Abstract
BACKGROUND
Limited population-based information is available on the co-occurrence of dementia and Parkinson disease (PD). However, projecting the prevalence of PD with and without dementia over the next 50 years is crucial for planning public-health and patient-care initiatives.
OBJECTIVES
To project the prevalence of PD with and without dementia in the United States by 2060.
METHODS
We used the Rochester Epidemiology Project medical records-linkage system to identify all persons with PD with or without dementia residing in Olmsted County, Minnesota on 1 January 2006. A movement-disorders specialist reviewed the complete medical records of each person to confirm the presence of PD. We calculated the age- and sex-specific prevalence of PD with and without dementia and projected US prevalence through 2060.
RESULTS
We identified 296 persons with PD with and without dementia on the prevalence date (187 men, 109 women); the overall prevalence increased with age from 0.01% (ages 30–39 years) to 2.83% (≥90 years). The prevalence of PD without dementia increased with age from 0.01% (30–39 years) to 1.25% (≥90 years). The prevalence of PD with dementia increased with age from 0.10% (60–69 years) to 1.59% (≥90 years). The prevalence was higher in men than in women for all subtypes and all age groups. We project by 2060 an approximate doubling of the number of persons with PD without dementia and a tripling of the number of persons with PD with dementia in the United States.
CONCLUSIONS
The prevalence of PD with and without dementia increases with age and is higher in men than women. We project that the number of persons with PD in the US will increase substantially by 2060.
Keywords: Parkinson’s disease, dementia, prevalence, Rochester Epidemiology Project (REP), Olmsted County, projections
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease (AD). The incidence of PD has been reported to be 14.2 per 100,000 person-years overall, to be higher in men than in women, and to increase with age [1]. According to a 2014 meta-analysis, the prevalence of PD increases with older age, from 41 per 100,000 (0.04%) in persons aged 40 to 49 years to 1,903 per 100,000 (1.9%) in persons aged 80 years and older [2]. Two studies have projected a dramatic increase in the number of persons living with PD and dementia with Lewy bodies (DLB) in the coming years [3, 4], and one study projected a significant economic burden of patients with PD in the United States [5]. Earlier recognition and better therapeutic management of the clinical symptoms of PD have increased the prevalence of PD in recent years due to improved survival. The somewhat inconsistent results across several prevalence studies can be explained by the different clinical criteria used to diagnose PD. On the other hand, the studies converge on reporting a lower prevalence of PD in women and in Asia [2]. Of note, there is limited information on the prevalence of PD with dementia (PDD), DLB, and PD with other dementia (PD/OD). These data are crucial to understand the impact of PD — with and without dementia —on public health.
To address questions regarding the burden of PD with and without dementia in the aging population, we calculated its prevalence on January 1, 2006, in Olmsted County, MN, and we projected the number of persons affected by PD from 2015 to 2060 in the US.
Methods
Ascertainment of Patients with Parkinsonism
Details about the ascertainment of parkinsonism patients are reported elsewhere [1, 6]. Briefly, we used the medical records-linkage system of the Rochester Epidemiology Project (REP) to identify all persons with parkinsonism residing in Olmsted County, Minnesota, between 1976 and 2010. The REP essentially indexes and links all medical information for the county population [7–10]. All medical diagnoses, surgical interventions, and other procedures are available as computerized indexes using the Hospital Adaptation of the International Classification of Diseases, Eighth Revision (H-ICDA) [11] and the International Classification of Diseases, Ninth Revision (ICD-9) [12].
We ascertained potential cases of parkinsonism using a computerized-screening phase and a subsequent clinical-confirmation phase as described in the original two incidence studies for the periods 1976–1990 and 1991–2005 [1, 13]. The complete medical records of all persons receiving at least one screening diagnostic code for Parkinsonism were reviewed by a movement-disorders specialist (JHB and RS) using specifically designed abstracting forms and instruction manuals. The movement-disorders specialist established the onset year and type of parkinsonism using specified diagnostic criteria [1, 13–16]. Further details about the case-finding procedures are published elsewhere [1, 13].
