Abstract
The microenvironment of pancreatic ductal adenocarcinoma (PDAC) is characterized by a dense fibrotic stroma (desmoplasia) generated by pancreatic cancer–associated fibroblasts (CAFs) derived from pancreatic stellate cells (PSCs) and pancreatic fibroblasts (PFs). Using an unbiased GPCRomic array approach, we identified 82 G-protein–coupled receptors (GPCRs) commonly expressed by CAFs derived from 5 primary PDAC tumors. Compared with PSCs and PFs, CAFs have increased expression of GPR68 (a proton-sensing GPCR), with the results confirmed by immunoblotting, The Cancer Genome Atlas data, and immunohistochemistry of PDAC tumors. Co-culture of PSCs with PDAC cells, or incubation with TNF-α, induced GPR68 expression. GPR68 activation (by decreasing the extracellular pH) enhanced IL-6 expression via a cAMP/PKA/cAMP response element binding protein signaling pathway. Knockdown of GPR68 by short interfering RNA diminished low pH-induced production of IL-6 and enhancement of PDAC cell proliferation by CAF conditioned media. CAFs from other gastrointestinal cancers also express GPR68. PDAC cells thus induce expression by CAFs of GPR68, which senses the acidic microenvironment, thereby increasing production of fibrotic markers and IL-6 and promoting PDAC cell proliferation. CAF-expressed GPR68 is a mediator of low-pH–promoted regulation of the tumor microenvironments, in particular to PDAC cell–CAF interaction and may be a novel therapeutic target for pancreatic and perhaps other types of cancers.—Wiley, S. Z., Sriram, K., Liang, W., Chang, S. E., French, R., McCann, T., Sicklick, J., Nishihara, H., Lowy, A. M., Insel, P. A. GPR68, a proton-sensing GPCR, mediates interaction of cancer-associated fibroblasts and cancer cells.
Keywords: GPCR signaling pathway, pancreatic cancer, cyclic AMP, tumor microenvironment
Pancreatic ductal adenocarcinoma (PDAC), the most common type of pancreatic cancer, is currently the third-leading cause of cancer death in the United States and is predicted to be the second such cause by 2020 (1–3). The current 5-yr survival rate of PDAC is ∼8%. Factors that contribute to that high death rate include the early phase of the disease, such that, at the time of diagnosis, many patients have locally advanced or metastatic disease (1). Surgical resection, the only curative treatment, is feasible in <20% of patients. Chemotherapy of PDAC has had limited effect. The epidermal growth factor receptor–inhibitor erlotinib, an approved, targeted therapy, produces minimal clinical benefits (4, 5). New, effective treatments for pancreatic cancer are thus a major, unmet medical need.
Abundant fibrotic stroma (desmoplasia), a characteristic feature of PDAC, can comprise up to 80% of the tumor mass (6, 7). The dense stroma creates a hypovascular, hypoxic environment that contributes to drug resistance by inhibiting delivery of chemotherapeutic agents to tumor cells (8, 9). Multiple cell types in the tumor microenvironment contribute to the regulation of the stroma in PDAC, including pancreatic fibroblasts (PFs), pancreatic stellate cells (PSCs), vascular cells, and inflammatory/immune cells (9, 10). Activation of PFs and PSCs (by cytokines, growth factors, and oxidative and metabolic stress) converts them into pancreatic cancer-associated fibroblasts (CAFs), myofibroblastic cells that express abundant α-smooth muscle actin (α-SMA) and contribute to PDAC progression (11–13).
G-protein–coupled receptors (GPCRs), the largest family of cell signaling receptors (∼3% of the human genome), are 7 transmembrane receptors that respond to numerous types of extracellular signals and regulate many physiologic processes (14). GPCRs are the molecular entities most commonly targeted by U.S. Food and Drug Administration–approved drugs (15, 16). Emerging evidence implicates GPCRs in cancer: certain GPCRs have increased expression in tumors and are involved in cancer initiation and/or progression (17, 18). GPCRs can contribute to fibroblast–myofibroblast conversion (19), and increases in cellular cAMP (a second messenger for certain GPCRs) can blunt the myofibroblastic phenotype (20). Little is known regarding the role of GPCRs in CAFs. We, thus, tested the hypothesis that GPCRs expressed by pancreatic CAFs regulate the activity of CAFs and PDAC cells. Our findings provide support for this hypothesis and identify a novel pH-sensing GPCR, GPR68, as a regulator of pancreatic CAFs and CAF–PDAC cell interaction.
MATERIALS AND METHODS
Cell isolation and culture
Patient-derived PDAC tumors were diced into small pieces (0.3–0.5 mm) and embedded in growth factor reduced Matrigel (Corning, Corning, NY, USA) on a 60-mm culture dish. Prewarmed CAF medium [4.5 g/L glucose DMEM, 30% fetal bovine serum (FBS), 1 µg/ml fetuin, 20 ng/ml epidermal growth factor, 2 mM glutamate, 1 mM sodium pyruvate, nonessential amino acids, 100 IU/ml penicillin (pen), 100 µg/ml streptomycin (strep), and 0.25 µg/ml amphotericin B] was added to immerse the Matrigel. Cells were incubated in 95% air/5% CO2. After ∼6 d, explants with CAF outgrowth were harvested, suspended in PBS, and incubated at room temperature with 0.025% trypsin for 15 min. Cells collected by centrifugation at 1000 rpm for 5 min were resuspended, then transferred to and cultured on 10-cm plates with CAF medium. Primary CAFs can be cultured for 10 ∼ 15 passages before senescence occurs; we used only low passage (<15) primary CAFs. PSCs and PFs were purchased from ScienCell Research Laboratories (3830; ScienCell Research Laboratories, Carlsbad, CA, USA) and Vitro Biopharma (SC00A5; Vitro Biopharma, Golden, CO, USA), respectively, and grown according to the manufacturers’ instructions. PDAC cell lines AsPC-1, BxPC-3, and MIA PaCa-2, were purchased from American Type Culture Collection (Manassas, VA, USA). AsPC-1 and BxPC-3 were cultured in RPMI-1640 medium with 10% FBS, nonessential amino acids, sodium pyruvate, and pen–strep. MIA PaCa-2 was grown in 4.5 g/L DMEM with 10% FBS, nonessential amino acids, sodium pyruvate, and pen–strep. Cells were grown at 37°C with 95% air/5% CO2.
TaqMan GPCR array
Cells were cultured on 6-well plates until ∼80% confluent, then washed twice with cold PBS. RNA was isolated using an RNeasy kit with DNase treatment (Qiagen, Hilden, Germany) and converted to cDNA using SuperScript III (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s protocol. cDNA was diluted with double-distilled H2O and mixed with TaqMan reaction buffer (Thermo Fisher Scientific) to a final concentration of 1 μg/ml. We assayed GPCR expression using TaqMan GPCR arrays (4367785; Thermo Fisher Scientific) by a 7900HT Fast Real-Time system (Thermo Fisher Scientific). Data were analyzed with RQ Manager software (Thermo Fisher Scientific). GPCR expression was quantified as the difference between cycle threshold values (∆Ct) with 18S rRNA as the reference gene. Comparison of expression by different cells was quantified as fold-change = 2(∆∆Ct).
RNA sequencing
RNA extracted from CAFs and PSCs underwent RNA sequencing by DNA Link (San Diego, CA, USA). Libraries were prepared using the Illumina (San Diego, CA, USA) TruSeq-stranded mRNA kit and sequenced at ∼25–30 million 75 base single-reads per sample on an Illumina Nextseq500 sequencer. FASTQ files were aligned using the STAR aligner (21) on Illumina’s Basespace cloud computing/analysis service, with reads mapped to the human hg38 reference genome (refseq). BAM files with aligned reads were analyzed using Cufflinks (v.2.2.1.0) (22, 23) via Galaxy to quantify gene expression in fragments per kilobase of transcript per million mapped reads (FPKM). Cufflinks effective length correction was used for length normalization, along with multiread corrections; fragments compatible with specified reference RNAs were counted to calculate FPKMs. Counts files generated by the STAR Basespace Application were input into edgeR (24) to determine counts per million (CPM) and to perform differential expression analysis. Pairwise comparison of fold-changes in GPCR expression between individual CAF samples and PSCs was calculated from their ratio of CPM values.
Data mining of public RNA sequencing data
Archived data in the public domain stored on the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) repository (25) were mined to obtain additional gene expression data. RNAseq data (12), stored at accession number GSE43770 in SRA format, were mined for expression of GPCRs in human PSCs. SRA files were imported into Illumina’s Basespace platform and extracted FastQ files were analyzed using the same bioinformatics analysis pipeline as above, to quantify gene expression in FPKM and CPM.
Real-time quantitative PCR
RNA was isolated using an RNeasy kit with DNase treatment (Qiagen) and converted to cDNA using an iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, USA). cDNA was mixed with gene-specific primers and SYBR green reagent (Quantabio, Beverly, MA, USA) for PCR amplification using a DNA Engine Opticon 2 system (MJ Research, St. Bruno, QC, Canada). Primers were designed using Primer Premier 6 software (Premier Biosoft, Palo Alto, CA, USA). The primer sequences are listed in Supplemental Table S5. Gene expression was quantified as ∆Ct using 18S rRNA as the reference gene. We compared expression of genes in different samples using fold-change = 2(∆∆Ct).
