Abstract
We investigated the effect of temperature and irradiance on leaf respiration (R, non-photorespiratory mitochondrial CO2 release) of snow gum (Eucalyptus pauciflora Sieb. ex Spreng). Seedlings were hydroponically grown under constant 20°C, controlled-environment conditions. Measurements of R (using the Laisk method) and photosynthesis (at 37 Pa CO2) were made at several irradiances (0–2,000 μmol photons m−2 s−1) and temperatures (6°C–30°C). At 15°C to 30°C, substantial inhibition of R occurred at 12 μmol photons m−2 s−1, with maximum inhibition occurring at 100 to 200 μmol photons m−2 s−1. Higher irradiance had little additional effect on R at these moderate temperatures. The irradiance necessary to maximally inhibit R at 6°C to 10°C was lower than that at 15°C to 30°C. Moreover, although R was inhibited by low irradiance at 6°C to 10°C, it recovered with progressive increases in irradiance. The temperature sensitivity of R was greater in darkness than under bright light. At 30°C and high irradiance, light-inhibited rates of R represented 2% of gross CO2 uptake (vc), whereas photorespiratory CO2 release was approximately 20% of vc. If light had not inhibited leaf respiration at 30°C and high irradiance, R would have represented 11% of vc. Variations in light inhibition of R can therefore have a substantial impact on the proportion of photosynthesis that is respired. We conclude that the rate of R in the light is highly variable, being dependent on irradiance and temperature.
Leaf respiration provides ATP, reducing equivalents, and carbon skeletons necessary for biosynthetic reactions. Leaf respiration may also help protect the photosynthetic apparatus from photoinhibitory damage by oxidizing excess photosynthetic reducing equivalents (Raghavendra et al., 1994; Saradadevi and Raghavendra, 1994; Hurry et al., 1995; Atkin et al., 2000b). Moreover, leaf respiration can provide ATP for Suc synthesis (Krömer, 1995) and may help repair photosynthetic proteins degraded by photoinhibition (in particular, the D1 protein of photosystem II) (Hoefnagel et al., 1998, and refs. therein). Leaf respiration is therefore a vital component of plant metabolism. However, leaf respiration also represents a major source of CO2 release in plants. Up to 35% of the CO2 fixed by photosynthesis each day is released back into the atmosphere by leaf respiration in plants grown under controlled-environment, constant-temperature conditions (Van Der Werf et al., 1994; Atkin and Lambers, 1998). Variations in the magnitude of leaf respiration could therefore have an important impact on the carbon economy of a plant.
While leaf respiration (R, non-photorespiratory mitochondrial CO2 release) occurs both in the light and in darkness, the extent to which it continues in the light appears to be highly variable. Most studies have reported that the rate of leaf respiration in the light (Rd or day respiration) is less than that in darkness (Rn or night respiration) (Brooks and Farquhar, 1985; Avelange et al., 1991; Krömer, 1995; Atkin et al., 1997, 1998a, 1998b), with the degree of inhibition ranging from 16% to 77%. The inhibition of R by light is rapid (within approximately 50 s) and occurs at irradiances as low as 3 μmol photons m−2 s−1 (Atkin et al., 1998a).
Most studies that have investigated the degree to which R is inhibited by light have done so at a single temperature (typically 25°C). In their natural habitat, plants are exposed to large temperature fluctuations, with leaf temperatures during the day often being 20°C to 30°C higher than those at night. It is not clear, however, if the degree of light inhibition is constant across a wide range of temperatures. Although Brooks and Farquhar (1985) reported that variations in temperature did not affect the degree of inhibition, they did not determine respiratory flux in the light at temperatures below 15°C. It is also not known if the effect of light on R at each temperature varies with irradiance; exposure to low temperatures and bright light may well have very different effects on R than exposure to low temperatures at low irradiance, particularly if mitochondria oxidize excess photosynthetic reducing equivalents under cold, bright conditions (Raghavendra et al., 1994; Saradadevi and Raghavendra, 1994; Hurry et al., 1995; Atkin et al., 2000a). To fully elucidate the degree to which respiration continues in the light, we need to determine the effect of temperature and irradiance on leaf respiration.
Our study investigates the interactive effects of temperature and irradiance on leaf respiration in snow gum (Eucalyptus pauciflora Sieb. ex Spreng). We used the Laisk (1977, as extended by Brooks and Farquhar, 1985) method to obtain estimates of Rd at each temperature and irradiance. The study also determines the impact of temperature/irradiance induced variations in Rd on net CO2 uptake in the light. Our results indicate that the degree of inhibition of R varies with both temperature and irradiance. The temperature sensitivity of leaf respiration at high irradiance is substantially lower than in darkness. Moreover, in leaves exposed to high temperatures, variations in the degree of light inhibition play an important role in determining the proportion of gross photosynthetic CO2 uptake that is respired.
MATERIALS AND METHODS
Snow gum (Eucalyptus pauciflora Sieb. ex Spreng) seedlings were raised from seed from a population collected in Gudgenby Valley in Namadgi National Park in southeastern Australia (35°45′S/148°59′E). The seeds were transported to Utrecht University in the Netherlands, vernalized at 4°C for 4 weeks, and then germinated on seed trays under controlled-environment conditions (constant 20°C temperature; 14 h/10 h day/night rhythm; 520 μmol photons m−2 s−1 photosynthetically active radiation [PAR]; 70% relative humidity). Germinants were transplanted 6 weeks later to 32-L hydroponics tanks containing a fully aerated modified Hoagland nutrient solution. Full details on the growth conditions and nutrient solution are given in Atkin et al. (1996). The seedlings were grown for a further 10 to 14 weeks. The plants reached a height of approximately 0.3 m.