For this study, we identified all persons affected by PD with or without dementia alive and residing in Olmsted County, MN, on January 1, 2006. Because we selected our prevalence day (January 1, 2006) at the end of a combined-incidence study period from 1976 to 2005, we included all persons with incident PD over the 30 years who survived to the prevalence day. In addition, we included persons who developed PD while living outside of Olmsted County but resided in the county on the prevalence day. These persons were not included in the incidence studies but were eligible for this prevalence study. The Institutional Review Boards of the Mayo Clinic and Olmsted Medical Center approved the study.
Diagnostic Criteria
Our diagnostic adjudication included two steps: the definition of parkinsonism as a syndrome and the definition of different types of parkinsonism within the syndrome. We defined parkinsonism as the presence of at least two of four cardinal signs: rest tremor, bradykinesia, rigidity, and impaired postural reflexes. Among persons fulfilling the parkinsonism criteria, we applied diagnostic criteria for specific types of parkinsonism and grouped parkinsonism patients into presumed proteinopathies [6].
PD was defined as parkinsonism with all three of the following: no other cause (e.g., repeated stroke with stepwise progression, repeated head injury, history of encephalitis, neuroleptic treatment within 6 months before symptom onset, hydrocephalus, brain tumor); no documented unresponsiveness to levodopa at doses of at least 1 g/d in combination with carbidopa (applicable only to treated patients); and no prominent or early (<1 year of onset) signs of more extensive nervous system involvement (e.g., dysautonomia) not explained otherwise [13]. We utilized previously published consensus criteria to define DLB, PDD, and PD/OD (Table 1) [1, 6, 17, 18].
Table 1.
Diagnostic criteria for specific types of Parkinson disease
Parkinson Disease without dementia (PD): All 4 of:
|
Parkinson Disease with Dementia (PDD): Both of:
|
Dementia with Lewy Bodies (DLB): Both of:
|
Parkinson Disease/Other Dementia (PD/OD): Both of:
|
To determine whether a person had dementia on the prevalence day, he/she had to fulfill one of two criteria: 1) a physician diagnosis of dementia before prevalence day; or 2) documentation in the medical record of loss of independence in everyday activities clearly related to cognitive difficulties (not motor), not in the context of delirium or other mental disorder, occurring before the prevalence day.
The case-finding procedures were valid and reliable as described more extensively elsewhere [1, 13]. In brief, an independent records review by the two movement-disorders specialists applying the same diagnostic criteria (JHB and RS) showed 90% agreement on the presence of parkinsonism and 70% agreement on the exclusion of parkinsonism (kappa = 0.60; 95% CI, 0.31–0.89; sample classified by RS as 30 patients with parkinsonism and 10 persons free of parkinsonism from the 1991–2005 incidence study) [1]. In general, the agreement on the year of onset of parkinsonism was also high (intra-class correlation coefficient, 0.85; 95% CI, 0.77–0.92) [1]. Finally, a comparison of clinical diagnoses of specific proteinopathies (synucleinopathies and tauopathies) with autopsy findings in the 65 patients who had died showed 81.5% overall agreement [1]. For synucleinopathies, the sensitivity was 97.8% (95% CI, 88.5–99.9), the specificity was 42.1% (95% CI, 20.3–66.5), the positive predictive value (PPV) was 80.4% (95% CI, 67.6–89.8), and the negative predictive value (NPV) was 88.9% (95% CI, 51.8–99.7). For tauopathies, the sensitivity was 40.0% (95% CI, 5.3–85.3), the specificity was 100.0% (95% CI, 94.0–100.0), the PPV was 100.0% (95% CI, 15.8–100.0), and the NPV was 95.2% (95% CI, 86.7–99.0).
Data Analysis
We chose January 1, 2006, as our prevalence date and excluded persons who denied authorization to use their medical records for research [10]. We included all persons who met criteria for PD with or without dementia, with symptom onset before the prevalence date, and with residence in Olmsted County on the prevalence date. We computed age- and sex-specific prevalence for PD overall and for specific subtypes of PD. Because our study was descriptive and involved the entire Olmsted County population, no sampling procedures were involved, and statistical tests were not necessary for the interpretation of the data. Prevalence was directly standardized by age and by sex to the 2010 US Census population (when applicable), and projections were made using age- and sex-specific prevalence and US population estimates available from the US Census [19]. All analyses utilized SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Table 2 shows the results of the prevalence of PD, PDD, DLB, PD/OD and all types of PD combined. We identified 296 persons with prevalent PD of all types; 187 (63.2%) were men, and 109 (36.8%) were women. The overall prevalence increased with age from 0.01% (ages 30–39 years) to 2.83% (ages 90 years and older). The prevalence of PD without dementia increased with age from 0.01% (ages 30–39 years) to 1.25% (ages 90 years and older). The prevalence of PD with dementia increased with age from 0.10% (ages 60–69 years) to 1.59% (ages 90 years and older) (Figure 1).
