Abstract
Objective
Paradoxical arthritis under tumour necrosis factor inhibitor (TNF-i) for inflammatory bowel disease (IBD) has been described. This study aims to evaluate the histological features of paired synovial tissue (ST) and colonic mucosa (CM) tissue in patients with IBD developing paradoxical arthritis under TNF-i.
Methods
Patients with IBD without history of coexisting joint involvement who developed arthritis under TNF-i were enrolled. Each patient underwent ST biopsy and ileocolonoscopy with CM biopsies. ST and CM paired samples were stained through immunohistochemistry (IHC) for CD68, CD21, CD20, CD3 and CD117. Clinical and immunological parameters (anticitrullinated peptides antibodies (ACPA)-immunoglobulin (Ig)M/IgA rheumatoid factor (RF)) were collected. Psoriatic arthritis (PsA) and ACPA/IgM-RF/IgA-RF negative rheumatoid arthritis (RA) were enrolled as comparison.
Results
10 patients with IBD (age 46.0±9.7 years, 13.2±9.9 years of disease duration, 2.5±1.6 years of TNF-i exposure, six with Crohn’s disease and four with ulcerative colitis, respectively) were studied. At ST level, IHC revealed that patients with IBD with paradoxical arthritis showed more similar histological findings in terms of synovial CD68+, CD21+, CD20+, CD3+ and CD117+ cells compared with PsA than ACPA/IgM-RF/IgA-RF negative RA. Analysing the CM specimens, patients with IBD showed the presence of CD68+, CD3+, CD117+ and CD20+ cells in 100%, 70%, 60% and 50% of cases, respectively, despite endoscopic remission. Finally, addition of conventional disease-modifying antirheumatic drugs and switch to ustekinumab were more effective than swapping into different TNF-i in patients with IBD with paradoxical arthritis.
Conclusion
Patients with IBD may develop histologically proven synovitis during TNF-i, comparable to PsA. The inhibition of inflammatory pathways alternative to TNF (IL12/1L23) may be an effective therapeutic option for severe paradoxical articular manifestations.
Keywords: inflammatory bowel disease, paradoxical arthritis, TNF-inhibition, synovial tissue, colonic mucosa tissue
Key messages.
What is already known about this subject?
Patients with inflammatory bowel diseases (IBD) may develop histologically proven paradoxical synovitis under tumour necrosis factor inhibitor (TNF-i) treatment.
What does this study add?
Patients with IBD with paradoxical arthritis under TNF-i show signs of subclinical inflammation of the colonic mucosa, despite endoscopic and clinical remission.
Paradoxical synovial inflammation in patients with IBD under TNF-i shows similarities compared with patients with psoriatic arthritis in terms of synovial inflammatory cells distribution.
How might this impact on clinical practice?
The inhibition of inflammatory pathways alternative to TNF (ie, interleukin (IL)12/IL23) may be an effective therapeutic option for severe paradoxical articular manifestations in patients with IBD.
Introduction
Articular manifestations are the most common extraintestinal clinical manifestations in patients with inflammatory bowel diseases (IBD) being present in ~30% of patients.1–4 Tumour necrosis factor inhibitors (TNF-i) are currently used for the treatment of rheumatoid arthritis (RA) and psoriatic arthritis (PsA) and were subsequently demonstrated to be effective on both intestinal and extraintestinal manifestations in patients with IBD.5 6 Despite effectiveness and good tolerance for TNF-i, more than 10% of treated patients discontinue this treatment because of the occurrence of adverse events, mainly infectious and paradoxical manifestations.7 8 Articular manifestations are defined as paradoxical when they occur during treatment as TNF-i, which are expected to prevent or treat them. They are defined as disabling arthralgia9 or arthritis occurring in patients with IBD in intestinal remission or low disease activity under TNF-i, and their onset in patients with IBD under TNF-i can lead to quality of life impairment and sometimes to discontinuation of effective treatment. To date, such manifestations are poorly described, and their prevalence and pathophysiology remain unknown.9 Moreover, there are currently no predictors of their occurrence and the optimal clinical management is still matter of debate.
Based on this, the aims of the study were (1) to define the histological characteristics in terms of CD68+, CD21+, CD3+, CD20+ and CD117+ cells of synovial tissue of patients with IBD in clinical remission with newly onset of arthritis and the ultrasonographic findings (greyscale (GS) and power-Doppler (PD) signal) during TNF-i treatment; (2) to assess the histological characteristics in terms of CD68+, CD21+, CD3+, CD20+ and CD117+ cells of colonic mucosa of the same patients with IBD at the time of onset of clinical arthritis under TNF-i treatment; (3) to identify the possible similarities/differences between the synovial and intestinal compartments and (4) to assess the clinical outcome after treatment modifications (conventional (c)-disease-modifying antirheumatic drugs (DMARDs) addition, TNF-i swap or biologic (b)-DMARDs switch).
Patients and methods
Patient’s enrolment
Consecutive patients with Crohn’s disease (CD)10 or ulcerative colitis (UC)11 in stable clinical and endoscopic remission, without history of coexisting joint involvement, who developed peripheral arthritis during maintenance therapy with TNF-i, were prospectively enrolled at the Fondazione Policlinico Agostino Gemelli, Rome, Italy (presidio Columbus) from January 2015 to April 2017. At study entry, demographic features, IBD features (type, extension and disease duration) and current medications were recorded for each patient. Disease extension was classified according to the ‘Montreal Classification’.12 Baseline clinical IBD activity was assessed using the Harvey-Bradshaw Index (HBI)13 or Partial Mayo Score (PMS),14 as appropriate for each patient. Baseline endoscopic and histological assessments were performed within 15 days from study entry. Endoscopic activity was determined according to the Mayo endoscopic subscore14 for UC and to Simple Endoscopic Score (SES) for CD (SES-CD).15 Endoscopic remission was defined as a Mayo Score ≤1 for UC and a SES-CD ≤2. At study entry, each patient underwent rheumatological (SA and LP) evaluation during which clinical parameters were collected (swollen joint count (SJC), tender joint count (TJC), Disease Activity Score (DAS)). Moreover, immunological parameters (antinuclear antibodies (ANA), anticitrullinated peptides antibodies (ACPA), immunoglobulin (Ig)M-rheumatoid factor (RF) and IgA-RF) and inflammatory soluble markers (C-reactive protein serum level and erythrocyte sedimentation rate) were recorded for each patient. Patients naive to any DMARDs treatment fulfilling classification criteria for PsA16 or for IgM/IgA-RF and ACPA (Ab) negative (Ab−) RA17 were enrolled as comparison groups. After study enrolment, patients with IBD were followed-up, in an outpatient setting, using a multidisciplinary approach (rheumatologist and gastroenterologist) to discuss treatment drug modifications. The multidisciplinary team proposed the following treatment modifications: (1) addition of c-DMARDs (eg, methotrexate 10 mg/weekly for 2 weeks then increased at 15 mg/weekly or sulphasalazine starting at a dose of 1 g/daily increased up to 3 g/daily) or (2) switch to another TNF-i or (3) swap to IL-12/IL-23 inhibitor, respectively. After clinical interventions, patients were prospectively followed-up with every 2 months scheduled visits for at least 6 months. At each visit, clinical rheumatological and gastroenterological evaluations were performed. All subjects provided signed informed consent.