Measurements of CO2 uptake and release in intact, attached leaves were conducted using an IR gas analyzer (LI-6262, LI-COR, Lincoln, NE) in the differential mode in an open system (Atkin et al., 1997; Poot et al., 1997). Three leaf cuvettes were connected to a data acquisition system (Keithley 575, Cleveland) and measured simultaneously. Air in each chamber was mixed with a fan, which resulted in boundary layer conductances of approximately 6 to 10 mol m−2 s−1. Different light intensities were obtained by placing small-mesh wire netting filters in front of slide projector lamps mounted above each cuvette (Atkin et al., 1997). Leaf temperatures were measured using two 0.08-mm type K thermocouples per cuvette, which were appressed to the underside of the leaves. Temperature was controlled by a thermostat-controlled circulating water bath. Water vapor pressure and CO2 partial pressures were controlled as previously described (Atkin et al., 1997). Gas-exchange parameters were calculated according to the method of von Caemmerer and Farquhar (1981).
Determinations of leaf gas exchange commenced after at least 2 h of photosynthesis in the growth cabinets. One of the labeled leaves on each of the three 20°C-grown plants was inserted into each temperature-controlled leaf chamber of the gas exchange system. Each of the three leaves was then allowed to equilibrate for 30 min, during which time they were exposed to a moderate irradiance (400 μmol photons m−2 s−1 PAR). The leaves were then exposed to a range of irradiances (0, 12, 100, 200, 400, 800, and finally 2,000 μmol photons m−2 s−1 PAR), and then left to adjust for 15 to 20 min at each new irradiance before the CO2 response was measured. The first measurements of Rn were conducted after 30 min of darkness; it takes 10 to 25 min for post-illumination respiration to stabilize in snow gum, with the time increasing with decreasing temperature (Atkin et al., 1998b). At each irradiance, net CO2 exchange rates were measured at four to eight decreasing internal CO2 partial pressure (pI) values (in the range of approximately 10–2.5 Pa CO2).
Leaves were then exposed to an atmospheric CO2 partial pressure of 37 Pa and the rate of net CO2 exchange determined. A linear regression of net CO2 exchange versus pi for the low CO2 partial pressure range (10–2.5 Pa) was then calculated for each irradiance. The point at which three regressions intersect was used to determine Γ* whenever possible. Γ* is the pi where CO2 uptake by carboxylation is matched by photorespiratory CO2 release, and where the rate of CO2 release is Rd (Laisk, 1977). In our study, the three linear regressions that were used to calculate the Γ* values were taken from leaves exposed to 100, 200, and 400 μmol photons m−2 s−1 for 6°C, 10°C, 15°C, 20°C, and 25°C. At 6°C and 10°C, the point at which the three regressions intersected yielded negative respiration values, i.e. CO2 uptake. Γ* could not, therefore, be determined at 6°C and 10°C. At 30°C, 200, 400, and 800 μmol photons m−2 s−1 data were used, as Rd was not constant until 200 μmol photons m−2 s−1. An assumption underlying the Laisk (1977) method is that R does not change with irradiance.
The above measurements were conducted at a single temperature on each measuring day, after which time the plants were returned to the controlled-environment growth cabinet. The measurement procedure was then repeated on the next day at a new temperature. The sequence of measurement temperatures was 25°C, 6°C, 30°C, 10°C, 20°C, and 15°C. Checks of gas exchange characteristics were made after the 3rd and 6th measuring day by measuring gas exchange at a common temperature (25°C); exposure to the different temperatures did not have any significant effect on the rates of respiration in darkness or the light-saturated rate of net photosynthesis at 25°C (data not shown).
The rate of leaf respiration in the light at each measurement temperature and irradiance was determined using the regressions for the net CO2 exchange versus pi over the low CO2 partial pressure range (see above). Rd was taken as the rate of CO2 efflux at Γ*. Rates of carboxylatory CO2 uptake (νc) and photorespiratory CO2 release (i.e. 0.5νo) were calculated according to the method of Farquhar and von Caemmerer (1982):
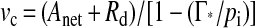 |
1 |
and
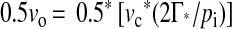 |
2 |
where Anet is the rate of net photosynthetic CO2 uptake in the presence of an atmospheric CO2 partial pressure of 37 Pa (von Caemmerer and Farquhar, 1981). Data from the CO2-response curves under light saturation were used to calculate Vcmax values according to the method of von Caemmerer and Farquhar (1981) using Michaelis-Menten constants for CO2 and O2 reported by von Caemmerer et al. (1994). Vcmax was calculated under the assumption that at low pi, photosynthesis was limited by Rubisco only.
The impact of CO2 partial pressure and temperature on leaf respiration rates measured in darkness was assessed using a two-way analysis of variance (Zar, 1996).
RESULTS
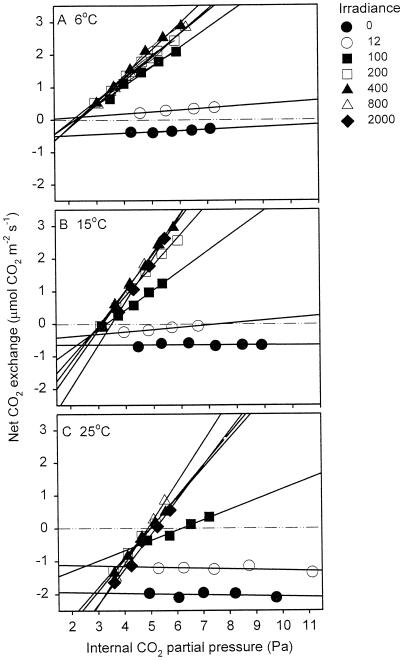
Figure 1 shows an example of the net CO2 exchange over the pi range of 3 to 10 Pa at several irradiances for a single leaf exposed to three temperatures (6°C, 15°C, and 25°C). Similar results were observed for the other three temperatures (10°C, 20°C, and 30°C; data not shown). The response at each irradiance was linear for all temperatures over the range of low pi values (e.g. Fig. 1, A–C). Exposure to very low irradiance (12 μmol photons m−2 s−1) resulted in a substantial decrease in the net release of CO2 at all temperatures (relative to darkness), suggesting that leaf respiration was inhibited even by this low irradiance. At 6°C (Fig. 1A), the intersection of the 100, 200, and 400 μmol photons m−2 s−1 regressions yielded negative respiration values (i.e. positive net CO2 exchange). Leaf respiration in darkness was significantly greater when measured at low (4–5 Pa) CO2 partial pressure compared with measurements at 37 Pa (F1, 36 = 35.9; P < 0.01; Table I).