Table 2.
Age- and sex-specific prevalence of Parkinson disease on January 1, 2006 in Olmsted County, MN.
| Type of PD | Prevalence, % (No. cases) *
|
All ages | Age- stand.† |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age group, years
| ||||||||||
| 0–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | 90+ | |||
| All types of Parkinson disease (PD, PDD, DLB, and PD/OD) | ||||||||||
| Men | 0 (0) | 0.01 (1) | 0.02 (2) | 0.11 (9) | 0.68 (33) | 2.40 (72) | 4.02 (56) | 6.64 (14) | 0.27 (187) | 0.38 |
| Women | 0 (0) | 0 (0) | 0 (0) | 0.07 (6) | 0.38 (20) | 0.82 (30) | 1.79 (42) | 1.64 (11) | 0.15 (109) | 0.15 |
| Both sexes | 0 (0) | 0.01 (1) | 0.01 (2) | 0.09 (15) | 0.52 (53) | 1.53 (102) | 2.62 (98) | 2.83 (25) | 0.21 (296) | 0.24 |
|
| ||||||||||
| Parkinson disease without dementia (PD) | ||||||||||
| Men | 0 (0) | 0.01 (1) | 0.02 (2) | 0.11 (9) | 0.54 (26) | 1.37 (41) | 2.30 (32) | 2.37 (5) | 0.17 (116) | 0.23 |
| Women | 0 (0) | 0 (0) | 0 (0) | 0.07 (6) | 0.32 (17) | 0.57 (21) | 0.85 (20) | 0.89 (6) | 0.10 (70) | 0.10 |
| Both sexes | 0 (0) | 0.01 (1) | 0.01 (2) | 0.09 (15) | 0.42 (43) | 0.93 (62) | 1.39 (52) | 1.25 (11) | 0.13 (186) | 0.15 |
|
| ||||||||||
| Parkinson disease with dementia (PDD, DLB, and PD/OD) | ||||||||||
| Men | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.14 (7) | 1.03 (31) | 1.72 (24) | 4.27 (9) | 0.10 (71) | 0.15 |
| Women | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.06 (3) | 0.25 (9) | 0.94 (22) | 0.74 (5) | 0.05 (39) | 0.05 |
| Both sexes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.10 (10) | 0.60 (40) | 1.23 (46) | 1.59 (14) | 0.08 (110) | 0.09 |
|
| ||||||||||
| Parkinson disease dementia (PDD) | ||||||||||
| Men | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.10 (5) | 0.47 (14) | 0.93 (13) | 0.95 (2) | 0.05 (34) | 0.07 |
| Women | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.02 (1) | 0.08 (3) | 0.56 (13) | 0.45 (3) | 0.03 (20) | 0.03 |
| Both sexes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.06 (6) | 0.26 (17) | 0.70 (26) | 0.57 (5) | 0.04 (54) | 0.04 |
|
| ||||||||||
| Dementia with Lewy bodies (DLB) | ||||||||||
| Men | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.02 (1) | 0.53 (16) | 0.65 (9) | 1.42 (3) | 0.04 (29) | 0.06 |
| Women | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.04 (2) | 0.14 (5) | 0.26 (6) | 0.15 (1) | 0.02 (14) | 0.02 |
| Both sexes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.03 (3) | 0.32 (21) | 0.40 (15) | 0.45 (4) | 0.03 (43) | 0.03 |
|
| ||||||||||
| Parkinson disease / Other dementia (PD/OD) | ||||||||||
| Men | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.02 (1) | 0.03 (1) | 0.14 (2) | 1.90 (4) | 0.01 (8) | 0.02 |
| Women | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.03 (1) | 0.13 (3) | 0.15 (1) | 0.01 (5) | 0.01 |
| Both sexes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.01 (1) | 0.03 (2) | 0.13 (5) | 0.57 (5) | 0.01 (13) | 0.01 |
The prevalence percent can be computed by dividing the number in parentheses by the corresponding denominator and multiplying by 100. Denominators for men were: ages 0–29, 30,738; ages 30–39, 9,471; ages 40–49, 10,508; ages 50–59, 8,011; ages 60–69, 4,851; ages 70–79, 2,996; ages 80–89, 1,394; ages 90+, 211; and all ages, 68,180. Denominators for women were ages 0–29, 31,975; ages 30–39, 9,821; ages 40–49, 11,140; ages 50–59, 8,591; ages 60–69, 5,333; ages 70–79, 3,653; ages 80–89, 2,342; ages 90+, 672; and all ages, 73,527. Denominators for both sexes were: ages 0–29, 62,713; ages 30–39, 19,292; ages 40–49, 21,648; ages 50–59, 16,602; ages 60–69, 10,184; ages 70–79, 6,649; ages 80–89, 3,736; ages 90+, 883; and all ages, 141,707.