Ultrasound assessment
All enrolled patients underwent ultrasound (US) assessment according to the same protocol.18 Briefly, each patient underwent US evaluation using GSUS and PDUS techniques in the following joint sites bilaterally: transverse and longitudinal scanning of dorsal and volar views of the second and third metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints and longitudinal and transverse scanning of the dorsal aspect of the wrist (radiocarpal–intercarpal), bilateral knee and second to fifth metatarsophalangeal (MTP) joints. US assessment was performed by one rheumatologist (LP) experienced in US who was unaware of the clinical and laboratory findings. A commercially available real-time scanner (MyLab Twice, Esaote) equipped with a multifrequency linear probe was used at 10–14 MHz. Intrareader reliability was 0.78.
The presence and location of any synovial hypertrophy (SH) were quantified as thickness expressed in millimetres. SH was graded on the basis of greyscale images using a semiquantitative scoring method consisting of a 0–3 scale where 0=no SH (defined as SH<2.0 mm for radiocarpal and intercarpal joints, SH<0.8 mm for second and third PIP joints and SH<0.5 mm for second and third MCP and second to fifth MTP joints), 1=mild SH (defined as 2.0 mm<SH<2.9 mm for radiocarpal and intercarpal joints, 0.8 mm<SH<1.4 mm for second and third PIP joints and 0.5 mm<SH<1.9 mm for second and third MCP and second to fifth MTP joints), 2=moderate SH (defined as 3.0 mm<SH<5 mm for radiocarpal and intercarpal joints, 1.5 mm<SH<3.0 mm for second and third PIP joints and 2.0 mm<SH<4.0 mm for second and third MCP and second to fifth MTP joints) and 3=severe hypertrophy (defined as SH>5 mm for radiocarpal and intercarpal joints, SH>3.0 mm for second and third PIP joints and SH>4.0 mm for second and third MCP and second to fifth MTP joints).
PD was recorded using a semiquantitative technique consisting of a 0–3 scale where 0=no PD signal, 1=mild PD signal, 2=moderate PD signal and 3=marked PD signal. Two overall SH and PD scores were calculated as the sum of scores obtained from each joint for SH and PD.18
Immunohistochemistry for CD68, CD21, CD20, CD3 and CD117 on synovial tissue and gut mucosa paired samples
At study entry, each patient with IBD, PsA and Ab− RA enrolled underwent ST biopsy of the knee. ST biopsy was performed under US guidance, following a standardised procedure19 as follows. All patients underwent ultrasound evaluation of the knee using an ultrasound machine with a multifrequency linear transducer (MyLab Twice, Esaote). Using the ultrasound view, the best point of entrance for the needle was identified on the lateral margin of the suprapatellar recess. Each patient was provided with a face mask and cap and the whole procedure was undertaken under sterile conditions. Skin disinfection was done with iodine solution (performed twice, starting from the point of needle entrance up to 25 cm proximally and distally). Arthrocentesis of the knee joint was performed using the lateral suprapatellar access if joint effusion was present. The skin, subcutaneous tissue and joint capsule was anaesthetised with 10 mL lidocaine 2%. Next, a 14G needle (Precisa 1410-HS Hospital Service Spa, Italy) was inserted into the joint. Regions of synovial hypertrophy were identified under greyscale guidance19 to ensure sampling of representative synovial tissue. All the synovial tissue specimens obtained (at least eight pieces for each analysis) were placed on a non-woven wet gauze for collection.19
Patients with IBD underwent a video-ileocolonoscopy according to a standard technique20 with segmental colonic mucosa (CM) and ileal biopsies, as appropriate.
Once collected, ST and CM paired samples were stained through H&E and immunohistochemistry (IHC) for CD68, CD21, CD20, CD3 and CD117 using the following protocol. Briefly, ST and CM samples were stained for CD68 mouse antihuman monoclonal antibody (clone 514H12), CD21 mouse antihuman monoclonal antibody (clone 2G9), CD20 mouse antihuman monoclonal antibody (clone L26), CD3 mouse antihuman monoclonal antibody (clone LN10) or CD117 mouse antihuman monoclonal antibody (clone EP10) (all from Leica Biosystem, Newcastle, UK) by automatic immunostainer BOND MAX III (Leica). Slides were examined by two independent evaluators using a light microscope (Leica DM 2000) and all tissues were evaluated using a numerical score based on the number of CD68+, CD21+, CD3+, CD20+ and CD117+ cells in the lining and sublining areas of the section (three different fields in each section), with a score of 0 indicating no positive cells; one indicating <10% positive cells; two indicating 10%–50% positive cells and three indicating >50% positive cells.21 22 CM staining for all the assessed markers were collected and recorded as presence/absence. The inter-rater agreement coefficient was assessed for each single IHC marker (see online Supplementary table 1).
rmdopen-2018-000667supp001.doc (238.5KB, doc)
Statistical analysis
Statistical analysis was performed using SPSS V.20.0 and Prism Software (Graph-Pad, San Diego, California, USA). Categorical and quantitative variables were described as frequencies, percentage and mean±SD as appropriate. Data on demographic and clinical features were compared between patients by the non-parametric Mann-Whitney U test or χ2 test, as appropriate. Spearman’s rank correlation test was used for correlation in all analyses. A p value <0.05 was considered statistically significant. All authors had access to the study data and reviewed and approved the final manuscript.
Results
Enrolled patients’ characteristics
Ten patients with IBD (four (40.0%) UC and six (60.0%) CD, respectively) with new peripheral arthritis onset under TNF-i were consecutively enrolled. Demographic, clinical and immunological characteristics of the study cohorts are summarised in table 1. The mean duration of IBD disease was 13.2±9.9 years. As far as the disease extent is concerned, all patients with UC had previous extensive colitis and all patients with CD had previous ileocolonic localisation. Clinical activity was defined as remission or mild disease activity for each patient (median HBI of 3.8±1.0 and PMS of 2.5±1.3, respectively). Endoscopic assessment revealed remission or mild disease activity for all patients. Patients with IBD showed similar joint involvement in terms of TJC and SJC than patients with PsA and Ab− RA (table 1). No one patient with IBD had spine involvement, neither familiar nor personal history of rheumatic diseases. Smoking habit was reported in two patients (20%, one UC and one CD, respectively). Five patients (50%) had a history of previous exposure to another TNF-i. Moreover, at study enrolment, patients with IBD were equally treated with infliximab (50.0%) or adalimumab (50.0%) with a mean exposure time of 2.5±1.6 years. Four (40.0%) patients with IBD were in combination therapy with sulphasalazine (each patient at 3 g/daily) at study enrolment. ANAs were detected in higher percentage of patients with IBD (90.0%) compared with patients with Ab− RA (13.3%, p<0.001) and PsA (41.7%, p=0.02). All enrolled patients were confirmed as ACPA and IgM/IgA-RF negative. Patients with IBD had poliarticular involvement comparable to patients with Ab− RA and PsA (table 1) and none of the patients had spine involvement.