Figure 1.
Example of the effect of irradiance on net CO2 exchange (μmol CO2 m−2 s−1) versus pi of a single leaf at three temperatures: 6°C (A), 15°C (B), and 25°C (C). Measurements were also conducted at 10°C, 20°C, and 30°C (not shown). The symbols represent the irradiances under which each set of measurements was made (in μmol photons m−2 s−1). Lines represent the linear regressions at each irradiance.
Table I.
Effect of temperature on maximum carboxylation rates (Vcmax) and Rn measured at ambient atmospheric CO2 partial pressure (pa) of 37 Pa and at a low (4–5 Pa) pi
| Temperature | Vcmax |
Rn
|
|
|---|---|---|---|
| Ambient CO2 | Low CO2 | ||
| °C | μmol CO2 m−2 s−1 | ||
| 6 | 21.1 ± 3.5 | 0.24 ± 0.03 | 0.44 ± 0.04 |
| 10 | 27.5 ± 4.6 | 0.37 ± 0.03 | 0.48 ± 0.03 |
| 15 | 47.6 ± 11.3 | 0.64 ± 0.07 | 0.74 ± 0.08 |
| 20 | 62.7 ± 13.6 | 1.06 ± 0.03 | 1.19 ± 0.02 |
| 25 | 72.6 ± 2.0 | 1.61 ± 0.11 | 1.72 ± 0.13 |
| 30 | 105.6 ± 2.04 | 2.48 ± 0.21 | 2.67 ± 0.04 |
The Vcmax values were estimated from fitted CO2-response curves similar to those shown in Figure 1 for measurements done at 2,000 μmol photons m−2 s−1. Vcmax values for each temperature were calculated according to the method of Von Caemmerer and Farquhar (1981), using data from the CO2-response curves (e.g. Fig. 1) and the Michaelis-Menten constants for CO2 and O2 according to the method of Von Caemmerer et al. (1994). The Γ*25 used in these calculations was 4.31 Pa (see “Results”). Vcmax was calculated under the assumption that at the low pi values shown in Figure 1, photosynthesis was limited by Rubisco only. Values are means of three replicate measurements (±se).
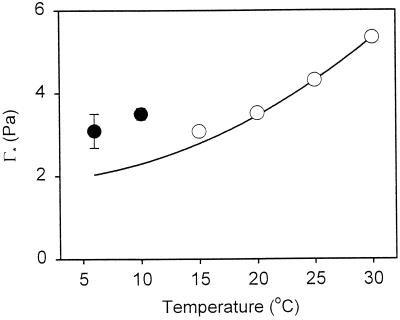
Figure 2 shows the temperature dependence of our experimentally derived Γ* values over the 15°C to 30°C range where the regressions of the net CO2 exchange versus pi at three irradiances intersected. The erroneous Γ* values at 6°C and 10°C are shown for comparison. The solid line shows the temperature dependence of Γ* calculated from data of Jordan and Ogren (1984) by Brooks and Farquhar (1985):
 |
3 |
where Γ*T is the Γ* value at a set temperature (T) and Γ*25 is Γ* at 25°C. With the exception of 15°C, our Γ* values were almost identical to those predicted by Jordan and Ogren (1984) as long as we used our experimentally derived Γ*25 value (i.e. 4.31 ± 0.04 Pa; n = 5; ±se). Given this match, and the erroneous nature of our Γ* values at 6°C and 10°C (Fig. 2), which yielded negative respiration values, we decided to estimate R values for all temperatures using Γ* values predicted by Equation 3 and our experimentally derived Γ*25 value of 4.31 Pa. Doing so provided positive estimates of R for both 6°C and 10°C cases.
Figure 2.
Effect of temperature on Γ*. ○, Γ* values calculated using the intercept of three linear regressions of net CO2 exchange data versus pi (e.g. Fig. 1) for leaves of 20°C-grown plants exposed to 15°C, 20°C, 25°C, and 30°C (e.g. Fig. 1, B and C). The three linear regressions used to calculate Γ* were for 100, 200, and 400 μmol photons m−2 s−1 for all temperatures except 30°C, where 200, 400, and 800 μmol photons m−2 s−1 were used. Values represent the mean of three individual leaves (±se); where the se values are not visible, they are smaller than the shown symbol. The erroneous Γ* values for leaves exposed to 6°C and 10°C are shown for comparison (●); it was not possible to accurately calculate the Γ* values at 6°C and 10°C because the common regression intercept for measurements at three irradiances yielded a negative R value. The solid line represents the temperature dependence of Γ* of spinach calculated from the data of Jordan and Ogren (1984) using our estimate of Γ* at 25°C (4.31 ± 0.04 Pa).