Transfection and short interfering RNA knockdown
For transfection, we mixed 2 µg of an empty pLX304 vector or a pLX304 vector containing GPR68 (DNASU Plasmid Repository, Tempe, AZ, USA) with 8 μl FuGene HD transfection reagent (Promega, Madison, WI, USA) in Opti-MEM reduced-serum medium at room temperature for 15 min. DNA–transfection reagent complex (100 μl) was added to PSCs and incubated for 24 h.
For GPR68 knockdown, we purchased siGenome non-targeting control short interfering RNA (siRNA) and SmartPool human GPR68 siRNA (mixture of 4 GPR68 siRNAs) from Dharmacon (Lafayette, CO, USA). We cultured CAFs overnight in 6-well plates (80,000 cells/well); 2.25 ml of fresh CAF medium was then added to each well, followed by 250 µl of opti-MEM containing 7.5 µl Lipofectamine reagent and 25 pM of siRNA (final concentration, 10 nM). The cells were then incubated for 72 h.
Immunoblot analysis
Lysates were collected from cells grown in 6-well plates by scraping wells in 80 μl RIPA buffer (Cell Signaling Technology, Danvers, MA, USA) containing phosphatase and protease inhibitors. Cell lysates were homogenized by sonication. Protein concentrations were determined by BCA protein assay (Thermo Fisher Scientific). We separated proteins by SDS/PAGE in 4 ∼ 12% polyacrylamide gels (Thermo Fisher Scientific) and transferred gels to PVDF membranes using an iBlot transfer machine (Thermo Fisher Scientific). Membranes were blocked using 5% bovine serum albumin (BSA; w/v) in PBS Tween-20 and incubated at 4°C overnight with primary antibodies diluted in 1% BSA (w/v) in PBS Tween-20. Blots were then incubated with secondary antibodies conjugated with horseradish peroxidase at room temperature for 1 h. Bands were visualized by adding chemiluminescent substrate (Lumigen, Southfield, MI, USA). Densitometry quantification was analyzed by ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA). Antibodies used in this study were for α-SMA (A5228; MilliporeSigma, Billerica, MA, USA), GPR68 (sc-98437; Santa Cruz Biotechnology, Santa Cruz, CA, USA), phospho-cAMP response element binding protein (CREB; Ser133) (9198S; Cell Signaling Technology), and CREB (9197S; Cell Signaling Technology).
Immunofluorescence staining
CAFs (without or with GPR68 knockdown) were seeded on 12-mm, round coverslips (Corning) in 24-well plates (20,000 cells/well) and incubated overnight. Wells were washed twice with PBS, fixed in 2% paraformaldehyde/PBS for 10 min, and then washed with 10 mM glycine (pH 7.4) in PBS for 5 min. The cells were permeabilized in 0.1% Triton-X/PBS for 10 min at room temperature. After washing with PBS/Tween 20 (0.1% Tween), the coverslips were blocked with the addition of 1% BSA/PBS/0.05% Tween for 20 min at room temperature. Primary antibodies were diluted in 1% BSA/PBS/0.05% Tween 20 with 1:100 ratio for GPR68 antibody (sc-98437; Santa Cruz Biotechnology) and 1:1000 ratio for IL-6 antibody (ab9324; Abcam, Cambridge, MA, USA) Coverslips were incubated with diluted primary antibodies for 48 h at 4°C. After 3 washes with 1% BSA/PBS/0.05% Tween, the coverslips were incubated with secondary antibodies at room temperature for 1 h and with DAPI for nuclear staining. Images were obtained using a Carl Zeiss (Oberkochen, Germany) AxioObserver D1 microscope equipped with an LD A-Plan ×20/0.35 Ph1 objective.
Immunohistochemistry
Paraffin-embedded tissue was sectioned and placed in a microwave oven for 5 min for antigen retrieval. Immunostaining for GPR68 was performed using an automated immunostainer (Agilent Technologies, Santa Clara, CA, USA) with anti-GPR68 rabbit pAb (ab61420; Abcam), peroxidase-labeled secondary antibodies and diaminobenzidine as substrate. For fluorescence immunohistochemistry, anti-GPR68 rabbit pAb (1:500, ab61420; Abcam) and anti–α−SMA mAb (1:50, clone 1A4, code MS-113-P; Thermo Fisher Scientific) were employed as primary antibodies. Anti-mouse Alexa Fluor 594 (1:300, ab150108; Abcam) and anti-rabbit Alexa Fluor 488 (1:300, ab150073; Abcam) were used as secondary antibodies.
Coculture of PSCs with PDAC cells
PSCs were cultured overnight on 6-well plates (50,000 cells/well). AsPC-1, BxPC-3, and MIA PaCa-2 cells were cultured overnight (80,000 cells/well) on 24-mm, 6-well, Transwell-permeable supports that have a 0.4-µm pore size (Corning). The next day the Transwell supports were placed on top of PSCs and cultured for 48 h in 4.5 g glucose/L DMEM with pen–strep in the absence or presence of 10 ng/ml TNF-α neutralizing antibody (InvivoGen, San Diego, CA, USA). PSCs were then removed for mRNA purification and quantitative PCR (qPCR).
Cell viability assay
The pH medium was DMEM containing 5 g/L glucose, 20 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), 20 mM NaHCO3, 2% FBS, 100 IU/ml pen and 100 µg/ml strep, with pH adjusted by HCl or NaOH at room temperature. CAFs transfected with control or GPR68 siRNA were grown overnight in 96-well plates (10,000 cells/well). The cells were then incubated for 48 h in low-serum pH medium. Cell viability was assessed with a CellTiter-Glo luminescent assay (Promega) and detected using a DXT 800 multimode plate reader (Beckman Coulter, Brea, CA, USA).
Cell proliferation assay
CAFs were placed onto 6-well plates (80,000 cells/well) and transfected with control or GPR68 siRNA for 72 h. Cell culture medium was then changed to pH medium, and incubation was continued for 48 h. The conditioned media (CM) were passed through a 0.45-μm filter, a portion was incubated with 10 ng/ml IL-6 neutralizing antibody (InvivoGen) for 30 min and then added to BxPC-3 cells seeded in 96-well plates (10,000 cells/well). The BxPC-3 cells were cultured with CAF CM for 72 h. Cell proliferation were assessed by a CellTiter-Glo Luminescent assay (Promega) with detection using a DTX 800 multimode plate reader.
IL-6 ELISA
We collected CAF CM as just above, assayed IL-6 protein using Human IL-6 ELISA Max standard set (BioLegend, San Diego, CA, USA) using the manufacturer’s protocol and detected absorbance at 450 nm with a DTX 800 multimode plate reader.
cAMP assay
CAFs transfected with control or GPR68 siRNA were grown overnight on a 96-well plate (10,000 cells/well). Culture medium was changed to pH medium with a pH between 6.4 and 7.4 and incubated for 30 min. The cyclic nucleotide phosphodiesterase inhibitor isobutylmethylxanthine (1 mM final concentration) was added to each well. Cells were incubated (37°C) for 10 min before quantifying cAMP with the HitHunter cAMP assay (DiscoverX, Fremont, CA, USA). Detection of the luminescence was with a DTX 800 multimode plate reader.
Statistical analysis
Data were analyzed with Prism 6.0 software (GraphPad Software, La Jolla, CA, USA). Data are presented as means ± sem. Statistical comparisons were calculated with a 2-tailed, unpaired Student’s t test, 1-way ANOVA, or 2-way ANOVA. A value of P < 0.05 was considered statistically significant.
Data availability
RNA sequencing FASTQ files and gene expression data in FPKM that support the findings of this study have been deposited in the NCBI GEO database with the accession code GSE101665.
RESULTS
GPCRs expressed by pancreatic CAFs, PFs, and PSCs
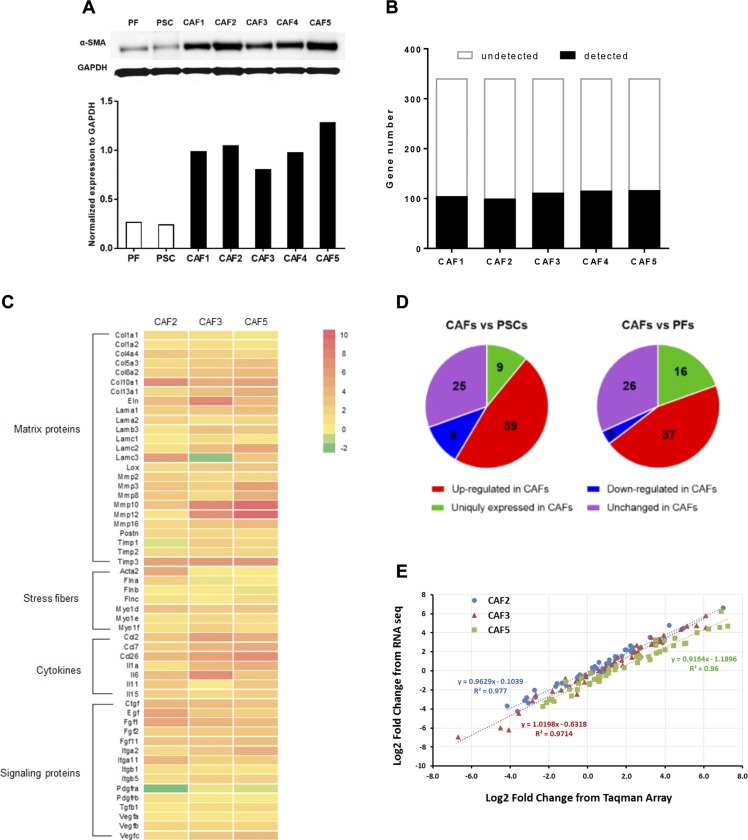
Pancreatic CAF cultures, which express substantial α-SMA, were generated from primary tumors of 5 patients with PDAC (Fig. 1A). RNA sequencing analysis confirmed that, compared with PSCs (CAF precursor cells), CAFs have a greater expression of numerous profibrotic genes, including matrix and stress-fiber proteins, cytokines, and growth factors (Fig. 1B) (12).