Table 1.
Demographic and clinical characteristics of the study cohorts
| IBD (n=10) | RA (n=15) | PsA (n=12) | P values* | P values† | |
| Age, years (mean±SD) | 46.0±9.7 | 55.5±17.1 | 54.7±17.2 | 0.06 | 0.20 |
| Female, n (%) | 9 (90.0) | 13 (86.7) | 4 (33.3) | 0.80 | 0.01 |
| Disease duration, years (mean±SD) | 13.2±9.9 | 0.8±0.9 | 0.8±0.2 | <0.001 | <0.001 |
| TJC44 (mean±SD) | 4.8±2.5 | 9.1±9.5 | 5.0±3.2 | 0.64 | 0.85 |
| SJC44 (mean±SD) | 4.0±2.3 | 9.0±9.3 | 4.0±2.6 | 0.28 | 0.91 |
| DAS (mean±SD) | 3.4±0.7 | 3.6±1.3 | 3.0±0.4 | 0.89 | 0.16 |
| CD/UC, n (%) | 6 (60.0)/4 (40.0) | – | – | – | – |
| Partial Mayo score (mean±SD) | 2.5±1.3 | – | – | – | – |
| Harvey-Bradshaw score (mean±SD) | 2.8±1.0 | – | – | – | – |
| ESR, mm/first hour (mean±SD) | 43.5±22.7 | 48.6±26.6 | 26.6±39.9 | 0.66 | 0.06 |
| CRP, mg/L (mean±SD) | 20.9±44.3 | 19.9±23.1 | 16.7±29.2 | 0.34 | 0.70 |
| ANA positivity, n (%) | 9 (90.0) | 2 (13.3) | 5 (41.7) | <0.001 | 0.02 |
| IgA-RF positivity, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – | – |
| IgM-RF positivity, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – | – |
| ACPA positivity, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – | – |
| Anti-TNF exposure, years (mean±SD) | 2.5±1.6 | – | – | – | – |
| Infliximab/adalimumab, n(%) | 5 (50.0)/5 (50.0) | – | – | – | – |
| Concomitant c-DMARDs, n(%) | 4 (40.0) | 0 (0.0) | 0 (0.0) | – | – |
*Patients with IBD versus patients with RA.
†Patients with IBD versus patients with PsA.
Bold values indicates p<0.05.
ACPA, anticitrullinated peptides antibodies; CD, Crohn’s disease; CRP, C-reactive protein; DAS, Disease Activity Score; DMARDs, disease-modifying antirheumatic drugs; ESR, erythrocyte sedimentation rate; IBD, inflammatory bowel disease; PsA, psoriatic arthritis; RA, rheumatoid arthritis; RF, rheumatoid factor; SJC, swollen joint count; TJC, tender joint count; TNF, tumour necrosis factor; UC, ulcerative colitis.
Ultrasonographic findings and synovitis pattern of IBD are not different compared with patients with PsA and Ab− RA
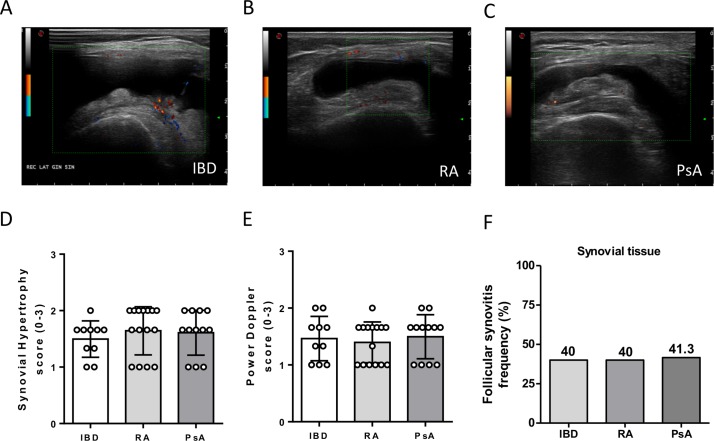
All enrolled patients underwent US assessment through GSUS and PDUS evaluation (figure 1A–C). There was no significant difference comparing patients with IBD developing arthritis during anti-TNF treatment and patients with Ab− RA or PsA naive to treatment in terms of GSUS (figure 1D) and PDUS (figure 1E) scores. Moreover, patients with IBD with paradoxical arthritis showed similar rate of follicular synovitis pattern compared with patients with PsA and Ab− RA (figure 1F).
Figure 1.
Ultrasonographic characteristics and synovitis pattern of patients with inflammatory bowel disease (IBD) with onset of peripheral arthritis under tumour necrosis factor inhibitor (TNF-i), patients with rheumatoid arthritis (RA) naive to treatment and patients with psoriatic arthritis (PsA) naive to treatment. (A) Example picture of ultrasound assessment of patient with IBD with onset of peripheral arthritis under TNF-i. (B) Example picture of ultrasound assessment of patients with RA naive to treatment. (C) Example picture of ultrasound assessment of patient with PsA naive to treatment. (D) Grey scale synovial hypertrophy score in patients with IBD (n=10) with onset of peripheral arthritis under TNF-i, patients with RA naive to treatment (n=15) and patients with PsA naive to treatment (n=12). (E) Power Doppler score of synovitis in patients with IBD (n=10) with onset of peripheral arthritis under TNF-i, patients with RA naive to treatment (n=15) and patients with PsA naive to treatment (n=12). (F) Follicular synovitis frequency in patients with IBD, RA and PsA.
Patients with IBD with paradoxical arthritis show similar histological findings in terms of synovial resident CD68+, CD21+, CD20+, CD3+ and CD117+ cells compared with patients with PsA than Ab− RA.