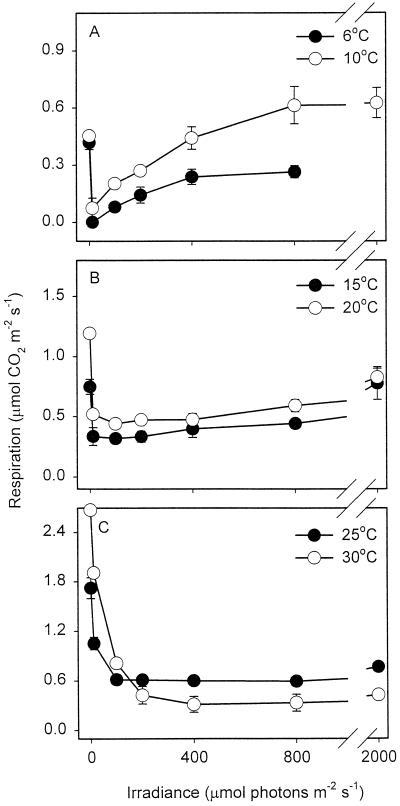
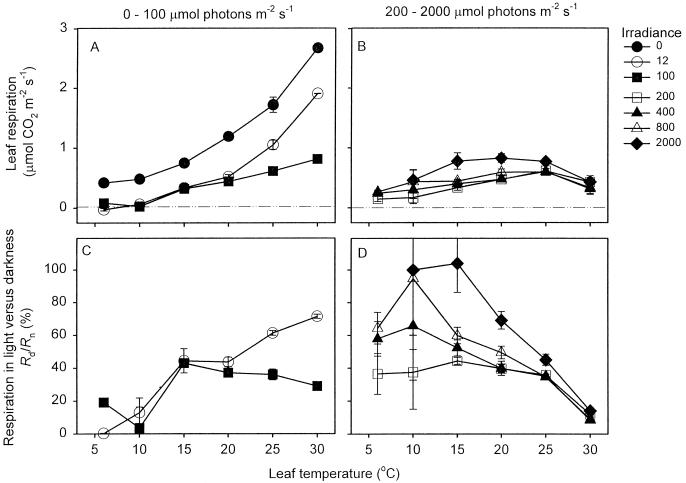
Figure 3 shows the effect of temperature and irradiance on leaf respiration. Rn increased with increasing temperature. At low temperatures, (i.e. 6°C and 10°C; Fig. 3A), Rn was inhibited by low quantum flux density, but then recovered with progressive increases in irradiance. Rn was also inhibited by low irradiance at moderate-to-high temperatures (i.e. 15°C–30°C; Fig. 3, B and C); however, higher irradiance had little additional effect on R at these temperatures. The irradiance necessary to maximally inhibit R increased with increases in leaf temperature (e.g. 12 μmol photons m−2 s−1 at 15°C [Fig. 3B] and 400 μmol photons m−2 s−1 at 30°C [Fig. 3C]).
Figure 3.
Relationship between R and irradiance at various temperatures. Values are ±se; n = 3. Values of R were calculated using the linear regressions of net CO2 exchange versus Pi at each irradiance (e.g. Fig. 1), our estimate of Γ*25 (4.31 Pa), and the temperature dependence of Γ* given in Equation 3.
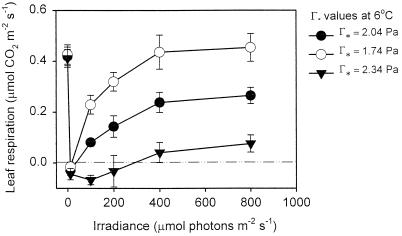
Was the apparent irradiance-dependent increase in R at 6°C and 10°C (Fig. 3A) real, or was it the result of errors in the value of Γ*? If the Γ* value for snow gum leaves in our system at 6°C were higher than that predicted by Jordan and Ogren (1984), then we would have overestimated the actual R value at each irradiance. To assess the impact of errors in Γ* on our estimates of R, we determined the impact of Γ* values at 6°C that were 0.3 Pa higher and 0.3 Pa lower (i.e. a ±15% change) than that used in our calculations (2.04 Pa) on the irradiance dependence of R at 6°C (Fig. 4). Figure 4 demonstrates that R increased in an irradiance-dependent manner when Γ* at 6°C was assumed to be 2.04 or 1.74 Pa. When Γ* was assumed to be 2.34 Pa (i.e. Γ*25 = 4.61), little increase in R occurred until 400 μmol photons m−2 s−1; the Γ* value therefore has a substantial impact on the degree to which the calculated rates of R increase with increasing irradiance.
Figure 4.
Determining the effect of different Γ* values on the relationship between R and irradiance at 6°C using the temperature dependence of Γ* given in Equation 3 (±se n = 3). Three different estimates of Γ* at 6°C were used in the calculations.
What effect did the interaction of irradiance and temperature have on the temperature response curves of leaf respiration? Figure 5, A and B, shows the temperature response of leaf respiration for leaves exposed to 0, 12, and 100 μmol photons m−2 s−1 (Fig. 5A) and 200, 400, 800, and 2,000 μmol photons m−2 s−1 (Fig. 5B). The Q10 (the proportional increase in respiration for each 10°C rise in temperature) of Rn was 2.21; a common Q10 could be applied over the range of temperatures used in our study, as plots of log10-transformed Rn against leaf temperature were linear. The degree of temperature sensitivity decreased, however, when leaves were exposed to irradiances greater than 12 μmol photons m−2 s−1. For example, the Q10 values over the 6°C to 25°C range (assuming a constant Q10) were 1.61 and 1.57 at 800 and 2,000 μmol photons m−2 s−1, respectively (Fig. 5B). Moreover, there was little difference in the rates of R at 6°C and 30°C in leaves exposed to 800 to 2,000 μmol photons m−2 s−1 (Fig. 5B).
Figure 5.
Effect of irradiance on the relationship between temperature and R. Values for 0 to 100 μmol photons m−2 s−1 are shown in A and C, whereas B and D show values for 200 to 2,000 μmol photons m−2 s−1. Values of R were calculated using the linear regressions of net CO2 exchange versus Pi at each irradiance (e.g. Fig. 1), our estimate of Γ*25 (4.31 Pa), and the temperature dependence of Γ* given in Equation 3. A and B show the absolute rates of leaf respiration, while C and D show rates in the light as a percentage of those in darkness.