Figure 1.
Myofibroblastic phenotype and GPCR expression of pancreatic CAFs. A) Immunoblot of α-SMA in PFs, PSCs, and 5 primary CAF cell lines (CAF1–5); expression of α-SMA was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). B) TaqMan GPCR array analysis of nonchemosensory GPCR expression in 5 CAF lines. GPCR expression was quantified as the ΔCt between a GPCR and 18S rRNA; detected GPCRs were those with a ΔCt ≤ 25. Supplemental Table S1 lists the GPCRs detected in CAFs, PSCs, and PFs. C) Heat map of the selected CAF marker genes based on RNA sequencing analysis of PSC and 3 primary CAF cell lines (CAF2, CAF3, and CAF5). Data are presented as log2 fold-change of gene expression in each CAF compared with PSCs. Genes were categorized by 4 profibrotic features: matrix proteins, stress fibers, cytokines, and signaling proteins. D) Expression of 82 GPCRs detected in all 5 CAFs compared with PSCs and PFs. Expression difference was quantified as fold-change (fold change = 2(ΔCt_PSC or ΔCt_PF −ΔCt_CAF)). GPCRs were grouped as up-regulated (fold-change >2), down-regulated (fold-change < 0.5), unchanged (0.5 ≤ fold change ≤ 2), or uniquely expressed in CAFs (i.e., not detected in PSCs/PFs.) E) Log2-fold change of the 50 most abundant GPCRs in CAFs compared with PSCs, as determined by RNA sequencing plotted against the same from TaqMan GPCR arrays; linear regressions and correlation coefficients are indicated.
Of the >800 GPCRs in the human genome, ∼350 are nonchemosensory (other than visual, odorant, or tastant) (26) receptors. We quantified GPCRs expressed in the 5 CAF samples with TaqMan GPCR arrays, which assessed the expression of 341 nonchemosensory GPCRs; 18S rRNA was used as reference gene and a ΔCt value ≤ 25 was set as the threshold for GPCR detection. The 5 CAF samples expressed 105, 100, 112, 116, and 117 GPCRs, respectively (Fig. 1C), of which 82 GPCRs (including 31 orphan GPCRs) were shared among the 5 samples. Supplemental Table S1 lists the CAF-expressed GPCRs.
PFs and PSCs expressed 84 and 100 GPCRs, respectively. Of the 82 commonly detected GPCRs in CAFs, 39 and 37 had >2-fold greater expression than in PSCs and PFs, respectively; 25 of those GPCRs were increased in CAFs compared with both PFs and PSCs. Few GPCRs had >50% decreased expression in CAFs; adenosine A2b (ADORA2B) was the only GPCR with consistently decreased expression in CAFs. Of note, 9 and 16 GPCRs were uniquely expressed by CAFs but not by PSCs or PFs, respectively (Fig. 1D and Supplemental Tables S2 and S3).
Log2-fold changes from the TaqMan GPCR array data correlated closely with RNA sequencing data for 3 CAF cell lines, CAF2, CAF3, and CAF5 (R2 > 0.96 for all 3), providing validation for the use of TaqMan GPCR arrays as a means to assess GPCR expression (Fig. 1E). A heat map of the log2-fold change of GPCRs in CAFs compared with PSCs showed similar patterns for GPCR array and RNA sequencing data (Supplemental Fig. S1A). Four GPCRs—GPR56, GPR68, somatostatin receptor 1 (SSTR1), and GPCR class C group 5 member A (GPRC5A)—were among the 10 most highly up-regulated GPCRs in CAFs compared with both PSCs (Table 1) and PFs (Table 2). The ∆Ct values of GPR56, GPR68, SSTR1, and GPRC5A in CAFs (18.7, 17.5, 17.2, and 15.2, respectively) indicated that they were also relatively highly expressed in CAFs.
TABLE 1.
GPCRs with the greatest increase in expression in CAFs compared to PSCs
| GPCR | Fold-change | Avg. fold change | Avg. ∆Ct in CAF | ||||
|---|---|---|---|---|---|---|---|
| CAF1 vs. PSC | CAF2 vs. PSC | CAF3 vs. PSC | CAF4 vs. PSC | CAF5 vs. PSC | |||
| OXTR | 790.2 | 128.0 | 35.2 | 284.2 | 113.6 | 270.2 | 15.6 |
| GPR68 | 20.3 | 18.5 | 68.8 | 55.4 | 118.4 | 56.3 | 17.5 |
| GPR56 | 2.6 | 1.7 | 69.2 | 4.1 | 150.9 | 45.7 | 18.7 |
| GPRC5A | 59.8 | 4.9 | 49.7 | 71.7 | 33.8 | 44.0 | 15.2 |
| SSTR1 | 29.4 | 9.4 | 27.9 | 66.8 | 76.1 | 41.9 | 17.2 |
| BDKRB1 | 3.1 | 13.0 | 13.1 | 25.8 | 50.5 | 21.1 | 16.6 |
| PPYR1 | 8.3 | 60.4 | 4.1 | 4.9 | 17.1 | 18.9 | 19.7 |
| GPR37 | 4.5 | 30.9 | 0.7 | 43.3 | 1.6 | 16.2 | 18.7 |
| BDKRB2 | 1.2 | 10.4 | 11.8 | 20.6 | 32.2 | 15.2 | 18.5 |
| ADRB2 | 11.2 | 1.0 | 2.7 | 38.4 | 12.7 | 13.2 | 20.0 |
GPCRs with the greatest increase in expression in CAFs compared to PSCs. The 10 GPCRs with the greatest increases in CAFs compared to PSCs are ranked by average-fold changes. Gene expression is presented as ΔCt (with18S rRNA as reference in this and other experiments). The expression differences between CAFs and control cells were quantified as fold changes (2ΔCt_PSC − ΔCt_CAF). Average ΔCt in CAFs was calculated by log2 of mean-fold changes relative to 18s eRNA. Supplemental Table S2 compares the commonly detected 82 GPCRs in CAFs with expression in PSCs.
TABLE 2.
GPCRs with the greatest increase in expression in CAFs compared to PFs
| GPCR | Fold change | Avg. Fold- change | Avg. ∆Ct in CAF | ||||
|---|---|---|---|---|---|---|---|
| CAF1 vs. PF | CAF2 vs. PF | CAF3 vs. PF | CAF4 vs. PF | CAF5 vs. PF | |||
| GPR56 | 3.5 | 2.2 | 91.6 | 5.4 | 199.7 | 60.5 | 18.7 |
| GPR68 | 7.0 | 6.4 | 23.8 | 19.1 | 40.9 | 19.4 | 17.5 |
| F2RL1 | 1.9 | 0.8 | 17.4 | 4.4 | 59.0 | 16.7 | 16.4 |
| EDG1 | 12.2 | 5.8 | 4.3 | 3.9 | 54.4 | 16.1 | 19.5 |
| SSTR1 | 10.3 | 3.3 | 9.7 | 23.3 | 26.5 | 14.6 | 17.2 |
| GPRC5A | 19.5 | 1.6 | 16.2 | 23.4 | 11.1 | 14.4 | 15.2 |
| GPR51 | 0.8 | 3.6 | 1.8 | 19.8 | 31.9 | 11.6 | 19.4 |
| FZD4 | 12.4 | 5.5 | 11.7 | 16.0 | 10.2 | 11.2 | 16.8 |
| GPR150 | 7.8 | 2.7 | 5.5 | 16.6 | 22.1 | 11.0 | 21.2 |
| F2RL2 | 6.9 | 18.9 | 6.4 | 4.4 | 15.2 | 10.4 | 20.1 |
GPCRs with the greatest increase in expression in CAFs compared to PFs. The 10 GPCRs with expression in the greatest increases in CAFs compared to PFs are ranked by average-fold changes. Gene expression is presented as ΔCt (with18S rRNA as reference in this and other experiments). The expression differences between CAFs and control cells were quantified as fold-changes (2ΔCt_PF − ΔCt_CAF). Average ΔCt in CAFs was calculated by log2 of mean-fold changes relative to 18s rRNA. Supplemental Table S3 compares the commonly detected 82 GPCRs in CAFs with PFs.