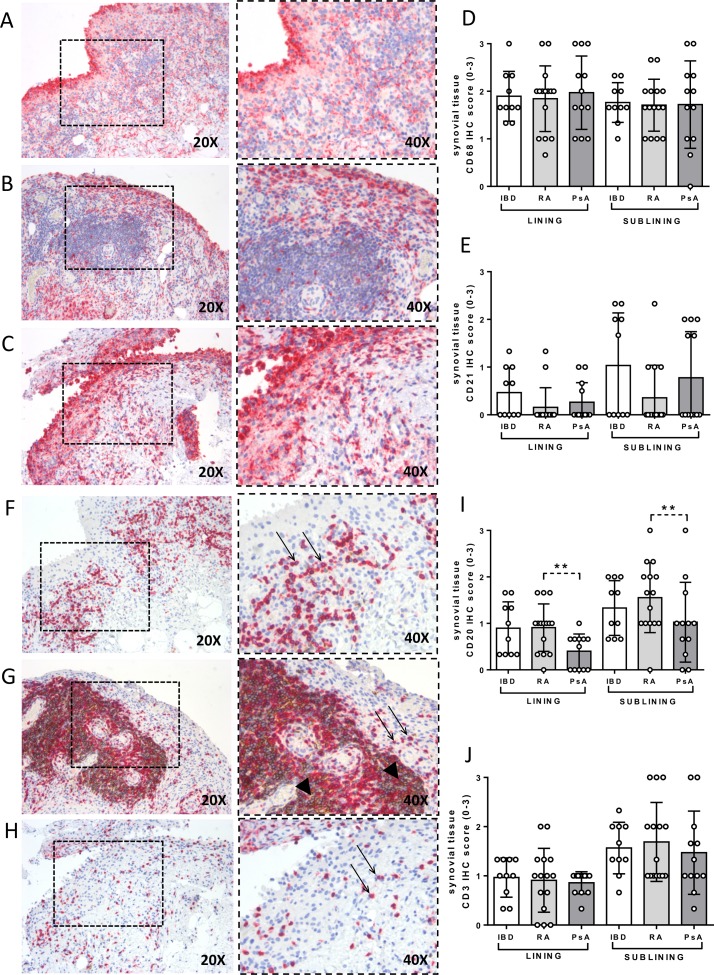
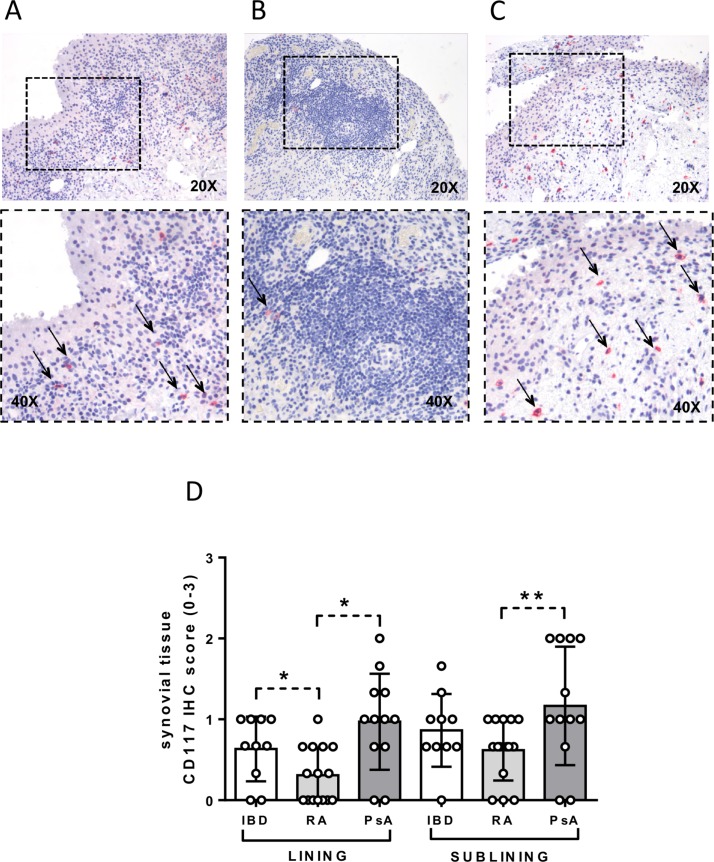
Each enrolled patient underwent US-guided ST biopsy of the knee, and IHC for CD68+, CD21+, CD20+ and CD3+ was performed to assess semiquantitative lining and sublining scores. As shown in figure2A–J, patients with IBD with paradoxical onset of arthritis showed similar IHC scores for CD68+, CD21+, CD20+ and CD3+ cells in the lining and sublining areas compared with patients with PsA and Ab− RA. However, patients with Ab− RA showed higher IHC score for CD20+ cells in the lining (0.9±0.5) and sublining area (1.6±0.8) compared with patients with PsA (lining: 0.4±0.4; p=0.01; sublining: 1.0±0.8; p=0.04) (figure 2I) (see online Supplementary table 2). Considering the presence of CD117+ cells, patients with IBD with paradoxical arthritis show higher IHC score for lining synovial CD117+ cells (0.6±0.4) compared with patients with Ab− RA (0.3±0.3; p=0.03) but similar to patients with PsA (0.9±0.2; p=0.14) (figure 3A–D). Moreover, patients with PsA showed higher IHC scores for CD117+ cells in the lining (1.0±0.6) and sublining (1.2±0.7) compared with patients with Ab− RA (lining: 0.3±0.3; p=0.001; sublining: 0.6±0.4; p=0.02) (figure 3B–D).
Figure 2.
Immunohistochemical (IHC) staining for CD68/CD21 and CD3/CD20 on synovial tissue (ST) of patients with inflammatory bowel disease (IBD) with onset of peripheral arthritis under tumour necrosis factor inhibitor (TNF-i), patients with rheumatoid arthritis (RA) naive to treatment and patients with psoriatic arthritis (PsA) naive to treatment. (A) CD68 (red)/CD21 (brown) staining of ST from patient with IBD with onset of peripheral arthritis under TNF-i treatment. (B) CD68 (red)/CD21 (brown) staining of ST from patient with RA naive to treatment. (C) CD68 (red)/CD21 (brown) staining of ST from patient with PsA naive to treatment (magnification 20× and 40× for the corresponding insert). (D) Lining and sublining IHC score for CD68+ and (E) CD21+ cells in patients with IBD (n=10) with onset of peripheral arthritis under TNF-i, patients with RA naive to treatment (n=15) and patients with PsA naive to treatment (n=12). (F) CD3 (red)/CD20 (brown) staining of ST from patient with IBD with onset of peripheral arthritis under TNF-i treatment. (G) CD3 (red)/CD20 (brown) staining of ST from patient with RA naive to treatment. (H) CD3 (red)/CD20 (brown) staining of ST from patient with PsA naive to treatment (magnification 20× and 40× for the corresponding insert). (I) Lining and sublining IHC score for CD20+ (lining IHC CD20 score in patients with RA naive to treatment vs patients with PsA naive to treatment, *p<0.05; sublining IHC CD20 score in patients with RA naive to treatment vs patients with PsA naive to treatment, *p<0.05) and (J) CD3+ cells in patients with IBD (n=10) with onset of peripheral arthritis under TNF-i, patients with RA naive to treatment (n=15) and patients with PsA naive to treatment (n=10).