Figure 5 also shows the rate of leaf respiration at each irradiance and temperature expressed as a percentage of the rate in darkness; a low percentage value indicates a high degree of light inhibition of R. The degree of inhibition at each irradiance varied substantially with temperature (Fig. 5, C and D). In leaves exposed to low irradiances (e.g. 12 and 100 μmol photons m−2 s−1; Fig. 5C), maximum inhibition of R occurred in the cold (i.e. 6°C and 10°C). In contrast, little or no inhibition occurred in the cold in leaves exposed to high irradiance (e.g. 800 and 2,000 μmol photons m−2 s−1; Fig. 5D). The degree of light inhibition at a set irradiance was therefore highly variable.
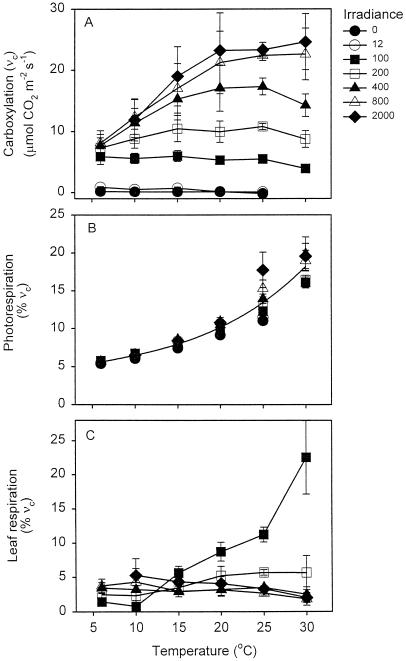
Figure 6 shows the effect of temperature and irradiance on gross photosynthetic CO2 uptake (i.e. νc) or the percentage of νc that is respired at each temperature and irradiance. In leaves exposed to ≥200 μmol photons m−2 s−1, increasing the temperature increased νc (Fig. 6A) but had little effect on the percentage of νc that was respired (Fig. 6C). Leaf respiration represented 2% to 5% of gross CO2 assimilation in leaves exposed to 200 to 2,000 μmol photons m−2 s−1 (Fig. 6C). This contrasts with the approximately 5% to 20% (at 6°C to 30°C, respectively) of νc that was released by photorespiration (i.e. 0.5 νo) (Fig. 6B). However, the percentage of CO2 fixed by νc that was subsequently released by Rd did increase with temperature in leaves exposed to 100 μmol photons m−2 s−1: at this low irradiance, Rd increased with temperature (Fig. 5A), whereas νc did not (Fig. 6A). Up to 23% of the CO2 fixed was respired by Rd at 30°C in leaves exposed to 100 μmol photons m−2 s−1 (Fig. 6C).
Figure 6.
Relationship between temperature and the Rubisco carboxylation rate (νc) (A), the ratio of photorespiratory CO2 release to Rubisco carboxylation (B), and the ratio of non-photorespiratory respiration to Rubisco carboxylation (R/νc) (C). Rates of νc, photorespiration, and R at each temperature and irradiance were calculated as described in the “Materials and Methods.” The line in B is fitted to all of the data; variations in photorespiration at a particular temperature were due to variations in pi.
DISCUSSION
Our study has demonstrated that leaf respiration rates in the light are highly variable, being dependent on irradiance and temperature. The degree to which light inhibited R was greatest at high irradiance and moderate-to-high temperatures, and lowest at high irradiance and low temperatures (Figs. 3 and 5). Using a 14C pulse-chase method to determine rates of R in the light and in darkness, Hurry et al. (1996) and Pärnik et al. (1998) also reported differences in the degree of light inhibition at different temperatures in controlled-environment-grown winter rye. In contrast, Brooks and Farquhar (1985) reported that the degree of inhibition at a set irradiance did not vary with temperature in spinach. Kirschbaum and Farquhar (1984) reported that light inhibited leaf respiration by a constant 40% in controlled-environment-grown snow gum when measured across a temperature range of 15°C to 35°C. Clearly, the effect of temperature on light inhibition of R does not always vary with temperature. Several factors may be responsible for the contrasting results, including the differences in plant species, growth conditions, and experimental protocols.
What effect do variations in irradiance and temperature have on the percentage of photosynthetic CO2 uptake released by leaf respiration compared with that released by photorespiration? Photorespiratory CO2 release can represent a large percentage of νc, particularly at high temperatures (Fig. 6B; Sage, 1995). In contrast, Rd represents a minor proportion of νc at all temperatures in leaves exposed to high irradiance values (e.g. only 2% at 30°C and 2,000 μmol photons m−2 s−1; Fig. 6C). A substantially greater proportion of νc would have been respired at high temperatures and high irradiance if leaf respiration had not been inhibited by light (e.g. at 30°C, leaf respiration rates in darkness were 11% of νc at 2,000 μmol photons m−2 s−1). At 40°C and high irradiance, this value would have been substantially higher if respiration continued to increase with temperature to a greater extent than νc. Incomplete inhibition of R by light contributed to the high percentage of νc that was respired (23%) in leaves exposed to 30°C and 100 μmol photons m−2 s−1 (Fig. 6C). Clearly, a high degree of light inhibition of R at high temperatures and high irradiance substantially reduces respiratory CO2 release.