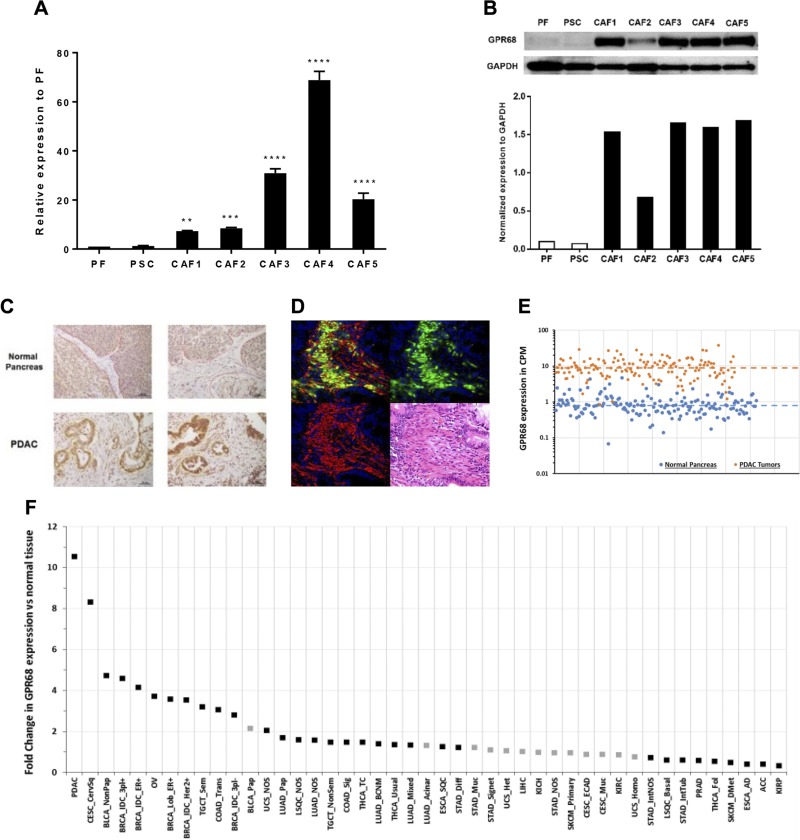
GPR68 is up-regulated in CAFs compared with PFs and PSCs and its fold-increase in PDAC compared with normal pancreas is greater than for other cancers relative to their normal precursor tissues
TaqMan GPCR array data revealed that GPR68 is the second most highly up-regulated GPCR in CAFs compared with PSCs (Table 1) and PFs (Table 2). GPR68 belongs to a family of proton-sensing GPCRs along with GPR4, GPR65, and GPR132 (27). We focused our interest on GPR68 because of the acidic microenvironment in PDAC tumors and because GPR68 has been implicated as a tumor suppressor or promoter in other types of cancer (28). Real-time qPCR confirmed that GPR68 expression is increased 7-70 fold in pancreatic CAFs compared with PSCs and PFs (Fig. 2A). Prior RNA sequencing data (12) also revealed greater GPR68 expression in pancreatic CAFs/activated PSCs (Supplemental Fig. S1B). Compared with PSCs and PFs, CAFs have increased GPR68 protein expression (Fig. 2B). The tumors of patients with PDAC (32/38 tumors; 84%) showed high GPR68 expression in the tumor stroma (Fig. 2C), CAFs in PDAC tumor stroma express both α-SMA and GPR68 (Fig. 2D), and most PDAC tumors without increased GPR68 had medullary invasion by PDAC cells and lacked CAFs (Supplemental Fig. S1C). Other proton-sensing GPCRs are not expressed by CAFs (Supplemental Fig. S2A). Analysis of publicly available RNA sequencing data showed that, on average, GPR68 expression is 10.5-fold higher in 147 PDAC tumors than in 165 healthy pancreases (Fig. 2E). PDAC has the highest fold-increase in GPR68 expression compared with healthy tissue among 45 types of cancer (Fig. 2F). GPR68 is also the only proton-sensing GPCR detected in 3 PDAC cell lines, AsPC-1, BxPC-3, and MIA PaCa-2 (Supplemental Fig. S2B).
Figure 2.
Increased GPR68 mRNA and protein expression in pancreatic CAFs and PDAC tumors. A) Real-time qPCR analysis of GPR68 expression in PSCs, PFs, and CAFs1–5. Expression of GPR68 was normalized to PFs by 2(ΔCt_PF − ΔCt_CAFs or ΔCt_PSCs). Data shown are means ± sem (n ≥ 3), 1-way ANOVA followed by Dunnett post hoc test. **P < 0.01, ***P < 0.001, ****P < 0.0001. B) Immunoblot of GPR68 in PFs, PSCs, and 5 CAF cell lines 1–5; GPR68 expression was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). C) Immunohistochemistry of GPR68 in normal (healthy) pancreatic tissues and in pancreases with PDAC. Top: healthy human pancreas with the stroma area highlighted with red, dotted lines. Bottom: PDAC tissue; cancer cells are highlighted with yellow, dotted lines. Increased GPR68 expression is seen in PDAC stromal cells and PDAC cells compared with control pancreas. D) Fluorescent immunohistochemical analysis of PDAC. Formalin-fixed, paraffin-embedded tissue was stained with anti-GPR68 Ab (green), anti-αSMA Ab (red), and DAPI (blue). The yellow signal indicates colocalization of GPR68 and αSMA in the same CAFs. E) GPR68 expression (CPM) from RNA sequencing data for PDAC tumors (from The Cancer Genome Atlas, n = 147; orange dots) and healthy pancreatic tissue [from the Genotype-Tissue Expression project (63), n = 165; blue dots]. GPR68 expression is 10.5-fold greater in PDAC tumors than in healthy tissue. [false discovery rate (FDR) = 7.37−233]. Median expression for each cohort is shown (dashed lines). GPR68 expression in PDAC tumors is greater than median expression in healthy tissue in all PDAC samples. F) Fold-changes in expression of GPR68 mRNA in 45 different types of tumors compared to normal tissue. Black squares indicate fold-changes that are (and gray squares that are not) statistically significant (FDR < 0.05). GPR68 has the greatest increase in expression in PDAC. The identity and number of replicates for each cancer type is in Supplemental Table S4.
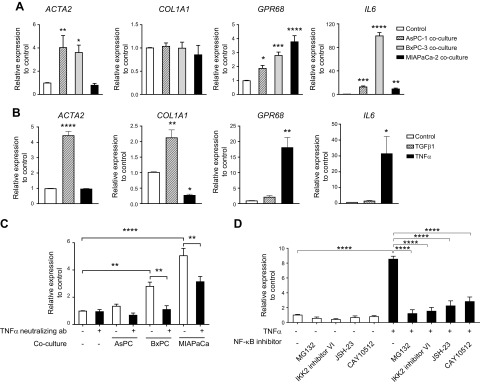
TNF-α increases GPR68 expression in PSCs
PDAC cells and PSCs have bidirectional crosstalk (29, 30). PDAC cells can activate PSCs and increase their proliferation and migration; TGF-β1 is a driver of PSC activation (31, 32). We used a Transwell coculture system to study interaction between PDACs and PSCs. Coculture of PSCs with AsPC-1, BxPC-3, or MIA PaCa-2 cells increased the expression of GPR68, α-SMA (ACTA2), and IL-6 (Fig. 3A). Incubation of PSCs with TGF-β1 increased expression of ACTA2 and COL1A1 but did not change GPR68 expression (Fig. 3B). By contrast, PSCs incubated with TNF-α had 18-fold greater expression of GPR68 and 31-fold greater expression of IL-6 but not ACTA2 or COL1A1 (Fig. 3B). These results suggest that regulation of GPR68 expression in PSCs depends on TNF-α but may be independent of TGF-β–promoted transformation of PSCs to myofibroblasts. Indeed, adding TNF-α–neutralizing antibody to the coculture system diminished the PDAC-induced increase in GPR68 expression in PSCs (Fig. 3C). In addition, MG132, IKK2 inhibitor VI, JSH-23, and CAY10512, which all inhibit NF-κB, reduced the TNF-α–promoted increase in GPR68 expression in PSCs (Fig. 3D). Together, the results imply that PDAC cells release TNF-α, which acts via NF-κB in PSCs to increase GPR68 expression, a pathway for GPR68 induction akin to that of monomac 6 cells (33). To test whether hypoxia up-regulated GPR68 expression (34), we cultured PSCs in 1% O2 for 48 h and found a 1.7-fold increase in GPR68 expression compared with that of normoxia-cultured PSCs, but hypoxia did not significantly change myofibroblast marker expression (Supplemental Fig. S2C).
Figure 3.