Figure 3.
Immunohistochemical (IHC) staining for CD117 on synovial tissue (ST) of patients with inflammatory bowel disease (IBD) with onset of peripheral arthritis under tumour necrosis factor inhibitor (TNF-i), patients with rheumatoid arthritis (RA) naive to treatment and patients with psoriatic arthritis (PsA) naive to treatment. (A) CD117 (red) staining of ST from patient with IBD with onset of peripheral arthritis under TNF-i treatment (magnification 20× and 40× for the corresponding insert) (thin black arrows indicate CD117+ cells). (B) CD117 (red) staining of ST from patient with RA naive to treatment (magnification 20× and 40× for the corresponding insert) (thin black arrow indicates CD117+ cells). (C) CD117 (red) staining of ST from patient with PsA naive to treatment (magnification 20× and 40× for the corresponding insert) (thin black arrows indicate CD117+ cells). (D) Lining and sublining IHC score for CD117+ cells in patients with IBD (n=10) with onset of peripheral arthritis under TNF-i, patients with RA naive to treatment (n=15) and patients with PsA naive to treatment (n=12); lining IHC CD117 score in patients with IBD versus patients with RA naive to treatment, *p<0.02; lining IHC CD117 score in patients with RA naive to treatment versus patients with PsA naive to treatment, *p<0.02; sublining IHC CD117 score in patients with RA naive to treatment versus patients with PsA naive to treatment, **p<0.05.
Colonic mucosa of patients with IBD with paradoxical arthritis under TNF-i showed histological signs of subclinical inflammation despite clinical and endoscopic remission
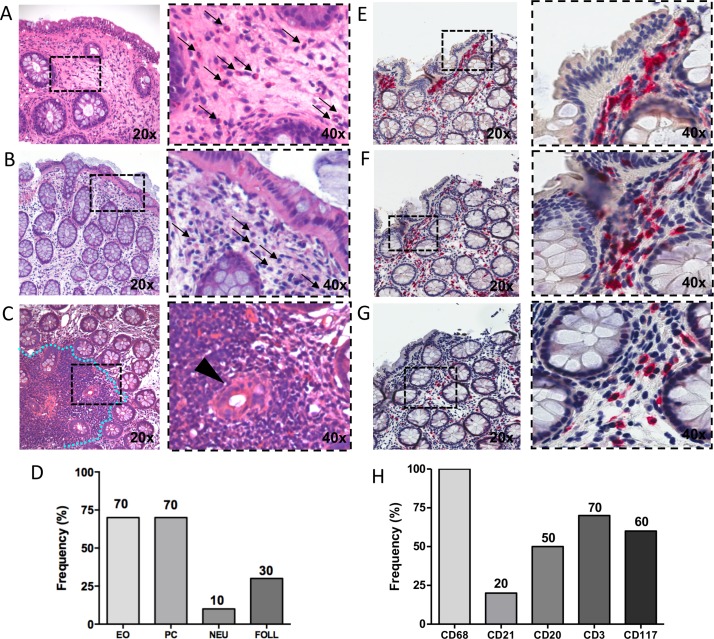
In this setting of clinical and endoscopic remission or mild disease activity, patients with IBD showed signs of histologically proven subclinical inflammation as shown in figure 4A–H. In detail, 70% of patients with IBD showed the presence of eosinophils in the lamina propria (figure 4A), 70% plasma cells (figure 4B) and 30% gut inflammatory follicles (figure 4C) at H&E staining (figure 4D). Moreover, the analysis of the inflammatory infiltrates by IHC revealed that all patients with IBD showed the presence of CD68+ (figure 4E), 70% of CD3+ (figure 4F) and 60% of CD117+ cells (figure 4G), respectively, within the lamina propria (figure 4H).
Figure 4.
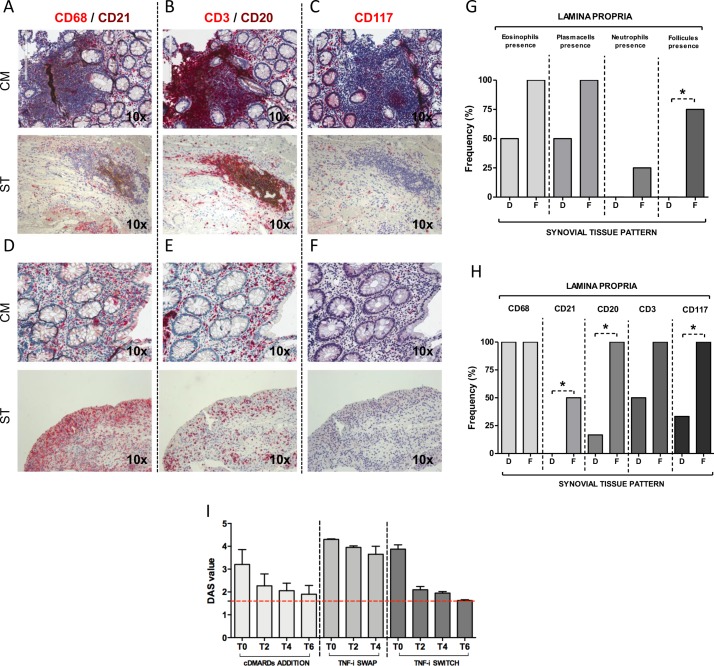
H&E and immunohistochemical (IHC) staining for CD68/CD21, CD3/CD20 and CD117 on colonic mucosa of patients with inflammatory bowel disease (IBD) in disease remission/low disease activity with onset of peripheral arthritis under TNF-i. (A) H&E staining of colonic mucosa of patient with IBD in disease remission with onset of peripheral arthritis under TNF-i showing the presence of eosinophils (thin black arrows). (B) Plasma cells (thin black arrows) and (C) gut follicles (blue dotted line) and cripts damage (back arrow head) (magnification 20× and 40× for the corresponding insert). (D) Frequency of eosinophils (EO), plasma cells (PC), neutrophils (NEU) and gut follicles (FOLL) presence in the colonic mucosa biopsies of patients with IBD (n=10) in disease remission/low disease activity with onset of peripheral arthritis under TNF-i. (E) CD68 (red)/CD21 (brown) staining of colonic mucosa from patient with IBD with onset of peripheral arthritis under TNF-i treatment. (F) CD3 (red)/CD20 (brown) staining of colonic mucosa from patient with IBD with onset of peripheral arthritis under TNF-i treatment. (G) CD117 (red) staining of colonic mucosa biopsy from patient with IBD with onset of peripheral arthritis under TNF-i treatment (magnification 20× and 40× for the corresponding insert). (H) Frequency of CD68+, CD21+, CD3+, CD20+ and CD117+ cells presence in the colonic mucosa biopsies of patients with IBD (n=10) in disease remission/low disease activity with onset of peripheral arthritis under TNF-i.