Our results demonstrate that the temperature sensitivity of R is greatest in darkness, decreasing as irradiance increased (Fig. 5). Leaf respiration was almost completely insensitive to temperature at high irradiance. What is the cause of this irradiance-dependent difference in temperature sensitivity? In darkness, low temperatures reduced R, probably as a result of reduced rates of carbon input into the mitochondria and/or increased adenylate control of mitochondrial electron transport (due to reduced demand for ATP at low temperatures). The activity of key enzymes that control substrate input into the mitochondria, such as the pyruvate dehydrogenase complex (PDC) and NAD+-malic enzyme (ME), is likely to be reduced at low temperatures. Reductions in the activity of PDC and ME may also explain why R is inhibited by low irradiance values at all temperatures (e.g. Fig. 3), as both are rapidly inactivated by light (Budde and Randall, 1990; Hill and Bryce, 1992). The timing of inactivation of ME (Hill and Bryce, 1992) and PDC (Budde and Randall, 1987) closely mirrors the time taken for light to inhibit R (Atkin et al., 1998a, 1998b). It is likely that the light inhibition of R is due to the rapid light inactivation of PDC and ME (Atkin et al., 1998a, 1998b, 1999b; Padmasree and Raghavendra, 1998). Exposure to low temperatures may accentuate the inhibitory effect of light on PDC and ME activity and explain why the degree of light inhibition of R at low irradiance (e.g. 12–100 μmol photons m−2 s−1) was greater at low than at high temperatures (Fig. 3C).
The suggested mechanism by which R is initially inhibited by light may also explain why the degree of inhibition remains relatively constant over a range of high irradiances when measured at moderate temperatures (i.e. the degree of inactivation of PDC and ME remains constant over a range of irradiances). However, if R did actually increase with increasing irradiance at low temperatures (as suggested when Γ* at 6°C was assumed to be 1.73 or 2.04 Pa; Fig. 4), then the above mechanism would not provide a complete explanation for our results. Irradiance-dependent increases in R at low temperatures could occur if photosynthetic redox equivalents were exported from the chloroplast and subsequently oxidized in the mitochondria with concomitant CO2 release.
While it is easy to see how the export of photosynthetic redox equivalents could be coupled to increased mitochondrial O2 consumption in the light (Saradadevi and Raghavendra, 1992; Raghavendra et al., 1994; Hurry et al., 1995; Xue et al., 1996), it is less clear how they could be coupled to increased non-photorespiratory CO2 release (R). For the export of excess photosynthetic redox equivalents to be coupled to increased rates of CO2 release (R) in the light (and thus lower degrees of light inhibition of R), two things would need to occur. First, flux through glycolysis would need to increase to replace the carbon lost during decarboxylation of compounds used to export the excess photosynthetic redox equivalents. This seems possible, as initial exposure to low temperatures often results in the accumulation of soluble carbohydrates (Stuiver et al., 1995; Strand et al., 1997). Second, the light inhibition of PDC would have to be overcome. The light-dependent inactivation of PDC can be overcome if concentrations of pyruvate or other positive effectors are sufficiently high. Thus, while we cannot be certain that respiration actually increased with increasing irradiance at low temperatures (due to our reliance on Eq. 3 to predict Γ* at low temperatures), increases could theoretically occur if chloroplasts exported excess redox equivalents to the mitochondria as described above.
Was our reliance on Equation 3 to predict the temperature dependence of Γ* at both high and low temperatures justified? Jordan and Ogren (1984) calculated the temperature dependence of Γ* from CO2/O2 specificity values obtained from spinach enzyme extracts using the solubilities of CO2 and O2 in solution at each temperature over the 5°C to 40°C range. Our estimates of Γ* using the Laisk (1977) method were almost identical to that predicted by Jordan and Ogren (1984) over the 20°C to 30°C range (Fig. 2), so long as our value of Γ* at 25°C (Γ*25) was used in Equation 3. However, we were not able to estimate Γ* below 15°C due to the negative respiration values occurring at the regression intercept (e.g. Fig. 1A). In the absence of sub-15°C estimates of Γ* using the Laisk (1977) method, we felt that the combined use of Γ*25 and Equation 3 was the most suitable way to provide estimates of Γ* at both high and low temperatures. When combined with an analysis of what effect errors in Γ* have on estimates of Rd (Fig. 4), this approach provides some insight into the potential impact of temperature and irradiance on R at low temperatures.
To determine the impact of irradiance on R using measurements of gas exchange at Γ*, the Laisk (1977) method assumes that Γ* does not vary with irradiance. Γ* reflects the specificity of Rubisco for CO2 relative to O2 and is the CO2 partial pressure where CO2 uptake by carboxylation is matched by photorespiratory CO2 release. Changes in irradiance, and thus ATP and NADPH production by photosynthetic electron transport, will have the same absolute impact on carboxylation as photorespiration; Γ* is therefore irradiance independent. Γ* also appears to be invariant among species, with woody species (Villar et al., 1994; Balaguer et al., 1996) exhibiting similar Γ* values as broad-leaved, non-woody species (Brooks and Farquhar, 1985; von Caemmerer et al., 1994). Moreover, Westbeek et al. (1999) reported that there was no systematic difference in Γ* among seven Poa species.
The use of low CO2 partial pressures to estimate R in the light raises two additional issues. First, R might be underestimated at Γ* if mitochondrial substrate supply is limiting. To assess whether this was the case, Atkin et al. (1998a) used a fast-response gas exchange system to rapidly expose illuminated leaves to Γ* following a period of photosynthesis at ambient CO2 partial pressure. If carbon supply limited R at Γ*, then R should be initially high when first exposed to Γ* and decrease with time as the substrate supply becomes limiting. This did not happen; rather, steady-state values of R were maintained over 10 min (Atkin et al., 1998a). Thus, as long as measurements of R are conducted during this time period, it seems likely that carbon supply does not limit R at Γ*.
A second concern about the use of low CO2 partial pressures is that R may be substantially greater at Γ* than at ambient CO2 concentrations. Rn is inhibited by high CO2 concentrations in short-term experiments (Bunce, 1990, 1995; Amthor, 1994; Ziska and Bunce, 1994; González-Meler et al., 1996). Conversely, Rn might be stimulated at low CO2 concentrations. If correct, then Rd may also be overestimated when measured at Γ*. Although we did not determine the impact of CO2 concentration on Rd, we did determine the effect of “normal” (atmospheric partial pressure of 37 Pa) and low CO2 partial pressure (near Γ*) on Rn at several temperatures (Table I). Rn was significantly higher at Γ*. However, the fact that the absolute differences between the Rn at 37 Pa and Γ* were small (Table I) suggests that Rd is unlikely to be substantially overestimated at Γ*. Moreover, it seems likely that the magnitude of any overestimate will be irradiance independent.