Directly standardized to the total 2010 US Census population. The prevalence directly standardized by age and by sex to the total 2010 US Census population was 0.24 for all types of PD, 0.15 for PD without dementia; 0.09 for PD with dementia; 0.04 for PDD; 0.04 for DLB; and 0.01 for PD/OD.
Figure 1.
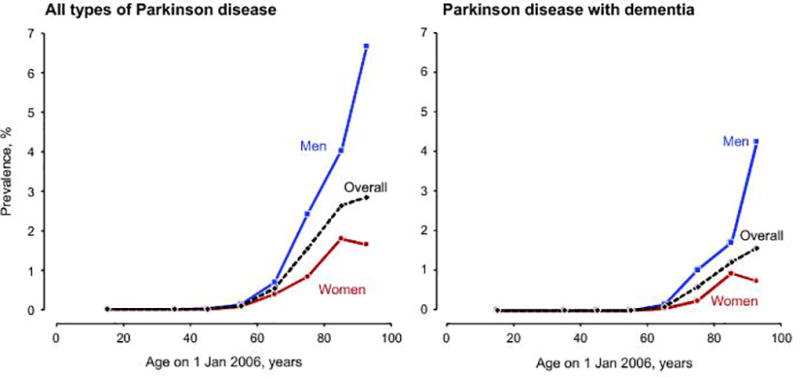
Age- and sex-specific prevalence of all types of Parkinson disease and of Parkinson disease with dementia on January 1, 2006 in Olmsted County, MN.
The overall prevalence was 0.03% (43 persons) for DLB and 0.04 (54 persons) for PDD; in both subtypes, men were more commonly affected than women, and the prevalence increased with older age. However, there was a small decline in the prevalence of PDD, DLB, and all types of PD with dementia in women 90 years old and older. The proportion of PD patients with dementia increased with older age (Figure 2). The prevalence of PD was higher in men than in women for all subtypes of PD and across all ages.
Figure 2.
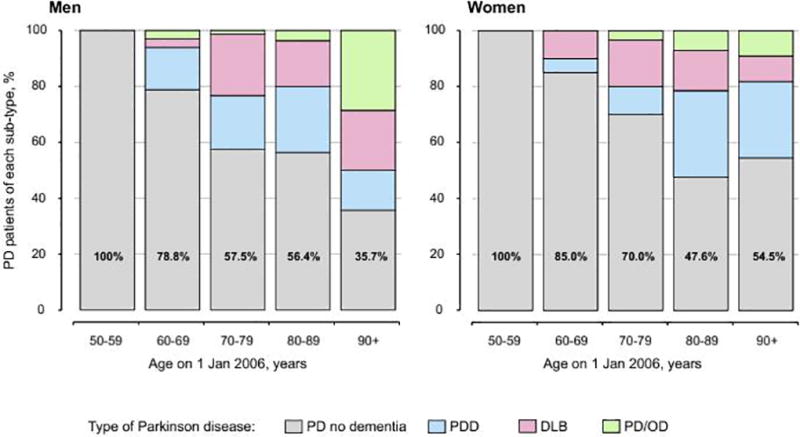
Age- and sex-specific percentages of subtypes of PD. The numbers displayed in the gray bars are the percentage of all PD patients without dementia.