Direct correlation between histological findings in ST and colonic mucosa paired samples of patients with IBD with paradoxical arthritis under TNF-i
Comparing the ST and colonic histological features using ST and CM paired samples of patients with IBD with paradoxical arthritis under TNF-i, there was a direct correlation between the inflammatory cells infiltrate in ST and CM in terms of CD68+, CD21+, CD20+, CD3+ and CD117+ cells (figure 5A−F). As shown in figure 5G, three (75.0%) patients with IBD with follicular synovitis compared with no patients with IBD with diffuse synovitis (p<0.05) showed gut follicular structures. Moreover, 10 (100.0%) patients with IBD with follicular synovitis showed the presence of CD20+ and CD117+ cells and 50.0% presence of CD21+ cells in the gut lamina propria, compared with patients with IBD with diffuse synovitis pattern showing presence in the gut lamina propria of CD20+ cells in two (20%), CD117+ cells in three (30.0%) and none with the presence of CD21+ cells (p<0.05 for each comparison) (figure 5H). c-DMARDs addition or b-DMARDs switch showed higher efficacy than TNF-i swap in patients with IBD with paradoxical arthritis under TNF-i.
Figure 5.
Correlations of CD68, CD21, CD3, CD20 and CD117 immunohistochemical staining between paired synovial tissue (ST) and colonic mucosa (CM) biopsies of patients with inflammatory bowel disease (IBD) in disease remission/low disease activity with onset of peripheral arthritis under tumour necrosis factor inhibitor (TNF-i) and clinical response to different treatment changes. (A, D) CD68 (red)/CD21 (brown); (B, E) CD3 (red)/CD20 (brown) staining and (C, F) CD117 (red) staining of CM and ST paired biopsies from patient with IBD with onset of arthritis under TNF-i (magnification 20×). (G) Frequency rate of eosinophils, plasma cells, neutrophils and gut follicles presence in the lamina propria of the CM based on the synovitis pattern; *p<0.05: gut follicles frequency in the lamina propria of CM of patients with IBD with follicular (F) versus diffuse (D) synovitis. (H) frequency of CD68+, CD21+, CD3+, CD20+ and CD117+ cells in the lamina propria of CM based on the synovitis pattern; *p<0.05 comparing patients with IBD with F versus D synovitis. (I) Disease Activity Score (DAS) in patients with IBD in disease remission/low disease activity with onset of peripheral arthritis under TNF-i after conventional-disease-modifying antirheumatic drugs (DMARDs) addition, TNF-i swap or switch. Data are reported as mean±SD. Dotted red line indicates reference for DAS<1.6. T0: study entry; T2, 4, 6: 2, 4 or 6 months follow-up.
Among patients with IBD, six (60.0%) patients added a c-DMARD to their previous TNF-i treatment, whereas four (40.0%) patients changed their TNF-i ((two (50.0%) patients swapped into another TNF-i and two (50.0%) switched from TNF-i into ustekinumab, respectively) (see online Supplementary table 3), based on the clinical indication provided after multidisciplinary (rheumatologist and gastroenterologist) discussion. As shown in Supplementary table 3, patients with IBD who changed their TNF-i (n=4) had more extensive joint involvement (SJC 6.0±2.3 and TJC 7.0±1.8) and higher disease activity (DAS 4.1±0.4) compared with patients with IBD receiving simple c-DMARDs addition (n=6) (SJC 2.7±0.8; TJC 3.3±1.5 and DAS 3.1±0.5; p=0.02 for each comparison). As shown in figure 5I, all patients with IBD receiving c-DMARDs (sulphasalazine and/or methotrexate) in addition to their previous TNF-i, showed reduction of DAS after 6 months follow-up. Moreover, among patients with IBD who changed their previous TNF-i treatment, only patients with IBD switching to ustekinumab showed reduction of DAS compared with patients with IBD swapping to another TNF-i. With regard to IBD activity, none of the patients experienced a disease worsening or a flare during study follow-up.
Discussion
In this cohort study, we have defined the histopathological features of tissue inflammation of synovial and colonic mucosa paired samples of patients with IBD in clinical and endoscopic remission who developed paradoxical arthritis under TNF-i treatment, showing histological similarities mainly with naive to treatment patients with PsA used as comparison. Moreover, we found a direct correlation between synovial and gut compartments in terms of microanatomical organisation of the inflammatory infiltrate which may support the good clinical response to IL12/IL23 inhibition for the treatment of severe articular paradoxical manifestations.
TNF-α is a proinflammatory cytokine with a known crucial role in the activation of immune cells.23 While its role in triggering inflammation is well known, some TNF-α mediated pathways still remain unknown. The introduction of selective pharmacological inhibitors of TNF-α has revolutionised the management of patients with IBD.24 However, patients with IBD treated with TNF-i may experience paradoxical clinical manifestations involving the joint and the skin.25 Articular manifestations are the most common extraintestinal clinical manifestations in patients with IBD, being detected in nearly one-third of patients.2–4 Articular manifestations are defined paradoxical when they occur during treatment, as TNF-i, that are expected to prevent or treat them, and in contrast to paradoxical cutaneous ones, they have been poorly described.