In conclusion, our measurements demonstrate that leaf respiration in the light is highly variable, being dependent on irradiance and temperature. Our results also demonstrate that variations in the degree of light inhibition of R have a substantial impact on the temperature sensitivity of leaf respiration. The high degree of light inhibition of R at high temperatures and high irradiance substantially reduces the proportion of photosynthetic CO2 release that is respired.
ACKNOWLEDGMENTS
The technical assistance of Nola McFarlane, Marc Bergkotte, and Rob Welschen is gratefully acknowledged.
Footnotes
This work was funded by an Australian Research Council Postdoctoral Fellowship Award to O.K.A. Financial assistance to O.K.A. was also provided by the Australian Department of Industry and Technology Bilateral Science and Technology Program.
LITERATURE CITED
- Amthor JS. Higher plant respiration and its relationship to photosynthesis. In: Schulze ED, Caldwell MM, editors. Ecophysiology of Photosynthesis. Ecological Studies. Berlin: Springer-Verlag; 1994. pp. 71–101. [Google Scholar]
- Atkin OK, Botman B, Lambers H. The causes of inherently slow growth in alpine plants: an analysis based on the underlying carbon economies of alpine and lowland Poaspecies. Funct Ecol. 1996;10:698–707. [Google Scholar]
- Atkin OK, Evans JR, Ball MC, Siebke K, Pons TL, Lambers H. Light inhibition of leaf respiration: the role of irradiance and temperature. In: Moller IM, Gardestrom P, Gliminius K, Glaser E, editors. Plant Mitochondria: From Gene to Function. Leiden, The Netherlands: Bluckhuys Publishers; 1998a. pp. 567–574. [Google Scholar]
- Atkin OK, Evans JR, Siebke K. Relationship between the inhibition of leaf respiration by light and enhancement of leaf dark respiration following light treatment. Aust J Plant Physiol. 1998b;25:437–443. [Google Scholar]
- Atkin OK, Holly C, Ball MC. Acclimation of snow gum (Eucalyptus pauciflora) leaf respiration to seasonal and diurnal variations in temperature: the importance of changes in the capacity and temperature sensitivity of respiration. Plant Cell Environ. 2000a;23:15–26. [Google Scholar]
- Atkin OK, Lambers H. Slow-growing alpine and fast-growing lowland species: a case study of factors associated with variation in growth rate among herbaceous higher plants under natural and controlled conditions. In: Lambers H, Poorter H, Van Vuuren MMI, editors. Inherent Variation in Plant Growth: Physiological Mechanisms and Ecological Consequences. Leiden, The Netherlands: Backhuys Publishers; 1998. pp. 259–288. [Google Scholar]
- Atkin OK, Millar AH, Gardeström P, Day DA. Relationships between photosynthesis, carbohydrate metabolism and respiration in higher plants. In: Leegood RC, Sharkey TT, Von Cammerer S, editors. Advances in Photosynthesis. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2000b. (in press) [Google Scholar]
- Atkin OK, Westbeek MHM, Cambridge ML, Lambers H, Pons TL. Leaf respiration in light and darkness: a comparison of slow- and fast-growing Poaspecies. Plant Physiol. 1997;113:961–965. doi: 10.1104/pp.113.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avelange M-H, Thiery JM, Sarrey F, Gans P, Rébeillé F. Mass-spectrometric determination of O2 and CO2gas exchange in illuminated higher plant cells: evidence for light-inhibition of substrate decarboxylations. Planta. 1991;183:150–157. doi: 10.1007/BF00197782. [DOI] [PubMed] [Google Scholar]
- Balaguer L, Afif D, Dizengremel P, Dreyer E. Specificity factor of ribulose carboxylase/oxygenase of Quercus robur. Plant Physiol Biochem. 1996;34:879–883. [Google Scholar]
- Brooks A, Farquhar GD. Effect of temperature on the CO2-O2specificity of ribulose-1,5- biphosphate carboxylase/oxygenase and the rate of respiration in the light: estimates from gas exchange measurements on spinach. Planta. 1985;165:397–406. doi: 10.1007/BF00392238. [DOI] [PubMed] [Google Scholar]
- Budde RJA, Randall DD. Regulation of pea mitochondrial pyruvate dehydrogenase complex activity: inhibition of ATP-dependent inactivation. Arch Biochem Biophys. 1987;258:600–606. doi: 10.1016/0003-9861(87)90382-1. [DOI] [PubMed] [Google Scholar]
- Budde RJA, Randall DD. Pea leaf mitochondrial pyruvate dehydrogenase complex is inactivated in vivoin a light-dependent manner. Proc Natl Acad Sci USA. 1990;87:673–676. doi: 10.1073/pnas.87.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce JA. Short- and long-term inhibition of respiratory carbon dioxide efflux by elevated carbon dioxide. Ann Bot. 1990;65:637–642. [Google Scholar]
- Bunce JA. Effects of elevated carbon dioxide concentration in the dark on the growth of soybean seedlings. Ann Bot. 1995;75:365–368. [Google Scholar]
- Farquhar GD, von Caemmerer S. Modelling of photosynthetic response to environmental conditions. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editors. Encyclopedia of Plant Physiology. 12B. Physiological Plant Ecology II. Water Relations and Carbon Assimilation. Berlin: Springer Verlag; 1982. pp. 551–587. [Google Scholar]
- González-Meler MA, Ribas-Carbó M, Siedow JN, Drake BG. Direct inhibition of plant mitochondrial respiration by elevated CO2. Plant Physiol. 1996;112:1349–1355. doi: 10.1104/pp.112.3.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SA, Bryce JH. Malate metabolism and light-enhanced dark respiration in barley mesophyll protoplasts. In: Lambers H, van der Plas LHW, editors. Molecular, Biochemical and Physiological Aspects of Plant Respiration. The Hague, The Netherlands: SPB Academic Publishing; 1992. pp. 221–230. [Google Scholar]