Table 3 shows the prevalence of PD overall and by subtypes for 6 segments of the population with a 10-years moving age cut-off: persons ≥ 40, ≥ 50, ≥ 60, ≥ 70, ≥ 80, and ≥ 90 years of age. The prevalence of PD increases with age within each decade, and men were affected more than women. In particular, the prevalence of all types of PD was 1.3% by age ≥60, 2.0% by ages ≥70 years, and 2.8% by ages ≥ 90 years.
Table 3.
Prevalence of Parkinson disease at a given age or older on January 1, 2006, in Olmsted County, MN.
| Type of PD | Prevalence rate, % (No. cases) *
|
||||||
|---|---|---|---|---|---|---|---|
| All ages | Age group (moving age cut-off)
|
||||||
| ≥ 40 y | ≥ 50 y | ≥ 60 y | ≥ 70 y | ≥ 80 y | ≥ 90 y | ||
| All types of Parkinson disease (PD, PDD, DLB, and PD/OD) | |||||||
| Men | 0.27 (187) | 0.66 (186) | 1.05 (184) | 1.85 (175) | 3.09 (142) | 4.36 (70) | 6.64 (14) |
| Women | 0.15 (109) | 0.34 (109) | 0.53 (109) | 0.86 (103) | 1.24 (83) | 1.76 (53) | 1.64 (11) |
| Both sexes | 0.21 (296) | 0.49 (295) | 0.77 (293) | 1.30 (278) | 2.00 (225) | 2.66 (123) | 2.83 (25) |
|
| |||||||
| Parkinson disease without dementia (PD) | |||||||
| Men | 0.17 (116) | 0.41 (115) | 0.65 (113) | 1.10 (104) | 1.70 (78) | 2.31 (37) | 2.37 (5) |
| Women | 0.10 (70) | 0.22 (70) | 0.34 (70) | 0.53 (64) | 0.70 (47) | 0.86 (26) | 0.89 (6) |
| Both sexes | 0.13 (186) | 0.31 (185) | 0.48 (183) | 0.78 (168) | 1.11 (125) | 1.36 (63) | 1.25 (11) |
|
| |||||||
| Parkinson disease with dementia (PDD, DLB, and PD/OD) | |||||||
| Men | 0.10 (71) | 0.25 (71) | 0.41 (71) | 0.75 (71) | 1.39 (64) | 2.06 (33) | 4.27 (9) |
| Women | 0.05 (39) | 0.12 (39) | 0.19 (39) | 0.33 (39) | 0.54 (36) | 0.90 (27) | 0.74 (5) |
| Both sexes | 0.08 (110) | 0.18 (110) | 0.29 (110) | 0.51 (110) | 0.89 (100) | 1.30 (60) | 1.59 (14) |
|
| |||||||
| Parkinson disease dementia (PDD) | |||||||
| Men | 0.05 (34) | 0.12 (34) | 0.19 (34) | 0.36 (34) | 0.63 (29) | 0.93 (15) | 0.95 (2) |
| Women | 0.03 (20) | 0.06 (20) | 0.10 (20) | 0.17 (20) | 0.28 (19) | 0.53 (16) | 0.45 (3) |
| Both sexes | 0.04 (54) | 0.09 (54) | 0.14 (54) | 0.25 (54) | 0.43 (48) | 0.67 (31) | 0.57 (5) |
|
| |||||||
| Dementia with Lewy bodies (DLB) | |||||||
| Men | 0.04 (29) | 0.10 (29) | 0.17 (29) | 0.31 (29) | 0.61 (28) | 0.75 (12) | 1.42 (3) |
| Women | 0.02 (14) | 0.04 (14) | 0.07 (14) | 0.12 (14) | 0.18 (12) | 0.23 (7) | 0.15 (1) |
| Both sexes | 0.03 (43) | 0.07 (43) | 0.11 (43) | 0.20 (43) | 0.35 (40) | 0.41 (19) | 0.45 (4) |
|
| |||||||
| Parkinson disease / Other dementia (PD/OD) | |||||||
| Men | 0.01 (8) | 0.03 (8) | 0.05 (8) | 0.08 (8) | 0.15 (7) | 0.37 (6) | 1.90 (4) |
| Women | 0.01 (5) | 0.02 (5) | 0.02 (5) | 0.04 (5) | 0.07 (5) | 0.13 (4) | 0.15 (1) |
| Both sexes | 0.01 (13) | 0.02 (13) | 0.03 (13) | 0.06 (13) | 0.11 (12) | 0.22 (10) | 0.57 (5) |
The prevalence percent can be computed by dividing the number in parentheses by the corresponding denominator and multiplying by 100. Denominators for men were: all ages, 68,180; ages ≥ 40, 27,971; ages ≥ 50, 17,463; ages ≥ 60, 9,452; ages ≥ 70, 4,601; ages ≥ 80, 1,605; ages ≥ 90, 211. Denominators for women were: all ages, 73,527; ages ≥ 40, 31,731; ages ≥ 50, 20,591; ages ≥ 60, 12,000; ages ≥ 70, 6,667; ages ≥ 80, 3,014; ages ≥ 90, 672. Denominators for both sexes were: all ages, 141,707; ages ≥ 40, 59,702; ages ≥ 50, 38,054; ages ≥ 60, 21,452; ages ≥ 70, 11,268; ages ≥ 80, 4,619; ages ≥ 90, 883.