Coculture of PDAC cells and PSCs increases expression by PSCs of GPR68 and fibrotic genes, effects mediated by TNF-α and TGF-β, respectively. A) Real-time qPCR analysis of gene expression in PSCs cocultured for 48 h with 3 PDAC cell lines: AsPC-1, BxPC-3, and MIA PaCa-2. ΔCt values were normalized to 18S rRNA. Relative expression was compared by 2(ΔCt_control − ΔCt_coculture). Data are means ± sem, n ≥ 3, 1-way ANOVA, followed by Dunnett post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. B) Real-time qPCR analysis of gene expression in PSCs incubated for 48 h with TGF-β1 or TNF-α (each 50 ng/ml). Relative expression was compared by 2(ΔCt_control − ΔCt_TGF-β1 or ΔCt_TNF-α). Data are means ± sem, n = 3, 1-way ANOVA, followed by Dunnett post hoc test. *P < 0.05, **P < 0.01, ****P < 0.0001. C) Real-time qPCR analysis of GPR68 expression in PSCs cocultured for 48 h with AsPC-1, BxPC-3, and MIA PaCa-2 cells, with/without 10 ng/ml TNF-α–neutralizing antibody. Relative expression was compared with control by 2(ΔCt_control − ΔCt_coculture). Data are means ± sem, n = 3; 2-way ANOVA, followed by Tukey post hoc test. **P < 0.01; ****P < 0.0001. D) Real-time qPCR analysis of GPR68 expression in PSCs incubated for 6 h with TNF-α (50 ng/ml) and NF-κB inhibitors MG-132 (20 μM), IKK2 inhibitor VI (5 µM), JSH-23 (20 μM), and CAY10512 (0.3 µM). Relative expression was normalized to control by 2(ΔCt_control − ΔCt_TGF-β1 or ΔCt_TNF-α). Data are means ± sem, n = 3, 2-way ANOVA followed by Tukey post hoc test. ****P < 0.0001.
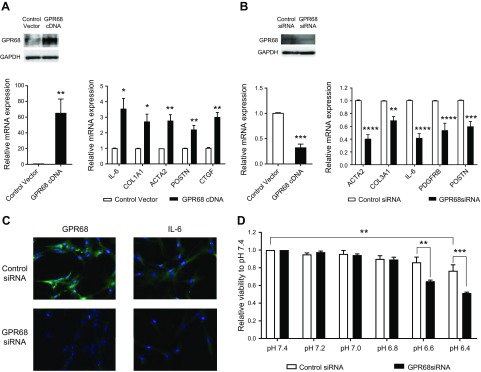
GPR68 regulates IL-6 production in CAFs
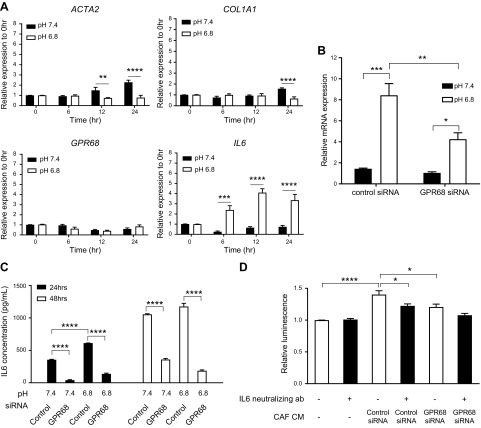
We used gain-of-function and loss-of-function approaches to assess the role of GPR68. Transfection of PSCs with GPR68 cDNA increased expression of myofibroblast markers: ACTA2, COL1A1, IL-6, POSTN, and CTGF (Fig. 4A). Knockdown of GPR68 in CAFs with siRNA decreased expression of ACTA2, COL3A1, IL-6, POSTN, and PDGFRB (Fig. 4B). IL-6 protein was reduced in CAFs with GPR68 knockdown (Fig. 4C). We tested the effect of GPR68 on cell viability in conditions that mimic the in vivo acidic tumor microenvironment (35, 36). After 48 h incubation, viability of CAFs at pH 6.4 was reduced compared with that at pH 7.4; CAFs with GPR68 knockdown had even less viability (Fig. 4D), suggesting that GPR68 may help maintain CAF viability in acidic conditions. GPR68 knockdown did not alter CAF viability at pH 7.4, 7.2, 7.0, or 6.8 (Fig. 4D) or affect growth of CAFs (for 96 h) at pH 7.4 or 6.8 (Supplemental Fig. S3A).
Figure 4.
Gain-of-function of GPR68 in PSCs and loss-of-function studies of GPR68 in pancreatic CAFs. A) PSCs transfected with GPR68 cDNA had increased GPR68 mRNA (bottom) and protein (top) expression compared with control (left). Real-time qPCR analysis of expression of fibrosis-related genes in PSCs transfected with control vector and GPR68 cDNA for 24 h. Expression was compared with the control vector. Data are means ± sem, n = 3, unpaired Student’s t test. *P < 0.05, **P < 0.01. B) CAFs transfected with GPR68 siRNA had decreased GPR68 mRNA (bottom) and protein (top) expression compared with control (left). Real-time qPCR analysis of expression of fibrosis-related genes in CAFs transfected with control siRNA or GPR68 siRNA for 72 h. Expression was compared with control siRNA. Data are means ± sem, n = 3, unpaired Student’s t test. **P < 0.01, ***P < 0.001, ****P < 0.0001. C) Immunofluorescent staining of GPR68 and IL-6 in CAFs transfected with control siRNA or GPR68 siRNA for 72 h. Green: GPR68/IL6; blue: DAPI. D) CAFs transfected with control or GPR68 siRNA were cultured at the indicated pH for 48 h. Data for cell viability are presented relative to viability at pH 7.4. Data are means ± sem, n = 3; 2-way ANOVA, followed by Tukey post hoc test. **P < 0.01, ***P < 0.001.
To further investigate the role of GPR68 in gene regulation, we incubated CAFs at pH 7.4 and pH 6.8 [a pH that activates GPR68 (27)]. At pH 6.8, expression of IL-6 increased, ACTA1 and COL1A1 decreased, and GPR68 was unchanged (Fig. 5A). GPR68 knockdown in CAFs diminished the pH 6.8–induced increase in IL-6 mRNA expression (Fig. 5B). GPR68 knockdown also decreased IL-6 protein present in the CM of CAFs cultured at pH 7.4 and 6.8 (Fig. 5C). Addition of CAF-CM enhanced PDAC (BxPC-3) cell proliferation; that response was reduced in CM treated with IL-6–neutralizing antibody or in CAFs with GPR68 knockdown (Fig. 5D). Activation of GPR68 by extracellular protons thus enhanced IL-6 expression in CAFs, and the resultant increase in IL-6 in CAF CM can stimulate PDAC cell proliferation.
Figure 5.
Effect of growth at low pH and of GPR68 on gene expression and IL-6 expression by CAFs and PDAC cell proliferation. A) Real-time qPCR analysis of expression of fibrosis-related genes in CAFs cultured in pH 7.4 or 6.8 medium for 6, 12, and 24 h. Expression was normalized to that at 0 h. Data are means ± sem, n = 3, unpaired Student’s t test. **P < 0.01, ***P < 0.001, ****P < 0.0001. B) CAFs transfected with control or GPR68 siRNA were cultured in pH 7.4 or 6.8 medium for 6 h. IL-6 expression was measured by real-time qPCR and normalized to expression with control siRNA at pH 7.4. Data are means ± sem, n = 3; 2-way ANOVA, followed by Tukey post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001. C) CAFs transfected with control or GPR68 siRNA were cultured in pH 7.4 or 6.8 medium for 24 and 48 h. IL-6 in CM was assessed by ELISA. Data are means ± sem, n = 3; 2-way ANOVA, followed by Tukey post hoc test. ****P < 0.0001. D) CEllTiter-Glo luminescent (proliferation) assay of BxPC cells cultured in CM from CAFs transfected with control or GPR68 siRNA for 72 h with or without IL-6 neutralizing antibody (ab). Data are means ± sem, n = 4; 2-way ANOVA, followed by Tukey post hoc test. *P < 0.05, ****P < 0.0001.
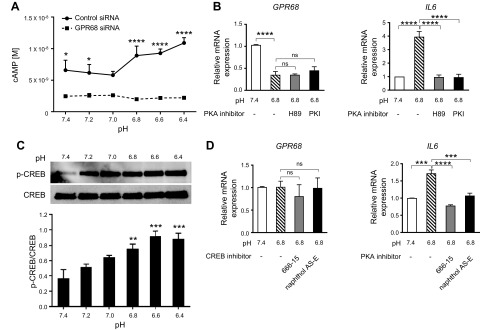
GPR68 regulates IL-6 production through cAMP-PKA-CREB in CAFs
GPR68 reportedly can couple to heterotrimeric G proteins that include Gs, Gi/o, Gq, and G12/13 (27, 37–40). Low pH increased cAMP in CAFs but not in CAFs with GPR68 knockdown, a result implying linkage of GPR68 to Gs in CAFs (Fig. 6A). Moreover, the pH 6.8–promoted increase in IL-6 expression by CAFs was blocked by 2 PKA inhibitors (H89 and PKI); neither of which altered GPR68 expression in CAFs (Fig. 6B). Low pH activation of GPR68 also increased Ser131 phosphorylation (the PKA phosphorylation site) of CREB (Fig. 6C). The CREB inhibitors naphthol AS-E (41) and 666-15 (42), which disrupt CREB–CREB-binding protein interaction and CREB-regulated gene expression, blunted pH 6.8–promoted IL-6 expression in CAFs without altering GPR68 expression (Fig. 6D).