- Hoefnagel MHN, Atkin OK, Wiskich JT. Interdependence between chloroplasts and mitochondria in the light and the dark. Biochim Biophys Acta. 1998;1366:235–255. [Google Scholar]
- Hurry VM, Keerberg O, Pärnik T, Öquist G, Gardeström P. Effect of cold hardening on the components of respiratory decarboxylation in the light and in the dark in leaves of winter rye. Plant Physiol. 1996;111:713–719. doi: 10.1104/pp.111.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurry VM, Tobiæson M, Krömer S, Gardeström P, Öquist G. Mitochondria contribute to increased photosynthetic capacity of leaves of winter rye (Secale cerealeL.) following cold-hardening. Plant Cell Environ. 1995;18:69–76. [Google Scholar]
- Jordan DB, Ogren WL. The CO2/O2specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase: dependence on ribulose bisphosphate concentration, pH and temperature. Planta. 1984;161:308–313. doi: 10.1007/BF00398720. [DOI] [PubMed] [Google Scholar]
- Kirschbaum MUF, Farquhar GD. Temperature dependence of whole-leaf photosynthesis in Eucalyptus paucifloraSieb. ex Spreng. Plant Physiol. 1984;11:519–538. [Google Scholar]
- Krömer S. Respiration during photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:45–70. [Google Scholar]
- Laisk AK. Kinetics of Photosynthesis and Photorespiration in C3-Plants. Moscow: Nauka; 1977. [Google Scholar]
- Padmasree K, Raghavendra AS. Interaction with respiration and nitrogen metabolism. In: Raghavendra AS, editor. Photosynthesis. A Comprehensive Treatise. Cambridge, UK: Cambridge University Press; 1998. pp. 197–211. [Google Scholar]
- Pärnik T, Gardeström P, Ivanova H, Keerberg O. Photosynthetic and respiratory CO2fluxes in winter rye leaves at low temperature. In: Moller IM, Gardestrom P, Gliminius K, Glaser E, editors. Plant Mitochondria: From Gene to Function. Leiden, The Netherlands: Bluckhuys Publishers; 1998. pp. 585–589. [Google Scholar]
- Poot P, Pilon J, Pons TL. Photosynthetic characteristics of leaves of male sterile and hermaphrodictic sex types of Plantago lanceolatagrown under conditions of contrasting nitrogen and light availabilities. Physiol Plant. 1997;98:780–790. [Google Scholar]
- Raghavendra AS, Padmasree K, Saradadevi K. Interdependence of photosynthesis and respiration in plant cells: interactions between chloroplasts and mitochondria. Plant Sci. 1994;97:1–14. [Google Scholar]
- Sage RF. Was low atmospheric CO2 during the pleistocene a limiting factor for the origin of agriculture? Global Change Biol. 1995;1:93–106. [Google Scholar]
- Saradadevi K, Raghavendra AS. Dark respiration protects photosynthesis against photoinhibition in mesophyll protoplasts of pea (Pisum sativum) Plant Physiol. 1992;99:1232–1237. doi: 10.1104/pp.99.3.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saradadevi K, Raghavendra AS. Inhibition of photosynthesis by osmotic stress in pea (Pisum sativum) mesophyll protoplasts is intensified by chilling or photoinhibitory light: intriguing responses of respiration. Plant Cell Environ. 1994;17:739–746. [Google Scholar]
- Strand A, Hurry V, Gustafsson P, Gardestrom P. Development of Arabidopsis thalianaleaves at low temperatures releases the suppression of photosynthesis and photosynthetic gene expression despite the accumulation of soluble carbohydrates. Plant J. 1997;12:605–614. doi: 10.1046/j.1365-313x.1997.00605.x. [DOI] [PubMed] [Google Scholar]
- Stuiver CEE, Dekok LJ, Clement JMAM, Kuiper PJC. How indicative are changes in major metabolites for freezing tolerance of wheat. Bot Acta. 1995;108:106–110. [Google Scholar]
- Van Der Werf A, Poorter H, Lambers H. Respiration as dependent on a species' inherent growth rate and on the nitrogen supply to the plant. In: Roy J, Garnier E, editors. A Whole Plant Perspective on Carbon-Nitrogen Interactions. The Hague, The Netherlands: SPB Academic Publishing bv; 1994. pp. 83–103. [Google Scholar]
- Villar R, Held AA, Merino J. Comparison of methods to estimate dark respiration in the light in leaves of two woody species. Plant Physiol. 1994;105:167–172. doi: 10.1104/pp.105.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR, Hudson GS, Andrews TJ. The kinetics of ribulose-1,5-bisphosphate carboxylase/oxygenase in vivoinferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta. 1994;195:88–97. [Google Scholar]
- Westbeek MHM, Pons TL, Cambridge ML, Atkin OK. Analysis of differences in photosynthetic nitrogen-use efficiency of alpine and lowland Poaspecies. Oecologia. 1999;120:19–26. doi: 10.1007/s004420050828. [DOI] [PubMed] [Google Scholar]
- Xue XP, Gauthier DA, Turpin DH, Weger HG. Interactions between photosynthesis and respiration in the green alga Chlamydomonas reinhardtii: characterization of light-enhanced dark respiration. Plant Physiol. 1996;112:1005–1014. doi: 10.1104/pp.112.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Ed 3. Upper Saddle River, NJ: Prentice-Hall; 1996. [Google Scholar]
- Ziska LH, Bunce JA. Direct and indirect inhibition of single leaf respiration by elevated CO2concentrations: interaction with temperature. Physiol Plant. 1994;90:130–138. [Google Scholar]