Figure 3 shows the projected number of persons with PD of all types and PD with dementia from 2015 to 2060 in the United States. We project that the number of persons living with PD of all types will increase significantly from approximately 866,000 persons in 2015 to 1.96 million by 2060 (a 2.26 times increase; approximately a doubling). During the same time period, we project that the number of persons living with PD with dementia (PDD, DLB, and PD/OD) will increase from 312,000 persons in 2015 to 810,000 by 2060 (a 2.60 times increase; approximately a tripling).
Figure 3.
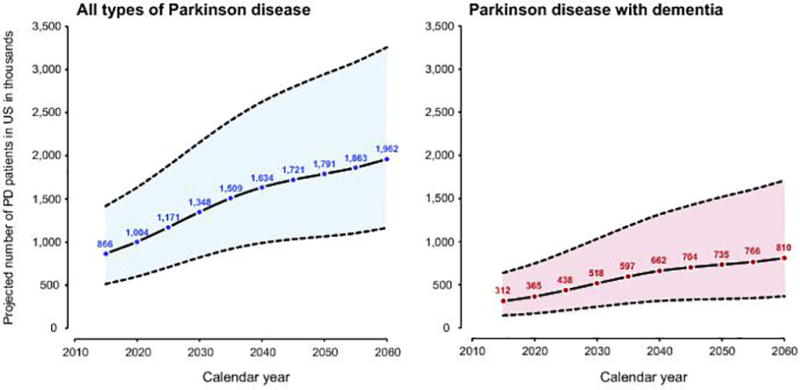
Projected number of persons with all types of Parkinson disease and with Parkinson disease with dementia from 2015 through 2060 in the US. The shaded area represents the upper and lower bounds of prevalence estimates based on the 95% confidence intervals of the age- and sex-specific prevalence from the Olmsted County, Minnesota population applied to the US Census projections.
Discussion
Our study suggests that the prevalence of PD without dementia and of PD with dementia (PDD, DLB, and PD/OD) increases with older age and is more common in men than women. We project the number of persons with PD of all types to approximately double and the number of persons with PD with dementia to approximately triple by 2060 in the United States.
Our findings are consistent with the findings from a recent meta-analysis comparing the prevalence of PD across several studies [2]. The prevalence of PD was report to increase from 41 per 100,000 (0.04%) in persons ages 40 to 49 years to 1,903 per 100,000 (1.9%) for persons ages 80 years older. In addition, the meta-analysis showed geographical differences with lower prevalence of PD in Asia compared with Europe, North America, and Australia [2].
The use of different clinical criteria may explain some of the variability in prevalence estimates [2]. The use of different diagnostic criteria may account for up to a 36% change in the identification of cases [20]. Similarly, variability may be a result of different medical personnel making the diagnosis of PD (e.g., specialists versus general practitioners). Interestingly, although major methodological differences are present in different studies, some epidemiological patterns are consistent. The prevalence of PD increases consistently with older age, and prevalence is consistently higher in men than women across almost all studies [2].