Figure 6.
pH-dependent activation of GPR68 acts via cAMP/PKA/CREB to increase IL-6 production by pancreatic CAFs. A) CAFs transfected with control or GPR68 siRNA were cultured in pH medium for 30 min, treated with 1 mM isobutylmethylxanthine for 10 min and cellular cAMP was measured by the HitHunter cAMP assay. Data are means ± sem, n = 3; 2-way ANOVA followed by Sidak post hoc test. *P < 0.05, ****P < 0.0001. B) Real-time qPCR analysis of GPR68 and IL-6 expression in CAFs cultured for 6 h in pH 7.4, pH 6.8, or pH 6.8 medium in the presence of the PKA inhibitors, H89 and PKI. Results were normalized to those at pH 7.4. Data are means ± sem, n ≥3; 1-way ANOVA, followed by Tukey post hoc test. ****P < 0.001(ns, nonsignificant). C) Immunoblot of CREB and Ser131 phosphorylated CREB (p-CREB) of CAFs cultured in pH medium (pH 7.4–6.4) for 6 h. Activation of CREB is shown as p-CREB/CREB. Data are means ± sem, n = 3; 1-way ANOVA, followed by Dunnett post hoc test. **P < 0.01, ***P < 0.001. D) Real-time qPCR analysis of GPR68 and IL-6 expression in CAFs cultured for 6 h in pH 7.4, pH 6.8, or pH 6.8 medium in the presence of the CREB inhibitors, naphthol AS-E and 666-15. Results are normalized to those at pH 7.4. Data are means ± sem, n = 3; 1-way ANOVA, followed by Tukey post hoc test. ***P < 0.001, ****P < 0.0001(ns, nonsignificant).
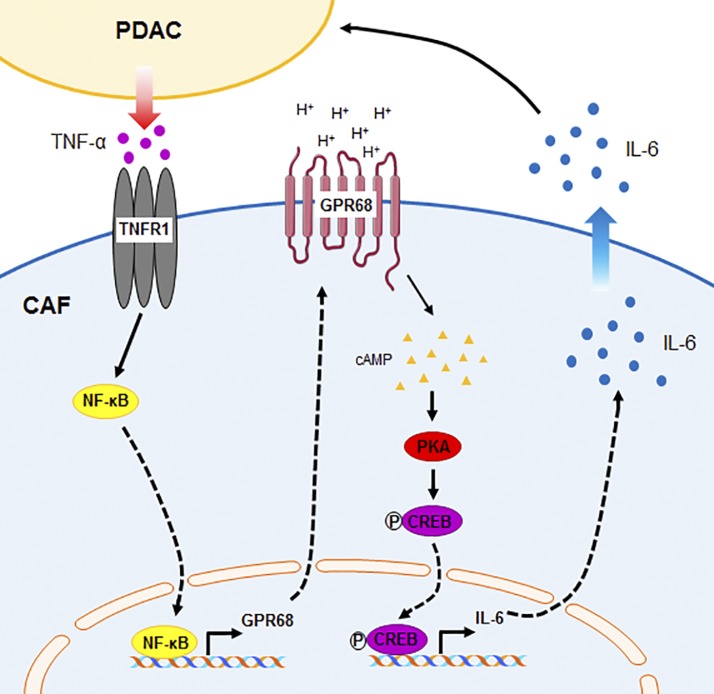
Collectively, our data reveal a novel mechanism of bidirectional interaction between PDAC cells and CAFs (Fig. 7): PDAC cells release TNF-α, which increases GPR68 expression in CAFs. Upon activation by extracellular protons, GPR68 increases expression of IL-6, which is secreted from CAFs and can stimulate PDAC cell proliferation.
Figure 7.
Schematic summary of PDAC cell–CAF interaction and GPR68. PDAC cells release TNF-α, which increases GPR68 expression by PSCs/CAFs. Extracellular protons activate GPR68 and increase IL-6 expression via the cAMP/PKA/CREB pathway. IL-6 released/secreted from CAFs can stimulate PDAC proliferation.
DISCUSSION
Prior studies have shown that GPCRs can regulate function in pancreatic cancer cells (43–46) but roles for GPCRs in CAFs and, in particular, in pancreatic CAFs, have not been known. Our use of TaqMan GPCR arrays and RNA sequencing as unbiased approaches to identify and quantify GPCRs in CAFs revealed that CAFs express many GPCRs. We observed an excellent correlation of GPCR expression data from TaqMan GPCR arrays and RNA sequencing but not with Affymetrix microarray data (not shown), which raises questions regarding the utility of such microarray data with respect to expression of GPCRs, especially because GPCRs tend express less than many other transcripts (47).
We identified several GPCRs with increased expression in CAFs compared with that of PSCs and PFs but focused on GPR68 as a proton-sensing GPCR. GPR68 is nearly undetectable in the healthy pancreas [Supplemental Fig. S3B and the Human Protein Atlas (48)] and in single-cell analysis of PSCs from patients without cancer (49). Other GPCRs with increased expression in CAFs may also have roles in pancreatic cancer (50–54). We did not assess for mutations of GPCRs because, unlike PDAC cells, CAFs do not have somatic mutations (55).
As in prior studies (29), we found that PSCs can be instructed by PDAC cells to become CAFs, at least in part by TGF-β. Because TNF-α, but not TGF-β, increases GPR68 expression in CAFs, the increase in fibrotic activity and the up-regulation of GPR68 appear to be independent events in the transformation of PSCs to CAFs.
Immunohistochemistry of tissue from patients revealed GPR68 expression in the stroma and in PDAC cells, a result consistent with GPR68 expression in 3 PDAC cells lines, AsPC-1, BxPC-3, and MIA PaCa-2, (Supplemental Fig. S2B), which suggests that GPR68 might also regulate PDAC cell function. Perhaps, in the development of pancreatic cancer, PDAC cells and CAFs both use the acidic tumor microenvironment and GPR68 to regulate features of the malignant phenotype. Furthermore, we found that gastrointestinal stromal tumor CAFs and appendix CAFs express GPR68 as the dominant member of the proton-sensing GPCRs (Supplemental Fig. S3C), indicating that CAFs from other gastrointestinal tumors, in addition to PDAC, express GPR68 suggesting roles for GPR68 in the acidic microenvironment of these and perhaps other types of tumors (56).
CAFs/activated PSCs, but not healthy PSCs, release IL-6 (54) (Fig. 4). GPR68 appears to be a previously unrecognized regulator of IL-6 production by CAFs and this appears to occur via cAMP/PKA/CREB signaling. That effect is observed at both pH 7.4 and pH 6.8 (Fig. 5C) and perhaps contributes to elevated serum IL-6 levels in patients with PDAC (57). Tumor-associated macrophages (58) and PDAC cells (59) also reportedly produce IL-6 in pancreatic cancer. It will be of interest to examine the contribution of GPR68 to IL-6 formation/secretion and that of other cytokines in other cell types found in pancreatic tumors.
Treatments directed at the PDAC stroma have been controversial, especially data implying that stromal components can restrain tumor growth (60, 61). Two subgroups of CAFs have been identified in pancreatic tumors: one adjacent to PDAC cells, and that has elevated α-SMA expression, and a second, located further from the PDAC cells, which secretes IL-6 (62). Based on those findings, the CAFs we studied, which express α-SMA and produce IL-6, are likely a mixture of the two types of CAFs.
The current results identify GPR68 as a potentially novel therapeutic target to blunt the activity of CAFs and thereby alter features of pancreatic cancer. More generally, it will be of interest to determine whether CAFs in additional types of cancer express GPR68 and use GPCR as a means to respond to their acidic microenvironments and regulate the malignant phenotype.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Jing Zhang (University of California, San Diego) for assisting us with statistical analysis. This work was supported by U.S. National Institutes of Health (NIH), National Cancer Institute (NCI) Therapeutic Training Grant 5T32CA121938, NIH/NCI Research Grants R21 CA189477, R01 CA155620, and Padres Pedal the Cause PTC2017, and by an American Society for Pharmacology and Experimental Therapeutics (ASPET)-David Lehr Research Award. The authors declare no conflicts of interest.
Glossary
- α-SMA
α smooth muscle actin
- BSA
bovine serum albumin
- CAF
cancer-associated fibroblast
- CM
conditioned medium
- CPM
counts per million
- CREB
cAMP response element binding protein
- FBS
fetal bovine serum
- FPKM
fragments per kilobase of transcript per million mapped reads
- GPCR
G-protein–coupled receptor
- GPR68
G-protein–coupled receptor 68
- PDAC
pancreatic ductal adenocarcinoma
- pen
penicillin
- PF
pancreatic fibroblast
- PSC
pancreatic stellate cell
- qPCR
quantitative PCR
- siRNA
short interfering RNA
- strep
streptomycin
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Z. Wiley, A. M. Lowy, and P. A. Insel designed the research; S. Z. Wiley, W. Liang, S. E. Chang, R. French, T. McCann, J. Sicklick, and H. Nishihara performed the research; K. Sriram performed the bioinformatics analysis; S. Z. Wiley, W. Liang, S. E. Chang, and H. Nishihara analyzed the data; and S. Z. Wiley, A. M. Lowy, and P. A. Insel wrote the paper.
