [PubMed] [Google Scholar]
- 44.Menzies F. M., Fleming A., Caricasole A., Bento C. F., Andrews S. P., Ashkenazi A., Füllgrabe J., Jackson A., Jimenez Sanchez M., Karabiyik C., Licitra F., Lopez Ramirez A., Pavel M., Puri C., Renna M., Ricketts T., Schlotawa L., Vicinanza M., Won H., Zhu Y., Skidmore J., Rubinsztein D. C. (2017) Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron 93, 1015–1034https://doi.org/10.1016/j.neuron.2017.01.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.