Paradoxical articular manifestations occur in patients with IBD in intestinal clinical remission or low disease activity and should be considered in case of exclusion of previous known rheumatic diseases.26 In a prospective study including 80 patients with IBD, Thiebault and colleagues reported a prevalence of 11% of paradoxical articular manifestations related to TNF-i usage.26
The mechanism of paradoxical inflammation induced by TNF-i is still a matter of debate. TNF-i are able to bind the Fab fragment to the membrane TNF-α promoting its reverse signalling inducing lymphocyte and macrophages apoptosis by activating the intrinsic mitochondrial apoptosis pathway.27 However, it has been found that, in addition to binding with their Fab fragment to TNF-α, infliximab and adalimumab are able to bind with their Fc region to the Fc receptor CD64 and CD161/32 expressed by monocytes and macrophages.28 The activation of these Fc receptors may lead to the paradoxical release of proinflammatory cytokines as IL-12 and IL-23 by immune cells.29 In particular, IL-12 was found to promote T-helper type 1 responses playing a crucial role in colitis30 and arthritis,31 while, IL-23 is a heterodimeric cytokine which orchestrate the inflammatory cascade enhancing increased levels of TNF-α, IL-6, IFN-γ and IL-17 within the gut–synovium axis.32 33 The presence of CD117+ cells in the synovial tissue (as well as in the lamina propria) suggests that mast cells may concur to the promotion of synovial much more than colonic mucosa inflammation, being a source of IL12/IL23.34
The majority of patients with IBD developing paradoxical arthritis under TNF-i treatment were found to be positive for antinuclear antibody26 35 supporting the notion that exposure to cell fragments due to TNF-i treatment may trigger the formation of organ non-specific autoantibodies such as ANA. In our study cohort, the majority (90%) of patients with IBD showed positivity for ANA, before the occurrence of arthritis, similarly to previously reported cohorts36 suggesting that paradoxical manifestations are more likely to occur in patients who are generally more susceptible to autoimmune phenomena. Moreover, in our study cohort, there was no correlation between the development of such manifestations and the presence of antibodies against TNF-i or over-rate drug plasma levels as previously described in a case series, by van Moerkercke, including 1300 patients receiving infliximab or adalimumab for IBD.37
To our knowledge, this is the first study, analysing the histological features of ST and CM paired samples in IBD patients developing paradoxical onset of arthritis during TNF-i treatment, despite clinical and endoscopic state of intestinal remission. At ST level, IHC revealed that IBD patients with paradoxical onset of arthritis under TNF-i are characterised by similar synovial infiltrates than PsA and ACPA/RF negative RA patients naïve to any pharmacological treatment, in terms of CD68+, CD21+, CD3+, CD20+ cells. However, a stronger similarity was found between IBD patients with paradoxical arthritis and naïve PsA patients analysing CD117+ cells synovial distribution, which was significantly higher than ACPA/RF negative RA patients. In addition, despite all IBD patients enrolled in the present study were in clinical and endoscopic remission, IHC assessment revealed that signs of subclinical inflammation were detected in almost all patients. To date, there is no agreement on the definition of histological remission of IBD with the development of several histological scores,38 but not all of them validated. Eosinophils and plasma cells presence are common findings at the histological evaluation of CM of IBD patients in clinical remission38 suggesting the presence of subclinical inflammation, despite absence of intestinal symptoms.
To date, there is no agreement on the management of paradoxical manifestations in patients with IBD under TNF-i. Tillack and colleagues reported that paradoxical psoriatic lesions did not improve after swapping infliximab to adalimumab, whereas switch to ustekinumab lead to clinical improvement in all treated patients without significant flare of CD.39 40 In our study, within a multidisciplinary (rheumatologist/gastroenterologist) discussion approach, patients with IBD who developed paradoxical arthritis under monotherapy with TNF-i were treated with addition of c-DMARDs (sulphasalazine or methotrexate) without TNF-i modification, whereas patients with IBD under already combined c-DMARDs+TNF-i treatment underwent TNF-i switch or TNF-i swap into IL12/IL23 inhibitor maintaining stable c-DMARDs therapy. Interestingly, addition of c-DMARDs and switch to ustekinumab were found to be more effective than swapping into different TNF-i in patients with IBD with paradoxical arthritis, suggesting that selective TNF inhibition may be directly responsible for the paradoxical manifestations onset. In particular, ST of patients with IBD with paradoxical arthritis showed high presence of CD117+ cells, similarly than patients with PsA, which were not found to be influenced by TNF inhibition.41 This finding suggests that the upstream inhibition of IL-17, by IL12/IL23 inhibitor, may be an efficacious option for the treatment of such manifestations, manly in patients with disabling arthritis.
In conclusion, paradoxical articular manifestations, developed in patients with IBD in intestinal remission under TNF-i, may include severe peripheral arthritis with similar joint burden as PsA and ACPA/RF negative RA. Moreover, despite clinical and endoscopic remission, patients with IBD with paradoxical articular manifestations show signs of subclinical gut inflammation and there is a direct correlation between the histological microanatomical organisation of synovial tissue and gut inflammation in such patients. Finally, despite the limited number of patients, c-DMARDs addition or b-DMARDs switch showed higher efficacy than TNF-i swap in patients with IBD with paradoxical arthritis under TNF-i.
Acknowledgments
We would like to thank Dr Maria Rita Gigante and Dr Clara Di Mario for assistance in synovial tissue biopsy performance and sample handling.
Footnotes
Contributors: SA, GF, AA and EG conceived the study. SA, DP, LP, LG and LM provided clinical support to patients follow-up. SA, BT, LB and FF performed experiments. SA, BT and GF performed statistical analysis. SA, DP, BT, GLR, FF, GF, AA and EG interpreted data. SA, DP, GF, AA and EG gave critical revision of the manuscript for important intellectual content. All authors have read and accepted the submitted version of the manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: This work was approved by local ethics committee
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional unpublished data are available.
References
- 1. Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol 2011;106:110–9. 10.1038/ajg.2010.343 [DOI] [PubMed] [Google Scholar]
- 2. Harbord M, Annese V, Vavricka SR, et al. The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis 2016;10:239–54. 10.1093/ecco-jcc/jjv213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brakenhoff LK, van der Heijde DM, Hommes DW, et al. The joint-gut axis in inflammatory bowel diseases. J Crohns Colitis 2010;4:257–68. 10.1016/j.crohns.2009.11.005 [DOI] [PubMed] [Google Scholar]
- 4. Orchard TR, Wordsworth BP, Jewell DP. Peripheral arthropathies in inflammatory bowel disease: their articular distribution and natural history. Gut 1998;42:387–91. 10.1136/gut.42.3.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herfarth H, Obermeier F, Andus T, et al. Improvement of arthritis and arthralgia after treatment with infliximab (Remicade) in a German prospective, open-label, multicenter trial in refractory Crohn’s disease. Am J Gastroenterol 2002;97:2688–90. 10.1111/j.1572-0241.2002.06064.x [DOI] [PubMed] [Google Scholar]