REFERENCES
- 1.American Cancer Society . (2017) Cancer Facts & Figures 2017, pp. 1–71, American Cancer Society, Atlanta, GA, USA [Google Scholar]
- 2.Rahib L., Smith B. D., Aizenberg R., Rosenzweig A. B., Fleshman J. M., Matrisian L. M. (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74, 2913–2921 [DOI] [PubMed] [Google Scholar]
- 3.Siegel R. L., Miller K. D., Jemal A. (2017) Cancer statistics, 2017. CA Cancer J. Clin. 67, 7–30 [DOI] [PubMed] [Google Scholar]
- 4.Ryan D. P., Hong T. S., Bardeesy N. (2014) Pancreatic adenocarcinoma. N. Engl. J. Med. 371, 1039–1049 [DOI] [PubMed] [Google Scholar]
- 5.Kleger A., Perkhofer L., Seufferlein T. (2014) Smarter drugs emerging in pancreatic cancer therapy. Ann. Oncol. 25, 1260–1270 [DOI] [PubMed] [Google Scholar]
- 6.Erkan M., Michalski C. W., Rieder S., Reiser-Erkan C., Abiatari I., Kolb A., Giese N. A., Esposito I., Friess H., Kleeff J. (2008) The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin. Gastroenterol. Hepatol. 6, 1155–1161 [DOI] [PubMed] [Google Scholar]
- 7.Hezel A. F., Kimmelman A. C., Stanger B. Z., Bardeesy N., Depinho R. A. (2006) Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 20, 1218–1249 [DOI] [PubMed] [Google Scholar]
- 8.Neesse A., Michl P., Frese K. K., Feig C., Cook N., Jacobetz M. A., Lolkema M. P., Buchholz M., Olive K. P., Gress T. M., Tuveson D. A. (2011) Stromal biology and therapy in pancreatic cancer. Gut 60, 861–868 [DOI] [PubMed] [Google Scholar]
- 9.Erkan M., Hausmann S., Michalski C. W., Fingerle A. A., Dobritz M., Kleeff J., Friess H. (2012) The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat. Rev. Gastroenterol. Hepatol. 9, 454–467 [DOI] [PubMed] [Google Scholar]
- 10.Feig C., Gopinathan A., Neesse A., Chan D. S., Cook N., Tuveson D. A. (2012) The pancreas cancer microenvironment. Clin. Cancer Res. 18, 4266–4276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wehr A. Y., Furth E. E., Sangar V., Blair I. A., Yu K. H. (2011) Analysis of the human pancreatic stellate cell secreted proteome. Pancreas 40, 557–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherman M. H., Yu R. T., Engle D. D., Ding N., Atkins A. R., Tiriac H., Collisson E. A., Connor F., Van Dyke T., Kozlov S., Martin P., Tseng T. W., Dawson D. W., Donahue T. R., Masamune A., Shimosegawa T., Apte M. V., Wilson J. S., Ng B., Lau S. L., Gunton J. E., Wahl G. M., Hunter T., Drebin J. A., O’Dwyer P. J., Liddle C., Tuveson D. A., Downes M., Evans R. M. (2014) Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159, 80–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roshani R., McCarthy F., Hagemann T. (2014) Inflammatory cytokines in human pancreatic cancer. Cancer Lett. 345, 157–163 [DOI] [PubMed] [Google Scholar]
- 14.Vassilatis D. K., Hohmann J. G., Zeng H., Li F., Ranchalis J. E., Mortrud M. T., Brown A., Rodriguez S. S., Weller J. R., Wright A. C., Bergmann J. E., Gaitanaris G. A. (2003) The G protein-coupled receptor repertoires of human and mouse. Proc. Natl. Acad. Sci. USA 100, 4903–4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierce K. L., Premont R. T., Lefkowitz R. J. (2002) Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3, 639–650 [DOI] [PubMed] [Google Scholar]
- 16.Overington J. P., Al-Lazikani B., Hopkins A. L. (2006) How many drug targets are there? Nat. Rev. Drug Discov. 5, 993–996 [DOI] [PubMed] [Google Scholar]
- 17.Dorsam R. T., Gutkind J. S. (2007) G-protein-coupled receptors and cancer. Nat. Rev. Cancer 7, 79–94 [DOI] [PubMed] [Google Scholar]
- 18.Lappano R., Maggiolini M. (2011) G protein-coupled receptors: novel targets for drug discovery in cancer. Nat. Rev. Drug Discov. 10, 47–60 [DOI] [PubMed] [Google Scholar]
- 19.Snead A. N., Insel P. A. (2012) Defining the cellular repertoire of GPCRs identifies a profibrotic role for the most highly expressed receptor, protease-activated receptor 1, in cardiac fibroblasts. FASEB J. 26, 4540–4547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu D., Aroonsakool N., Yokoyama U., Patel H. H., Insel P. A. (2013) Increase in cellular cyclic AMP concentrations reverses the profibrogenic phenotype of cardiac myofibroblasts: a novel therapeutic approach for cardiac fibrosis. Mol. Pharmacol. 84, 787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T. R. (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts A., Trapnell C., Donaghey J., Rinn J. L., Pachter L. (2011) Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 12, R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trapnell C., Williams B. A., Pertea G., Mortazavi A., Kwan G., van Baren M. J., Salzberg S. L., Wold B. J., Pachter L. (2011) Transcript assembly and abundance estimation from RNA-seq reveals thousands of new transcripts and switching among isoforms. Nat. Biotechnol. 28, 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson M. D., McCarthy D. J., Smyth G. K. (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edgar R., Domrachev M., Lash A. E. (2002) Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bjarnadóttir T. K., Gloriam D. E., Hellstrand S. H., Kristiansson H., Fredriksson R., Schiöth H. B. (2006) Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics 88, 263–273 [DOI] [PubMed] [Google Scholar]
- 27.Ludwig M.-G., Vanek M., Guerini D., Gasser J. A., Jones C. E., Junker U., Hofstetter H., Wolf R. M., Seuwen K. (2003) Proton-sensing G-protein-coupled receptors. Nature 425, 93–98 [DOI] [PubMed] [Google Scholar]
- 28.Damaghi M., Wojtkowiak J. W., Gillies R. J. (2013) pH sensing and regulation in cancer. Front. Physiol. 4, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haqq J., Howells L. M., Garcea G., Metcalfe M. S., Steward W. P., Dennison A. R. (2014) Pancreatic stellate cells and pancreas cancer: current perspectives and future strategies. Eur. J. Cancer 50, 2570–2582 [DOI] [PubMed] [Google Scholar]
- 30.Apte M. V., Wilson J. S., Lugea A., Pandol S. J. (2013) A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology 144, 1210–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apte M. V., Haber P. S., Darby S. J., Rodgers S. C., McCaughan G. W., Korsten M. A., Pirola R. C., Wilson J. S. (1999) Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut 44, 534–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kordes C., Brookmann S., Häussinger D., Klonowski-Stumpe H. (2005) Differential and synergistic effects of platelet-derived growth factor-BB and transforming growth factor-β1 on activated pancreatic stellate cells. Pancreas 31, 156–167 [DOI] [PubMed] [Google Scholar]
- 33.De Vallière C., Wang Y., Eloranta J. J., Vidal S., Clay I., Spalinger M. R., Tcymbarevich I., Terhalle A., Ludwig M.-G., Suply T., Fried M., Kullak-Ublick G. A., Frey-Wagner I., Scharl M., Seuwen K., Wagner C. A., Rogler G. (2015) G Protein-coupled pH-sensing receptor OGR1 is a regulator of intestinal inflammation. Inflamm. Bowel Dis. 21, 1269–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Vallière C., Cosin-Roger J., Simmen S., Atrott K., Melhem H., Zeitz J., Madanchi M., Tcymbarevich I., Fried M., Kullak-Ublick G. A., Vavricka S. R., Misselwitz B., Seuwen K., Wagner C. A., Eloranta J. J., Rogler G., Ruiz P. A. (2016) Hypoxia positively regulates the expression of pH-sensing G-protein-coupled receptor OGR1 (GPR68). Cell. Mol. Gastroenterol. Hepatol. 2, 796–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gillies R. J., Liu Z., Bhujwalla Z. (1994) 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am. J. Physiol. 267, C195–C203 [DOI] [PubMed] [Google Scholar]
- 36.Van Sluis R., Bhujwalla Z. M., Raghunand N., Ballesteros P., Alvarez J., Cerdán S., Galons J. P., Gillies R. J. (1999) In vivo imaging of extracellular pH using 1H MRSI. Magn. Reson. Med. 41, 743–750 [DOI] [PubMed] [Google Scholar]
- 37.Huang X.-P., Karpiak J., Kroeze W. K., Zhu H., Chen X., Moy S. S., Saddoris K. A., Nikolova V. D., Farrell M. S., Wang S., Mangano T. J., Deshpande D. A., Jiang A., Penn R. B., Jin J., Koller B. H., Kenakin T., Shoichet B. K., Roth B. L. (2015) Allosteric ligands for the pharmacologically dark receptors GPR68 and GPR65. Nature 527, 477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mogi C., Tomura H., Tobo M., Wang J. Q., Damirin A., Kon J., Komachi M., Hashimoto K., Sato K., Okajima F. (2005) Sphingosylphosphorylcholine antagonizes proton-sensing ovarian cancer G-protein-coupled receptor 1 (OGR1)-mediated inositol phosphate production and cAMP accumulation. J. Pharmacol. Sci. 99, 160–167 [DOI] [PubMed] [Google Scholar]