The prevalence of PD with dementia (including PDD, DLB, and PD/OD) has not been as extensively studied as the prevalence of PD without dementia. Studies have likely been hampered by difficulties in identification of cases due to complex clinical criteria and by the limited access to complete records. DLB has been reported as the second most common type of degenerative dementia, accounting for approximately 10 to 15% of autopsy cases [21]. However, in population-based studies of the elderly (age 65 and older), the prevalence of DLB has varied widely from 0.1% [22, 23] to 30.5% of the population [24, 25]. The prevalence of DLB increases with age and affects men more than women. Limited and unstable prevalence data are available for PDD and PD/OD.
Our study supports previous reports projecting a major increase in the number of persons living with PD in the US in the coming years. The pivotal study by Dorsey et al. predicted that the number of persons living with PD (ages 50 years or older) in the 15 most populous countries was between 4.1 and 4.6 million in 2005 and would double to between 8.7 and 9.3 million by 2030 [3]. Similarly, a more recent study projected substantial increases in the number of persons living with PD in the US, Canada, and Europe by 2050 [4]. This more recent study also projected that DLB (a sub-type of PD with dementia) would have greater percent increases than PD. We projected similar increases (nearly a triplication) in the number of persons living with PD associated with dementia.
The aging of the population, the improved management of PD, and the development of disease modifying agents (leading to prolonged survival), are likely to affect the projected increase in the number of persons affected by PD over the next several decades. However, a recent study by our group reported worse survival in persons with DLB and PDD than in persons with PD without dementia [26]. In addition, there may be other factors contributing to increased prevalence beyond the aging of the population and improved treatment and survival. It is also plausible that the future introduction of preventive interventions or environmental and social changes may decrease the incidence of PD and related disorders. However, we have recently reported an increase of the incidence of PD over 30 years in this same Olmsted County population [27]. If this trend is confirmed in other US populations, the projections should be modified accordingly [27].
Our study has several strengths. First, our study explored PD without dementia, and PD with dementia of all types combined (including PDD, DLB, and PD/OD) and also separately. Therefore, we were able to provide novel information beyond the pooled definition of all PD. Second, we studied a relatively stable population, using a population-based, medical records-linkage system encompassing nearly all care facilities in Olmsted County, Minnesota. A movement-disorders specialist adjudicated all persons by reviewing their complete medical records to reduce differences over time in diagnostic criteria.
Our study also has limitations. First, assessing the precise chronology of symptoms and the time of onset of clinical features from an historical review of medical records was difficult for some patients. Second, cases of DLB presenting without parkinsonian symptoms were not identified by our methods. However, given that parkinsonism is present in the majority of patients with DLB, especially after some years into the disease process, we likely captured most persons living with DLB. Lastly, our projections assume that the incidence of PD will remain steady to 2060. This assumption may result in our prevalence projections being either underestimated (if the incidence is truly increasing) or overestimated (if the incidence will decrease due to some new preventive interventions or environmental and social changes introduced before 2060).
Conclusions
We found that the prevalence of PD overall and of PD with dementia increases with age and affects men more than women. In addition, we projected a dramatic increase in the number of persons with PD living in the US through 2060. Our study contributes important new projections for the future burden of PD and, in particular, for PD with dementia. These projections have important public health implications.
Acknowledgments
We thank Mrs. Lea Dacy for editing and formatting the manuscript.
Funding/Support: This study was supported by the Parkinson’s Disease Foundation, Inc. It was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health grant numbers R01 AG034676 and R01 AG052425 and by the Mayo Foundation for Medical Education and Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr. Savica had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Savica, Bower.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Savica, Bower.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Grossardt.
Obtained funding: Bower, Savica.
Administrative, technical, or material support: Bower, Savica
Study supervision: Savica, Bower.
Disclosures
Dr. Savica receives funding from the Parkinson’s Disease Foundation, Inc
Mr. Grossardt: none
Dr. Bower receives funding from the Parkinson ’s Disease Foundation, Inc.