- 6. Kaufman I, Caspi D, Yeshurun D, et al. The effect of infliximab on extraintestinal manifestations of Crohn’s disease. Rheumatol Int 2005;25:406–10. 10.1007/s00296-004-0467-8 [DOI] [PubMed] [Google Scholar]
- 7. Cleynen I, Van Moerkercke W, Billiet T, et al. Characteristics of skin lesions associated with anti-tumor necrosis factor therapy in patients with Inflammatory bowel disease: a cohort study. Ann Intern Med 2016;164:10–22. 10.7326/M15-0729 [DOI] [PubMed] [Google Scholar]
- 8. Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut 2009;58:492–500. 10.1136/gut.2008.155812 [DOI] [PubMed] [Google Scholar]
- 9. Sondag M, Verhoeven F, Guillot X, et al. “Paradoxical” arthralgia occurring under anti-TNFα treatment for inflammatory bowel disease. Joint Bone Spine 2018;85:133–4. 10.1016/j.jbspin.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 10. Gomollón F, Dignass A, Annese V, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis 2017;11:3–25. 10.1093/ecco-jcc/jjw168 [DOI] [PubMed] [Google Scholar]
- 11. Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis 2017;11:649–70. 10.1093/ecco-jcc/jjx008 [DOI] [PubMed] [Google Scholar]
- 12. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19:5A–36. 10.1155/2005/269076 [DOI] [PubMed] [Google Scholar]
- 13. Harvey RF, Bradshaw JM. A simple index of Crohn’S-disease activity. The Lancet 1980;315:514 10.1016/S0140-6736(80)92767-1 [DOI] [PubMed] [Google Scholar]
- 14. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625–9. 10.1056/NEJM198712243172603 [DOI] [PubMed] [Google Scholar]
- 15. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc 2004;60:505–12. 10.1016/S0016-5107(04)01878-4 [DOI] [PubMed] [Google Scholar]
- 16. Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. 10.1002/art.21972 [DOI] [PubMed] [Google Scholar]
- 17. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. 10.1136/ard.2010.138461 [DOI] [PubMed] [Google Scholar]
- 18. Alivernini S, Peluso G, Fedele AL, et al. Tapering and discontinuation of TNF-α blockers without disease relapse using ultrasonography as a tool to identify patients with rheumatoid arthritis in clinical and histological remission. Arthritis Res Ther 2016. 18:39 10.1186/s13075-016-0927-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van de Sande MG, Gerlag DM, Lodde BM, et al. Evaluating antirheumatic treatments using synovial biopsy: a recommendation for standardisation to be used in clinical trials. Ann Rheum Dis 2011;70:423–7. 10.1136/ard.2010.139550 [DOI] [PubMed] [Google Scholar]
- 20. Fefferman DS, Farrell RJ. Endoscopy in inflammatory bowel disease: indications, surveillance, and use in clinical practice. Clin Gastroenterol Hepatol 2005;3:11–24. 10.1016/S1542-3565(04)00441-0 [DOI] [PubMed] [Google Scholar]
- 21. Alivernini S, Tolusso B, Petricca L, et al. Synovial features of patients with rheumatoid arthritis and psoriatic arthritis in clinical and ultrasound remission differ under anti-TNF therapy: a clue to interpret different chances of relapse after clinical remission? Ann Rheum Dis 2017;76:1228–36. 10.1136/annrheumdis-2016-210424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alivernini S, Kurowska-Stolarska M, Tolusso B, et al. MicroRNA-155 influences B-cell function through PU.1 in rheumatoid arthritis. Nat Commun 2016;7:12970 10.1038/ncomms12970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 2008;117:244–79. 10.1016/j.pharmthera.2007.10.001 [DOI] [PubMed] [Google Scholar]
- 24. Ford AC, Sandborn WJ, Khan KJ, et al. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2011;106:644–59. 10.1038/ajg.2011.73 [DOI] [PubMed] [Google Scholar]
- 25. Cleynen I, Vermeire S. Paradoxical inflammation induced by anti-TNF agents in patients with IBD. Nat Rev Gastroenterol Hepatol 2012;9:496–503. 10.1038/nrgastro.2012.125 [DOI] [PubMed] [Google Scholar]
- 26. Thiebault H, Boyard-Lasselin P, Guignant C, et al. Paradoxical articular manifestations in patients with inflammatory bowel diseases treated with infliximab. Eur J Gastroenterol Hepatol 2016;28:876–81. 10.1097/MEG.0000000000000643 [DOI] [PubMed] [Google Scholar]
- 27. Watson AJ. In vivo single-photon emission computed tomography imaging of apoptosis in Crohn’s disease and anti-tumour necrosis factor therapy. Gut 2007;56:461–3. 10.1136/gut.2006.111286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wojtal KA, Rogler G, Scharl M, et al. Fc gamma receptor CD64 modulates the inhibitory activity of infliximab. PLoS One 2012;7:e43361 10.1371/journal.pone.0043361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Niess JH, Danese S. Anti-TNF and skin inflammation in IBD: a new paradox in gastroenterology? Gut 2014;63:533–5. 10.1136/gutjnl-2013-304683 [DOI] [PubMed] [Google Scholar]
- 30. Simpson SJ, Shah S, Comiskey M, et al. T cell-mediated pathology in two models of experimental colitis depends predominantly on the interleukin 12/Signal transducer and activator of transcription (Stat)-4 pathway, but is not conditional on interferon gamma expression by T cells. J Exp Med 1998;187:1225–34. 10.1084/jem.187.8.1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gottlieb A, Menter A, Mendelsohn A, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet 2009;373:633–40. 10.1016/S0140-6736(09)60140-9 [DOI] [PubMed] [Google Scholar]
- 32. Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest 2006;116:1310–6. 10.1172/JCI21404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med 2003;198:1951–7. 10.1084/jem.20030896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nakano N, Nishiyama C, Kanada S, et al. Involvement of mast cells in IL-12/23 p40 production is essential for survival from polymicrobial infections. Blood 2007;109:4846–55. 10.1182/blood-2006-09-045641 [DOI] [PubMed] [Google Scholar]
- 35. Van Moerkercke W, Ackaert C, Kasran A. Severe auto-immune driven arthralgia as a new side effect in anti-TNFα treated IBD patients? J Crohns Colitis 2010;4:S11. [Google Scholar]
- 36. Vermeire S, Noman M, Van Assche G, et al. Autoimmunity associated with anti-tumor necrosis factor alpha treatment in Crohn’s disease: a prospective cohort study. Gastroenterology 2003;125:32–9. 10.1016/S0016-5085(03)00701-7 [DOI] [PubMed] [Google Scholar]
- 37. Fiorino G, Danese S, Pariente B, et al. Paradoxical immune-mediated inflammation in inflammatory bowel disease patients receiving anti-TNF-α agents. Autoimmun Rev 2014;13:15–19. 10.1016/j.autrev.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 38. Bryant RV, Winer S, Travis SP, et al. Systematic review: histological remission in inflammatory bowel disease. Is ’complete' remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis 2014;8:1582–97. 10.1016/j.crohns.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 39. Tillack C, Ehmann LM, Friedrich M, et al. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-γ-expressing Th1 cells and IL-17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut 2014;63:567–77. 10.1136/gutjnl-2012-302853 [DOI] [PubMed] [Google Scholar]
- 40. Pugliese D, Guidi L, Ferraro PM, et al. Paradoxical psoriasis in a large cohort of patients with inflammatory bowel disease receiving treatment with anti-TNF alpha: 5-year follow-up study. Aliment Pharmacol Ther 2015;42:880–8. 10.1111/apt.13352 [DOI] [PubMed] [Google Scholar]
- 41. Noordenbos T, Yeremenko N, Gofita I, et al. Interleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum 2012;64:99–109. 10.1002/art.33396 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2018-000667supp001.doc (238.5KB, doc)