- 39.Li J., Guo B., Wang J., Cheng X., Xu Y., Sang J. (2013) Ovarian cancer G protein coupled receptor 1 suppresses cell migration of MCF7 breast cancer cells via a Gα12/13-Rho-Rac1 pathway. J. Mol. Signal. 8, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh L. S., Berk M., Oates R., Zhao Z., Tan H., Jiang Y., Zhou A., Kirmani K., Steinmetz R., Lindner D., Xu Y. (2007) Ovarian cancer G protein-coupled receptor 1, a new metastasis suppressor gene in prostate cancer. J. Natl. Cancer Inst. 99, 1313–1327 [DOI] [PubMed] [Google Scholar]
- 41.Li B. X., Xiao X. (2009) Discovery of a small-molecule inhibitor of the KIX-KID interaction. ChemBioChem 10, 2721–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie F., Li B. X., Kassenbrock A., Xue C., Wang X., Qian D. Z., Sears R. C., Xiao X. (2015) Identification of a potent inhibitor of CREB-mediated gene transcription with efficacious in vivo anticancer activity. J. Med. Chem. 58, 5075–5087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryder N. M., Guha S., Hines O. J., Reber H. A., Rozengurt E. (2001) G protein-coupled receptor signaling in human ductal pancreatic cancer cells: neurotensin responsiveness and mitogenic stimulation. J. Cell. Physiol. 186, 53–64 [DOI] [PubMed] [Google Scholar]
- 44.Guha S., Eibl G., Kisfalvi K., Fan R. S., Burdick M., Reber H., Hines O. J., Strieter R., Rozengurt E. (2005) Broad-spectrum G protein-coupled receptor antagonist, [D-Arg1,D-Trp5,7,9,Leu11]SP: a dual inhibitor of growth and angiogenesis in pancreatic cancer. Cancer Res. 65, 2738–2745 [DOI] [PubMed] [Google Scholar]
- 45.Matsuo Y., Raimondo M., Woodward T. A., Wallace M. B., Gill K. R., Tong Z., Burdick M. D., Yang Z., Strieter R. M., Hoffman R. M., Guha S. (2009) CXC-chemokine/CXCR2 biological axis promotes angiogenesis in vitro and in vivo in pancreatic cancer. Int. J. Cancer 125, 1027–1037 [DOI] [PubMed] [Google Scholar]
- 46.Arafat H. A., Gong Q., Chipitsyna G., Rizvi A., Saa C. T., Yeo C. J. (2007) Antihypertensives as novel antineoplastics: angiotensin-I-converting enzyme inhibitors and angiotensin II type 1 receptor blockers in pancreatic ductal adenocarcinoma. J. Am. Coll. Surg. 204, 996–1005, discussion 1005–1006 [DOI] [PubMed] [Google Scholar]
- 47.Insel P. A., Wilderman A., Zambon A. C., Snead A. N., Murray F., Aroonsakool N., McDonald D. S., Zhou S., McCann T., Zhang L., Sriram K., Chinn A. M., Michkov A. V., Lynch R. M., Overland A. C., Corriden R. (2015) G protein-coupled receptor (GPCR) expression in native cells: “novel” endoGPCRs as physiologic regulators and therapeutic targets. Mol. Pharmacol. 88, 181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uhlén M., Fagerberg L., Hallström B. M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A., Olsson I., Edlund K., Lundberg E., Navani S., Szigyarto C. A., Odeberg J., Djureinovic D., Takanen J. O., Hober S., Alm T., Edqvist P. H., Berling H., Tegel H., Mulder J., Rockberg J., Nilsson P., Schwenk J. M., Hamsten M., von Feilitzen K., Forsberg M., Persson L., Johansson F., Zwahlen M., von Heijne G., Nielsen J., Pontén F. (2015) Proteomics: tissue-based map of the human proteome. Science 347, 1260419. [DOI] [PubMed] [Google Scholar]
- 49.Segerstolpe Å., Palasantza A., Eliasson P., Andersson E.-M., Andréasson A.-C., Sun X., Picelli S., Sabirsh A., Clausen M., Bjursell M. K., Smith D. M., Kasper M., Ämmälä C., Sandberg R. (2016) Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 24, 593–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang Y., Fan J., Yang J., Zhu G. Z. (2008) Characterization of GPR56 protein and its suppressed expression in human pancreatic cancer cells. Mol. Cell. Biochem. 308, 133–139 [DOI] [PubMed] [Google Scholar]
- 51.Ke N., Sundaram R., Liu G., Chionis J., Fan W., Rogers C., Awad T., Grifman M., Yu D., Wong-Staal F., Li Q.-X. (2007) Orphan G protein-coupled receptor GPR56 plays a role in cell transformation and tumorigenesis involving the cell adhesion pathway. Mol. Cancer Ther. 6, 1840–1850 [DOI] [PubMed] [Google Scholar]
- 52.Zhou H., Rigoutsos I. (2014) The emerging roles of GPRC5A in diseases. Oncoscience 1, 765–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li M., Wang X., Li W., Li F., Yang H., Wang H., Brunicardi F. C., Chen C., Yao Q., Fisher W. E. (2010) Somatostatin receptor-1 induces cell cycle arrest and inhibits tumor growth in pancreatic cancer. Cancer Sci. 99, 2218–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duluc C., Moatassim-Billah S., Chalabi-Dchar M., Perraud A., Samain R., Breibach F., Gayral M., Cordelier P., Delisle M.-B., Bousquet-Dubouch M.-P., Tomasini R., Schmid H., Mathonnet M., Pyronnet S., Martineau Y., Bousquet C. (2015) Pharmacological targeting of the protein synthesis mTOR/4E-BP1 pathway in cancer-associated fibroblasts abrogates pancreatic tumour chemoresistance. EMBO Mol. Med. 7, 735–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walter K., Omura N., Hong S. M., Griffith M., Goggins M. (2008) Pancreatic cancer associated fibroblasts display normal allelotypes. Cancer Biol. Ther. 7, 882–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pilon-Thomas S., Kodumudi K. N., El-Kenawi A. E., Russell S., Weber A. M., Luddy K., Damaghi M., Wojtkowiak J. W., Mulé J. J., Ibrahim-Hashim A., Gillies R. J. (2016) Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. 76, 1381–1390; erratum: 77, 2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okada S., Okusaka T., Ishii H., Kyogoku A., Yoshimori M., Kajimura N., Yamaguchi K., Kakizoe T. (1998) Elevated serum interleukin-6 levels in patients with pancreatic cancer. Jpn. J. Clin. Oncol. 28, 12–15 [DOI] [PubMed] [Google Scholar]
- 58.Lesina M., Kurkowski M. U., Ludes K., Rose-John S., Treiber M., Klöppel G., Yoshimura A., Reindl W., Sipos B., Akira S., Schmid R. M., Algül H. (2011) Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 19, 456–469 [DOI] [PubMed] [Google Scholar]
- 59.Bellone G., Carbone A., Smirne C., Scirelli T., Buffolino A., Novarino A., Stacchini A., Bertetto O., Palestro G., Sorio C., Scarpa A., Emanuelli G., Rodeck U. (2006) Cooperative induction of a tolerogenic dendritic cell phenotype by cytokines secreted by pancreatic carcinoma cells. J. Immunol. 177, 3448–3460 [DOI] [PubMed] [Google Scholar]
- 60.Rhim A. D., Oberstein P. E., Thomas D. H., Mirek E. T., Palermo C. F., Sastra S. A., Dekleva E. N., Saunders T., Becerra C. P., Tattersall I. W., Westphalen C. B., Kitajewski J., Fernandez-Barrena M. G., Fernandez-Zapico M. E., Iacobuzio-Donahue C., Olive K. P., Stanger B. Z. (2014) Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25, 735–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Özdemir B. C., Pentcheva-Hoang T., Carstens J. L., Zheng X., Wu C.-C., Simpson T. R., Laklai H., Sugimoto H., Kahlert C., Novitskiy S. V., De Jesus-Acosta A., Sharma P., Heidari P., Mahmood U., Chin L., Moses H. L., Weaver V. M., Maitra A., Allison J. P., LeBleu V. S., Kalluri R. (2014) Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734; erratum: 28, 831–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Öhlund D., Handly-Santana A., Biffi G., Elyada E., Almeida A. S., Ponz-Sarvise M., Corbo V., Oni T. E., Hearn S. A., Lee E. J., Chio I. I., Hwang C. I., Tiriac H., Baker L. A., Engle D. D., Feig C., Kultti A., Egeblad M., Fearon D. T., Crawford J. M., Clevers H., Park Y., Tuveson D. A. (2017) Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 214, 579–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lonsdale J., Thomas J., Salvatore M., Phillips R., Lo E., Shad S., Hasz R., Walters G., Garcia F., Young N., Foster B. (2013) The genotype-tissue expression (GTEx) project. Nat. Genet. 45, 580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA sequencing FASTQ files and gene expression data in FPKM that support the findings of this study have been deposited in the NCBI GEO database with the accession code GSE101665.