Dr. Rocca receives funding from the National Institutes of Health
References
- 1.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Incidence and pathology of synucleinopathies and tauopathies related to parkinsonism. JAMA Neurol. 2013;70:859–866. doi: 10.1001/jamaneurol.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2014;29:1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 3.Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 4.Bach JP, Ziegler U, Deuschl G, Dodel R, Doblhammer-Reiter G. Projected numbers of people with movement disorders in the years 2030 and 2050. Mov Disord. 2011;26:2286–2290. doi: 10.1002/mds.23878. [DOI] [PubMed] [Google Scholar]
- 5.Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson's disease in the United States. Mov Disord. 2013;28:311–318. doi: 10.1002/mds.25292. [DOI] [PubMed] [Google Scholar]
- 6.Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, Rocca WA. Incidence of Dementia With Lewy Bodies and Parkinson Disease Dementia. JAMA Neurol. 2013 doi: 10.1001/jamaneurol.2013.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Commission on Professional and Hospital Activities. H-ICDA, hospital adaptation of ICDA. 2. Ann Arbor, MI: 1973. National Center for Health Statistics. [Google Scholar]
- 12.Geneva, Switzerland: World Health Organization; 1977. Manual of the International Classification of Diseases, Injuries, and Causes of Death, based on the Recommendations of the Ninth Revision Conference, 1975, and Adopted by the Twenty-ninth World Health Assembly. [Google Scholar]
- 13.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976–1990. Neurology. 1999;52:1214–1220. doi: 10.1212/wnl.52.6.1214. [DOI] [PubMed] [Google Scholar]
- 14.Collins SJ, Ahlskog JE, Parisi JE, Maraganore DM. Progressive supranuclear palsy: neuropathologically based diagnostic clinical criteria. J Neurol Neurosurg Psychiatry. 1995;58:167–173. doi: 10.1136/jnnp.58.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilman S, Low P, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. American Autonomic Society and American Academy of Neurology. Clin Auton Res. 1998;8:359–362. doi: 10.1007/BF02309628. [DOI] [PubMed] [Google Scholar]
- 16.McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9:417–423. doi: 10.3233/jad-2006-9s347. [DOI] [PubMed] [Google Scholar]
- 17.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 19.Projections of the Population by Sex and Age for the United States: 2015 5o 2060 [Google Scholar]
- 20.de Rijk MC, Rocca WA, Anderson DW, Melcon MO, Breteler MM, Maraganore DM. A population perspective on diagnostic criteria for Parkinson's disease. Neurology. 1997;48:1277–1281. doi: 10.1212/wnl.48.5.1277. [DOI] [PubMed] [Google Scholar]
- 21.McKeith IG, Mosimann UP. Dementia with Lewy bodies and Parkinson's disease. Parkinsonism Relat Disord. 2004;10(Suppl 1):S15–18. doi: 10.1016/j.parkreldis.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Yamada T, Hattori H, Miura A, Tanabe M, Yamori Y. Prevalence of Alzheimer's disease, vascular dementia and dementia with Lewy bodies in a Japanese population. Psychiatry Clin Neurosci. 2001;55:21–25. doi: 10.1046/j.1440-1819.2001.00779.x. [DOI] [PubMed] [Google Scholar]
- 23.de Silva HA, Gunatilake SB, Smith AD. Prevalence of dementia in a semi-urban population in Sri Lanka: report from a regional survey. Int J Geriatr Psychiatry. 2003;18:711–715. doi: 10.1002/gps.909. [DOI] [PubMed] [Google Scholar]
- 24.Stevens T, Livingston G, Kitchen G, Manela M, Walker Z, Katona C. Islington study of dementia subtypes in the community. Br J Psychiatry. 2002;180:270–276. doi: 10.1192/bjp.180.3.270. [DOI] [PubMed] [Google Scholar]
- 25.Rahkonen T, Eloniemi-Sulkava U, Rissanen S, Vatanen A, Viramo P, Sulkava R. Dementia with Lewy bodies according to the consensus criteria in a general population aged 75 years or older. J Neurol Neurosurg Psychiatry. 2003;74:720–724. doi: 10.1136/jnnp.74.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savica R, Grossardt BR, Bower JH, et al. Survival and Causes of Death Among People With Clinically Diagnosed Synucleinopathies With Parkinsonism: A Population-Based Study. JAMA Neurol. 2017;74:839–846. doi: 10.1001/jamaneurol.2017.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Time Trends in the Incidence of Parkinson Disease. JAMA Neurol. 2016;73:981–989. doi: 10.1001/jamaneurol.2016.0947. [DOI] [PMC free article] [PubMed] [Google Scholar]


